Reusing Ceramic Waste as a Precursor in Alkali-Activated Cements: A Review
Abstract
:1. Introduction
2. Physico-Chemical Properties of CW
3. Ceramic Waste as a Precursor in Alkali-Activated Cements
3.1. AA Cement Using Only Ceramic Precursors
3.2. Hybrid Alkaline Cements
3.3. Combination of Different Precursors
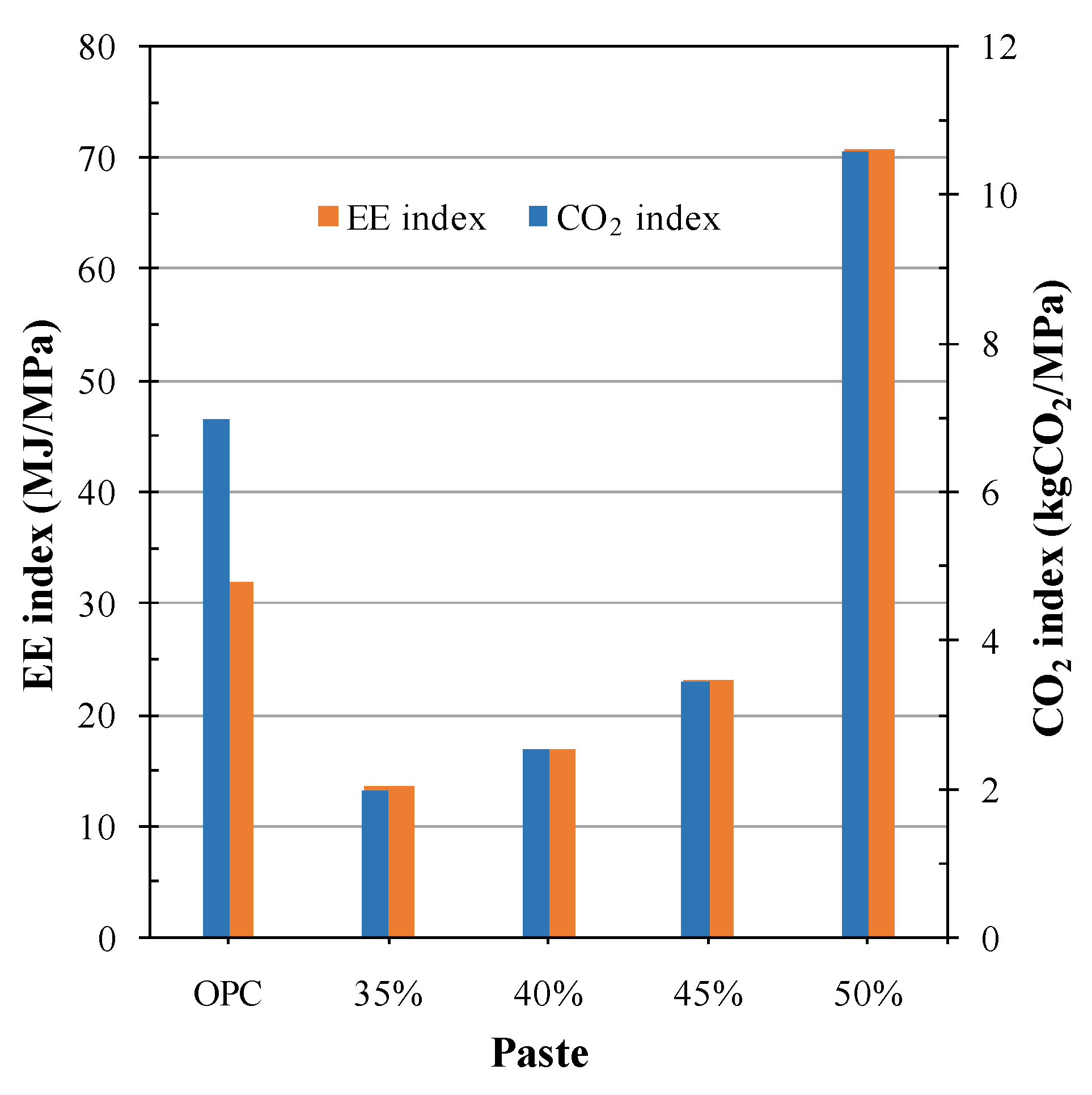
4. Sustainability and Carbon Footprint of AA CW Cements
5. Conclusions
- Research into using CW as the only precursor in AA cements has shown that most CWs require high temperatures and moderately high concentrations of activators to initiate alkaline reactions;
- Sometimes the setting time of AA CW systems can be delayed at room temperature. Although this could be seen as a disadvantage, it can be resolved by using hybrid systems or combining CW with other precursors;
- Although the contribution of an additional source of calcium favors AA reactions, very few studies have developed AA hybrid cements that combine the use of CW precursors with traditional binders, such as PC, CAC or Ca(OH)2;
- The mechanical properties of AA CW hybrid systems generally improve by incorporating different sources of calcium. Additionally, PC and CAC follow a different reaction mechanism than that generally observed when hydrated in water. Similarly, the combination of different precursors generally enhances AA CW systems’ mechanical properties.
- The growing demand for natural resources and energy has led to unsustainable development. This review evidences that CW can be successfully used as a precursor to develop more sustainable cements by AA, with appropriate properties to be employed in construction applications. This is a promising reutilization alternative for CW that would contribute to circular economy, helping to reduce not only PC consumption and its associated CO2 emissions but also the amount of landfilled waste.
6. Future Trends
- Given that the quality of CW materials significantly influences their behavior as a precursor in AA systems, separating CW materials when they are generated would facilitate their valorization;
- Although only one study has been found to combine the use of CW as an AA precursor and as a recycled aggregate, promising results with a wide variety of mechanical properties are reported [63]. The combined use of CW as both a recycled aggregate and a precursor in AA systems would allow larger amounts of CW to be reused, which would offer maximum environmental benefits;
- No previous studies have been found on the AA of CW with alternative activators to commercial SS. The viability of activating CW with more environmentally friendly activators derived from silica-rich waste materials, such as rice husk ash, diatomaceous earth or waste glass, should be explored;
- It is important to compare the AA of CW provided by the ceramic industry with that obtained from CDW, which may be mixed with other construction materials like cement mortar and gypsum. Although this review focuses only on the AA of CW with no impurities, it is necessary to investigate any potential differences in the activation process for both types of waste;
- An artificial neural network can be used to develop algorithms that allow the strength and properties of AA materials to be estimated depending on the CW employed as a precursor, the type of activator and the AA solution concentration;
- Standardization and long-term durability studies are required for real-scale applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CW | Ceramic waste |
| CWP | Ceramic waste powder |
| BCW | Brick ceramic waste |
| CSW | Ceramic sanitary ware |
| TCW | Tile ceramic waste |
| PTCW | Ceramic waste from polishing tiles |
| CDW | Construction and demolition waste |
| PC | Portland cement |
| SCM | Supplementary cementitious material |
| CAC | Calcium aluminate cement |
| FA | Fly ash |
| BFS | Blast furnace slag |
| MK | Metakaolin |
| FCC | Fluid catalytic cracking residue |
| CWA | Ceramic waste aggregate |
| SEM | Scanning electron microscopy |
| wt.% | Weight percentage |
| vol.% | Volume percentage |
| AA | Alkali activation or alkali-activated |
| SS | Sodium silicate |
References
- Reig, L.; Pitarch, Á.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.M.; Payá, J.; Tashima, M.M. Ceramic Waste: Reuse as a Recycled Aggregate. In Building Engineering Facing the Challenges of the 21st Century: Holistic Study from the Perspectives of Materials, Construction, Energy and Sustainability; Springer Science and Business Media: Singapore, 2023; pp. 533–551. [Google Scholar] [CrossRef]
- Rambaldi, E. Pathway towards a high recycling content in traditional ceramics. Ceramics 2021, 4, 486–501. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Burns, S.; Simmons, D.; Francis, E.; Chai, H.K.; Koutsos, V.; Huang, Y. Chemical, microstructural and mechanical properties of ceramic waste blended cementitious systems. J. Clean. Prod. 2019, 211, 1228–1238. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). Construction and Demolition Waste: Challenges and Opportunities in a Circular Economy. Available online: https://www.eea.europa.eu/publications/construction-and-demolition-waste-challenges (accessed on 13 October 2022).
- Zito, S.V.; Cordoba, G.P.; Irassar, E.F.; Rahhal, V.F. Durability of eco-friendly blended cements incorporating ceramic waste from different sources. J. Sustain. Cem.-Based Mater. 2021, 12, 13–23. [Google Scholar] [CrossRef]
- Alsaif, A. Utilization of ceramic waste as partially cement substitute—A review. Constr. Build. Mater. 2021, 300, 124009. [Google Scholar] [CrossRef]
- Nwankwo, C.O.; Bamigboye, G.O.; Davies, I.E.E.; Michaels, T.A. High volume Portland cement replacement: A review. Constr. Build. Mater. 2020, 260, 120445. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Potential use of ceramic sanitary ware waste as pozzolanic material. Boletín Soc. Española De Cerámica Y Vidr. 2021, 61, 611–621. [Google Scholar] [CrossRef]
- Heidari, A.; Tavakoli, S.; Tavakoli, D. Reusing waste ceramic and waste sanitary ware in concrete as pozzolans with nano-silica and metakaolin. Int. J. Sustain. Constr. Eng. Technol. 2019, 10, 55–67. [Google Scholar] [CrossRef]
- Jaskulski, R.; Jóźwiak-Niedźwiedzka, D.; Yakymechko, Y. Calcined clay as supplementary cementitious material. Materials 2020, 13, 4734. [Google Scholar] [CrossRef]
- Mohammed, S. Processing, effect and reactivity assessment of artificial pozzolans obtained from clays and clay wastes: A review. Constr. Build. Mater. 2017, 140, 10–19. [Google Scholar] [CrossRef]
- Reig, L.; Pitarch, Á.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.M.; Payá, J.; Tashima, M.M. Reutilization of Ceramic Waste as Supplementary Cementitious Material. In Building Engineering Facing the Challenges of the 21st Century: Holistic Study from the Perspectives of Materials, Construction, Energy and Sustainability; Lecture Notes in Civil Engineering (LNCE); Springer Science and Business Media: Frankfurt, Germany, 2023; pp. 553–576. [Google Scholar] [CrossRef]
- Jain, P.; Gupta, R.; Chaudhary, S. A literature review on the effect of using ceramic waste as supplementary cementitious material in cement composites on workability and compressive strength. Mater. Today Proc. 2022, 65, 871–876. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Cheng, S.; Xu, X.; Zhao, C.; Wang, X.; Wu, Q.; Bai, X. Sustainable reuse of ceramic waste powder as a supplementary cementitious material in recycled aggregate concrete: Mechanical properties, durability and microstructure assessment. J. Build. Eng. 2022, 52, 104418. [Google Scholar] [CrossRef]
- Gautam, L.; Jain, J.K.; Kalla, P.; Choudhary, S. A review on the utilization of ceramic waste in sustainable construction products. Mater. Today Proc. 2020, 43, 1884–1891. [Google Scholar] [CrossRef]
- Lim, N.H.A.S.; Mohammadhosseini, H.; Tahir, M.M.; Samadi, M.; Sam, A.R.M. Microstructure and Strength Properties of Mortar Containing Waste Ceramic Nanoparticles. Arab. J. Sci. Eng. 2018, 43, 5305–5313. [Google Scholar] [CrossRef]
- De Matos, P.R.; Sakata, R.D.; Onghero, L.; Uliano, V.G.; de Brito, J.; Campos, C.E.M.; Gleize, P.J.P. Utilization of ceramic tile demolition waste as supplementary cementitious material: An early-age investigation. J. Build. Eng. 2021, 38, 102187. [Google Scholar] [CrossRef]
- Hoppe Filho, J.; Pires, C.A.O.; Leite, O.D.; Garcez, M.R.; Medeiros, M.H.F. Red ceramic waste as supplementary cementitious material: Microstructure and mechanical properties. Constr. Build. Mater. 2021, 296, 123653. [Google Scholar] [CrossRef]
- Pereira-De-Oliveira, L.A.; Castro-Gomes, J.P.; Santos, P.M.S. The potential pozzolanic activity of glass and red-clay ceramic waste as cement mortars components. Constr. Build. Mater. 2012, 31, 197–203. [Google Scholar] [CrossRef]
- Jackiewicz-Rek, W.; Załęgowski, K.; Garbacz, A.; Bissonnette, B. Properties of cement mortars modified with ceramic waste fillers. Procedia Eng. 2015, 108, 681–687. [Google Scholar] [CrossRef]
- Pitarch, A.M.; Reig, L.; Tomás, A.E.; Forcada, G.; Soriano, L.; Borrachero, M.V.; Payá, J.; Monzó, J.M. Pozzolanic activity of tiles, bricks and ceramic sanitary-ware in eco-friendly Portland blended cements. J. Clean. Prod. 2021, 279, 123713. [Google Scholar] [CrossRef]
- Mas, M.A.; Monzó, J.M.; Payá, J.; Reig, L.; Borrachero, M.V. Ceramic tiles waste as replacement material in Portland cement. Adv. Cem. Res. 2016, 28, 221–232. [Google Scholar] [CrossRef]
- Fořt, J.; Vejmelková, E.; Keppert, M.; Rovnaníková, P.; Bezdička, P.; Černý, R. Alkaline activation of low-reactivity ceramics: Peculiarities induced by the precursors’ dual character. Cem. Concr. Compos. 2020, 105, 103440. [Google Scholar] [CrossRef]
- Cosa, J.; Soriano, L.; Borrachero, M.V.; Reig, L.; Payá, J.; Monzó, J.M. The compressive strength and microstructure of Alkali-activated binary cements developed by combining ceramic sanitaryware with fly ash or blast furnace slag. Minerals 2018, 8, 337. [Google Scholar] [CrossRef]
- Harkishan Joshi, P.; Parekh, D.N. Evaluación de la utilización de desechos cerámicos como sustituto de los componentes del hormigón—Una revisión. Rev. Ing. Constr. 2022, 37, 69–78, (In Spanish with English Version). [Google Scholar]
- Zhao, Y.; Gao, J.; Liu, C.; Chen, X.; Xu, Z. The particle-size effect of waste clay brick powder on its pozzolanic activity and properties of blended cement. J. Clean. Prod. 2020, 242, 118521. [Google Scholar] [CrossRef]
- Gautam, L.; Kalla, P.; Jain, J.K.; Choudhary, R.; Jain, A. Robustness of self-compacting concrete incorporating bone china ceramic waste powder along with granite cutting waste for sustainable development. J. Clean. Prod. 2022, 367, 132969. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, R.; Tyrer, M.; Wong, H.; Cheeseman, C. Sustainable infrastructure development through use of calcined excavated waste clay as a supplementary cementitious material. J. Clean. Prod. 2017, 168, 1180–1192. [Google Scholar] [CrossRef]
- Zanelli, C.; Raimondo, M.; Guarini, G.; Dondi, M. The vitreous phase of porcelain stoneware: Composition, evolution during sintering and physical properties. J. Non-Cryst. Solids 2011, 357, 3251–3260. [Google Scholar] [CrossRef]
- Pavesi, T.B.; Rohden, A.B.; Garcez, M.R. Supporting circular economy through the use of red ceramic waste as supplementary cementitious material in structural concrete. J. Mater. Cycles Waste Manag. 2021, 23, 2278–2296. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.M.; Cheeseman, C.R.; Payá, J. Properties and microstructure of alkali-activated red clay brick waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Influence of calcium aluminate cement (CAC) on alkaline activation of red clay brick waste (RCBW). Cem. Concr. Compos. 2016, 65, 177–185. [Google Scholar] [CrossRef]
- Sánchez De Rojas, M.I.; Frías, M.; Rodríguez, O.; Rivera, J. Durability of blended cement pastes containing ceramic waste as a pozzolanic addition. J. Am. Ceram. Soc. 2014, 97, 1543–1551. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Influence of the activator concentration and calcium hydroxide addition on the properties of alkali-activated porcelain stoneware. Constr. Build. Mater. 2014, 63, 214–222. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Saccani, A. Ceramic waste as aggregate and supplementary cementing material: A combined action to contrast alkali silica reaction (ASR). Cem. Concr. Compos. 2012, 34, 1141–1148. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; You, Q.; Chen, M.; Zeng, Q. Waste ceramic powder as a pozzolanic supplementary filler of cement for developing sustainable building materials. J. Clean. Prod. 2020, 259, 120853. [Google Scholar] [CrossRef]
- Reig, L.; Borrachero, M.V.; Monzó, J.M.; Savastano, H.; Tashima, M.M.; Payá, J. Use of ceramic sanitaryware as an alternative for the development of new sustainable binders. Key Eng. Mat. 2016, 668, 172–180. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Influence of calcium additions on the compressive strength and microstructure of alkali-activated ceramic sanitary-ware. J. Am. Ceram. Soc. 2018, 101, 3094–3104. [Google Scholar] [CrossRef]
- Cosa, J.; Soriano, L.; Borrachero, M.V.; Reig, L.; Payá, J.; Monzó, J.M. Influence of addition of fluid catalytic cracking residue (FCC) and the SiO2 concentration in alkali-activated ceramic sanitary-ware (CSW) binders. Minerals 2018, 8, 123. [Google Scholar] [CrossRef]
- Medina, C.; Banfill, P.F.G.; De Rojas, M.I.S.; Frías, M. Rheological and calorimetric behaviour of cements blended with containing ceramic sanitary ware and construction/demolition waste. Constr. Build. Mater. 2013, 40, 822–831. [Google Scholar] [CrossRef]
- Zito, S.V.; Irassar, E.F.; Rahhal, V.F. Management of sanitary ware wastes as supplementary cementing materials in concretes. J. Sustain. Cem. Mater. 2020, 9, 35–49. [Google Scholar] [CrossRef]
- Brekailo, F.; Pereira, E.; Pereira, E.; Farias, M.M.; Medeiros-Junior, R.A. Red ceramic and concrete waste as replacement of portland cement: Microstructure aspect of eco-mortar in external sulfate attack. Clean. Mater. 2022, 3, 100034. [Google Scholar] [CrossRef]
- Mohit, M.; Sharifi, Y. Ceramic waste powder as alternative mortar-based cementitious material. ACI Mater. J. 2019, 116, 107–116. [Google Scholar] [CrossRef]
- Sharifi, Y.; Ranjbar, A.; Mohit, M. Acid resistance of cement mortars incorporating ceramic waste powder as cement replacement. ACI Mater. J. 2020, 117, 145–156. [Google Scholar] [CrossRef]
- UNE-EN 450-1:2013; Fly Ash for Concrete. Part 1: Definition, Specifications and Conformity Criteria. UNE Normalización Española: Madrid, Spain, 2013. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0051480 (accessed on 13 October 2022).
- Reig, L.; Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Alkaline activation of ceramic waste materials. Waste Biomass Valorization 2013, 4, 729–736. [Google Scholar] [CrossRef]
- Rahhal, V.F.; Trezza, M.A.; Tironi, A.; Castellano, C.C.; Pavlíková, M.; Pokorný, J.; Irassar, E.F.; Jankovský, O.; Pavlík, Z. Complex characterization and behavior of waste fired brick powder-Portland cement system. Materials 2019, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.N.T.; Nguyen, D.T.; Nguyen, D.L. Potential use of clay brick waste powder and ceramic waste aggregate in mortar. Constr. Build. Mater. 2021, 313, 125516. [Google Scholar] [CrossRef]
- Medina_Martínez, C.; Sáez-del-Bosque, I.F.; Asensio-de-Lucas, E.; Caneda-Martínez, L.; Frías-Rojas, M.; Sánchez-de-Rojas, M.I. Recycled ceramics in concrete. Encycl. Renew. Sustain. Mater. 2020, 2, 483–489. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Dhinakaran, G. Strength and durability studies on high strength concrete using ceramic waste powder. Struct. Eng. Mech. 2017, 61, 171–181. [Google Scholar] [CrossRef]
- Xu, K.; Huang, W.; Zhang, L.; Fu, S.; Chen, M.; Ding, S.; Han, B. Mechanical properties of low-carbon ultrahigh-performance concrete with ceramic tile waste powder. Constr. Build. Mater. 2021, 287, 123036. [Google Scholar] [CrossRef]
- Pereira, V.M.; Camarini, G. Fresh and Hardened Properties of Self-Leveling Mortars with Porcelain and Red Ceramic Wastes. Adv. Civ. Eng. 2018, 2018, 6378643. [Google Scholar] [CrossRef]
- Agrawal, A.; Saravanan, T.J.; Asce, A.M.; Bisht, K.; Syed, K.I.; Kabeer, A. Synthesis of Cement Composites Utilizing Ceramic Waste as a Partial Replacement for Portland Cement: Literature Review. J. Hazard. Toxic Radioact. Waste 2021, 25, 03121003. [Google Scholar] [CrossRef]
- Pavlík, Z.; Trník, A.; Kulovaná, T.; Scheinherrová, L.; Rahhal, V.; Irassar, E.; Černý, R. DSC and TG Analysis of a Blended Binder Based on Waste Ceramic Powder and Portland Cement. Int. J. Thermophys 2016, 37, 32. [Google Scholar] [CrossRef]
- Friol Guedes de Paiva, F.; Tamashiro, J.R.; Pereira Silva, L.H.; Kinoshita, A. Utilization of inorganic solid wastes in cementitious materials—A systematic literature review. Constr. Build. Mater. 2021, 285, 122833. [Google Scholar] [CrossRef]
- Ali, A.H.; Al-Attar, A.A.; Kasm, Z.E. Effect of solid ceramic waste powder in partial replacement of cement on mechanical properties and sorptivity of cement mortar. Int. J. Civ. Eng. Technol. 2019, 10, 3055–3066. [Google Scholar]
- Bumanis, G.; Vaičiukynienė, D. Alkali Activation of Milled Red Brick Waste and Calcined Illite Clay with Silica Gel Addition. Materials 2022, 15, 3195. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef]
- Fořt, J.; Vejmelková, E.; Koňáková, D.; Alblová, N.; Čáchová, M.; Keppert, M.; Rovnaníková, P.; Černý, R. Application of waste brick powder in alkali activated aluminosilicates: Functional and environmental aspects. J. Clean. Prod. 2018, 194, 714–725. [Google Scholar] [CrossRef]
- Scheinherrová, L.; Vejmelková, E.; Keppert, M.; Doleželová, M.; Rovnaníková, P.; Černý, R. Effects of accelerated carbonation on properties of ceramic-based geopolymers. J. Therm. Anal. Calorim. 2021, 145, 2951–2966. [Google Scholar] [CrossRef]
- Pommer, V.; Vejmelková, E.; Černý, R.; Keppert, M. Alkali-activated waste ceramics: Importance of precursor particle size distribution. Ceram. Int. 2021, 47, 31574–31582. [Google Scholar] [CrossRef]
- Gado, R.A.; Hebda, M.; Lach, M.; Mikula, J. Alkali activation of waste clay bricks: Influence of the silica modulus, SiO2/Na2O, H2O/Na2O molar ratio, and liquid/solid ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Reig, L.; Sanz, M.A.; Borrachero, M.V.; Monzó, J.M.; Soriano, L.; Payá, J. Compressive strength and microstructure of alkali-activated mortars with high ceramic waste content. Ceram. Int. 2017, 43, 13622–13634. [Google Scholar] [CrossRef]
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of alkali activator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. Part B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Zhang, Z.; Wong, Y.C.; Arulrajah, A.; Sofi, M.; Sabri, Y. Reaction mechanism of alkali-activated brick clay mill residues. Constr. Build. Mater. 2022, 341, 127817. [Google Scholar] [CrossRef]
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Formulating eco-friendly geopolymer foam concrete by alkali-activation of ground brick waste. J. Clean. Prod. 2021, 325, 129180. [Google Scholar] [CrossRef]
- Sassoni, E.; Pahlavan, P.; Franzoni, E.; Bignozzi, M.C. Valorization of brick waste by alkali-activation: A study on the possible use for masonry repointing. Ceram. Int. 2016, 42, 14685–14694. [Google Scholar] [CrossRef]
- Horvat, B.; Ducman, V. Potential of green ceramicswaste for alkali activated foams. Materials 2019, 12, 3563. [Google Scholar] [CrossRef] [PubMed]
- Keppert, M.; Vejmelková, E.; Bezdička, P.; Doleželová, M.; Čáchová, M.; Scheinherrová, L.; Pokorný, J.; Vyšvařil, M.; Rovnaníková, P.; Černý, R. Red-clay ceramic powders as geopolymer precursors: Consideration of amorphous portion and CaO content. Appl. Clay Sci. 2018, 161, 82–89. [Google Scholar] [CrossRef]
- Keppert, M.; Scheinherrová, L.; Doleželová, M.; Vejmelková, E.; Černý, R. Phase composition of ceramic-based alkali-activated polymers: Combination of X-ray diffraction and thermal analysis. J. Therm. Anal. Calorim. 2020, 142, 157–166. [Google Scholar] [CrossRef]
- Mendes, J.P.; Elyseu, F.; Nieves, L.J.J.; Zaccaron, A.; Bernardin, A.M.; Angioletto, E. Synthesis and characterization of geopolymers using clay ceramic waste as source of aluminosilicate. Sustain. Mater. Technol. 2021, 28, e00264. [Google Scholar] [CrossRef]
- Amin, S.K.; El-Sherbiny, S.A.; El-Magd, A.A.M.A.; Belal, A.; Abadir, M.F. Fabrication of geopolymer bricks using ceramic dust waste. Constr. Build. Mater. 2017, 157, 610–620. [Google Scholar] [CrossRef]
- Öztürk, Z.B.; Atabey, İ.İ. Mechanical and microstructural characteristics of geopolymer mortars at high temperatures produced with ceramic sanitaryware waste. Ceram. Int. 2022, 48, 12932–12944. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; MacPhee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Maltseva, O.; Palomo, A.; Fernández-Jiménez, A. Hybrid alkaline cements: Part I. Fundamentals. Rom. J. Mater. 2012, 42, 330–335. [Google Scholar]
- Chirkova, V.V. Materials based on Blass-like Calcium-Free aluminosilicates and sodium compounds. Ph.D. Thesis, Kiev Civil Engineering Institute, Kiev, Ukraine, 1974. (In Russian). [Google Scholar]
- Palomo, A.; Fernández-Jiménez, A.; Kovalchuk, G.; Ordoñez, L.M.; Naranjo, M.C. OPC-fly ash cementitious systems: Study of gel binders produced during alkaline hydration. J. Mater. Sci. 2007, 42, 2958–2966. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing Ltd.: Cambridge, UK, 2015. [Google Scholar] [CrossRef]
- Murillo, L.M.; Delvasto, S.; Gordillo, M. A study of a hybrid binder based on alkali-activated ceramic tile wastes and portland cement. In Sustainable and Nonconventional Construction Materials Using Inorganic Bonded Fiber Composites; Woodhead Publishing Ltd.: Cambridge, UK, 2017; pp. 291–311. [Google Scholar] [CrossRef]
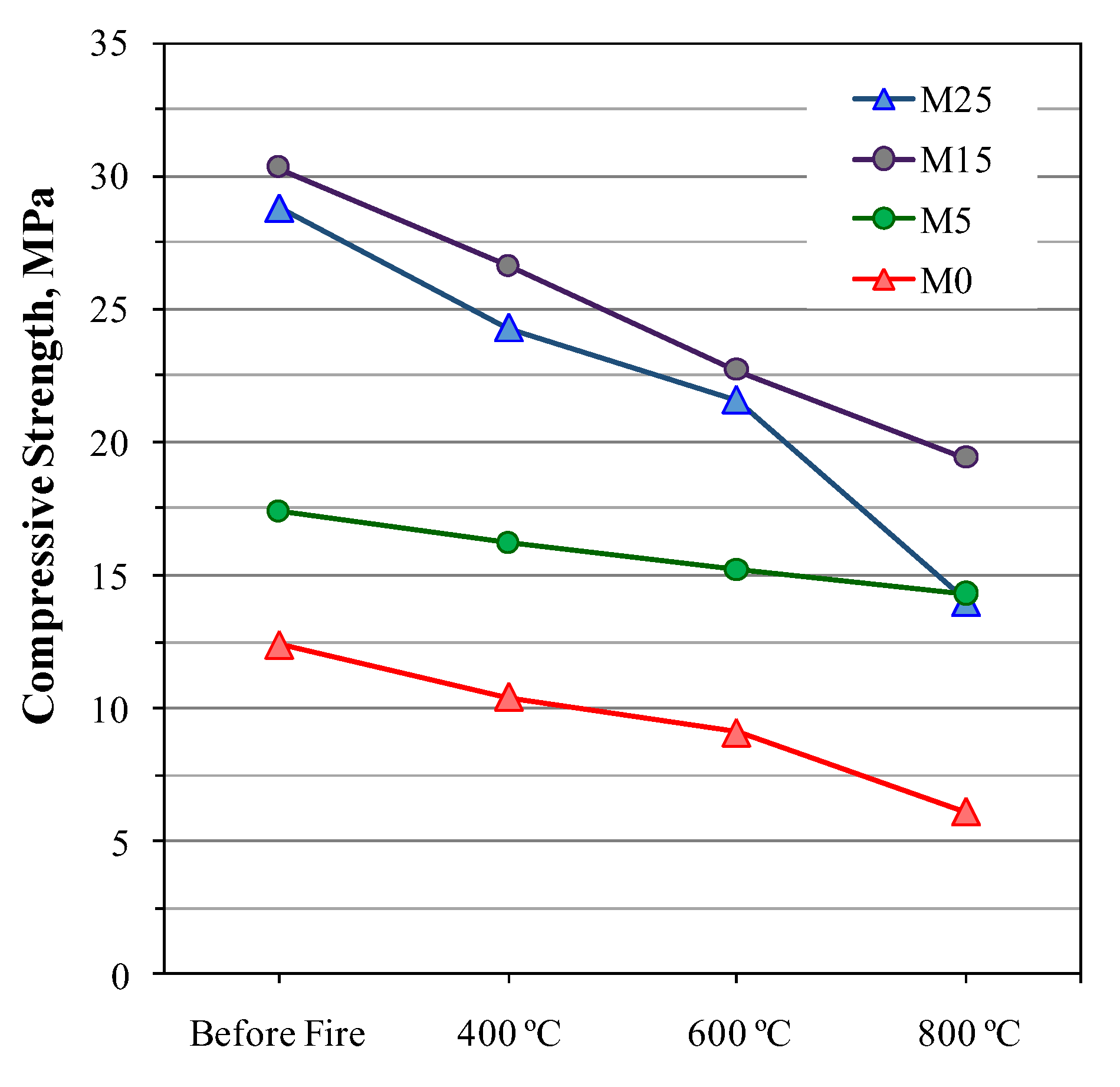
- Atabey, İ.İ. Influence of Ca and Al source on elevated temperature behavior of waste ceramic sanitaryware-based alkali-activated mortars. J. Aust. Ceram. Soc. 2022, 58, 949–962. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Luukkonen, T.; Mastali, M.; Kinnunen, P.; Illikainen, M. Development of one-part alkali activated ceramic/slag binders containing recycled ceramic aggregates. J. Mater. Civ. Eng. 2019, 31, 04018386. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J.; Tahir, M.M. Evaluation of alkali-activated mortars containing high volume waste ceramic powder and fly ash replacing GBFS. Constr. Build. Mater. 2019, 210, 78–92. [Google Scholar] [CrossRef]
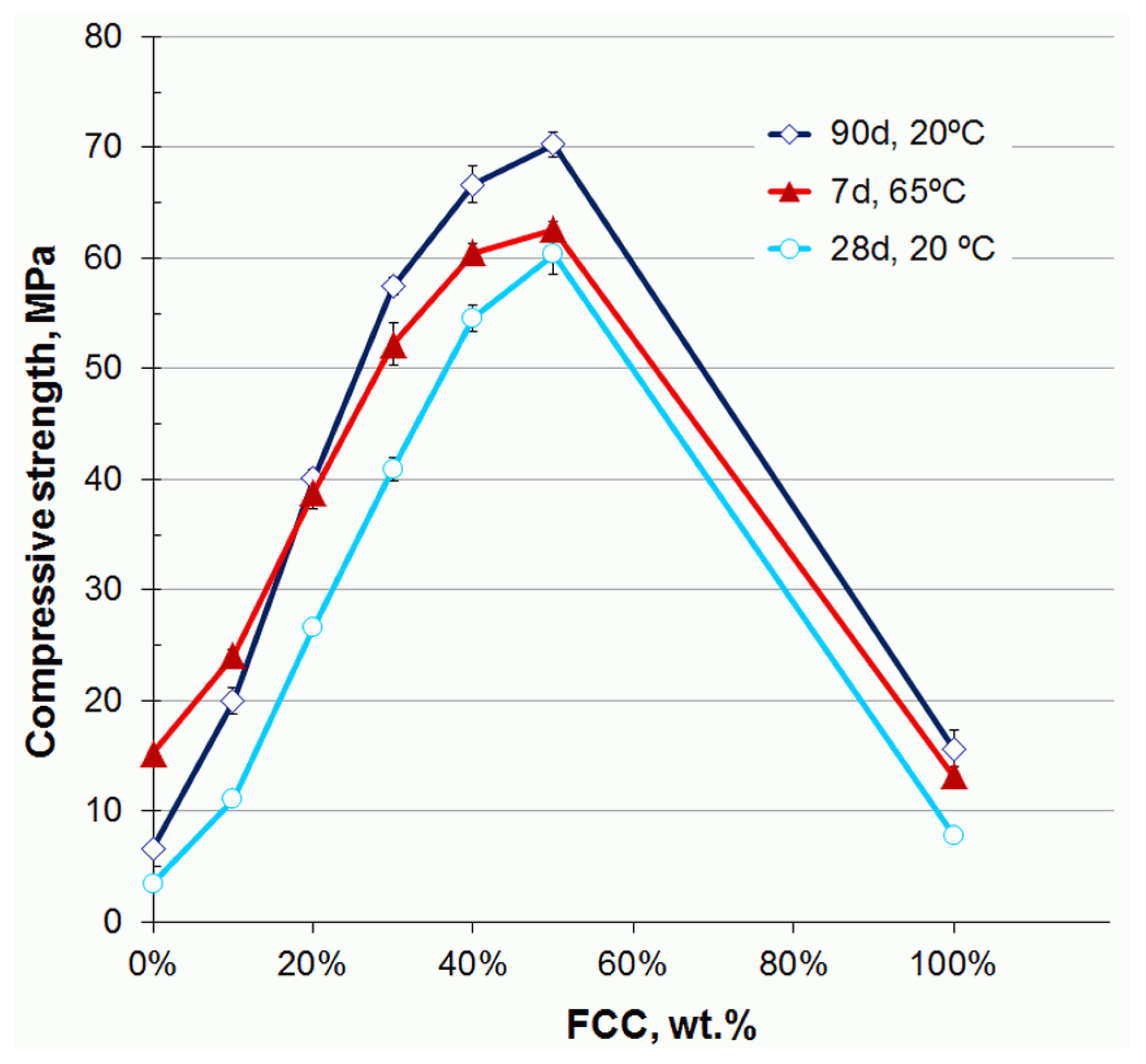
- Rashad, A.M.; Essa, G.M.F. Effect of ceramic waste powder on alkali-activated slag pastes cured in hot weather after exposure to elevated temperature. Cem. Concr. Compos. 2020, 111, 103617. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yehualaw, M.D.; Vo, D.H.; Huynh, T.P.; Largo, A. Performance evaluation of alkali activated mortar containing high volume of waste brick powder blended with ground granulated blast furnace slag cured at ambient temperature. Constr. Build. Mater. 2019, 223, 657–667. [Google Scholar] [CrossRef]
- Rakhimova, N.R.; Rakhimov, R.Z. Alkali-activated cements and mortars based on blast furnace slag and red clay brick waste. Mater. Des. 2015, 85, 324–331. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Ahn, Y.H.; Lin, R.S.; Wang, X.Y. Effect of waste ceramic powder on properties of alkali-activated blast furnace slag paste and mortar. Polymers 2021, 13, 2817. [Google Scholar] [CrossRef]
- Sedira, N.; Castro-Gomes, J.; Magrinho, M. Red clay brick and tungsten mining waste-based alkali-activated binder: Microstructural and mechanical properties. Constr. Build. Mater. 2018, 190, 1034–1048. [Google Scholar] [CrossRef]
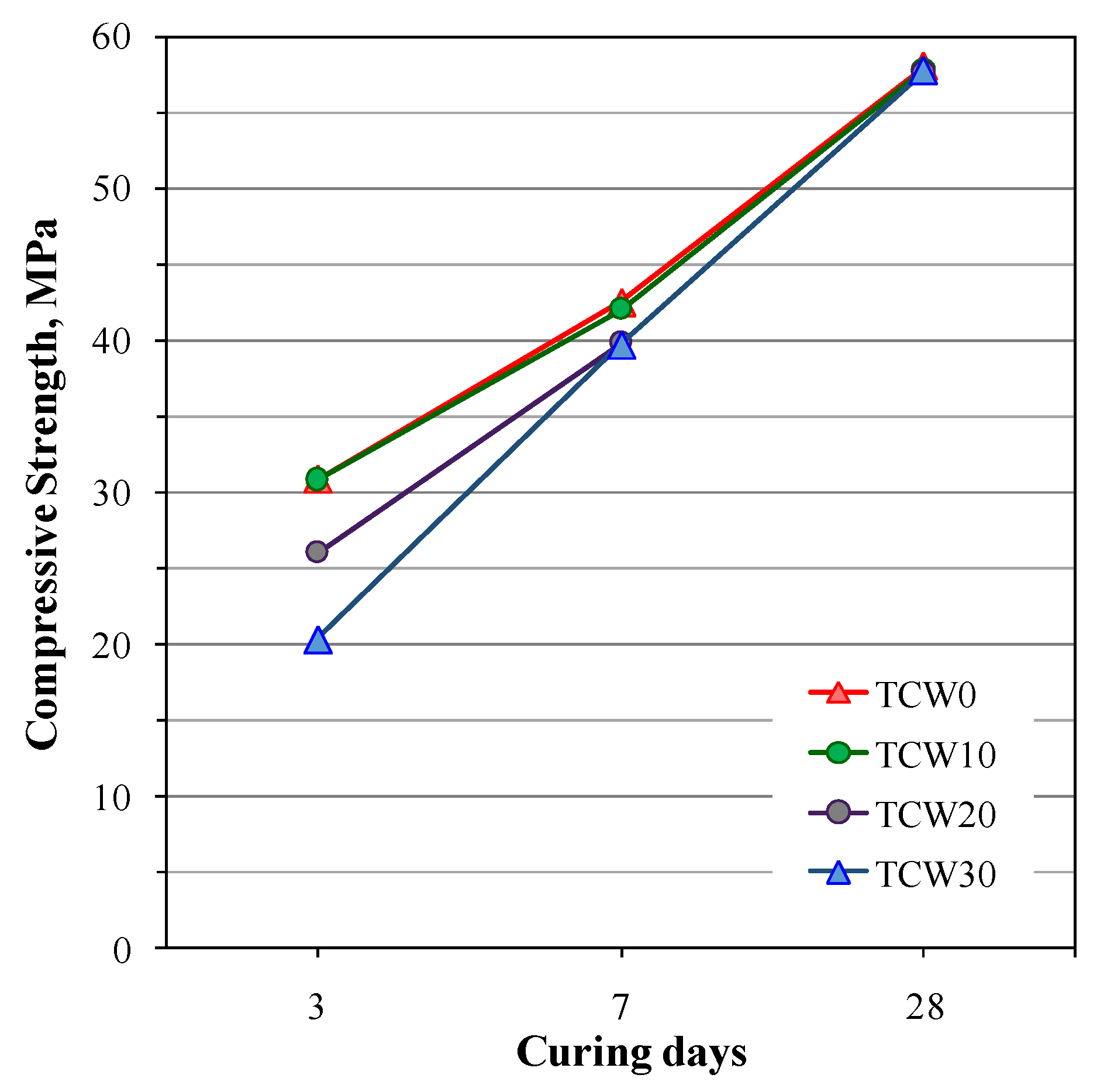
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J. Effects of ceramic tile powder waste on properties of self-compacted alkali-activated concrete. Constr. Build. Mater. 2020, 236, 117574. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Development of optimized binary ceramic tile and concrete wastes geopolymer binders for in-situ applications. J. Build. Eng. 2021, 43, 102906. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Development and characterization of binary recycled ceramic tile and brick wastes-based geopolymers at ambient and high temperatures. Constr. Build. Mater. 2021, 301, 124138. [Google Scholar] [CrossRef]
- Habert, G.; D’Espinose De Lacaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Saxena, R.; Gupta, T. Assessment of mechanical, durability and microstructural properties of geopolymer concrete containing ceramic tile waste. J. Mater. Cycles Waste Manag. 2022, 24, 725–742. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Şahmaran, M. Optimized application of ternary brick, ceramic and concrete wastes in sustainable high strength geopolymers. J. Clean. Prod. 2022, 338, 130650. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Mejía-Arcila, J.M.; Mejía de Gutiérrez, R. Eco-efficient alkali-activated cement based on red clay brick wastes suitable for the manufacturing of building materials. J. Clean. Prod. 2017, 166, 242–252. [Google Scholar] [CrossRef]



| Pozzolan or AA Precursor | ||
|---|---|---|
| CW | Density, g/cm3 | Mean Particle Size µm |
| BCW | 2.5–2.7 | 3.4–48.5 |
| [5,16,18,21,24,25] | ||
| TCW | 2.4–2.6 | <1–20 |
| [9,16,17,22,23,25] | ||
| CSW | 2.6 | 20–31.2 |
| [5,9,16,19,26] | ||
| Not specified | 2.3–3.0 | 2.3–35 |
| [6,14,15,20] | ||
| Feldspars | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CW | Q | M | C | Ca | H | R | D | K | Z | T | m | A | B | S | O | Reference |
| SiO2 | Al6Si2O13 | CaCO3 | CaO2 | Fe2O3 | 2SiO2·3CaO | CaMg(SiO3)2 | KAl2Si3AlO10(OH)2 | ZrSiO4 | Montmorillonite | KalSi3O8 | CaAl2Si2O8 | NaAlSi3O8 | (K,Na)(Si,Al)4O8 | KAlSi3O8 | ||
| BCW | X | - | - | - | X | - | - | - | - | - | - | X | - | - | - | [5] |
| BCW | X | - | - | - | X | - | X | X | - | - | X | - | X | - | X | [18] |
| BCW | X | - | X | - | - | - | - | - | - | - | X | X | X | - | [31] | |
| BCW | X | - | - | - | - | X | - | - | - | - | X | X | - | - | - | [32] |
| BCW | X | - | - | - | - | X | - | - | - | - | X | - | X | X | - | [16] |
| TCW | X | - | X | - | - | - | - | X | - | X | X | - | - | - | - | [33] |
| TCW | X | X | - | - | - | - | X | - | - | - | - | - | X | - | - | [17] |
| TCW | X | X | - | - | - | - | - | - | - | - | - | - | - | - | - | [34] |
| PTCW * | X | X | X | - | - | - | - | - | - | - | - | - | X | - | - | [35] |
| PTCW * | X | X | - | - | - | - | - | - | - | - | - | - | - | - | - | [36] |
| CSW | X | X | - | - | - | - | - | - | - | - | X | - | - | - | - | [8,16,37,38] |
| CSW | X | X | - | - | - | - | - | - | - | - | - | X | - | - | - | [19,39] |
| CSW | X | X | - | - | X | - | - | - | X | - | - | - | - | - | X | [40] |
| CSW | X | X | - | - | - | - | - | - | - | - | - | - | - | - | [5,41] | |
| Not specified | X | - | - | - | X | - | - | X | - | - | - | - | - | - | - | [42] |
| Not specified | X | - | - | X | - | - | - | - | - | - | - | - | X | - | - | [42,43,44] |
| CW | SiO2 % | Al2O3 % | Fe2O3 % | CaO % | Na2O % | K2O % | LOI * % | Amorph % | References |
|---|---|---|---|---|---|---|---|---|---|
| BCW | 49.9–64.6 | 16.6–20.0 | 5.6–14.2 | 0.6–11.5 | 0.5–5.2 | 2.3–4.4 | 0.2–2.4 | 20.1–46.7 | [5,16,18,21,31,46,47,48] |
| TCW | 55.8–78.3 | 14.4–20.0 | 1.1–7.9 | 0.1–9.6 | 0.2–4.7 | 1.4–3.5 | 0.1–1.1 | 46–60 | [9,17,22,33,34,46,49,50,51] |
| CSW | 66.0–70.8 | 19.0–24.1 | 0.6–1.3 | 0.1–1.2 | 1.3–2.7 | 2.8–4.6 | 0.2–1.1 | 45.6–46.0 | [5,9,16,19,37,39] |
| Porcelain insulators | 70.9–73.9 | 14.1–21.1 | 0.8–2.1 | 0.8–1.6 | 0.5–1.5 | 3.6–5.6 | - | - | [49,52] |
| Flower pots | 61.7 | 22.3 | 1.2 | 6.7 | 1.0 | 1.6 | 4.0 | - | [36] |
| Not specified | 28.8–78.3 | 9.8–45.2 | 0.5–16.6 | 0.02–25.2 | 0.2–13.5 | 0.2–13.5 | 0.0–7.5 | - | [6,7,15,25,53,54,55,56,57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano, L.; Tashima, M.M.; Reig, L.; Payá, J.; Borrachero, M.V.; Monzó, J.M.; Pitarch, Á.M. Reusing Ceramic Waste as a Precursor in Alkali-Activated Cements: A Review. Buildings 2023, 13, 3022. https://doi.org/10.3390/buildings13123022
Soriano L, Tashima MM, Reig L, Payá J, Borrachero MV, Monzó JM, Pitarch ÁM. Reusing Ceramic Waste as a Precursor in Alkali-Activated Cements: A Review. Buildings. 2023; 13(12):3022. https://doi.org/10.3390/buildings13123022
Chicago/Turabian StyleSoriano, Lourdes, Mauro M. Tashima, Lucía Reig, Jordi Payá, María V. Borrachero, José M. Monzó, and Ángel M. Pitarch. 2023. "Reusing Ceramic Waste as a Precursor in Alkali-Activated Cements: A Review" Buildings 13, no. 12: 3022. https://doi.org/10.3390/buildings13123022









