Experimental and Three-Dimensional Numerical Investigations of Dehydration and Pyrolysis in Wood under Elevated and High Temperatures
Abstract
1. Introduction
2. Modeling of Thermo-Physical Properties of Wood
2.1. Mass Transfer Model Considering Dehydration and Pyrolysis
2.2. Density
2.3. Specific Heat Capacity
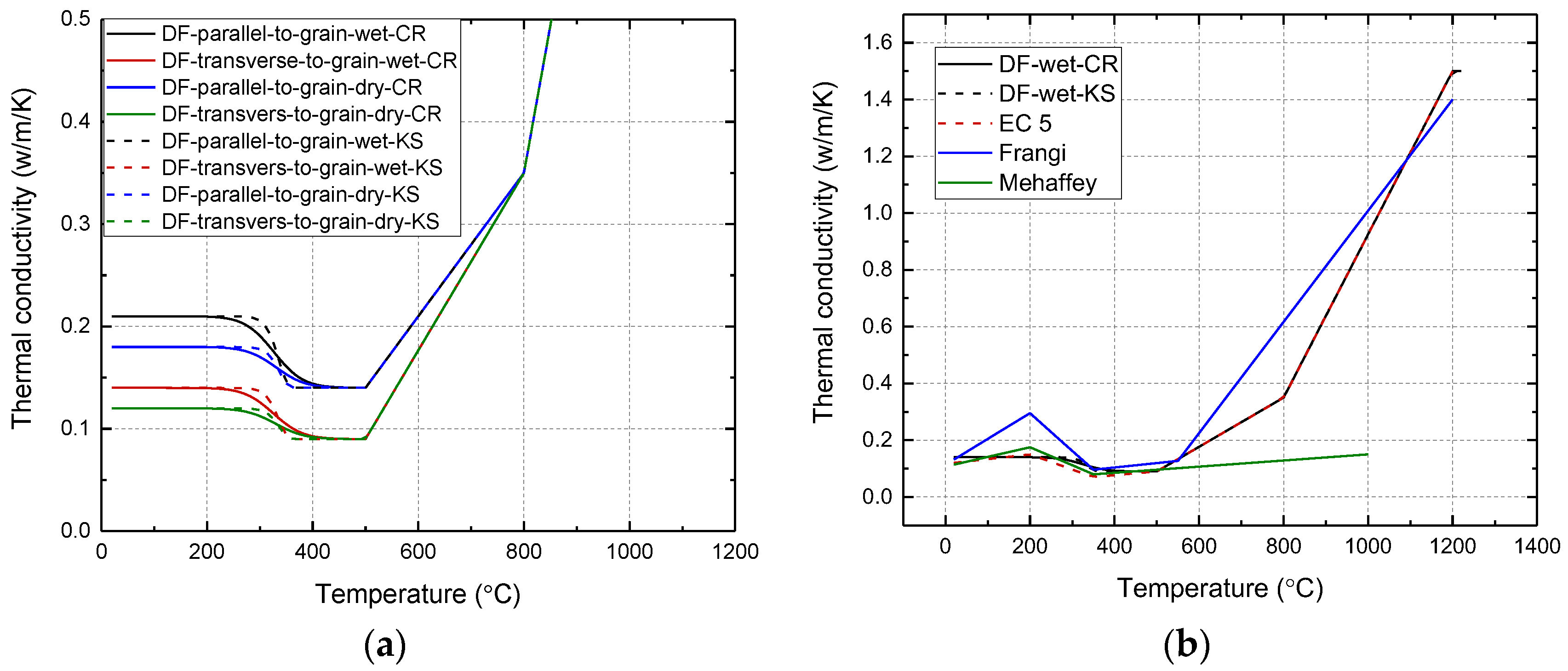
2.4. Thermal Conductivity
3. Numerical Modeling in Abaqus and OpenFOAM
3.1. Heat Transfer Model in Abaqus
3.2. Implementation in Abaqus
- Internal thermal energy, U;
- Variation of U with respect to temperature, ∂U/∂T;
- Variation of U with respect to the spatial gradients of temperature, ∂U/∂(∂T/∂xi) (i = 1, 2, 3);
- Heat flux vector, F;
- Variation of F with respect to temperature, ∂F/∂T;
- Variation of F with respect to the spatial gradients of temperature, ∂F/∂(∂T/∂xi) (i = 1, 2, 3).
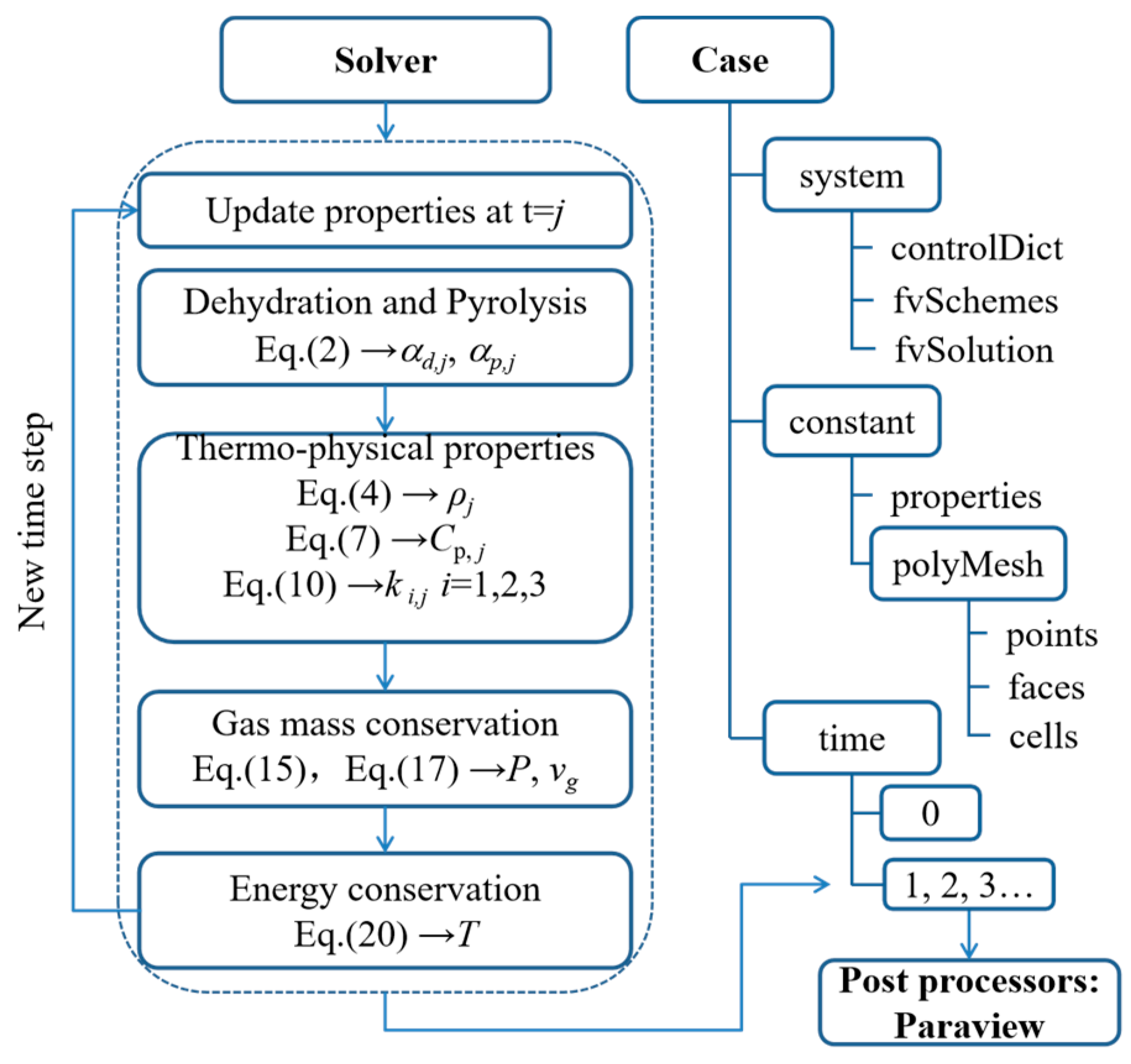
3.3. Heat Transfer Model in OpenFOAM
3.4. Implementation in OpenFOAM
4. Dehydration Experiments and Validation of Numerical Models
4.1. Dehydration Experiments under Elevated Temperature
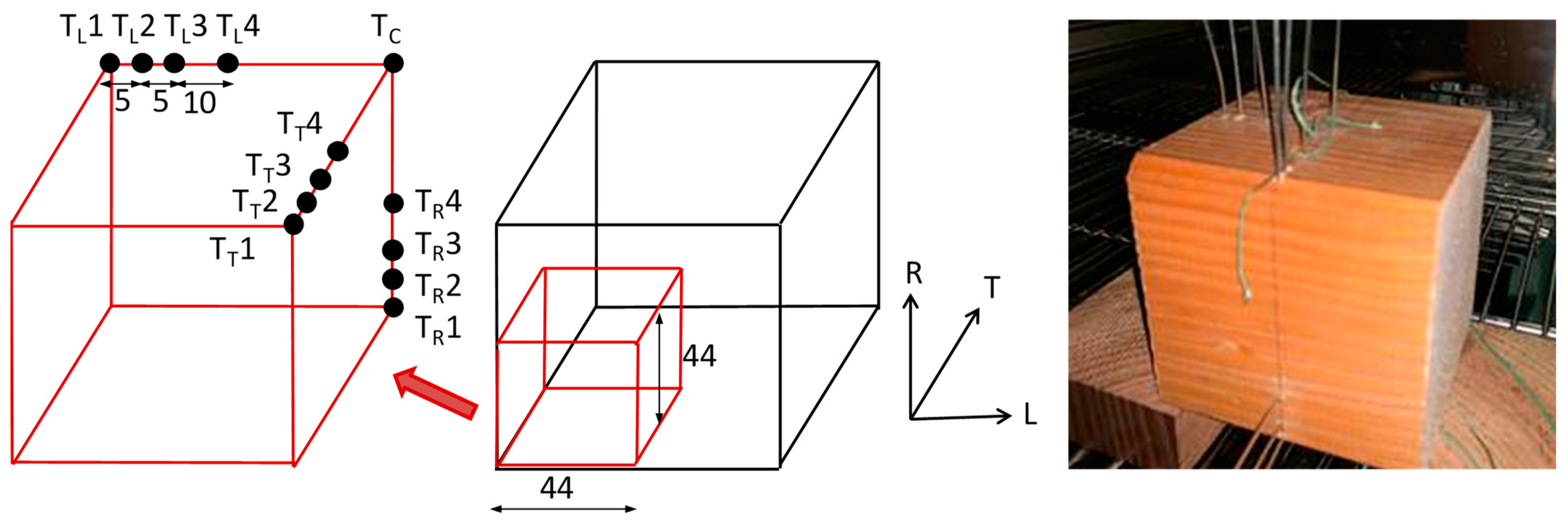
4.1.1. Materials and Specimens
4.1.2. Set-Up and Experimental Program
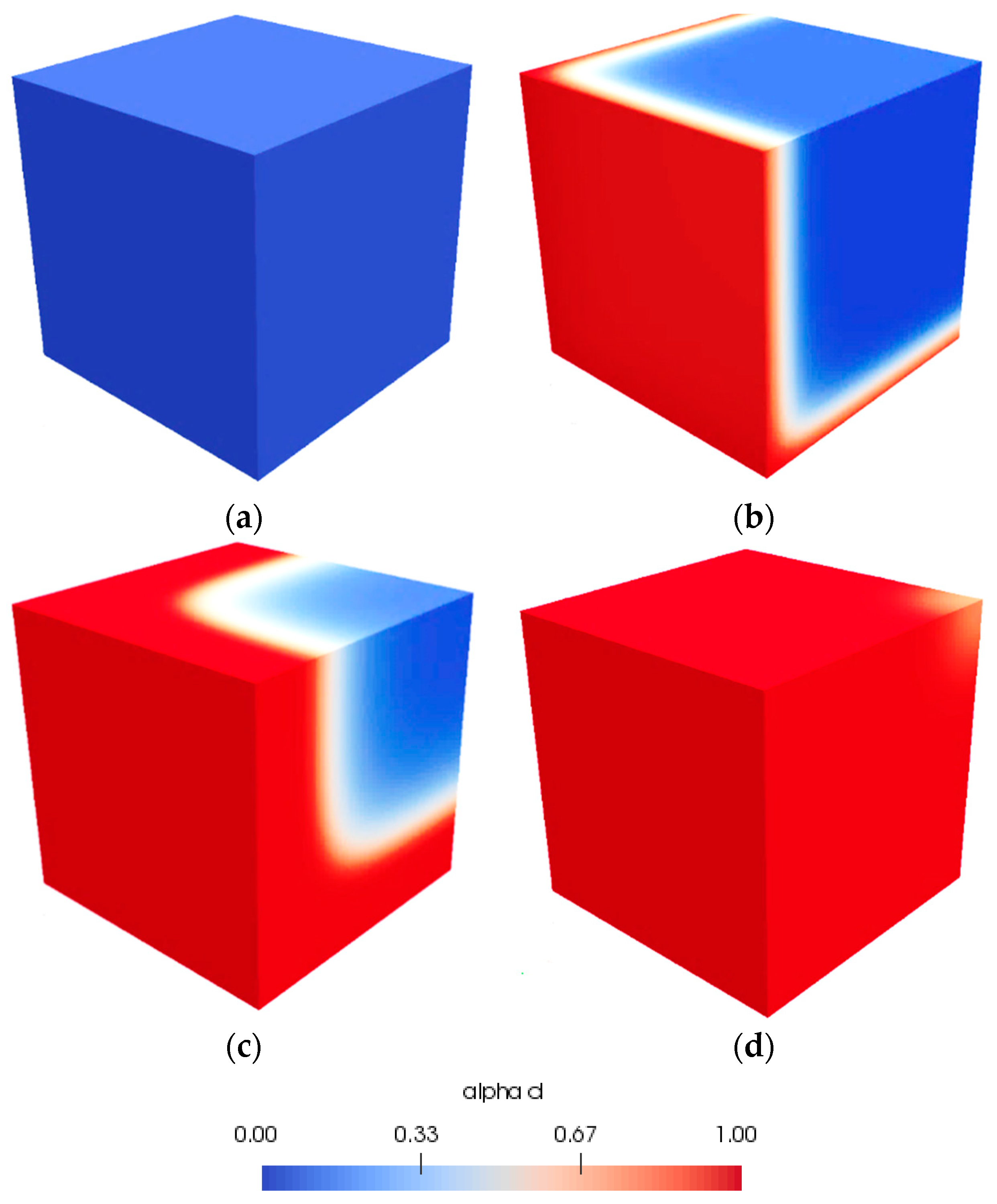
4.1.3. Description of Numerical Modeling
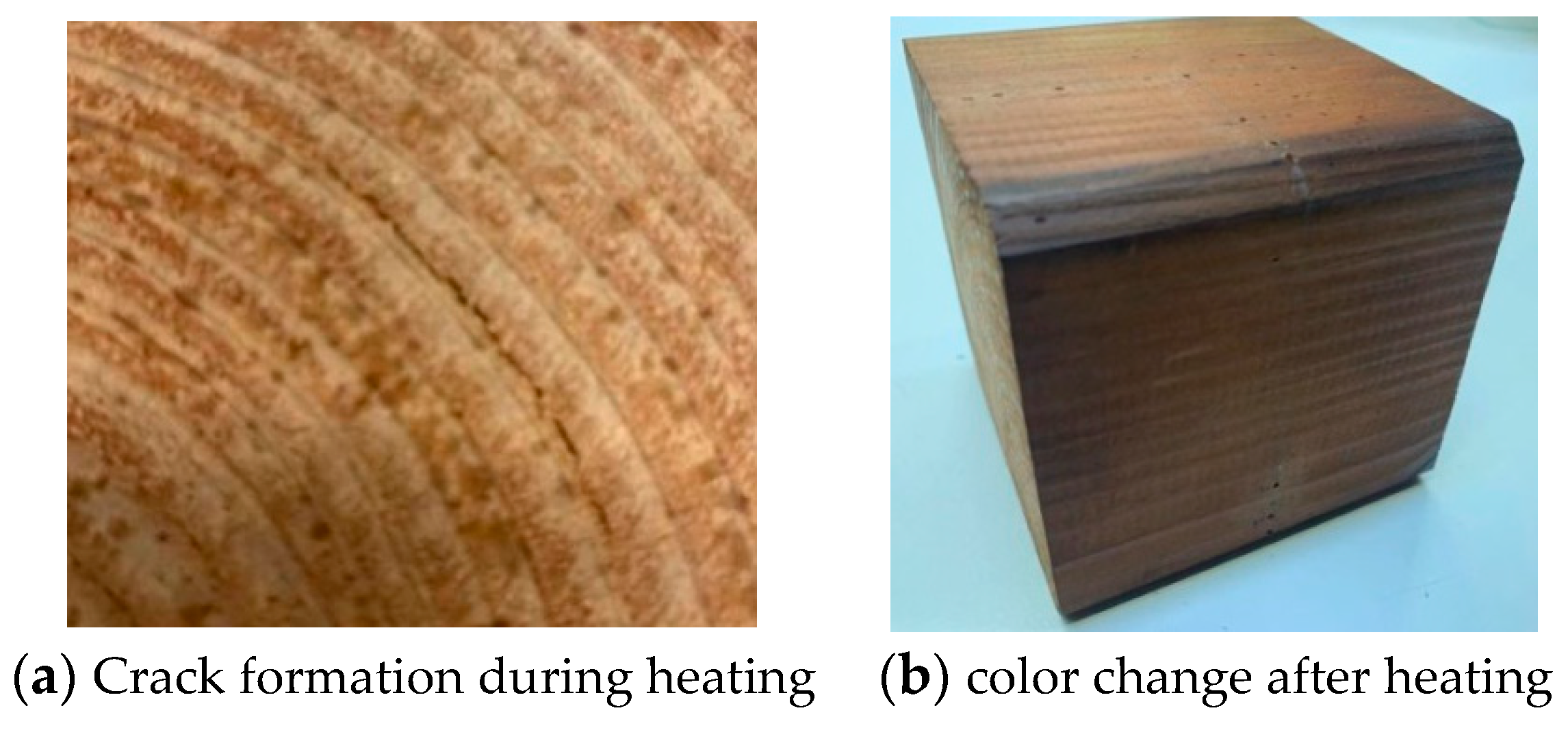
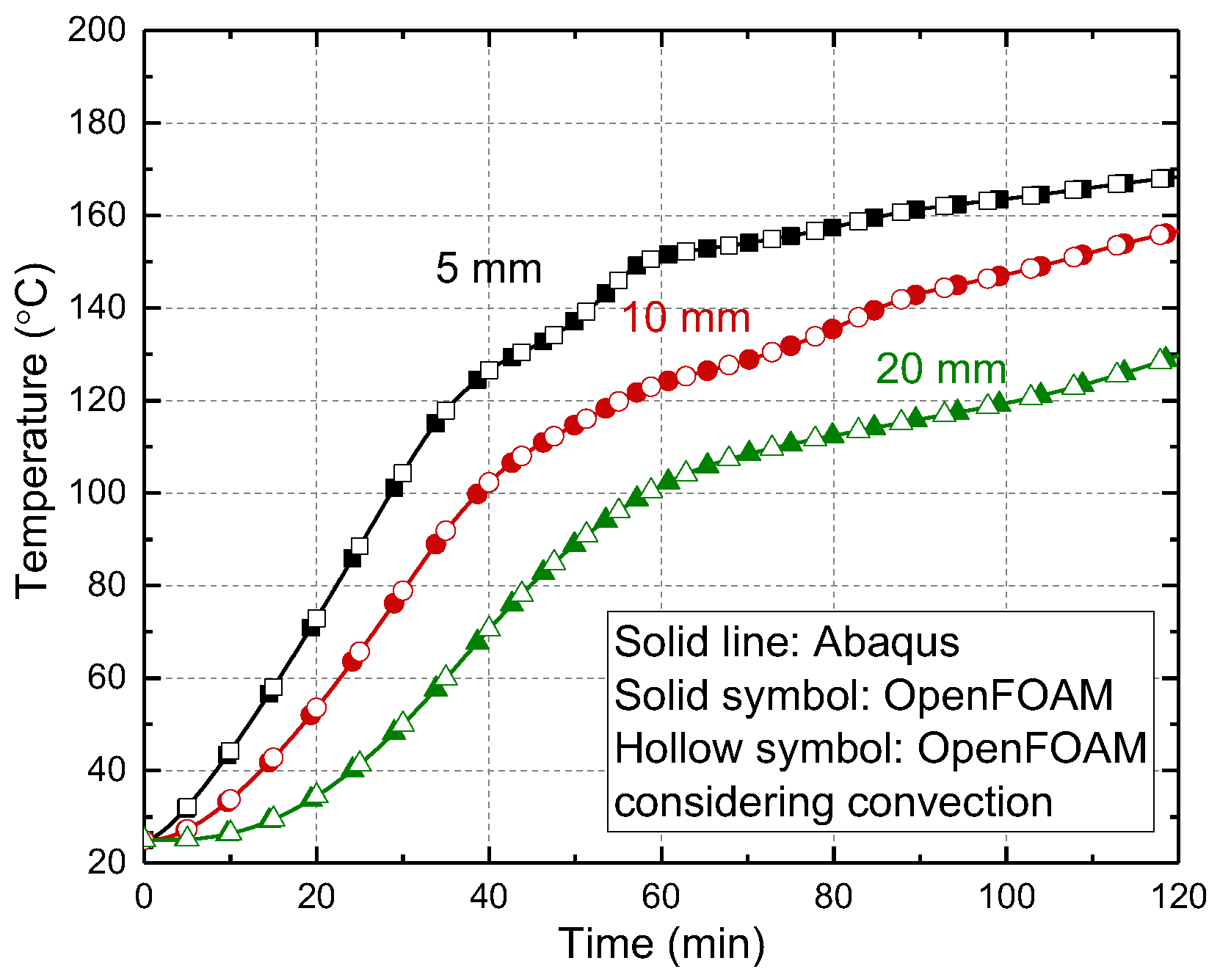
4.1.4. Comparison between Experimental and Numerical Results
4.2. Fire Experiments on Full-Scale CLT Panel
4.2.1. Description of Experimental Program
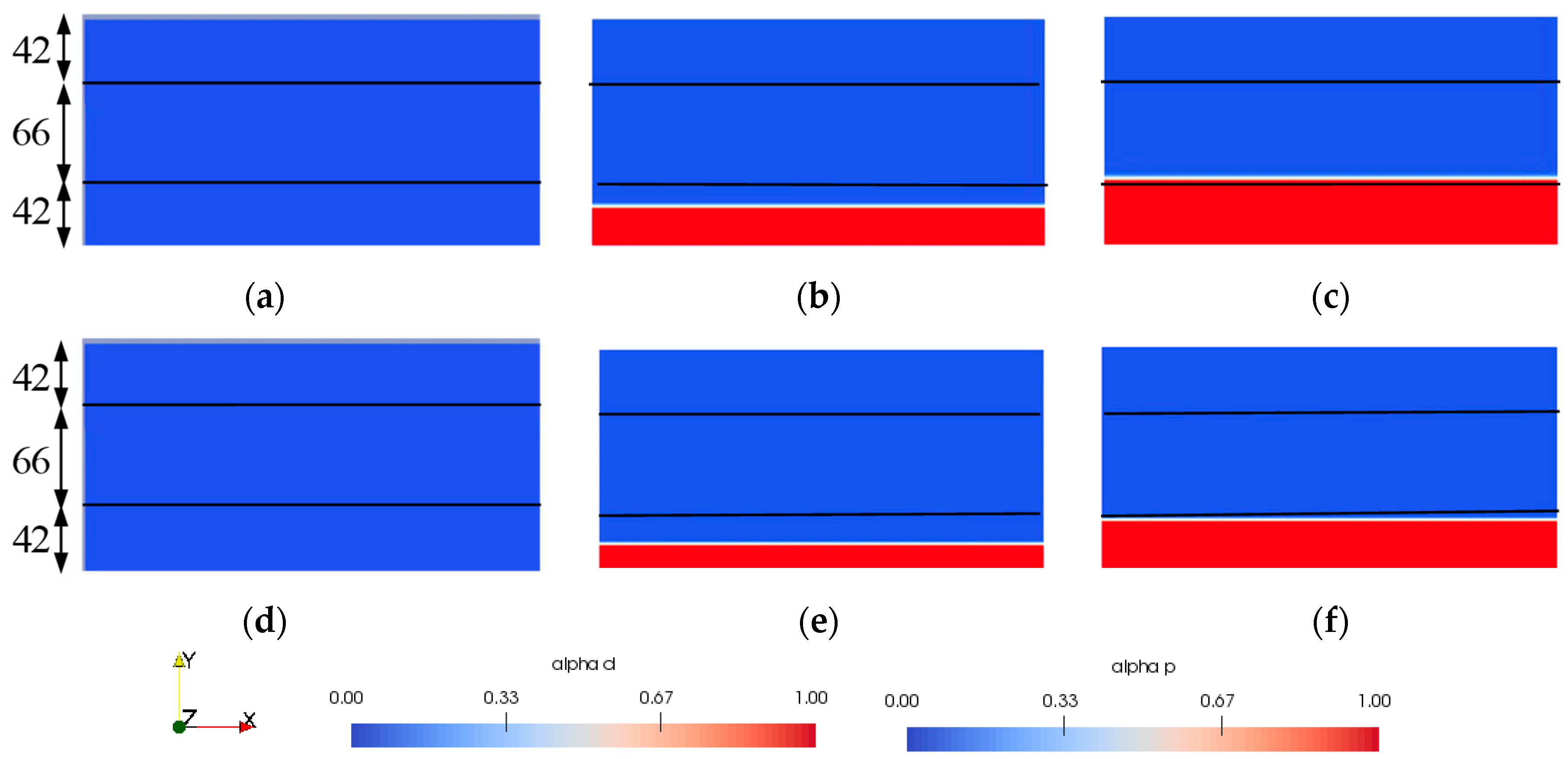
4.2.2. Description of Numerical Modeling
4.2.3. Comparison between the Experimental and Numerical Results
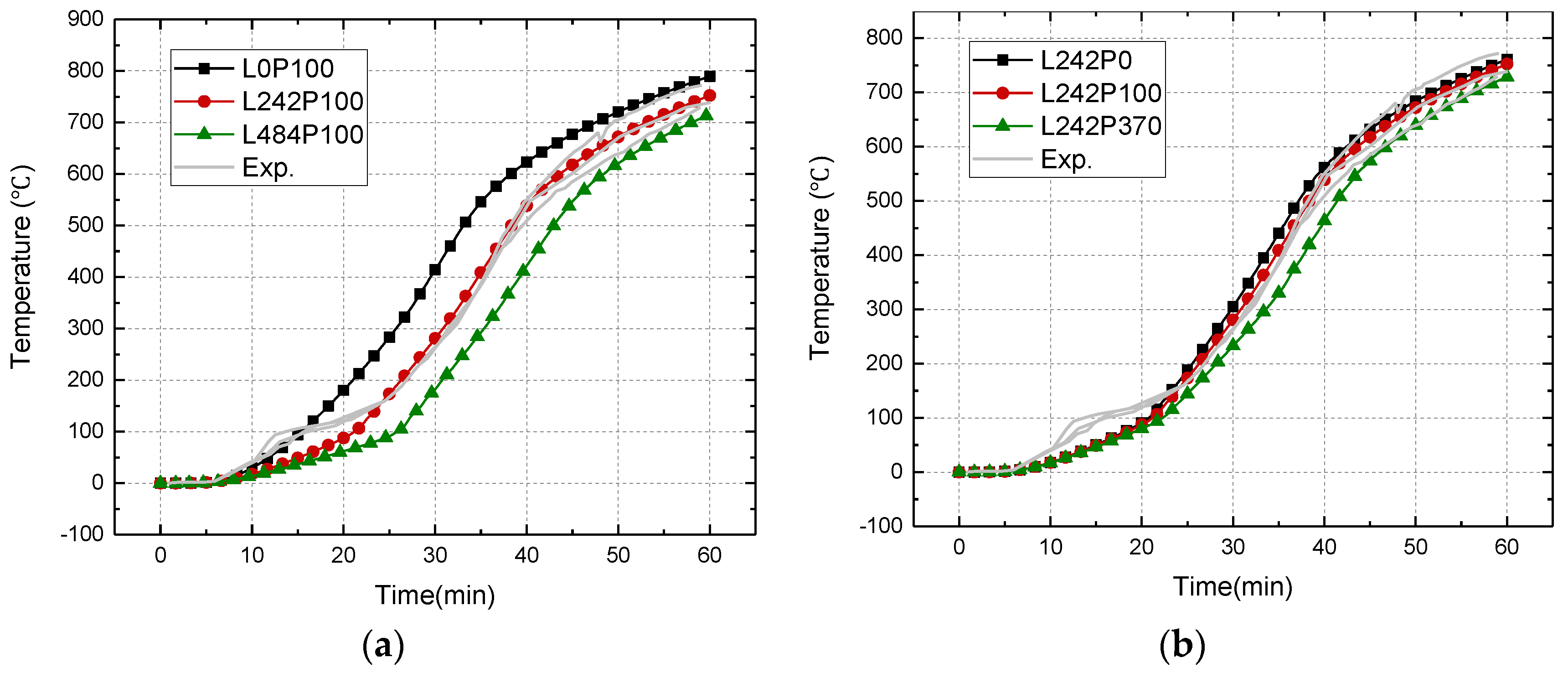
4.2.4. Effect of Latent Heat and Pyrolysis Heat
5. Conclusions
- The thermo-physical model of wood considering moisture content was developed based on the kinetic theory. Unlike the frequently used thermo-physical properties based on the piecewise linear functions of temperature, the proposed thermo-physical properties are continuous and exhibit obvious physical meaning.
- The apparent specific heat capacity model of wood with different moisture contents was validated by the differential scanning calorimetry experiments on the Douglas fir wood. The apparent specific heat capacity of the Douglas fir wood obviously increased from approximately 3700 kJ/(kg·K) to 6000 kJ/(kg·K) as the moisture content increased from 4% to 12%. Moreover, the apparent specific heat capacity also significantly increased during the pyrolysis process.
- Apparent specific heat capacity was recommended in the simulation of thermal responses of wood since both latent heat and pyrolysis heat have significant effects on the thermal responses of wood. The wood with high moisture content absorbed more latent heat through dehydration compared to that with low moisture content, exhibiting a relatively low increasement in temperature near 100 °C during the heating process. The temperature progression is more sensitive to the latent heat than to the pyrolysis heat.
- The thermo-physical model was successfully implemented in open-source finite volume software, OpenFOAM, which can simulate three-dimensional gas flow inside porous wood. The effect of convection of gas on the temperature progressions depends on the gas velocity and enthalpy. The low gas pressure led to a low gas velocity in the dehydration and pyrolysis stages. Therefore, the gas convection seems to have limited influence on the temperature progressions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, R.J. Wood Handbook: Wood as an Engineering Material; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010.
- Zhang, F.; Lu, Z.; Wang, D.; Fang, H. Mechanical properties of the composite sandwich structures with cold formed pro-filed steel plate and balsa wood core. Eng. Struct. 2024, 300, 117256. [Google Scholar] [CrossRef]
- Grønli, M.G.; Várhegyi, G.; Di Blasi, C. Thermogravimetric analysis and devolatilization kinetics of wood. Ind. Eng. Chem. Res. 2002, 41, 4201–4208. [Google Scholar] [CrossRef]
- Fredlund, B. Modelling of heat and mass transfer in wood structures during fire. Fire Saf. J. 1993, 20, 39–69. [Google Scholar] [CrossRef]
- Shen, D.K.; Fang, M.X.; Luo, Z.Y.; Cen, K.F. Modeling pyrolysis of wet wood under external heat flux. Fire Saf. J. 2007, 42, 210–217. [Google Scholar] [CrossRef]
- Park, W.C.; Atreya, A.; Baum, H.R. Experimental and theoretical investigation of heat and mass transfer processes during wood pyrolysis. Combust. Flame 2010, 157, 481–494. [Google Scholar] [CrossRef]
- Janssens, M.L. Modeling of the thermal degradation of structural wood members exposed to fire. Fire Mater. 2004, 28, 199–207. [Google Scholar] [CrossRef]
- König, J. Effective thermal actions and thermal properties of timber members in natural fires. Fire Mater. 2006, 30, 51–63. [Google Scholar] [CrossRef]
- Frangi, A. Brandverhalten von Holz-Beton-Verbunddecken; ETH: Zürich, Switzerland, 2001. [Google Scholar]
- Mehaffey, J.R.; Cuerrier, P.; Carisse, G. A model for predicting heat transfer through gypsum-board/wood-stud walls exposed to fire. Fire Mater. 1994, 18, 297–305. [Google Scholar] [CrossRef]
- EN 1995-1-2; Eurocode 5: Design of Timber Structures—Part 1–2: General—Structural Fire Design. European Committee for standardization (CEN): Brussels, Belgium, 2004.
- Janssens, M. Thermo-physical properties for wood pyrolysis models. In Proceedings of the Pacific Timber Engineering Conference, Gold Coast, Australia, 11–15 July 1994. [Google Scholar]
- Thomas, G.C. Fire Resistance of Light Timber Framed Walls and Floors; University of Canterbury: Christchurch, New Zealand, 1996. [Google Scholar]
- Naser, M.Z. Fire resistance evaluation through artificial intelligence—A case for timber structures. Fire Saf. J. 2019, 105, 1–18. [Google Scholar] [CrossRef]
- Díaz, A.R.; Flores, E.I.S.; Yanez, S.J.; Vasco, D.A.; Pina, J.C.; Guzmán, C.F. Multiscale modeling of the thermal conductivity of wood and its application to cross-laminated timber. Int. J. Therm. Sci. 2019, 144, 79–92. [Google Scholar] [CrossRef]
- Bai, Y.; Vallée, T.; Keller, T. Modeling of thermo-physical properties for FRP composites under elevated and high temperature. Compos. Sci. Technol. 2007, 67, 3098–3109. [Google Scholar]
- Fragiacomo, M.; Menis, A.; Clemente, I.; Bochicchio, G.; Ceccotti, A. Fire resistance of cross-laminated timber panels loaded out of plane. J. Struct. Eng. 2013, 139, 4013018. [Google Scholar] [CrossRef]
- Van, L.T.; Legrand, V.; Jacquemin, F. Thermal decomposition kinetics of balsa wood: Kinetics and degradation mechanisms comparison between dry and moisturized materials. Polym. Degrad. Stab. 2014, 110, 208–215. [Google Scholar]
- Wang, S.; Luo, Z. Pyrolysis of Biomass; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Gammon, B.W. Reliability Analysis of Wood-Frame Wall Assemblies Exposed to Fire; University of California: Berkeley, CA, USA, 1988. [Google Scholar]
- Aseeva, R.; Serkov, B.; Sivenkov, A. Fire Behavior and Fire Protection in Timber Buildings; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Spearpoint, M.J.; Quintiere, J.G. Predicting the piloted ignition of wood in the cone calorimeter using an integral model—Effect of species, grain orientation and heat flux. Fire Saf. J. 2001, 36, 391–415. [Google Scholar] [CrossRef]
- Hankalin, V.; Ahonen, T.; Raiko, R. On thermal properties of a pyrolysing wood particle. In Proceedings of the Finnish-Swedish Flame Days 2009, Naantali, Finland, 28–29 January 2009; pp. 1–16. [Google Scholar]
- Yapici, F.I.; Ozcifci, A.; Esen, R.; Kurt, S. The effect of grain angle and species on thermal conductivity of some selected wood species. BioResources 2011, 6, 2757–2762. [Google Scholar] [CrossRef]
- Fonseca, E.M.M.; Barreira, L.M.S. High temperatures in parallel or perpendicular wood grain direction: A numerical and experimental study. In Proceedings of the Fourth International Conference on Safety and Security Engineering IV, Antwerp, Belgium, 4–6 July 2011; pp. 171–183. [Google Scholar]
- Zhang, J.; Liu, Z.; Xu, Y.; Ma, S.; Xu, Q. An experimental and numerical study on the charring rate of timber beams exposed to three-side fire. Sci. China Technol. Sci. 2012, 55, 3434–3444. [Google Scholar] [CrossRef]
- Mindeguia, J.-C.; Cueff, G.; Dréan, V.; Auguin, G. Simulation of charring depth of timber structures when exposed to non-standard fire curves. Fire Eng. 2018, 9, 63–76. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Omar, A.A.; Ling, Z.; Yang, D.; Liu, Y. Postfire performance of pultruded wood-cored GFRP sandwich beams. Thin-Walled Struct. 2023, 193, 111240. [Google Scholar] [CrossRef]
- Keller, T.; Tracy, C.; Zhou, A. Structural response of liquid-cooled GFRP slabs subjected to fire—Part II: Thermo-chemical and thermo-mechanical modeling. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1296–1308. [Google Scholar] [CrossRef]
- OpenFOAM. Available online: http://www.openfoam.com (accessed on 1 January 2019).
- Weng, H.; Bailey, S.C.C.; Martin, A. Numerical study of iso-Q sample geometric effects on charring ablative materials. Int. J. Heat Mass Transf. 2015, 80, 570–596. [Google Scholar] [CrossRef]
- Lachaud, J.; Mansour, N.N. Porous-material analysis toolbox based on OpenFOAM and applications. J. Thermophys. Heat Transf. 2014, 28, 191–202. [Google Scholar] [CrossRef]
- Gentile, G.; Debiagi, P.E.A.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Faravelli, T. A computational framework for the pyrolysis of anisotropic biomass particles. Chem. Eng. J. 2017, 321, 458–473. [Google Scholar] [CrossRef]
- Lachaud, J.; Scoggins, J.B.; Magin, T.E.; Meyer, M.G.; Mansour, N.N. A generic local thermal equilibrium model for porous reactive materials submitted to high temperatures. Int. J. Heat Mass Transf. 2017, 108, 1406–1417. [Google Scholar] [CrossRef]
- ParaView. Available online: http://www.paraview.org (accessed on 1 January 2019).
- ASTM D4442-15; Standard Test Methods for Direct Moisture Content Measurement of Wood and Wood-Base Materials. ASTM International: West Conshohocken, PA, USA, 2015.
- Pozzobon, V.; Salvador, S.; Bézian, J.J.; El-Hafi, M.; Le Maoult, Y.; Flamant, G. Radiative pyrolysis of wet wood under intermediate heat flux: Experiments and modelling. Fuel Process. Technol. 2014, 128, 319–330. [Google Scholar] [CrossRef]
- Grønli, M.G.; Melaaen, M.C. Mathematical model for wood pyrolysis—Comparison of experimental measurements with model predictions. Energy Fuels 2000, 14, 791–800. [Google Scholar] [CrossRef]
- ISO 834-1; Fire Resistance Tests—Elements of Building Construction. International Organization for Standardization: Geneva, Switzerland, 1999.
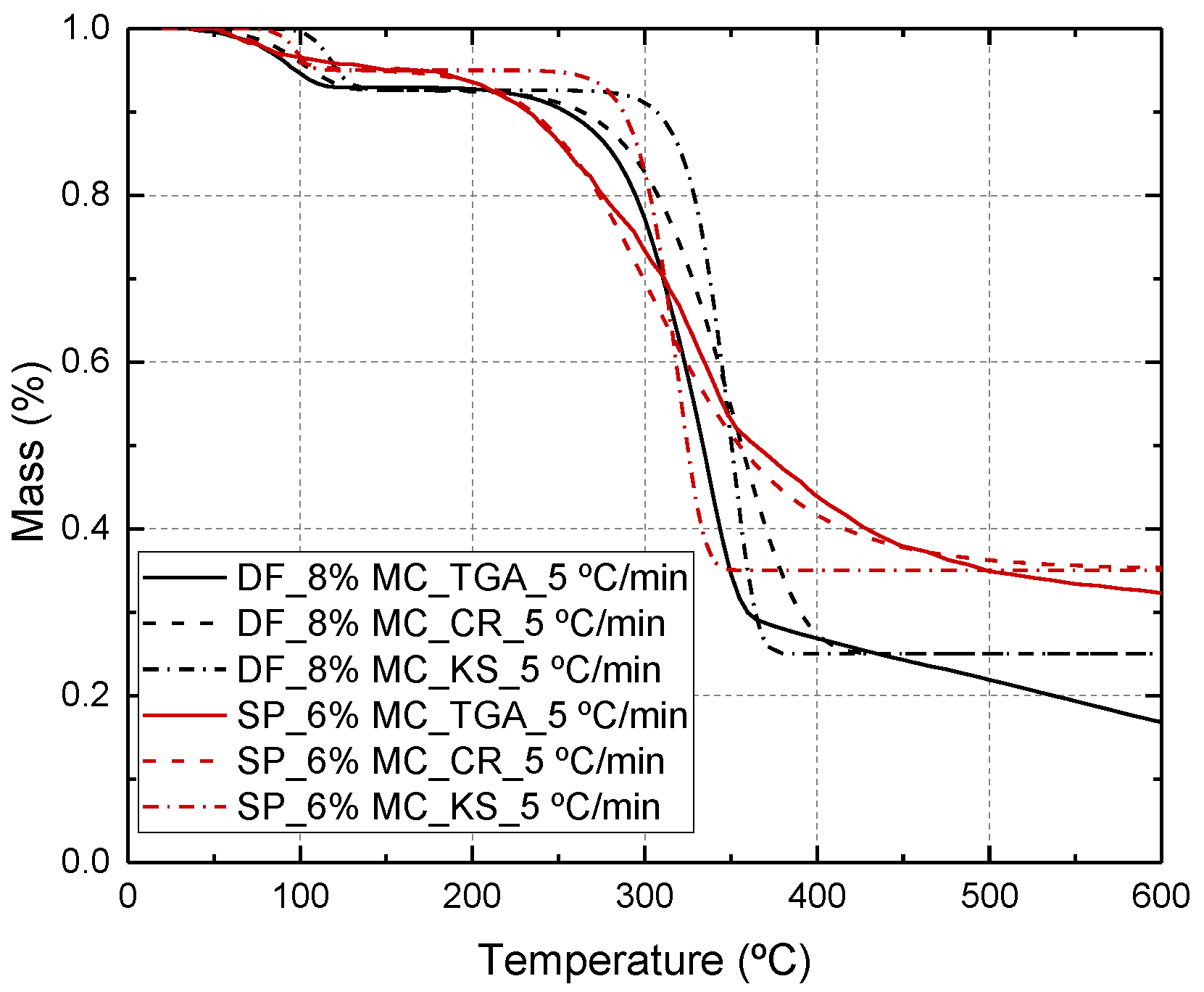
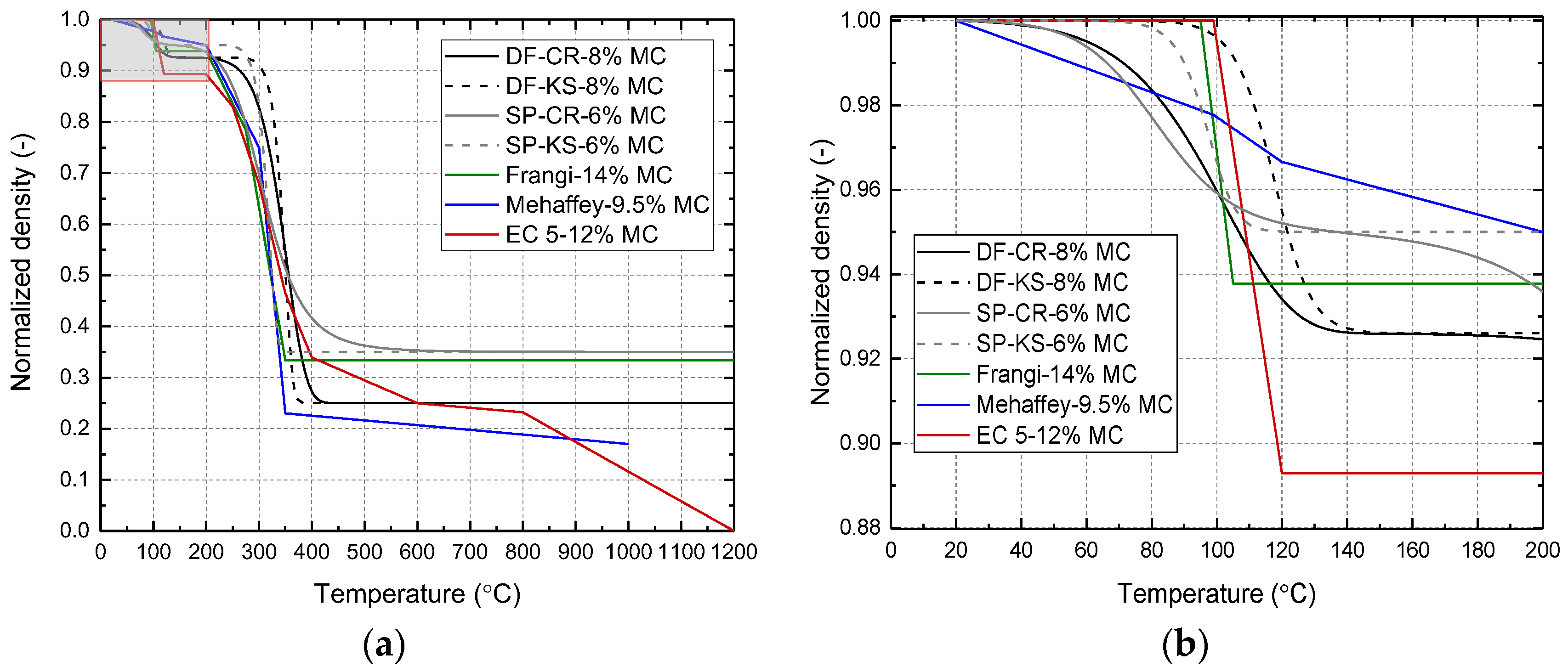
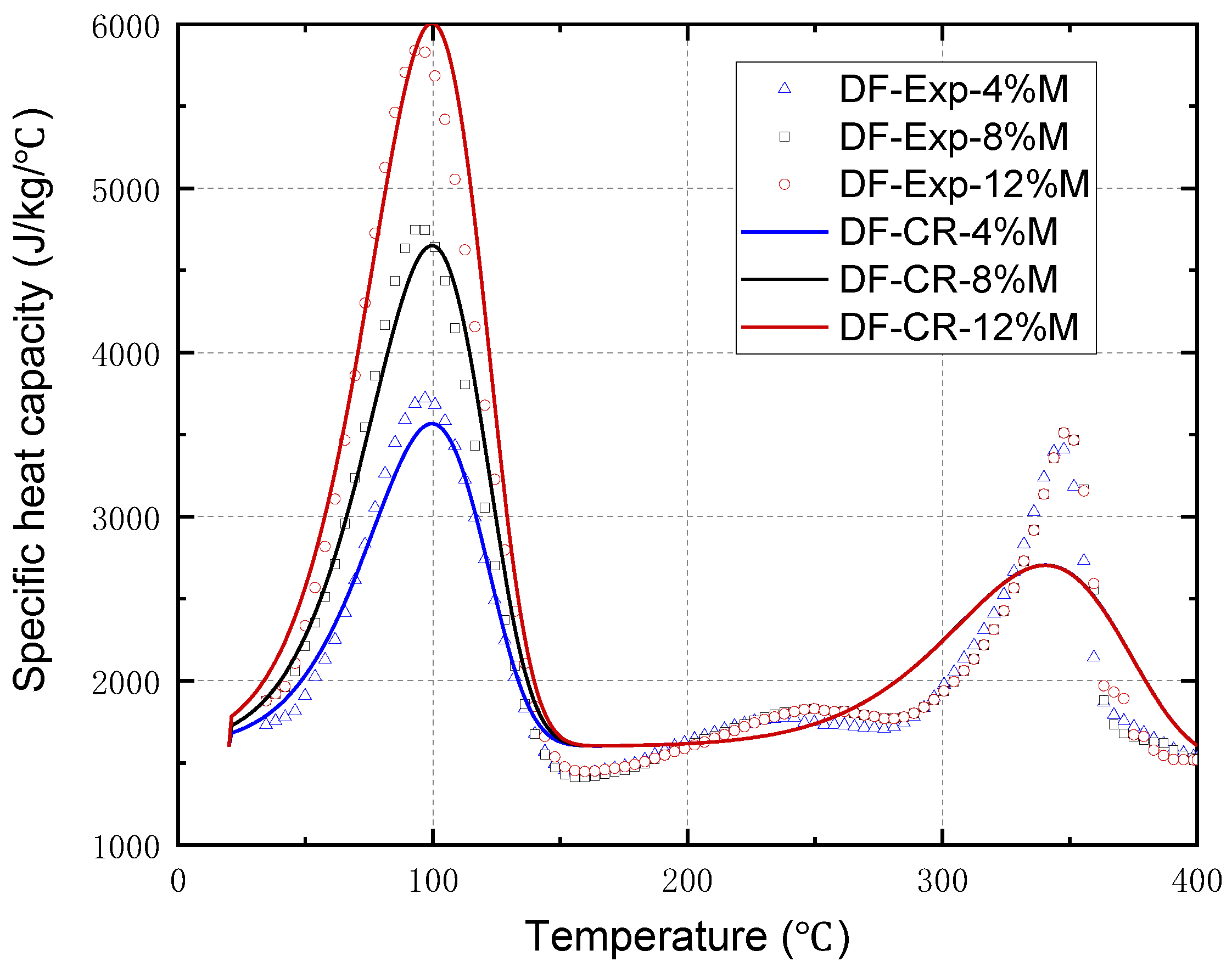
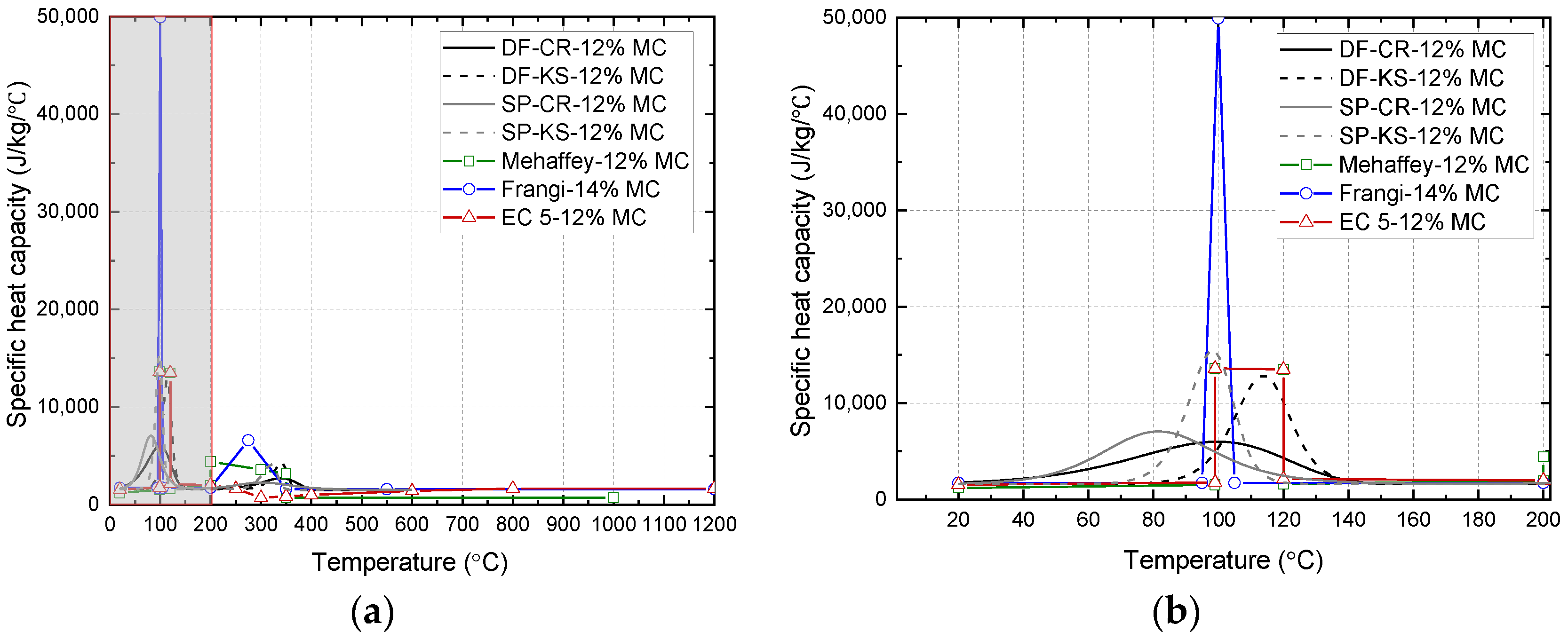




| Specie | Model | Ad (Hz) | Ed (kJ/mol) | nd (-) | Ap (Hz) | Ep (kJ/mol) | np (-) |
|---|---|---|---|---|---|---|---|
| DF (8% MC) | CR | 1.21 × 104 | 4.66 × 104 | 1.01 | 4.88 × 104 | 8.60 × 104 | 1.00 |
| DF (8% MC) | KS | 6.50 × 1022 | 1.83 × 105 | 1.70 | 8.20 × 1015 | 2.13 × 105 | 1.00 |
| SP (6% MC) | CR | 2.97 × 109 | 7.92 × 104 | 1.90 | 3.10 × 103 | 6.86 × 104 | 2.05 |
| SP (6% MC) | KS | 6.28 × 1025 | 1.96 × 105 | 1.52 | 2.08 × 1015 | 1.99 × 105 | 1.05 |
| Specie | Cp,v J/(kg·K) | Cp,d J/(kg·K) | Cp,c J/(kg·K) | Cd kJ/kg | Cpy kJ/kg |
|---|---|---|---|---|---|
| DF | 1600 | 1600 | 1500 | Equation (9) | 100 |
| SP | 1600 | 1600 | 1500 | Equation (9) | 100 |
| Specie | w (%) | kL,v W/(m·K) | kL,d W/(m·K) | kL,c W/(m·K) | kT/R,v W/(m·K) | kT/R,d W/(m·K) | kT/R,c W/(m·K) |
|---|---|---|---|---|---|---|---|
| DF (12% MC) | 12 | 0.21 | 0.21 | 0.14 | 0.14 [1] | 0.14 | 0.09 [11] |
| DF (0% MC) | 0 | 0.18 | 0.18 | 0.14 | 0.12 [1] | 0.12 | 0.09 [11] |
| SP (12% MC) | 12 | 0.18 | 0.18 | 0.14 | 0.12 [1] | 0.12 | 0.09 [11] |
| SP (0% MC) | 0 | 0.15 | 0.15 | 0.14 | 0.10 [1] | 0.10 | 0.09 [11] |
| Specimen | Exposed Surfaces | Dimension mm | Specie |
|---|---|---|---|
| DF block (13.7%MC) | 6 | 88 × 88 × 88 | Douglas fir |
| DF block (0% MC) | 6 | 88 × 88 × 88 | Douglas fir |
| CLT panel (12% MC) | 1 | 2500 × 600 × 150 | Spruce |
| ID | Moisture Content (%) | Heating Time (min) | Target Temperature (°C) |
|---|---|---|---|
| S1-13.7% | 13.7 | 120 | 200 |
| S2-13.7% | 13.7 | 120 | 200 |
| S3-0% | 0 | 120 | 200 |
| S4-0% | 0 | 120 | 200 |
| Symbol | Parameter | Value | Units | Refs. | ||
|---|---|---|---|---|---|---|
| Wet DF | Dry DF | SP | ||||
| ρv | Density of virgin wood | 581 | 510 | 449 | kg/m3 | [17] |
| ρd | Density of dehydrated wood | 510 | 510 | 401 | kg/m3 | |
| ρc | Density of char | 116 | 116 | 90 | kg/m3 | |
| w | Moisture content | 13.7 | 0 | 12 | % | [17] |
| Cp,v | Specific heat of virgin wood | 1600 | 1600 | 1600 | kJ/(kg·K) | |
| Cp,d | Specific heat of dehydrated wood | 1600 | 1600 | 1600 | kJ/(kg·K) | |
| Cp,c | Specific heat of char | 1500 | 1500 | 1500 | kJ/(kg·K) | |
| Cd | Latent heat | 272 | 0 | 242 | kJ/kg | |
| Cpy | Pyrolysis heat | 100 | 100 | 100 | kJ/kg | |
| kL,v | Longitudinal wood conductivity | 0.21 | 0.18 | 0.15 | W/(m·K) | |
| kR/T,v | Transverse wood conductivity | 0.14 | 0.12 | 0.10 | W/(m·K) | [1] |
| kL,c | Longitudinal char conductivity | 0.14 | 0.14 | 0.14 | W/(m·K) | |
| kR/T,c | Transverse char conductivity | 0.09 | 0.09 | 0.09 | W/(m·K) | [11] |
| KL,v | Longitudinal wood permeability | 7.5 × 10−13 | 7.5 × 10−13 | 7.5 × 10−13 | m2 | [39] |
| KR/T,v | Transverse wood permeability | 7.5 × 10−13 | 7.5 × 10−13 | 7.5 × 10−13 | m2 | [39] |
| KL,c | Longitudinal char permeability | 1.0 × 10−11 | 1.0 × 10−11 | 1.0 × 10−11 | m2 | [40] |
| KR/T,c | Transverse char permeability | 1.0 × 10−11 | 1.0 × 10−11 | 1.0 × 10−11 | m2 | [40] |
| εg,v | Porosity of virgin wood | 0.51 | 0.51 | 0.51 | [39] | |
| εg,c | Porosity of char | 0.85 | 0.85 | 0.85 | [39] | |
| μ | Viscosity coefficient of gas | 1.85 × 10−5 | 1.85 × 10−5 | 1.85 × 10−5 | kg/(m s) | |
| M | Mean molar mass of gas | 0.028 | 0.028 | 0.028 | kg/mol | |
| R | Ideal gas constant | 8.314 | 8.314 | 8.314 | J/(mol·K) | |
| σsb | Stefan–Boltzmann constant | 5.67 × 10−8 | 5.67 × 10−8 | 5.67 × 10−8 | W/(m2·K4) | |
| Group | Combination | MC (%) | Latent Heat (kJ/kg) | Pyrolysis Heat (kJ/kg) |
|---|---|---|---|---|
| 1 | L0P100 | 0 | 0 | 100 |
| L242P100 | 12 | 242 | 100 | |
| L484P100 | 24 | 484 | 100 | |
| 2 | L242P0 | 12 | 242 | 0 |
| L242P100 | 12 | 242 | 100 | |
| L242P370 | 12 | 242 | 370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Xu, B.; Chen, K.; Cui, Z.; Liu, Y.; Zhang, L. Experimental and Three-Dimensional Numerical Investigations of Dehydration and Pyrolysis in Wood under Elevated and High Temperatures. Buildings 2024, 14, 1547. https://doi.org/10.3390/buildings14061547
Li Q, Xu B, Chen K, Cui Z, Liu Y, Zhang L. Experimental and Three-Dimensional Numerical Investigations of Dehydration and Pyrolysis in Wood under Elevated and High Temperatures. Buildings. 2024; 14(6):1547. https://doi.org/10.3390/buildings14061547
Chicago/Turabian StyleLi, Qianyi, Biao Xu, Kaixi Chen, Zhaoyan Cui, Yan Liu, and Lingfeng Zhang. 2024. "Experimental and Three-Dimensional Numerical Investigations of Dehydration and Pyrolysis in Wood under Elevated and High Temperatures" Buildings 14, no. 6: 1547. https://doi.org/10.3390/buildings14061547
APA StyleLi, Q., Xu, B., Chen, K., Cui, Z., Liu, Y., & Zhang, L. (2024). Experimental and Three-Dimensional Numerical Investigations of Dehydration and Pyrolysis in Wood under Elevated and High Temperatures. Buildings, 14(6), 1547. https://doi.org/10.3390/buildings14061547






