Corrosion Performance of Buried Corrugated Galvanized Steel under Accelerated Wetting/Drying Cyclic Corrosion Test
Abstract
1. Introduction
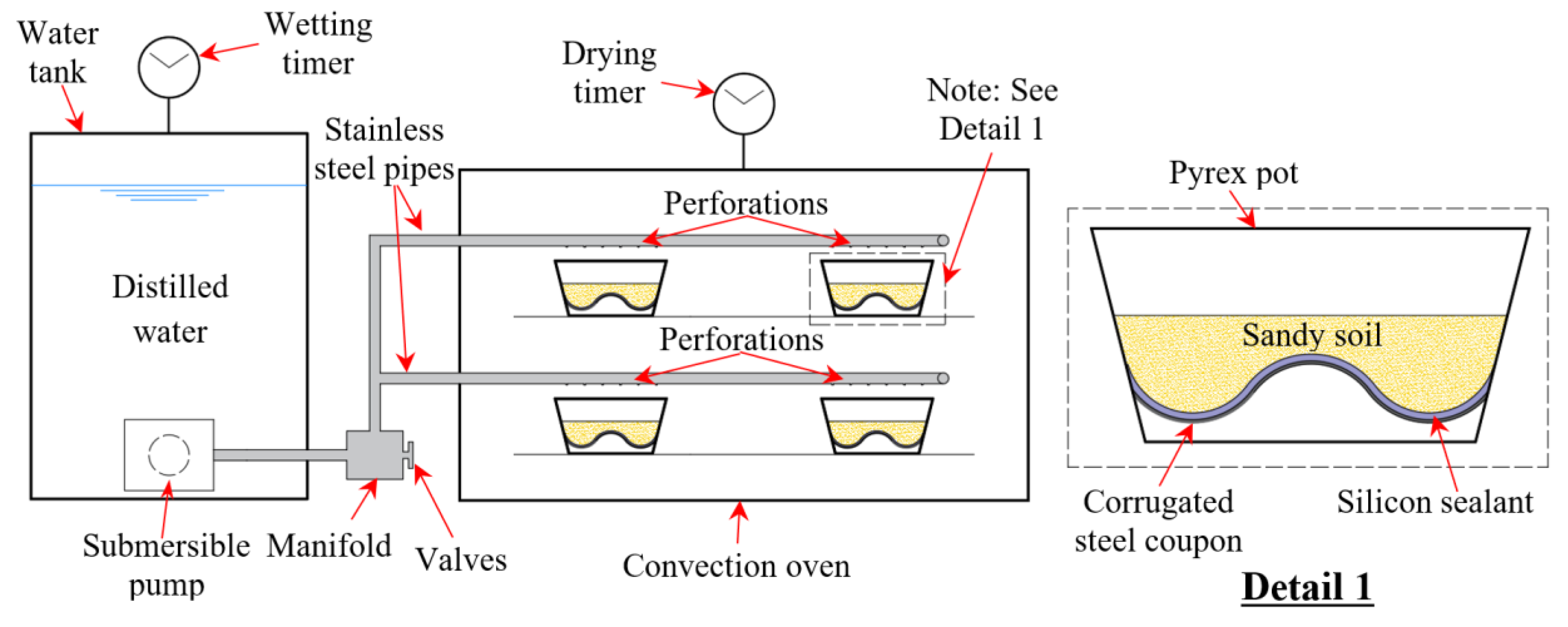
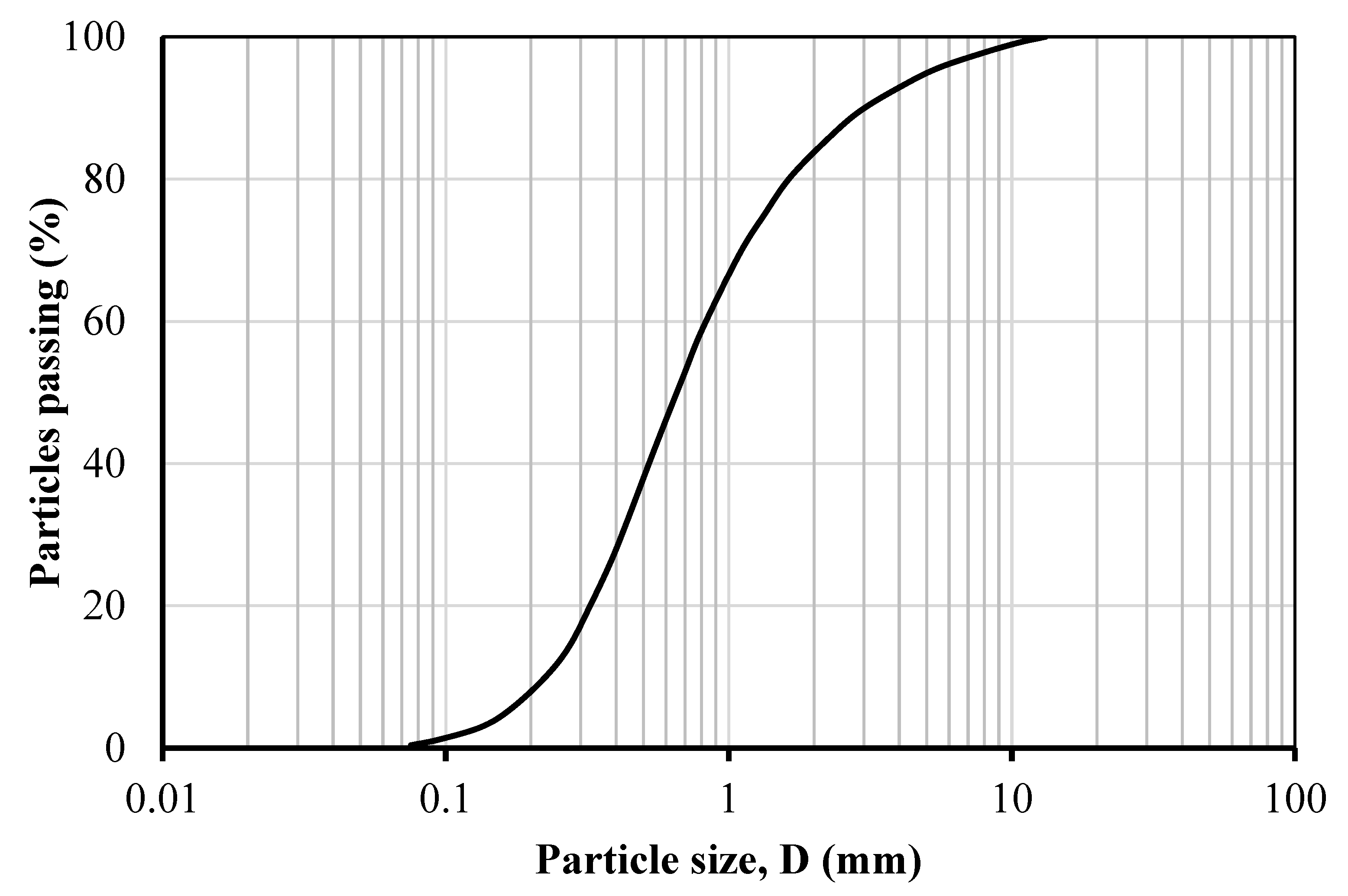
2. Experimental Procedures
3. Results
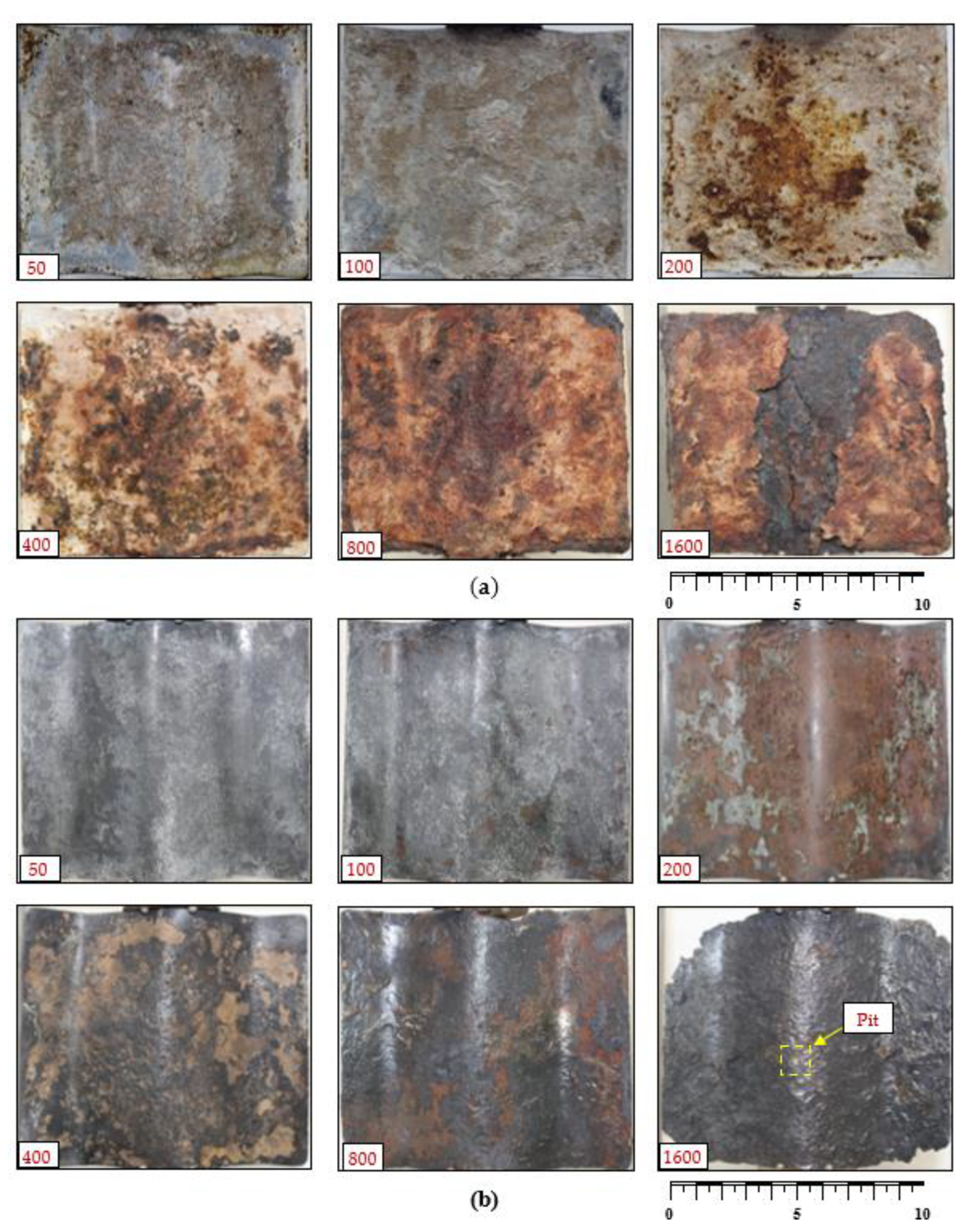
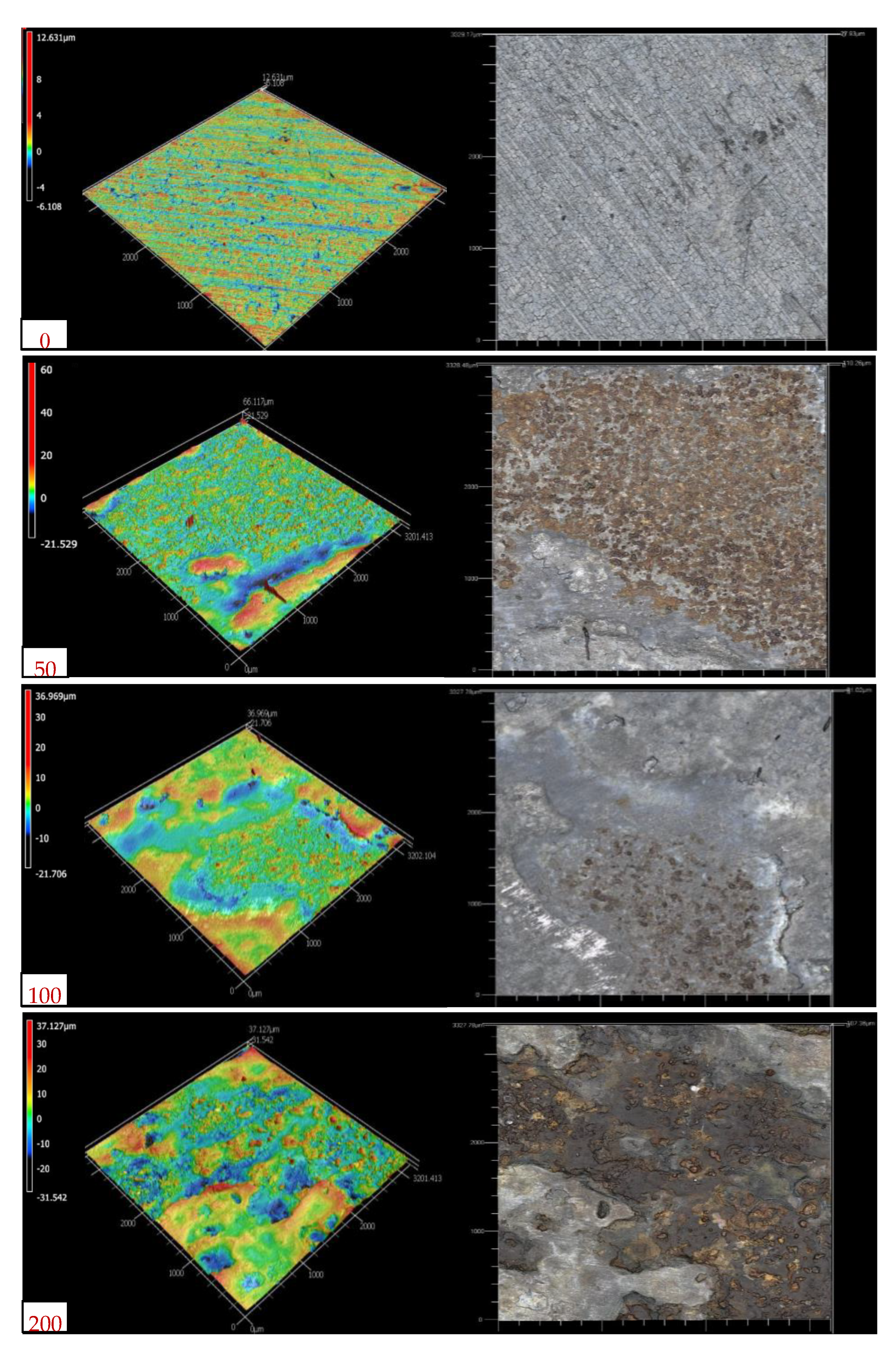
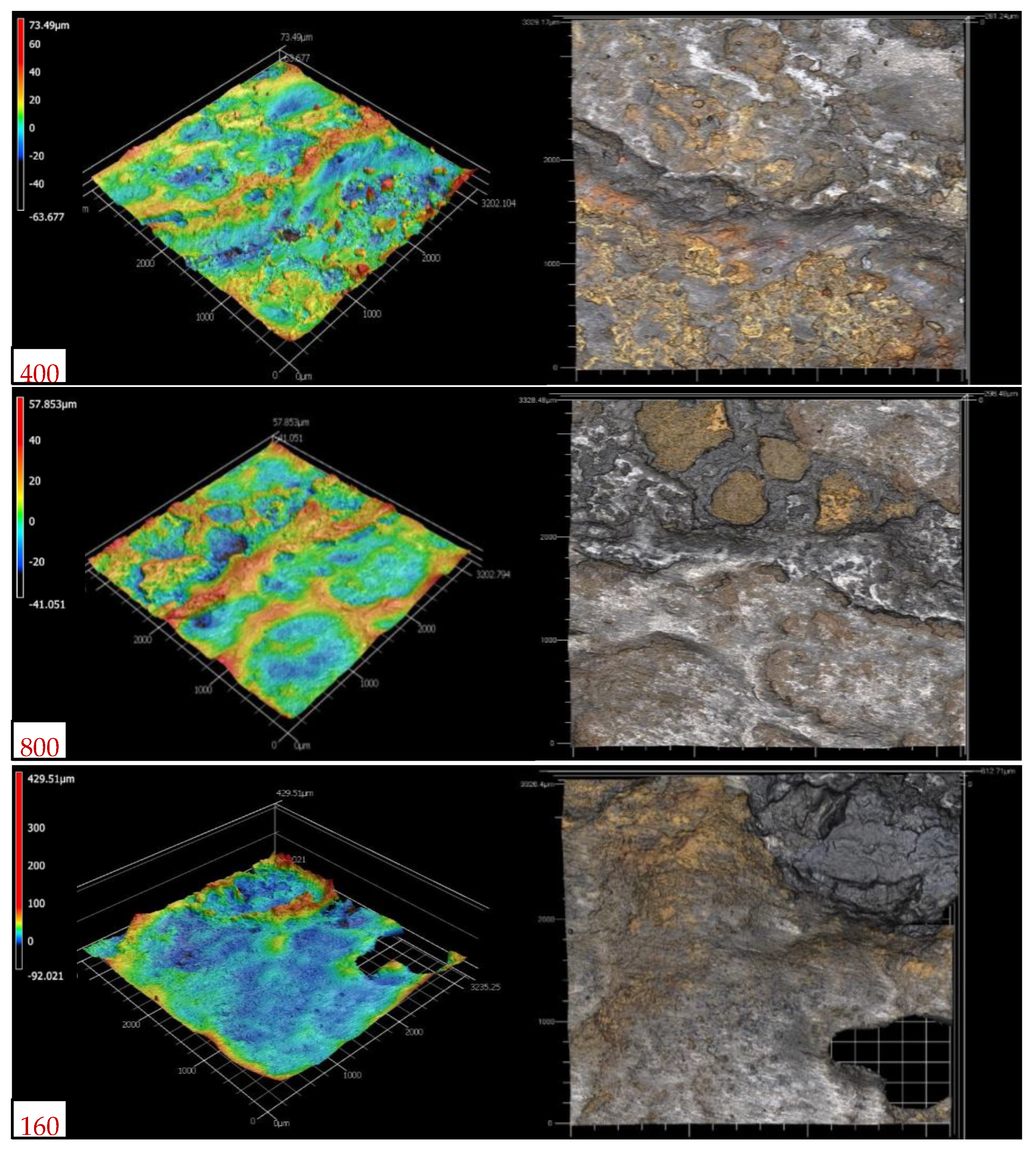
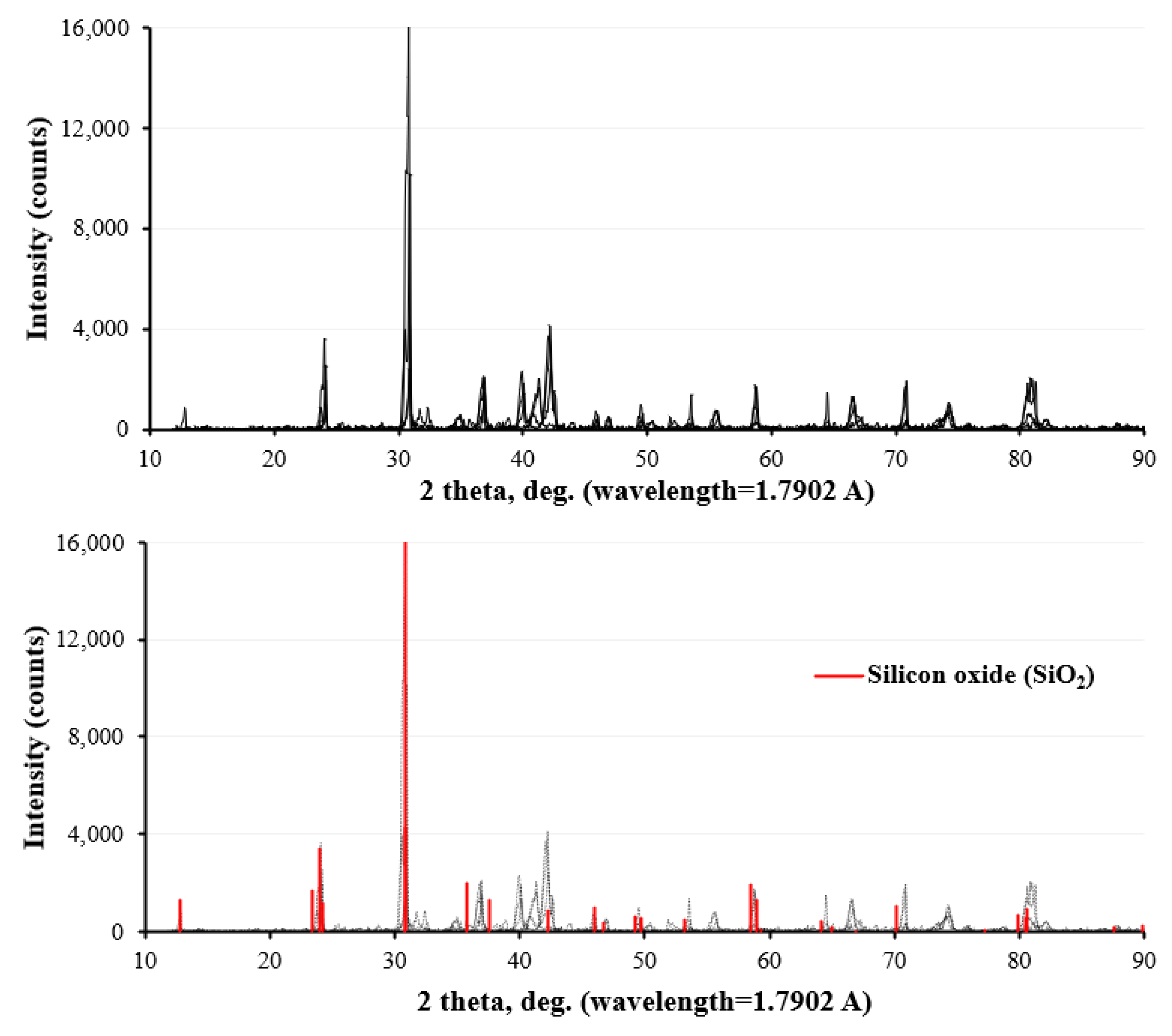
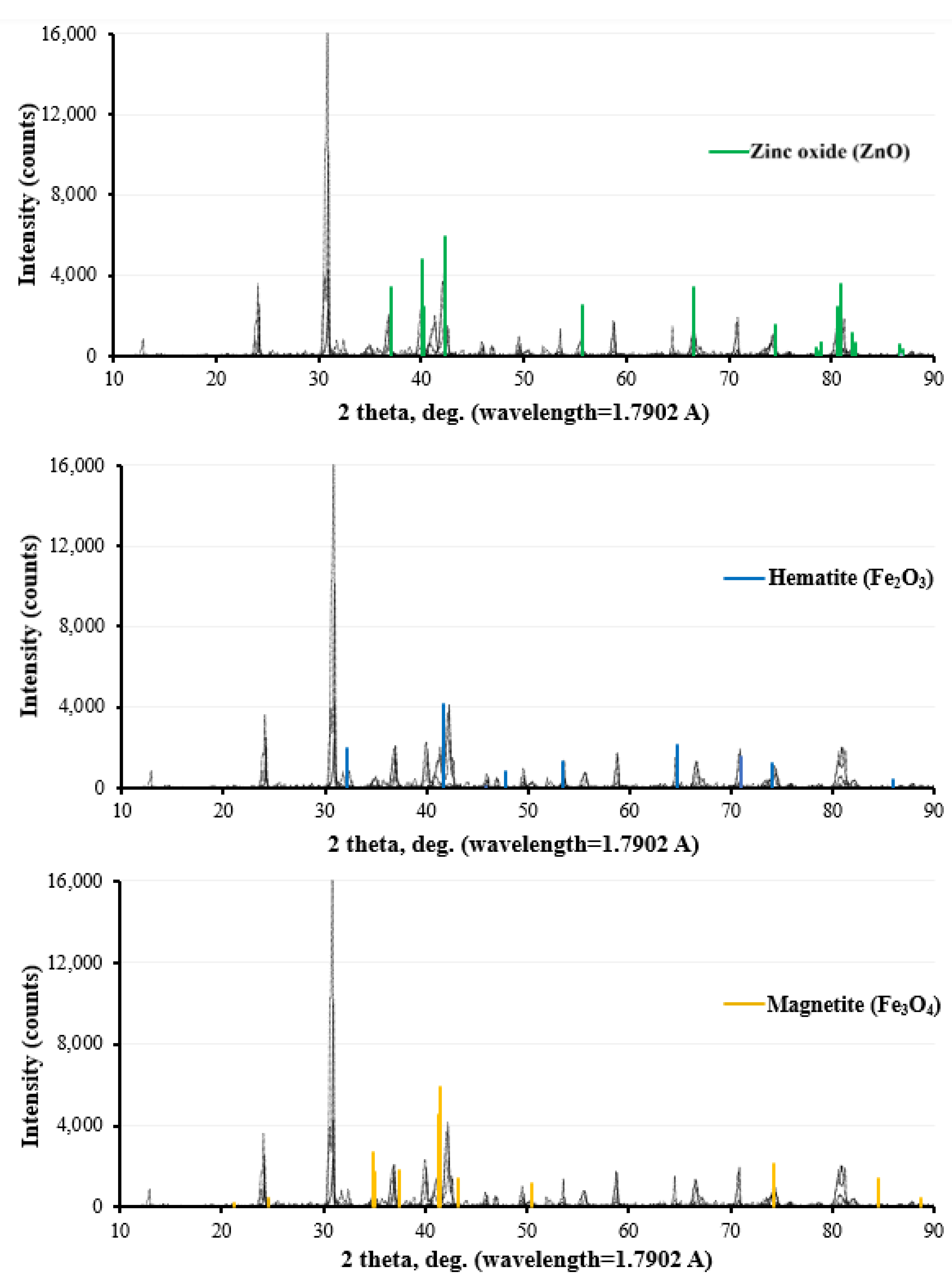
3.1. Surface Morphology and Corrosion Products
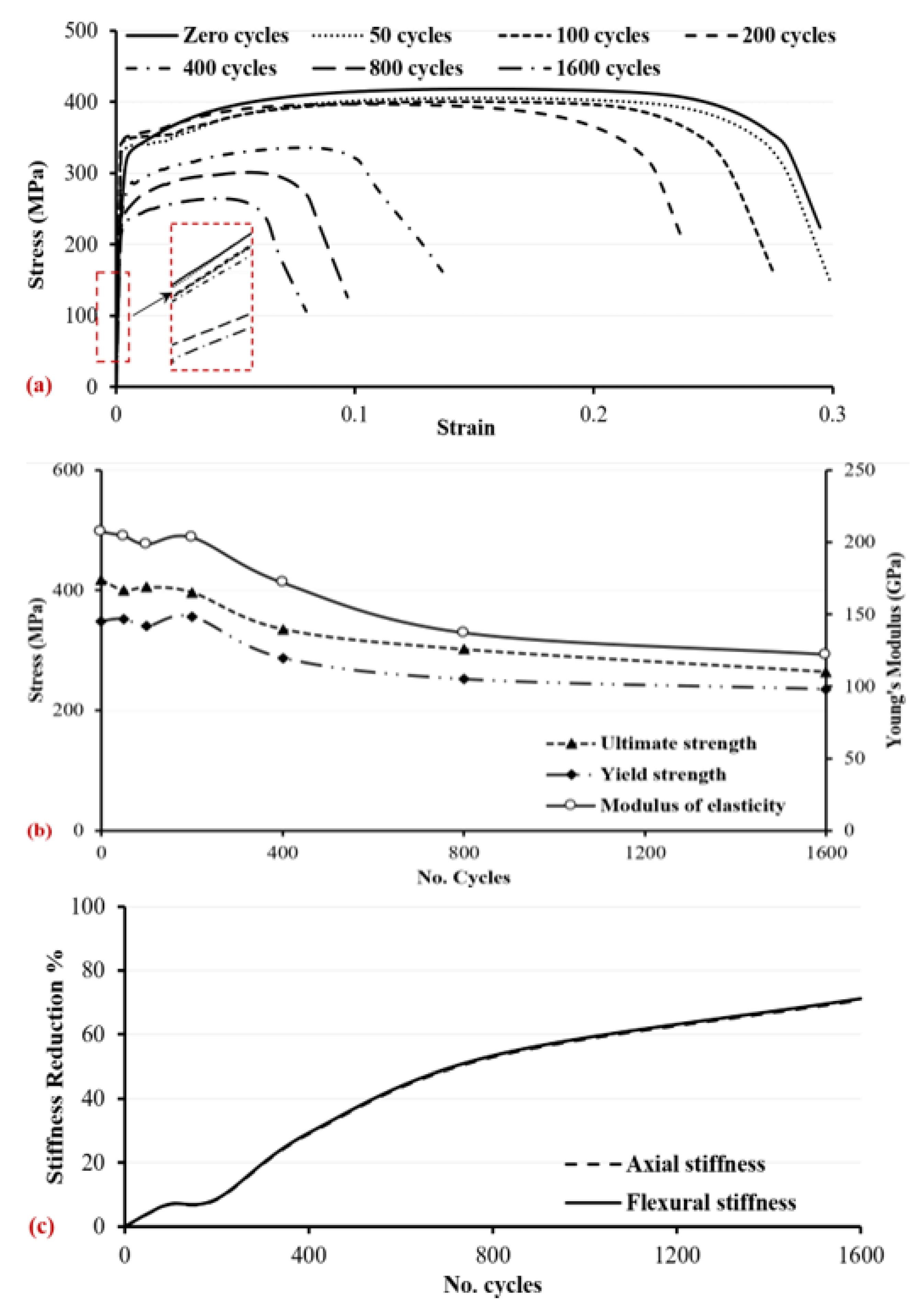
3.2. Mechanical Characteristics
4. Discussion
4.1. Corrosion Analysis
4.2. Mechanical Analysis
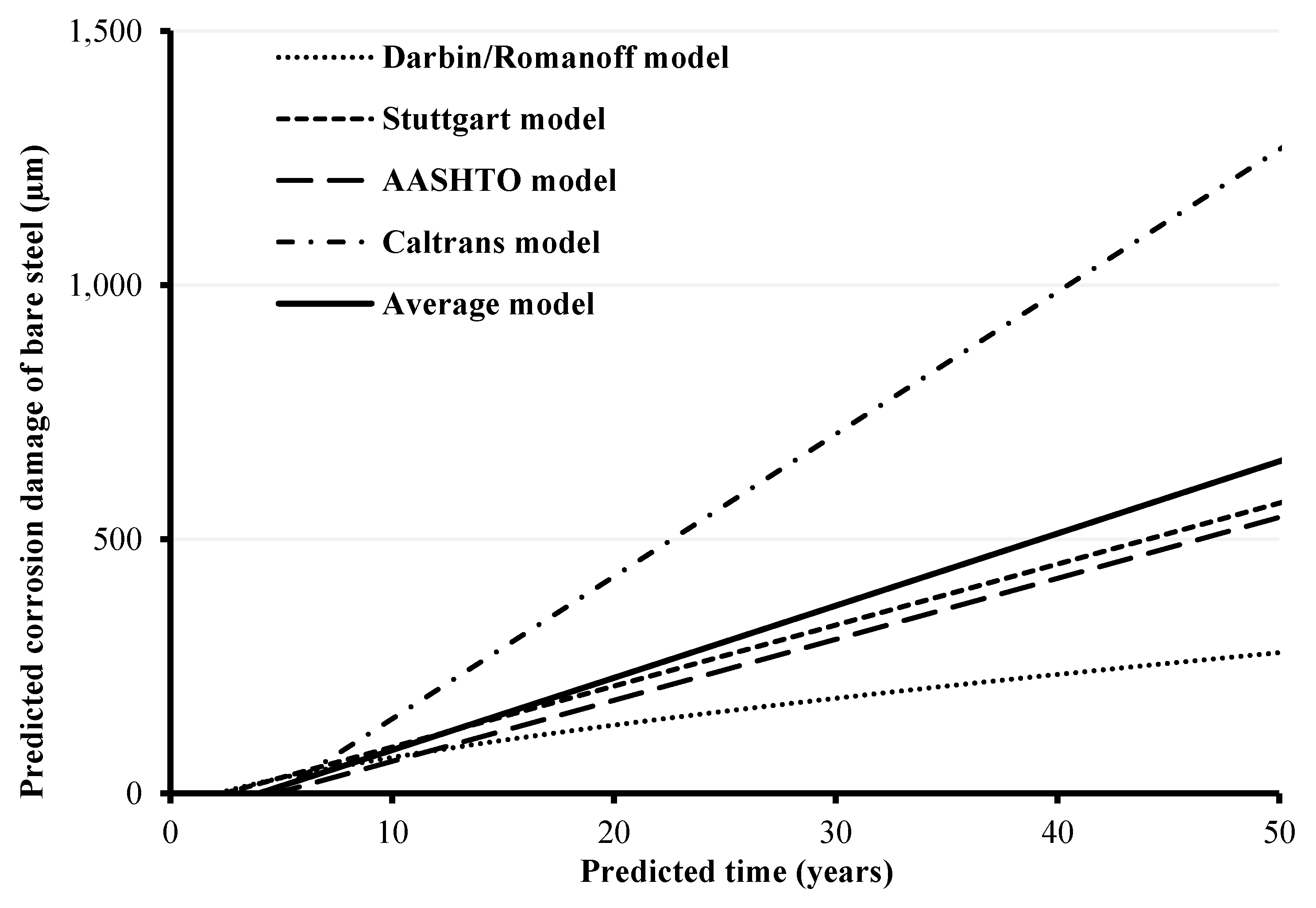
4.3. Prediction Models
5. Conclusions
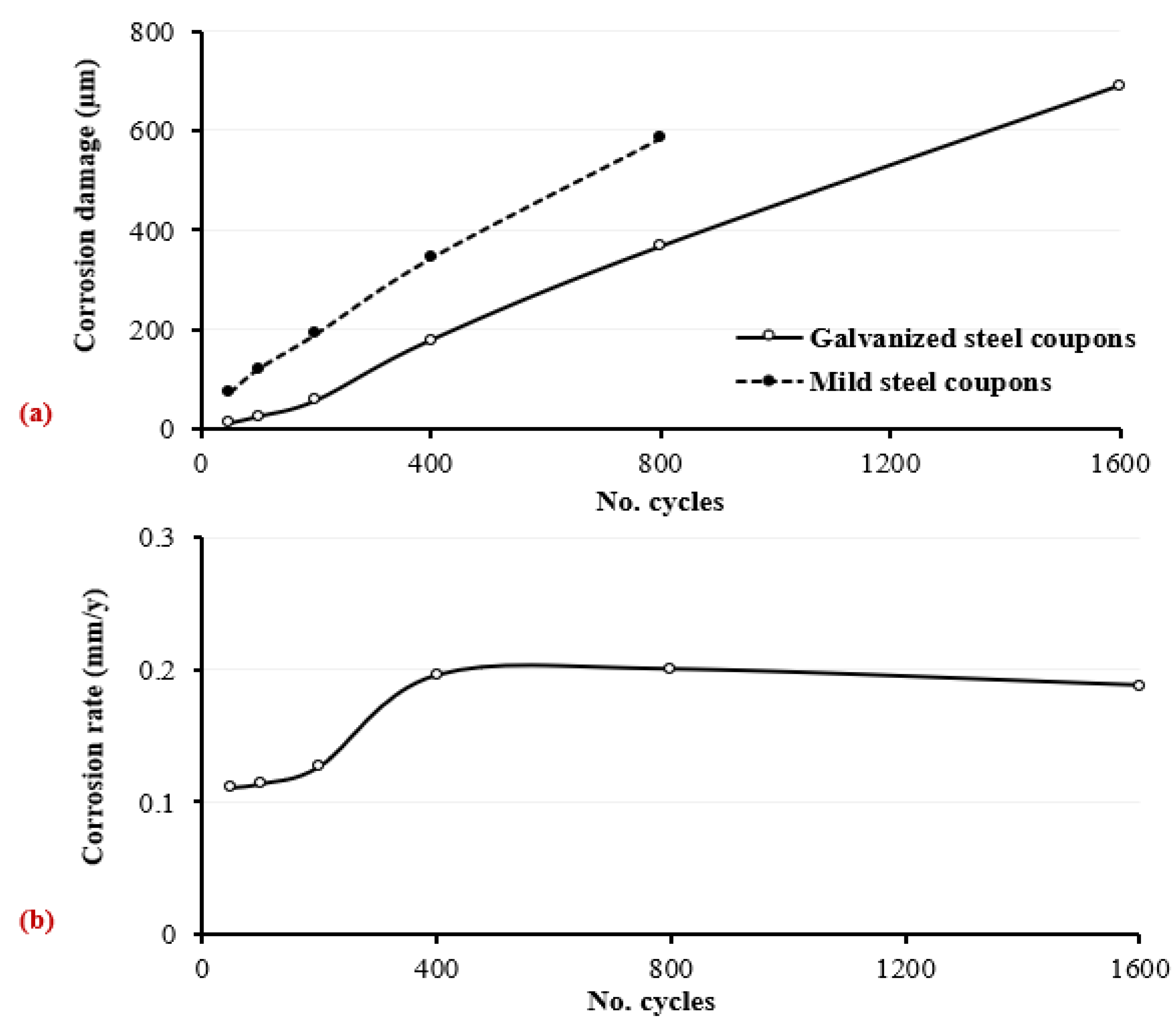
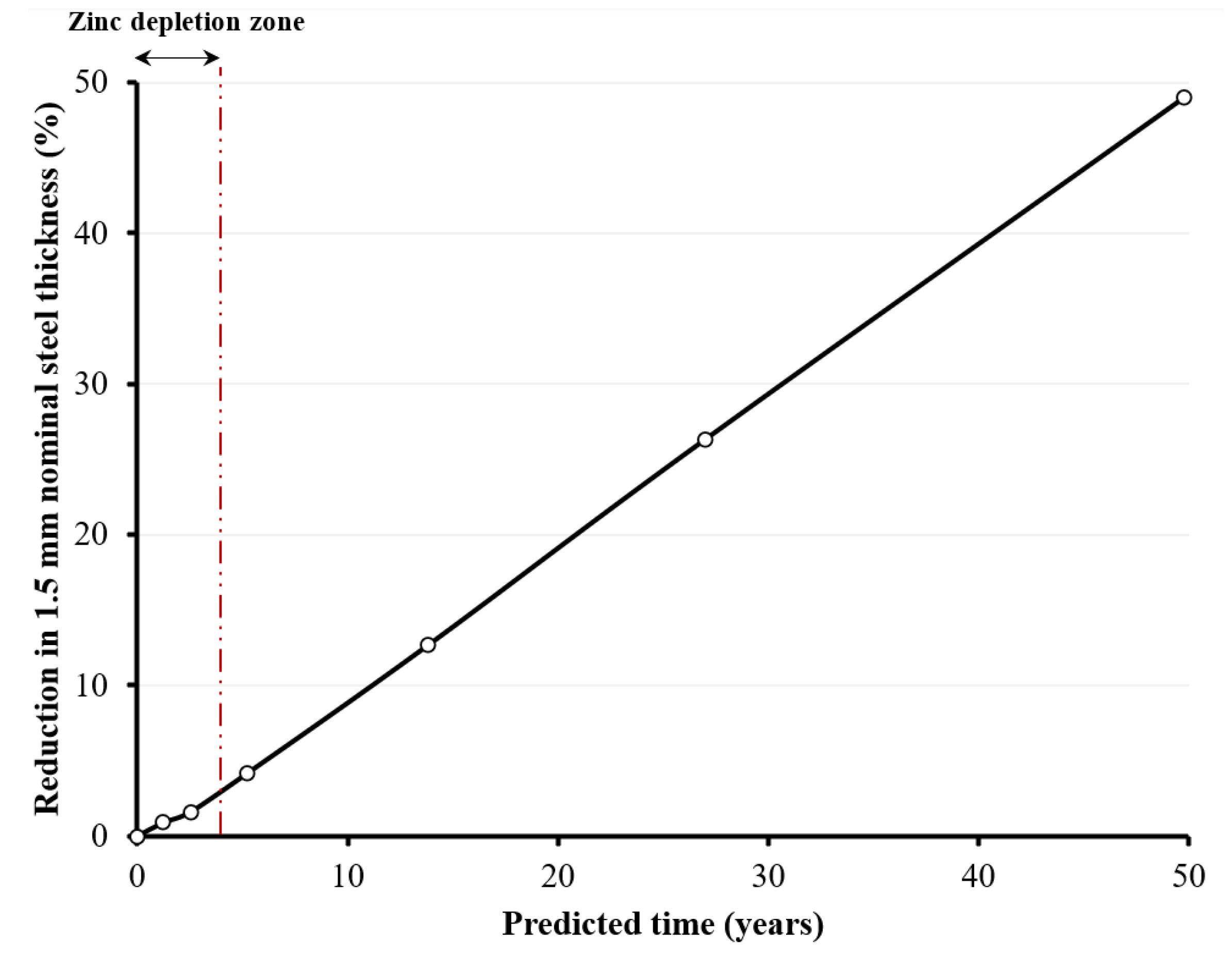
- The existence of the zinc coating layer significantly delayed the impact of corrosion, until the zinc layer was almost fully depleted (i.e., at around 200 wetting/drying cycles), resulting in the direct exposure of bare steel to the aggressive surrounding corrosive environment. For instance, under the worst corrosive state, corresponding to 1600 wetting/drying cycles, the maximum thickness loss was around 691 µm (around 49% of the nominal thickness).
- The corrosion rates at lower numbers of wetting/drying cycles (i.e., 50, 100, and 200 cycles) increased only slightly, with insignificant variations, demonstrating the corrosion resistance provided by the zinc coating. However, following zinc depletion and exposure to the bare steel surface, a substantial sharp increase in corrosion was observed for coupons exposed to 400 wetting/drying cycles. The developed corrosion modes included a combination of uniform and pitting corrosions, extending to cavities in the case of the coupons exposed to a higher number of wetting/drying cycles (e.g., 1600 cycles).
- The analysis of the rust layers showed similar corrosion product morphologies for all of the galvanized steel coupons. The XRD analysis results included four main chemical compounds: zinc oxide, quartz, magnetite, and hematite; while the SEM analysis with EDS indicated major elements such as iron, oxygen, zinc, and silicon.
- With regard to the mechanical properties of the corroded specimens, at a higher number of wetting/drying cycles, the tensile strengths, i.e., ultimate strength and yield strength, calculated based on the measured reduced cross-sectional area, hardness, and material ductility, decreased. The damage loss of the profile thickness and the deterioration of mechanical properties resulted in reduced axial stiffness and flexural stiffness, e.g., around 71% reductions corresponding to 1600 wetting/drying cycles, which weakened the loading capacity of the steel.
- Lastly, a simplified approximate corrosion prediction model, based on average predictions of four standard mathematical models, was presented to predict the corrosion of the bare steel in the galvanized coupons. The model predicts the corrosion time in years, corresponding to the thickness loss of the bare steel in the galvanized coupons following the depletion of the zinc coating. For instance, the average predictions corresponding to the corrosion developed in the coupons subjected to 1600 wetting/drying cycles gave around 50 years including the zinc depletion time. However, for a safer design, it is recommended that the predicted corrosion time for bare steel only should be used (around 46 years in the case of the 1600-cycle coupons), without adding the time required for depletion of the zinc coating. This would provide a conservative estimate for the timing of potential future damage that could lead to failure due to deterioration of the steel. The objective is to facilitate the implementation of measures necessary for avoiding failure, by providing rehabilitation solutions for damaged structures, or full replacement in severe cases.
6. Future Works
- Investigating the influence of corrosion propagation on the performance of buried steel pipes and culverts;
- Studying different rehabilitation measures to fix in-service deteriorated (damaged) steel structures;
- Lastly, investigating the effects of applying the proposed accelerated corrosion tests on coated metal specimens to evaluate the painting process on the steel surface with different primers, e.g., epoxy polyamide primer, at variable thicknesses to obtain their potential resistance against corrosion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Dai, Y.; Cheng, Y. Corrosion of underground pipelines in clay soil with varied soil layer thicknesses and aerations. Arab. J. Chem. 2020, 13, 3601–3614. [Google Scholar] [CrossRef]
- Wasim, M.; Li, C.; Robert, D.; Mahmoodian, M. Corrosion behaviour of pipes in soil and in simulated soil solution. In Proceedings of the Corrosion and Prevention Conference, Sydney, Australia, 12–15 November 2017; pp. 1–12. [Google Scholar]
- Ezzeldin, I.M.; El Naggar, H. Three-dimensional finite element modeling of corrugated metal pipes. Transp. Geotech. 2021, 27, 100467. [Google Scholar] [CrossRef]
- Ezzeldin, I.M.; El Naggar, H. Earth pressure distribution around flexible arch pipes. Eng. Struct. 2021, 237, 112226. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Huang, J.; Luo, L. Effect of corrosion on soil-structure interfacial shearing property and bearing capacity of steel foundation in submarine soil environment. J. Comput. Geotech. 2023, 156, 105269. [Google Scholar] [CrossRef]
- Ezzeldin, I.M.; El Naggar, H.; Newhook, J.; Jarjoura, G. Accelerated wetting/drying corrosion test for buried corrugated mild steel. Case Stud. Constr. Mater. 2022, 17, e01152. [Google Scholar]
- Artigas, A.; Monsalve, A.; Sipos, K.; Bustos, O.; Mena, J.; Seco, R.; Garza-Montes-de-Oca, N. Development of accelerated wet–dry cycle corrosion test in marine environment for weathering steels. Corros. Eng. Sci. Technol. 2015, 50, 628–632. [Google Scholar] [CrossRef]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Plenum Press: New York, NY, USA, 1996; pp. 183–213. [Google Scholar]
- Wasim, M.; Shoaib, S.; Mubarak, N.; Asiri, A.I. Factors influencing corrosion of metal pipes in soils. Environ. Chem. Lett. 2018, 16, 861–879. [Google Scholar] [CrossRef]
- El-Shamy, A.M.; Shehata, M.F.; Metwally, H.I.M.; Melegy, A. Corrosion and corrosion inhibition of steel pipeline in montmorillonitic soil filling material. Silicon J. 2018, 10, 2809–2815. [Google Scholar] [CrossRef]
- Wang, W.; Yang, W.; Shi, W.; Li, C. Modeling of corrosion pit growth for buried pipeline considering spatial and temporal variability. ASCE J. Eng. Mech. 2021, 147, 04021065. [Google Scholar] [CrossRef]
- Kim, C.; Chen, L.; Wang, H.; Castaneda, C. Global and local parameters for characterizing and modeling external corrosion in underground coated steel pipelines: A review of critical factors. J. Pipeline Sci. Eng. 2021, 1, 17–35. [Google Scholar] [CrossRef]
- Arriba-Rodriguez, L.; Ortega-Fernandez, F.; Villanueva-Balsera, J.; Rodriguez-Montequin, V. Corrosion predictive model in hot-dip galvanized steel buried in soil. Hindawi Complex. 2021, 2021, 9275779. [Google Scholar] [CrossRef]
- Bao, J.; Zhou, W. A random field model of external metal-loss corrosion on buried pipelines. J. Struct. Saf. 2021, 91, 102095. [Google Scholar] [CrossRef]
- Velázquez Altamirano, J.C. Modelación Estadística de Crecimiento de Picaduras en Tuberías Enterradas. Master’s Thesis, ESIQUIE, IPN, Ciudad de México, Mexico, 2006. [Google Scholar]
- Denison, I.A.; Romanoff, M. Corrosion of galvanized steels in soils. J. Res. Natl. Bur. Stand 1952, 49, 299–314. [Google Scholar] [CrossRef]
- Romanoff, M. Underground Corrosion; National Bureau of Standards, Circular 579; US Government Printing Office: Washington, DC, USA, 1957.
- Manning, P.E.; Duquette, D.J.; Savage, W.F. The role of sulfide inclusion morphology in pit initiation of several type 300 series stainless steels. Corrosion 1980, 36, 313–319. [Google Scholar] [CrossRef]
- Xia, D.; Deng, C.; Macdonald, D.; Jamali, S.; Mills, D.; Luo, J.; Strebl, M.G.; Amiri, M.; Jin, W.; Song, S.; et al. Electrochemical measurements used for assessment of corrosion and protection of metallic materials in the field: A critical review. J. Mater. Sci. Technol. 2022, 112, 151–183. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, G.; Muthumani, A. Corrosion of metals exposed to 25% magnesium chloride solution and tensile stress: Field and laboratory studies. J. Case Stud. Constr. Mater. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Li, X.; Hu, J.; Pan, Y.; Jin, Z. Indoor accelerated corrosion test and marine field test of corrosion-resistant low-alloy steel rebars. J. Case Stud. Constr. Mater. 2016, 5, 87–99. [Google Scholar] [CrossRef]
- Calero, J.; Alcantara, J.; Chico, B.; Diaz, I.; Simancas, J.; Fuente, D.; Morcillo, M. Wet/dry accelerated laboratory test to simulate the formation of multilayered rust on carbon steel in marine atmospheres. Corros. Eng. Sci. Technol. 2017, 52, 178–187. [Google Scholar] [CrossRef]
- Qian, S.; Cheng, Y.F. Accelerated corrosion of pipeline steel and reduced cathodic protection effectiveness under direct current interference. J. Constr. Build. Mater. 2017, 148, 675–685. [Google Scholar] [CrossRef]
- Suzumura, K.; Nakamurs, S. Environmental factors affecting corrosion of galvanized steel wires. J. Mater. Civ. Eng. 2004, 16, 1–7. [Google Scholar] [CrossRef]
- Lambert, M.R.; Townsend, H.E.; Hart, R.G.; Frydrych, D.J. Accelerated Corrosion Tests of Precoated Sheet Steels for Automobiles. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 378–384. [Google Scholar] [CrossRef]
- Lyon, S.B.; Thompson, G.E.; Johnson, J.B.; Wood, G.C.; Ferguson, J.M. Accelerated Atmospheric Corrosion Testing Using a Cyclic Wetting/drying Exposure Test: Aluminum, Galvanized Steel, and Steel. Natl. Assoc. Corros. Eng. 1987, 43, 719–726. [Google Scholar]
- Itoh, Y.; Kim, I. Accelerated cyclic corrosion testing of structural steels and its application to assess steel bridge coatings. Anti-Corros. Methods Mater. 2006, 53, 374–381. [Google Scholar] [CrossRef]
- Fishman, K.L.; Withiam, J.L. LRFD Metal Loss and Service-Life Strength Reduction Factors for Metal-Reinforced Systems; National Cooperative Highway Research Program (NCHRP), Report No. 675; Transportation Research Board: Washington, DC, USA, 2011. [Google Scholar]
- CAN/CSA-G401; Corrugated Steel Pipe Products. Canadian Standards Association (CSA): Mississauga, ON, Canada, 2014.
- CAN/CSA-S6-14; Canadian Highway Bridge Design Code. Canadian Standards Association (CSA): Mississauga, ON, Canada, 2014.
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2003.
- ASTM G1-03(2017); Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM A370-17a; Standard Test Methods and Definitions for Mechanical Testing of Steel Products. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM E8/E8M-21; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM E18-20; Standard Test Methods for Rockwell Hardness of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM B695-21; Standard Specification for Coatings of Zinc Mechanically Deposited on Iron and Steel. ASTM International: West Conshohocken, PA, USA, 2021.
- Sabzi, M.; Dezfuli, S.M.; Asadian, M.; Tafi, A.; Mahaab, A. Study of the effect of temperature on corrosion behavior of galvanized steel in seawater environment by using potentiodynamic polarization and EIS methods. Mater. Res. Express 2019, 6, 076508. [Google Scholar] [CrossRef]
- Cox, G.L. Effect of Temperature on the Corrosion of Zinc; Research Laboratory of Applied Chemistry, Department of Chemical Engineering, Massachusetts Institute of Technology: Cambridge, MA, USA, 1931; Volume 23, pp. 902–904. [Google Scholar]
- GM9540P; General Motors Engineering Standards, Materials and Processes-Procedures. Accelerated Corrosion Test: Detroit, MI, USA, 1997.
- Hiroshi, K.; Sakae, F.; Masaeu, S. New Accelerated Corrosion Test “ACTE” for Zinc-Coated Steel Sheets Used in Electrical Appliances; JFE Technical Report No. 12; JFE: Tokyo, Japan, 2006; pp. 36–41. [Google Scholar]
- Meade, C.L. Accelerated Corrosion Testing; National Exposure Testing: Sylvania, OH, USA, 1999; pp. 526–531. [Google Scholar]
- Song, Y.; Jiang, G.; Chen, Y. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Sci. Rep. 2017, 7, 6865. [Google Scholar] [CrossRef] [PubMed]
- Persson, D.; Thierry, D.; Karlsson, O. Corrosion and corrosion products of hot dipped galvanized steel during long term atmospheric exposure at different sites world-wide. Corros. Sci. 2017, 126, 152–165. [Google Scholar] [CrossRef]
- Cachet, C.; Ganne, F.; Maurin, G.; Petitjean, J.; Vivier, V.; Wiart, R. EIS investigation of zinc dissolution in aerated sulfate medium. Part I: Bulk zinc. Electrochem Acta 2001, 47, 509–518. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Sun, L. A comparative study on the corrosion of galvanized steel under simulated rust layer solution with and without 3.5wt%NaCl. Int. J. Electrochem. Sci. 2013, 8, 3494–3509. [Google Scholar] [CrossRef]
- Pojtanabuntoeng, K.; Machuca, L.; Salasi, M.; Kinsella, B.; Cooper, M. New experimental rig to investigate corrosion under insulation at different climate conditions. Corros. Mater. 2015, 40, 46–51. [Google Scholar]
- Rana, A.R.K.; Yang, M.; Umer, J.; Veret, T.; Brigham, G. Influence of robust drain openings and insulation standoffs on corrosion under insulation behavior of carbon steel. Corros. J. 2021, 77, 681–692. [Google Scholar] [CrossRef]
- Saad-Eldeen, S.; Garbatov, Y.; Guedes Soares, C. Effect of corrosion severity on the ultimate strength of a steel box girder. Eng. Struct. 2013, 49, 560–571. [Google Scholar] [CrossRef]
- Nikoloy, N.; Marcheva, Y.; Tsonev, V. Methods for preparation of corroded steel specimens for mechanical testing. MATEC Web Conf. 2019, 287, 07004. [Google Scholar] [CrossRef]
- Lee, H.; Cho, Y. Evaluation of the mechanical properties of steel reinforcement embedded in concrete specimen as a function of the degree of reinforcement corrosion. Int. J. Fract. 2009, 157, 81–88. [Google Scholar] [CrossRef]
- Hou, Y.; Lei, D.; Li, S.; Yang, W.; Li, C.-Q. Experimental investigation on corrosion effect on mechanical properties of buried metal pipes. Int. J. Corros. 2016, 2016, 5808372. [Google Scholar] [CrossRef]
- Cárdenas-Arias, C.; Rincón-Quintero, A.D.; Santos-Jaimes, A.; Sandoval Rodriguez, C.L.; Rojas-Gomez, D.; Ardila-Galvis, S.J. Elasticity modulus variation of the AISI SAE 1045 steel exposed to corrosion process by chloride using tension test destructive. IOP Conf. Ser. Mater. Sci. Eng. 2020, 844, 012059. [Google Scholar] [CrossRef]
- Kim, H.; Tae, S.; Lee, H.; Lee, S.; Noguchi, T. Evaluation of mechanical performance of corroded reinforcement considering the surface shape. ISIJ Int. 2009, 49, 1392–1400. [Google Scholar] [CrossRef]
- Chen, B.Q.; Garbatov, Y.; Guedes Soares, C. Mechanical properties assessment of specimens subjected to random non-uniform general corrosion and tensile load. In Maritime Engineering and Technology; Soares, C.G., Garbatov, Y., Sutulo, S., Santos, T.A., Eds.; Taylor & Francis Group: London, UK, 2012; ISBN 978-0-415-62146-5. [Google Scholar]
- Jurisic, P.; Parnuov, J. Influence of corrosion-related degradation of mechanical properties of shipbuilding steel on collapse strength of plates and stiffened panels. In Towards Green Marine Technology and Transport; Soares, C.G., Dejhalla, R., Pavletic, D., Eds.; Taylor & Francis Group: London, UK, 2015; ISBN 978-1-138-02887-6. [Google Scholar]
- Yu, H.; Zeng, X.; Ma, H.; Yue, P. Evolution law of reinforcement corrosion and mechanical properties under the conditions of basic magnesium sulfate cement concrete. KSCE J. Civ. Eng. 2022, 26, 4697–4708. [Google Scholar] [CrossRef]



| Fe | C | Mn | P | S |
|---|---|---|---|---|
| ~0.99 | 0.1–0.15 | 0.5–0.6 | 0.08 | 0.05 |
| Number of Wetting/Drying Cycles | 0 | 50 | 100 | 200 | 400 | 800 | 1600 |
|---|---|---|---|---|---|---|---|
| Maximum pit depth (Sv, µm) | 7.0 | 21.7 | 25.0 | 33.8 | 67.7 | 81.6 | 125.3 |
| Maximum surface height (Sz, µm) | 22.0 | 60.6 | 57.9 | 70.7 | 139.8 | 229.0 | 680.5 |
| No. Cycles | Sample | Elastic Modulus (GPa) | Ultimate Strength (MPa) | Yield Strength (MPa) |
|---|---|---|---|---|
| 0 | 1 | 201 | 407 | 331 |
| 2 | 205 | 415 | 343 | |
| 3 | 212 | 432 | 365 | |
| 4 | 208 | 418 | 355 | |
| Mean | 207 | 418 | 349 | |
| Standard Deviation | 4 | 9 | 13 | |
| 50 | 1 | 202 | 394 | 351 |
| 2 | 198 | 397 | 342 | |
| 3 | 206 | 407 | 352 | |
| 4 | 208 | 401 | 361 | |
| Mean | 204 | 400 | 352 | |
| Standard Deviation | 4 | 5 | 7 | |
| 100 | 1 | 204 | 397 | 342 |
| 2 | 194 | 410 | 332 | |
| 3 | 201 | 405 | 343 | |
| 4 | 192 | 408 | 341 | |
| Mean | 198 | 405 | 340 | |
| Standard Deviation | 5 | 5 | 4 | |
| 200 | 1 | 199 | 394 | 355 |
| 2 | 205 | 395 | 356 | |
| 3 | 211 | 411 | 362 | |
| 4 | 197 | 389 | 349 | |
| Mean | 203 | 397 | 356 | |
| Standard Deviation | 5 | 8 | 5 | |
| 400 | 1 | 160 | 324 | 274 |
| 2 | 172 | 336 | 292 | |
| 3 | 182 | 332 | 282 | |
| 4 | 175 | 352 | 301 | |
| Mean | 172 | 336 | 287 | |
| Standard Deviation | 8 | 10 | 10 | |
| 800 | 1 | 137 | 304 | 258 |
| 2 | 129 | 312 | 260 | |
| 3 | 139 | 289 | 238 | |
| 4 | 144 | 304 | 251 | |
| Mean | 137 | 302 | 252 | |
| Standard Deviation | 5 | 8 | 9 | |
| 1600 | 1 | 122 | 264 | 235 |
| 2 | 110 | 248 | 224 | |
| 3 | 135 | 281 | 245 | |
| Mean | 122 | 264 | 235 | |
| Standard Deviation | 10 | 13 | 9 | |
| Galvanized Steel Coupons | Mild Steel Coupons | |||||
|---|---|---|---|---|---|---|
| No. Cycles | Corrosion Damage (%) | Axial Stiffness (%) | Flexural Stiffness (%) | Corrosion Damage (%) | Axial Stiffness (%) | Flexural Stiffness (%) |
| 50 | 0.9 | 4.0 | 4.1 | 4.9 | 10.7 | 13.8 |
| 100 | 1.6 | 7.0 | 7.1 | 8.0 | 18.3 | 22.0 |
| 200 | 4.1 | 8.5 | 8.7 | 12.8 | 29.1 | 32.4 |
| 400 | 12.6 | 29.0 | 29.3 | 22.3 | 43.1 | 45.8 |
| 800 | 26.3 | 52.9 | 53.3 | 37.5 | 72.8 | 74.1 |
| 1600 | 49.0 | 70.7 | 71.1 | - | - | - |
| Wetting/drying accelerated test (cycles) | 0 | 50 | 100 | 200 | 400 | 800 | 1600 |
| Corrosion damage of total steel (with 41 µm zinc coating) (µm) | 0 | 12.8 | 26.3 | 58.5 | 179.6 | 367.8 | 691.4 |
| Corrosion damage of zinc coating (41 µm thickness) | 0 | 12.8 | 26.3 | 41 | 41 | 41 | 41 |
| Corrosion damage of bare steel (without 41 µm zinc coating) (µm) | 0 | 0 | 0 | 17.5 | 138.6 | 326.8 | 650.4 |
| Reduction in nominal steel thickness (zinc coating + bare steel) (%) | 0 | 0.9 | 1.63 | 4.13 | 12.63 | 26.3 | 49 |
| Predicted time corresponding to corrosion damage of steel (zinc coating + bare steel) (years) * | 0 | 1.25 | 2.57 | 5.2 | 13.8 | 27 | 49.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezzeldin, I.; El Naggar, H.; Newhook, J. Corrosion Performance of Buried Corrugated Galvanized Steel under Accelerated Wetting/Drying Cyclic Corrosion Test. Buildings 2024, 14, 1079. https://doi.org/10.3390/buildings14041079
Ezzeldin I, El Naggar H, Newhook J. Corrosion Performance of Buried Corrugated Galvanized Steel under Accelerated Wetting/Drying Cyclic Corrosion Test. Buildings. 2024; 14(4):1079. https://doi.org/10.3390/buildings14041079
Chicago/Turabian StyleEzzeldin, Islam, Hany El Naggar, and John Newhook. 2024. "Corrosion Performance of Buried Corrugated Galvanized Steel under Accelerated Wetting/Drying Cyclic Corrosion Test" Buildings 14, no. 4: 1079. https://doi.org/10.3390/buildings14041079
APA StyleEzzeldin, I., El Naggar, H., & Newhook, J. (2024). Corrosion Performance of Buried Corrugated Galvanized Steel under Accelerated Wetting/Drying Cyclic Corrosion Test. Buildings, 14(4), 1079. https://doi.org/10.3390/buildings14041079







