Impacts of Static Lighting in Confined Spaces on the Circadian Parameters, Alertness, Performance and Well-Being
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
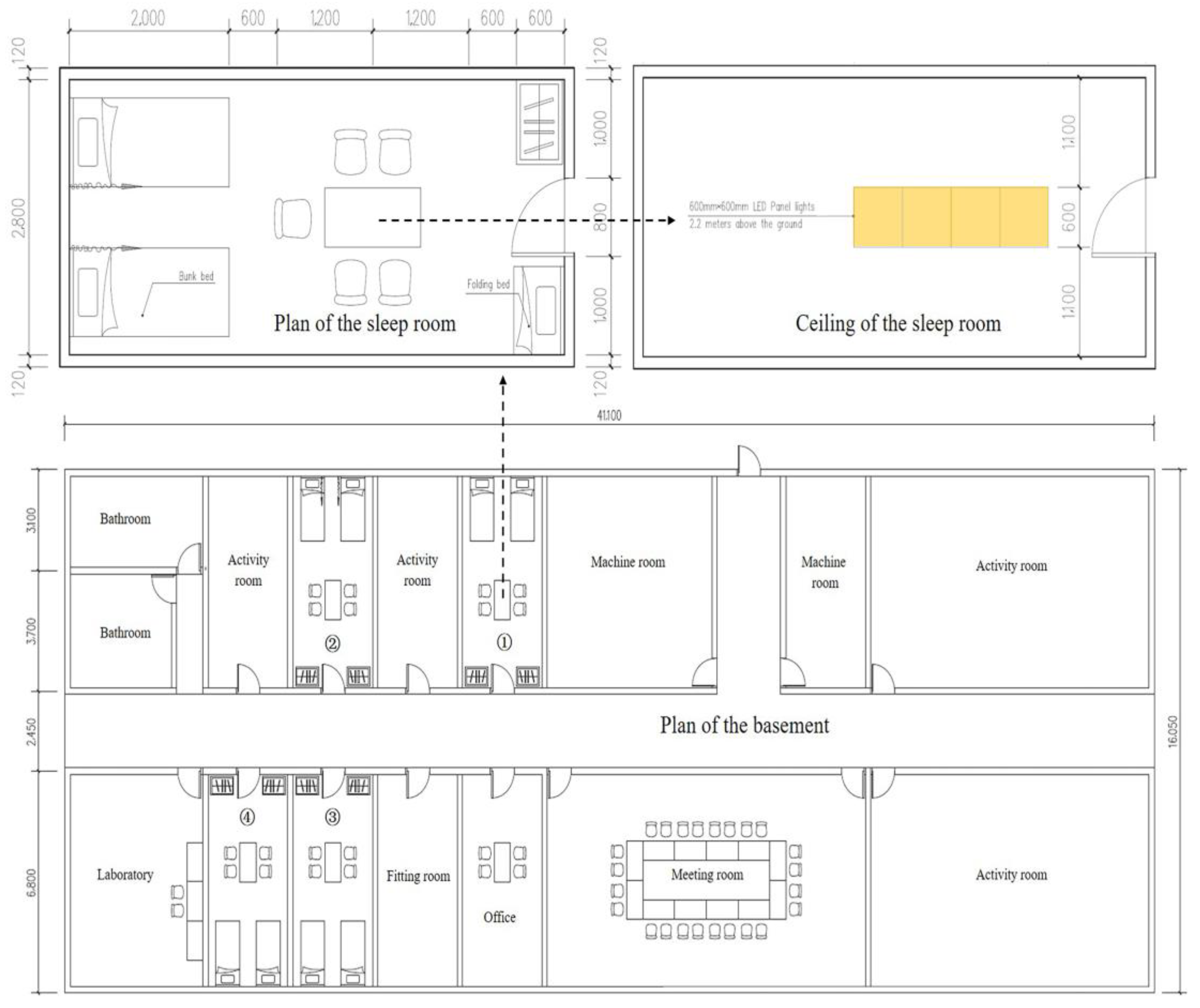
2.2. Experimental Setup
2.3. Light Treatment
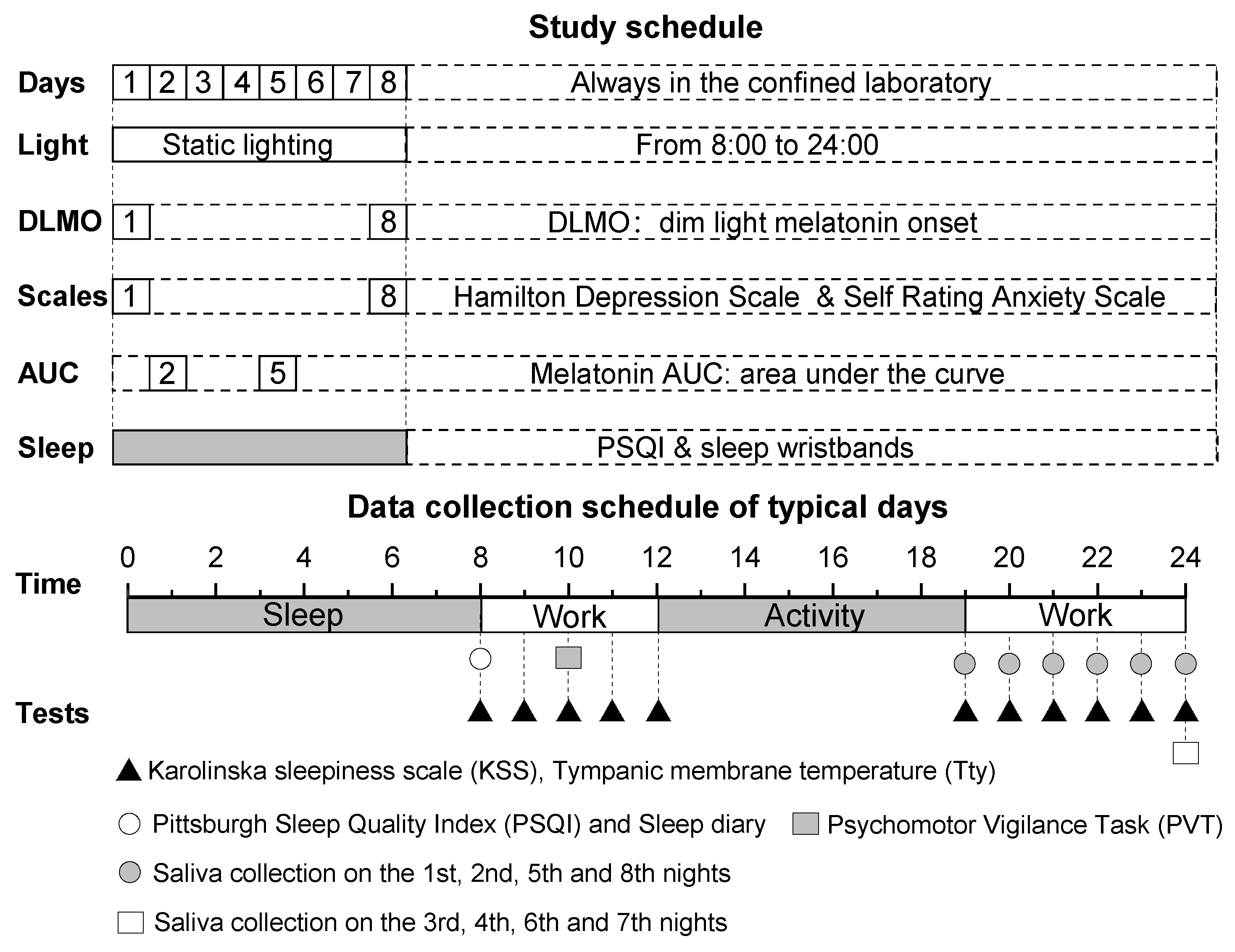
2.4. Experimental Protocol
2.5. Testing Methods
2.5.1. Subjective Variables
2.5.2. Tympanic Membrane Temperature (Tty)
2.5.3. Melatonin
2.5.4. Sleep Quality
2.5.5. Cognitive Performance
2.5.6. Emotional State
2.6. Data Statistics and Analysis Methods
3. Results
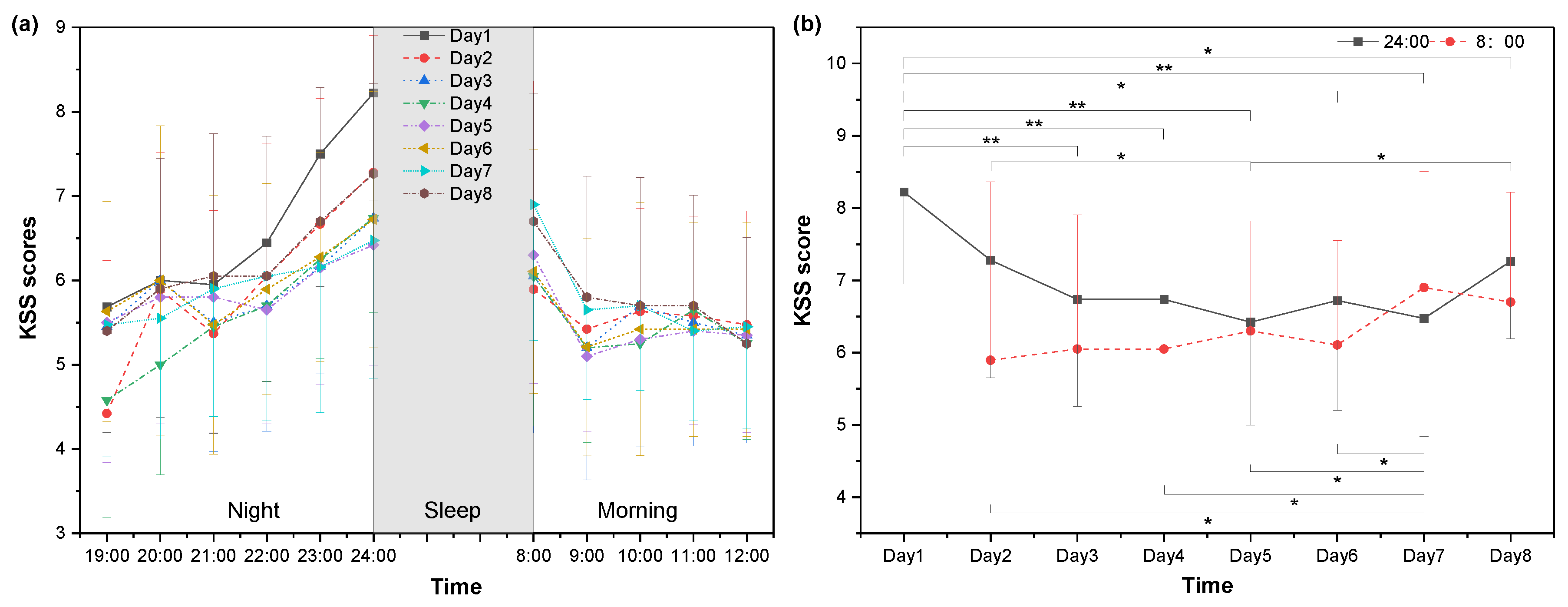
3.1. Subjective Sleepiness
3.2. Core Body Temperature
3.3. Melatonin
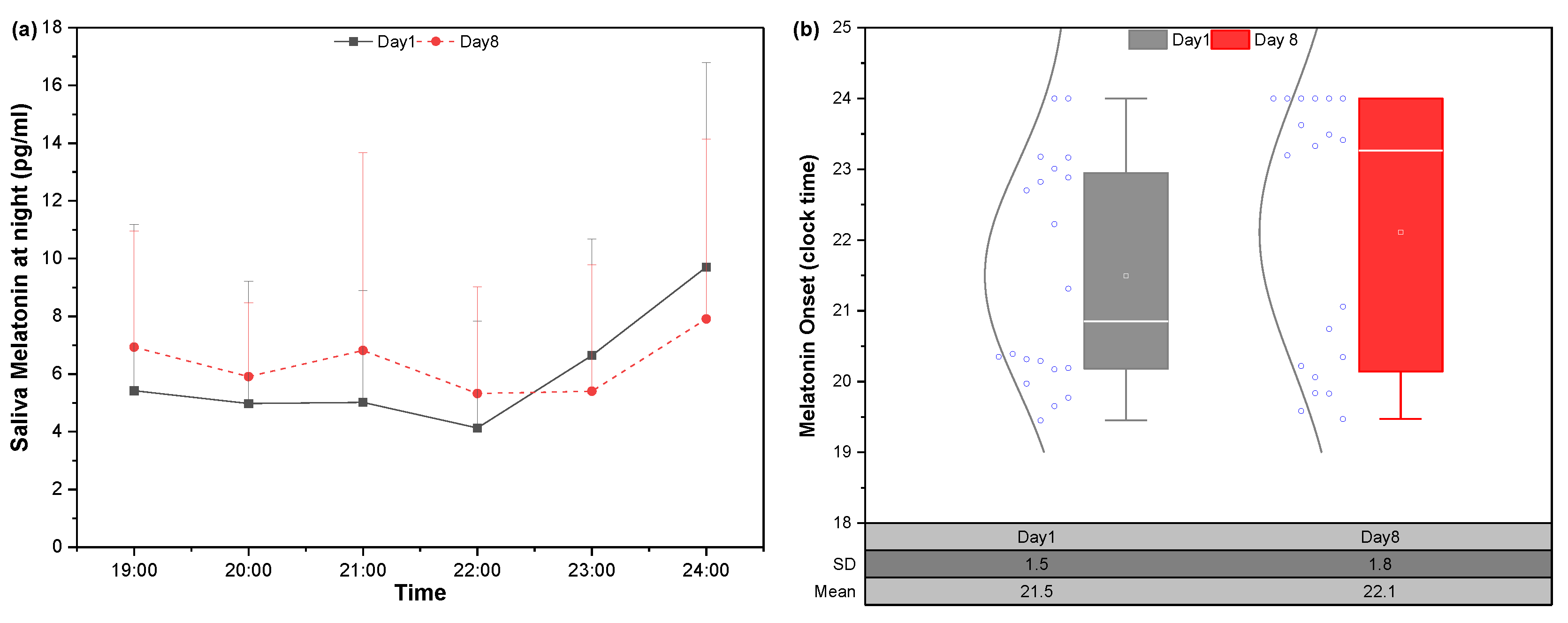
3.3.1. Salivary Melatonin and DLMO Changes in Dim Lighting Condition
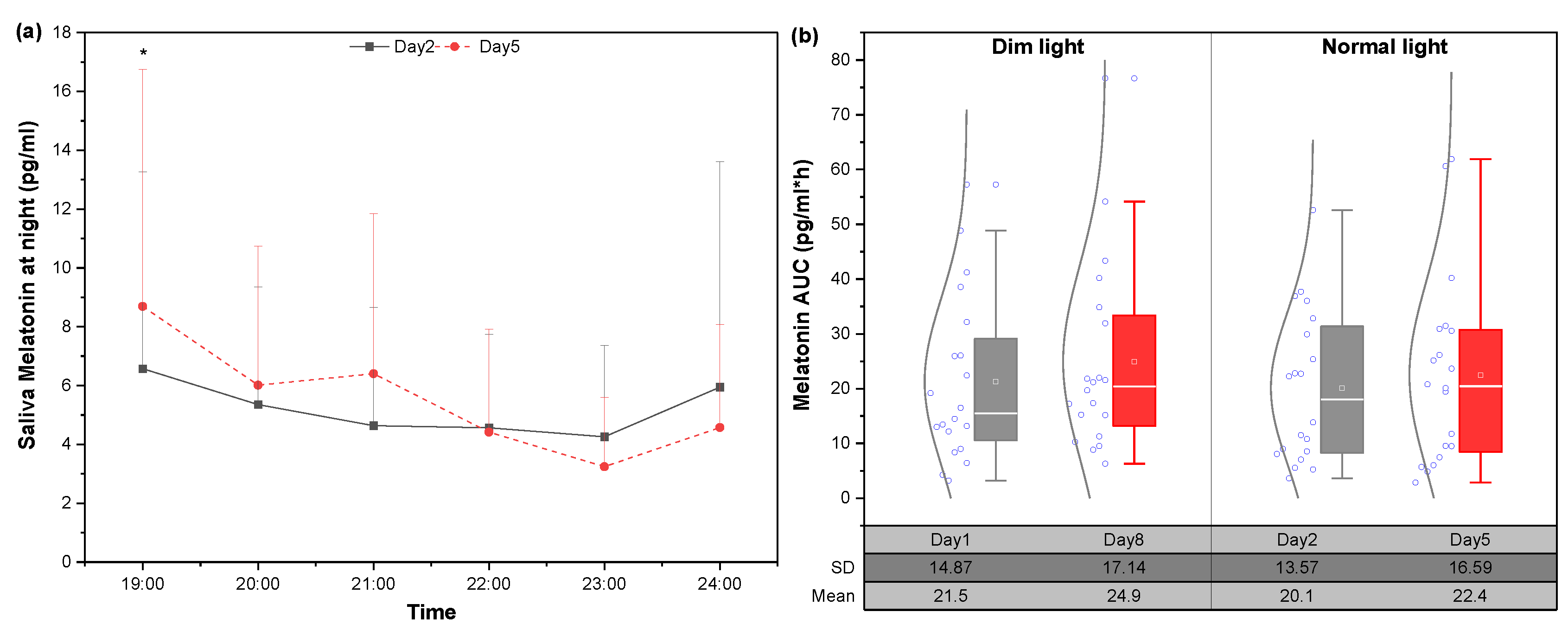
3.3.2. Changes in Melatonin under Static Lighting Conditions
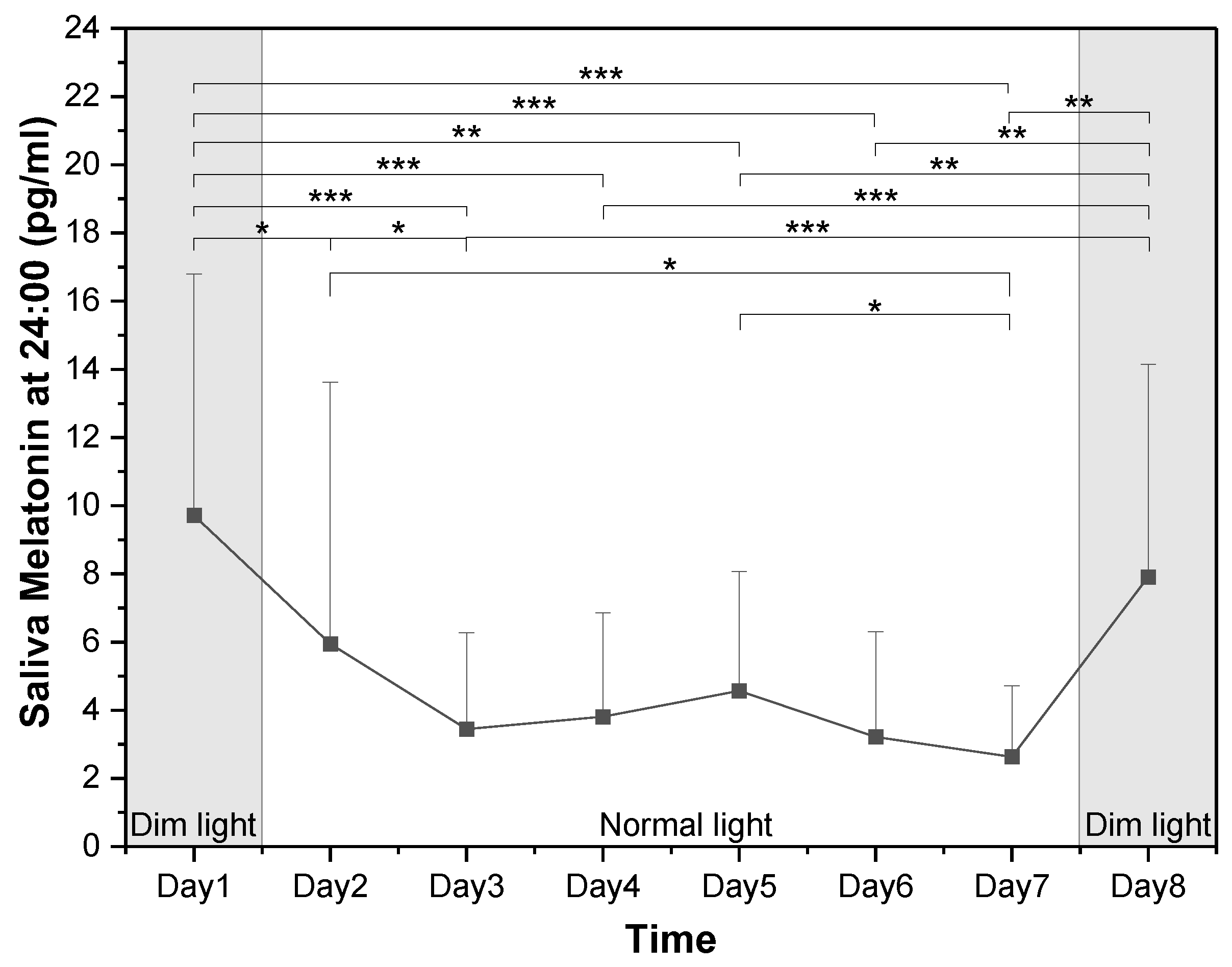
3.3.3. Changes in Melatonin Concentrations at Midnight
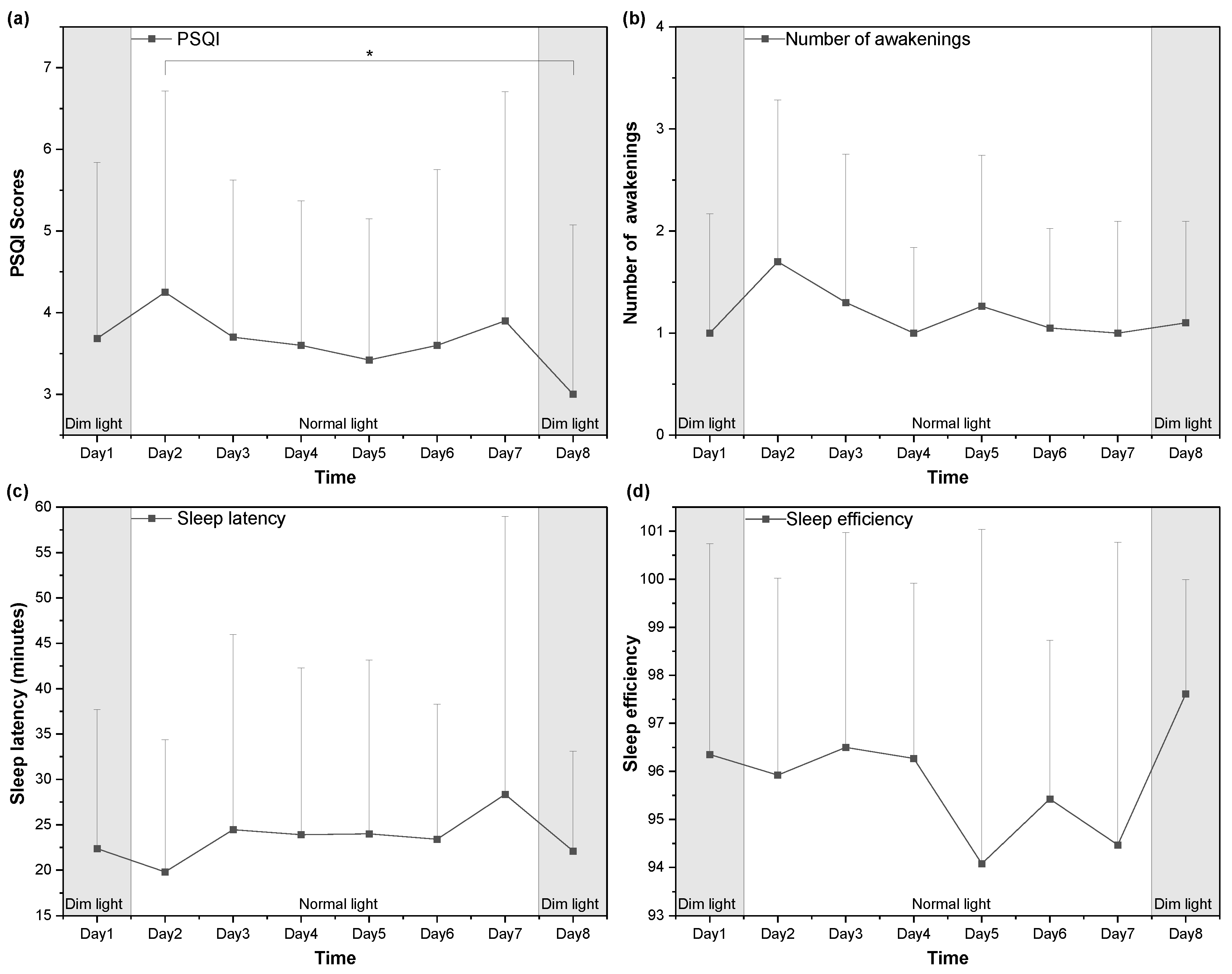
3.4. Sleep Quality
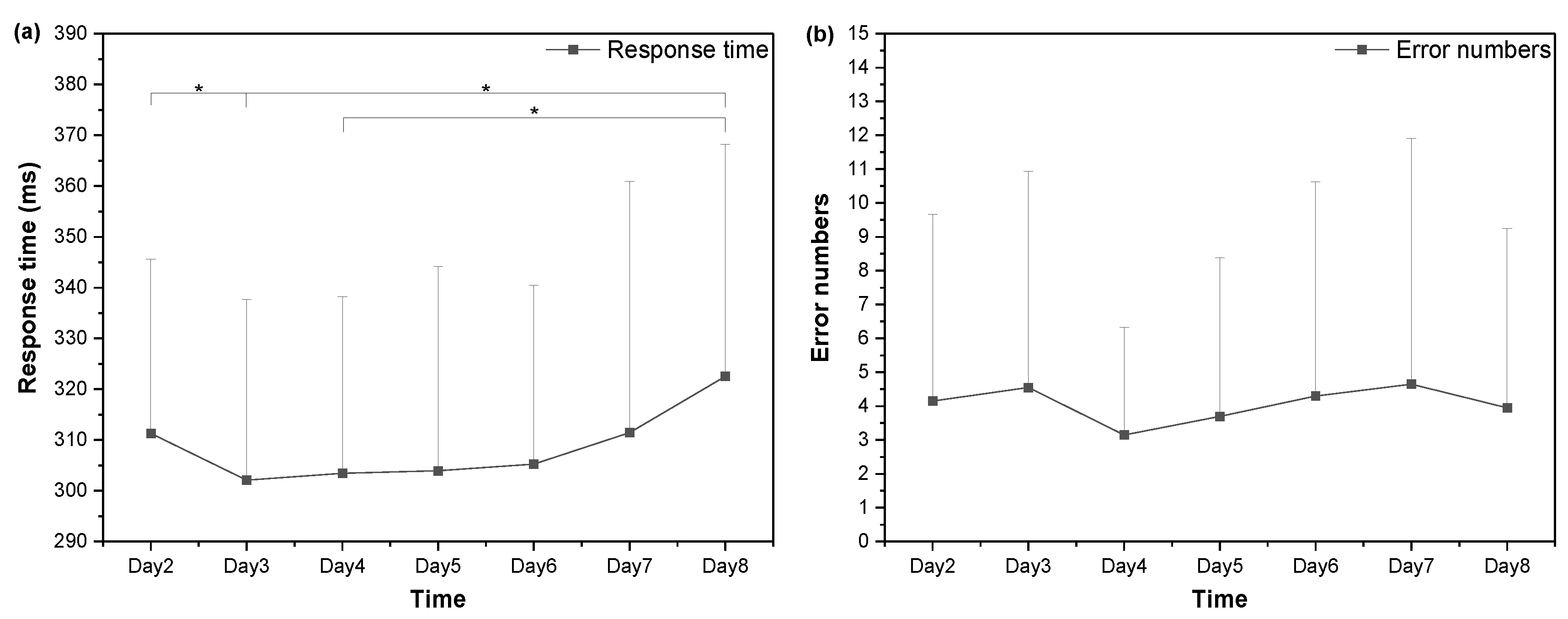
3.5. Cognitive Performance
3.6. Emotion Scale
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Häfker, N.S.; Tessmar-Raible, K. Rhythms of behavior: Are the times changin’? Curr. Opin. Neurobiol. 2020, 60, 55–66. [Google Scholar] [CrossRef]
- Brown, T.; Wright, K. Opinion: How do we define ‘healthy’ lighting? Light. Res. Technol. 2022, 54, 310. [Google Scholar] [CrossRef]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Vetter, C.; Pattison, P.M.; Houser, K.; Herf, M.; Phillips, A.J.; Wright, K.P.; Skene, D.J.; Brainard, G.C.; Boivin, D.B.; Glickman, G. A review of human physiological responses to light: Implications for the development of integrative lighting solutions. Leukos 2022, 18, 387–414. [Google Scholar] [CrossRef]
- CIE. CIE 158: 2009 Ocular Lighting Effects on Human Physiology and Behaviour; Commission Internationale de l’Eclairage: Vienna, Austria, 2009. [Google Scholar]
- Fernandez, F.X. Current insights into optimal lighting for promoting sleep and circadian health: Brighter days and the importance of sunlight in the built environment. Nat. Sci. Sleep 2022, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Palinkas, L.A.; Suedfeld, P. Psychosocial issues in isolated and confined extreme environments. Neurosci. Biobehav. Rev. 2021, 126, 413–429. [Google Scholar] [CrossRef]
- Jiang, N.; LI, Y.; Lai, H.; Yao, J.; Zhu, C.; Zhou, H.; WU, J. A tracking study on sleep characteristics of submariners during a long-term voyage. Med. J. Chin. People’s Lib. 2017, 42, 723. [Google Scholar]
- Nieuwenhuys, A.; Dora, J.; Melanie, K.-M.; Beckers, D.; Rietjens, G.; Helmhout, P. “20,000 leagues under the sea”: Sleep, cognitive performance, and self-reported recovery status during a 67-day military submarine mission. Appl. Ergon. 2021, 91, 103295. [Google Scholar] [CrossRef]
- Schlangen, L.J.; Price, L.L. The lighting environment, its metrology, and non-visual responses. Front. Neurol. 2021, 12, 624861. [Google Scholar] [PubMed]
- Smolensky, M.H.; Sackett-Lundeen, L.L.; Portaluppi, F. Nocturnal Light Pollution and Underexposure to Daytime Sunlight: Complementary Mechanisms of Circadian Disruption and Related Diseases; Taylor & Francis: Oxford, UK, 2015; Volume 32, pp. 1029–1048. [Google Scholar]
- Basner, M.; Dinges, D.F.; Mollicone, D.J.; Savelev, I.; Ecker, A.J.; Di Antonio, A.; Jones, C.W.; Hyder, E.C.; Kan, K.; Morukov, B.V. Psychological and behavioral changes during confinement in a 520-day simulated interplanetary mission to mars. PLoS ONE 2014, 9, e93298. [Google Scholar] [CrossRef] [PubMed]
- Pagel, J.I.; Choukèr, A. Effects of isolation and confinement on humans-implications for manned space explorations. J. Appl. Physiol. 2016, 120, 1449–1457. [Google Scholar] [CrossRef]
- Meng, C.; Wang, W.; Hao, Z.; Liu, H. Investigation on the influence of isolated environment on human psychological and physiological health. Sci. Total Environ. 2020, 716, 136972. [Google Scholar] [CrossRef]
- Mills, J. Circadian rhythms during and after three months in solitude underground. J. Physiol. 1964, 174, 217. [Google Scholar] [CrossRef]
- Leger, D.; Bayon, V.; Elbaz, M.; Philip, P.; Choudat, D. Underexposure to light at work and its association to insomnia and sleepiness: A cross-sectional study of 13,296 workers of one transportation company. J. Psychosom. Res. 2011, 70, 29–36. [Google Scholar] [CrossRef]
- Boubekri, M.; Cheung, I.N.; Reid, K.J.; Wang, C.; Zee, P.C. Impact of windows and daylight exposure on overall health and sleep quality of office workers: A case-control pilot study. J. Clin. Sleep Med. 2014, 10, 603–611. [Google Scholar] [CrossRef]
- LoPresti, M.L.; Anderson, J.A.; Saboe, K.N.; McGurk, D.L.; Balkin, T.J.; Sipos, M.L. The impact of insufficient sleep on combat mission performance. Mil. Behav. Health 2016, 4, 356–363. [Google Scholar] [CrossRef]
- Luyster, F.S.; Strollo, P.J.; Zee, P.C.; Walsh, J.K. Sleep: A health imperative. Sleep 2012, 35, 727–734. [Google Scholar] [CrossRef]
- Jiang, N.; Sheng, X.; Jiang, D.; Chen, W. Relationship between work stress and health in submariners. Jie Fang Jun Yi Xue Za Zhi 2013, 38, 665. [Google Scholar]
- Kawasaki, A.; Wisniewski, S.; Healey, B.; Pattyn, N.; Kunz, D.; Basner, M.; Münch, M. Impact of long-term daylight deprivation on retinal light sensitivity, circadian rhythms and sleep during the Antarctic winter. Sci. Rep. 2018, 8, 16185. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, S.; Kong, Z.; Chen, N. Sleep architecture, periodic breathing and mood disturbance of expeditioners at Kunlun Station (4087 m) in Antarctica. Adv. Polar Sci. 2020, 31, 215–223. [Google Scholar]
- Sandal, G.M.; van de Vijver, F.J.; Smith, N. Psychological hibernation in Antarctica. Front. Psychol. 2018, 9, 2235. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.A.; Bower, J.L.; Connaboy, C.; Agha, N.H.; Baker, F.L.; Smith, K.A.; So, C.J.; Simpson, R.J. Mental health, physical symptoms and biomarkers of stress during prolonged exposure to Antarctica’s extreme environment. Acta Astronaut. 2021, 181, 405–413. [Google Scholar] [CrossRef]
- Kuwabara, T.; Naruiwa, N.; Kawabe, T.; Kato, N.; Sasaki, A.; Ikeda, A.; Otani, S.; Imura, S.; Watanabe, K.; Ohno, G. Human change and adaptation in Antarctica: Psychological research on Antarctic wintering-over at Syowa station. Int. J. Circumpolar Health 2021, 80, 1886704. [Google Scholar] [CrossRef] [PubMed]
- Palinkas, L.A.; Suedfeld, P. Psychological effects of polar expeditions. Lancet 2008, 371, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wu, Q.; Xiong, Y.; Chen, G.; Song, D.; Xu, C. Circadian rhythm and sleep during prolonged Antarctic residence at Chinese Zhongshan station. Wilderness Environ. Med. 2016, 27, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Palinkas, L.A.; Houseal, M. Stages of change in mood and behavior during a winter in Antarctica. Environ. Behav. 2000, 32, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Korman, M.; Tkachev, V.; Reis, C.; Komada, Y.; Kitamura, S.; Gubin, D.; Kumar, V.; Roenneberg, T. Outdoor daylight exposure and longer sleep promote wellbeing under COVID-19 mandated restrictions. J. Sleep Res. 2022, 31, e13471. [Google Scholar] [CrossRef] [PubMed]
- Theberath, M.; Bauer, D.; Chen, W.; Salinas, M.; Mohabbat, A.B.; Yang, J.; Chon, T.Y.; Bauer, B.A.; Wahner-Roedler, D.L. Effects of COVID-19 pandemic on mental health of children and adolescents: A systematic review of survey studies. SAGE Open Med. 2022, 10, 20503121221086712. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.; Léger, D.; Fressard, L.; Peretti-Watel, P.; Verger, P.; Group, C. COVID-19 health crisis and lockdown associated with high level of sleep complaints and hypnotic uptake at the population level. J. Sleep Res. 2021, 30, e13119. [Google Scholar] [CrossRef]
- Sharma, M.; Aggarwal, S.; Madaan, P.; Saini, L.; Bhutani, M. Impact of COVID-19 pandemic on sleep in children and adolescents: A systematic review and meta-analysis. Sleep Med. 2021, 84, 259–267. [Google Scholar] [CrossRef]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the circadian system in shift work and its health impact. J. Biol. Rhythm. 2022, 37, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E. Melatonin, sleep disturbance and cancer risk. Sleep Med. Rev. 2009, 13, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Blaxton, J.M.; Bergeman, C.S.; Whitehead, B.R.; Braun, M.E.; Payne, J.D. Relationships among nightly sleep quality, daily stress, and daily affect. J. Gerontol. Ser. B 2017, 72, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.P.; van Der Helm, E. Overnight therapy? The role of sleep in emotional brain processing. Psychol. Bull. 2009, 135, 731. [Google Scholar] [CrossRef] [PubMed]
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ 2016, 355, 5210. [Google Scholar] [CrossRef] [PubMed]
- Proper, K.I.; Van De Langenberg, D.; Rodenburg, W.; Vermeulen, R.C.; Van Der Beek, A.J.; Van Steeg, H.; Van Kerkhof, L.W. The relationship between shift work and metabolic risk factors: A systematic review of longitudinal studies. Am. J. Prev. Med. 2016, 50, e147–e157. [Google Scholar] [CrossRef] [PubMed]
- Livingston, W.S.; Rusch, H.L.; Nersesian, P.V.; Baxter, T.; Mysliwiec, V.; Gill, J.M. Improved sleep in military personnel is associated with changes in the expression of inflammatory genes and improvement in depression symptoms. Front. Psychiatry 2015, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Vyas, M.V.; Garg, A.X.; Iansavichus, A.V.; Costella, J.; Donner, A.; Laugsand, L.E.; Janszky, I.; Mrkobrada, M.; Parraga, G.; Hackam, D.G. Shift work and vascular events: Systematic review and meta-analysis. BMJ 2012, 345, e4800. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yeung, K.; Chan, W.; Kwok, C.; Leung, S.; Wu, C.; Chan, E.; Yu, I.; Yang, X.; Tse, L. A meta-analysis on dose–response relationship between night shift work and the risk of breast cancer. Ann. Oncol. 2013, 24, 2724–2732. [Google Scholar] [CrossRef]
- Benke, K.K.; Benke, K.E. Uncertainty in health risks from artificial lighting due to disruption of circadian rhythm and melatonin secretion: A review. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 916–929. [Google Scholar] [CrossRef]
- Van de Putte, E.; Kindt, S.; Bracke, P.; Stevens, M.; Vansteenkiste, M.; Vandevivere, L.; Ryckaert, W.R. The influence of integrative lighting on sleep and cognitive functioning of shift workers during the morning shift in an assembly plant. Appl. Ergon. 2022, 99, 103618. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, J.; Wang, Y.; Dai, S.; Shao, R.; Hao, L. Active interventions of dynamic lighting on human circadian rhythm and sleep quality in confined spaces. Build. Environ. 2022, 226, 109766. [Google Scholar] [CrossRef]
- Zhao, J.P. GB 50034-2013; Standard for Lighting Design of Buildings. China Architecture & Building Press: Beijing, China, 2013.
- Pandi-Perumal, S.R.; Smits, M.; Spence, W.; Srinivasan, V.; Cardinali, D.P.; Lowe, A.D.; Kayumov, L. Dim light melatonin onset (DLMO): A tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Simón, D.; Pannunzio, B.; Casaravilla, C.; Díaz, Á.; Tassino, B. Chronotype-dependent changes in sleep habits associated with dim light melatonin onset in the antarctic summer. Clocks Sleep 2019, 1, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, M.G.; Bullough, J.D.; Bierman, A.; Fay, C.R.; Rea, M.S. On light as an alerting stimulus at night. Acta Neurobiol. Exp. 2007, 67, 171. [Google Scholar] [CrossRef] [PubMed]
- Stefani, O.; Freyburger, M.; Veitz, S.; Basishvili, T.; Meyer, M.; Weibel, J.; Kobayashi, K.; Shirakawa, Y.; Cajochen, C. Changing color and intensity of LED lighting across the day impacts on circadian melatonin rhythms and sleep in healthy men. J. Pineal Res. 2021, 70, e12714. [Google Scholar] [CrossRef] [PubMed]
- Kaida, K.; Takahashi, M.; Åkerstedt, T.; Nakata, A.; Otsuka, Y.; Haratani, T.; Fukasawa, K. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin. Neurophysiol. 2006, 117, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.T.; Kane, N.L.; Soos, G.; Gisolfi, C.V.; Kondo, N.; Sato, K. Reexamination of tympanic membrane temperature as a core temperature. J. Appl. Physiol. 1996, 80, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; He, S.; Dai, C.; Chen, S.; Chen, H.; Lin, Y. Lighting scheme recommendation for interior workplace to adjust the phase-advance jet lag. Build. Environ. 2021, 198, 107913. [Google Scholar] [CrossRef]
- Wang, T.; Shao, R.; Hao, L. Effects of Different Nocturnal Lighting Stimuli on Melatonin, Sleep and Cognitive Performance of Workers in Confined Spaces. Buildings 2023, 13, 2112. [Google Scholar] [CrossRef]
- Zitser, J.; Allen, I.E.; Falgàs, N.; Le, M.M.; Neylan, T.C.; Kramer, J.H.; Walsh, C.M. Pittsburgh Sleep Quality Index (PSQI) responses are modulated by total sleep time and wake after sleep onset in healthy older adults. PLoS ONE 2022, 17, e0270095. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kahathuduwa, C.; Vazsonyi, A.T. The Pittsburgh Sleep Quality Index (PSQI): Psychometric and clinical risk score applications among college students. Psychol. Assess. 2021, 33, 816. [Google Scholar] [CrossRef] [PubMed]
- Thomann, J.; Baumann, C.R.; Landolt, H.-P.; Werth, E. Psychomotor vigilance task demonstrates impaired vigilance in disorders with excessive daytime sleepiness. J. Clin. Sleep Med. 2014, 10, 1019–1024. [Google Scholar] [PubMed]
- Mendt, S.; Maggioni, M.A.; Nordine, M.; Steinach, M.; Opatz, O.; Belavy, D.; Felsenberg, D.; Koch, J.; Shang, P.; Gunga, H.C.; et al. Circadian rhythms in bed rest: Monitoring core body temperature via heat-flux approach is superior to skin surface temperature. Chronobiol. Int. 2017, 34, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Lack, L.C.; Gradisar, M.; Van Someren, E.J.; Wright, H.R.; Lushington, K. The relationship between insomnia and body temperatures. Sleep Med. Rev. 2008, 12, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X. What constitutes a physiological concentration of melatonin? J. Pineal Res. 2003, 34, 79–80. [Google Scholar] [PubMed]
- Molina, T.A.; Burgess, H.J. Calculating the dim light melatonin onset: The impact of threshold and sampling rate. Chronobiol. Int. 2011, 28, 714–718. [Google Scholar] [PubMed]
- Benloucif, S.; Burgess, H.J.; Klerman, E.B.; Lewy, A.J.; Middleton, B.; Murphy, P.J.; Parry, B.L.; Revell, V.L. Measuring melatonin in humans. J. Clin. Sleep Med. 2008, 4, 66–69. [Google Scholar] [PubMed]
- Nagtegaal, E.; Peeters, T.; Swart, W.; Smits, M.; Kerkhof, G.; van der Meer, G. Correlation between concentrations of melatonin in saliva and serum in patients with delayed sleep phase syndrome. Ther. Drug Monit. 1998, 20, 181–183. [Google Scholar] [CrossRef]
- Voultsios, A.; Kennaway, D.J.; Dawson, D. Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J. Biol. Rhythm. 1997, 12, 457–466. [Google Scholar]
- Figueiro, M.G.; Rea, M.S. Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students. Neuro. Endocrinol. Lett. 2010, 31, 92. [Google Scholar] [PubMed]
- Höhn, C.; Schmid, S.R.; Plamberger, C.P.; Bothe, K.; Angerer, M.; Gruber, G.; Pletzer, B.; Hoedlmoser, K. Preliminary Results: The Impact of Smartphone Use and Short-Wavelength Light during the Evening on Circadian Rhythm, Sleep and Alertness. Clocks Sleep 2021, 3, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Hébert, M.; Martin, S.K.; Lee, C.; Eastman, C.I. The effects of prior light history on the suppression of melatonin by light in humans. J. Pineal Res. 2002, 33, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ru, T.; Kompier, M.E.; Chen, Q.; Zhou, G.; Smolders, K.C.H.J. Temporal tuning of illuminance and spectrum: Effect of a full-day dynamic lighting pattern on well-being, performance and sleep in simulated office environment. Build. Environ. 2023, 228, 109842. [Google Scholar]
- Schledermann, K.M.; Bjørner, T.; West, A.S.; Hansen, T.S. Evaluation of staff’s perception of a circadian lighting system implemented in a hospital. Build. Environ. 2023, 242, 110488. [Google Scholar] [CrossRef]
- Brasher, K.; Sparshott, K.; Weir, A.; Day, A.; Bridger, R. Two year follow-up study of stressors and occupational stress in submariners. Occup. Med. 2012, 62, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wu, Q.; Li, H.; Zhang, T.; Xu, C. Different adaptations of Chinese winter-over expeditioners during prolonged Antarctic and sub-Antarctic residence. Int. J. Biometeorol. 2016, 60, 737–747. [Google Scholar] [PubMed]
- Arendt, J.; Middleton, B. Human seasonal and circadian studies in Antarctica (Halley, 75 S). Gen. Comp. Endocrinol. 2018, 258, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kantermann, T.; Juda, M.; Vetter, C.; Allebrandt, K.V. Light and the human circadian clock. Circadian Clocks 2013, 217, 311–331. [Google Scholar]
- Kanas, N.; Sandal, G.M.; Boyd, J.E.; Gushin, V.I.; Manzey, D.; North, R.; Leon, G.R.; Suedfeld, P.; Bishop, S.L.; Fiedler, E.R. Psychology and culture during long-duration space missions. In On Orbit and Beyond; Springer: Berlin/Heidelberg, Germany, 2013; pp. 153–184. [Google Scholar]
- Suedfeld, P. Antarctica and space as psychosocial analogues. Reach 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Ahmad, M.; Md. Din, N.S.B.; Tharumalay, R.D.; Che Din, N.; Ibrahim, N.; Amit, N.; Farah, N.M.; Osman, R.A.; Abdul Hamid, M.F.; Ibrahim, I.A. The effects of circadian rhythm disruption on mental health and physiological responses among shift workers and general population. Int. J. Environ. Res. Public Health 2020, 17, 7156. [Google Scholar] [CrossRef] [PubMed]
- Young, C.R.; Jones, G.E.; Figueiro, M.G.; Soutière, S.E.; Keller, M.W.; Richardson, A.M.; Lehmann, B.J.; Rea, M.S. At-sea trial of 24-h-based submarine watchstanding schedules with high and low correlated color temperature light sources. J. Biol. Rhythm. 2015, 30, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Trousselard, M.; Leger, D.; van Beers, P.; Coste, O.; Vicard, A.; Pontis, J.; Crosnier, S.-N.; Chennaoui, M. Sleeping under the ocean: Despite total isolation, nuclear Submariners maintain their sleep and wake patterns throughout their under sea mission. PLoS ONE 2015, 10, e0126721. [Google Scholar]
- Zhang, T.; Li, W.; Cheng, Z. Design of Scene Illumination System in Large Manned Spacecraft. Manned Spacefl. 2018, 24, 178–182. [Google Scholar]
- Dong, X.; Wu, Y.; Chen, X.; Li, H.; Cao, B.; Zhang, X.; Yan, X.; Li, Z.; Long, Y.; Li, X. Effect of thermal, acoustic, and lighting environment in underground space on human comfort and work efficiency: A review. Sci. Total Environ. 2021, 786, 147537. [Google Scholar]
- Francis, G.; Bishop, L.; Luke, C.; Middleton, B.; Williams, P.; Arendt, J. Sleep during the Antarctic winter: Preliminary observations on changing the spectral composition of artificial light. J. Sleep Res. 2008, 17, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhou, T.; Chen, Z.; Dang, W.; Jiao, F.; Zhan, J.; Chen, Y.; Chen, Y.; Pan, Z.; Kang, X.; et al. Investigation on entraining and enhancing human circadian rhythm in closed environments using daylight-like LED mixed lighting. Sci. Total Environ. 2020, 732, 139334. [Google Scholar] [CrossRef] [PubMed]
- Lowden, A.; Kecklund, G. Considerations on how to light the night-shift. Light. Res. Technol. 2021, 53, 437–452. [Google Scholar]
- Van Bommel, W.J. Non-visual biological effect of lighting and the practical meaning for lighting for work. Appl. Ergon. 2006, 37, 461–466. [Google Scholar] [CrossRef]
- Lowden, A.; Akerstedt, T. Assessment of a new dynamic light regimen in a nuclear power control room without windows on quickly rotating shiftworkers--effects on health, wakefulness, and circadian alignment: A pilot study. Chronobiol. Int. 2012, 29, 641–649. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Pedler, D. Red light: A novel, non-pharmacological intervention to promote alertness in shift workers. J. Saf. Res. 2020, 74, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Beaulieu, C. Light exposure in the natural environment: Relevance to mood and sleep disorders. Sleep Med. 2007, 8, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Cai, H.; Li, X. Non-visual effects of indoor light environment on humans: A review. Physiol. Behav. 2021, 228, 113195. [Google Scholar] [CrossRef] [PubMed]
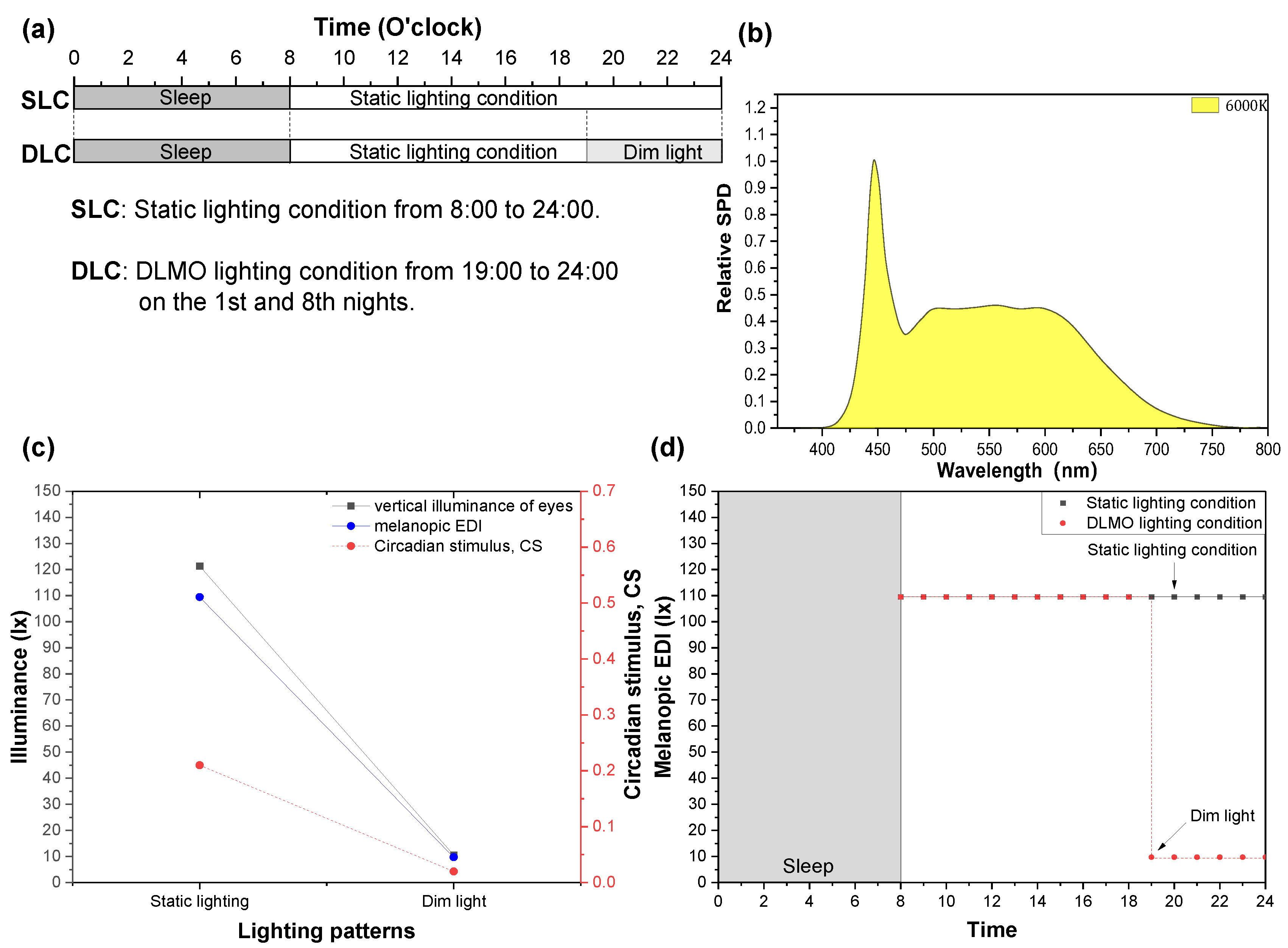


| Light Patterns | Static Lighting | Dim Lighting |
|---|---|---|
| Illuminance on the table/lx | 310.76 | 29.39 |
| Illuminance at the eyes/lx | 121.38 | 10.61 |
| Melanopic EDI/lx | 109.45 | 9.75 |
| S-cone-opic EDI/lx | 110.99 | 9.48 |
| M-cone-opic EDI/lx | 118.7 | 10.28 |
| L-cone-opic EDI/lx | 122.62 | 10.62 |
| Rhodopic EDI/lx | 112.07 | 9.86 |
| CLA/lx | 168 | 12 |
| CS | 0.21 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Shao, R.; Wang, Y.; Li, J.; Hao, L. Impacts of Static Lighting in Confined Spaces on the Circadian Parameters, Alertness, Performance and Well-Being. Buildings 2024, 14, 1115. https://doi.org/10.3390/buildings14041115
Wang T, Shao R, Wang Y, Li J, Hao L. Impacts of Static Lighting in Confined Spaces on the Circadian Parameters, Alertness, Performance and Well-Being. Buildings. 2024; 14(4):1115. https://doi.org/10.3390/buildings14041115
Chicago/Turabian StyleWang, Tongyue, Rongdi Shao, Yanni Wang, Juanjie Li, and Luoxi Hao. 2024. "Impacts of Static Lighting in Confined Spaces on the Circadian Parameters, Alertness, Performance and Well-Being" Buildings 14, no. 4: 1115. https://doi.org/10.3390/buildings14041115






