Compressive Strength and Microstructure of Carbide Slag and Alkali-Activated Blast Furnace Slag Pastes in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixture Proportions
2.3. Experimental Method (Sample Preparation) (Figure 3)
- (1)
- Water-to-Solid Ratio Test
- (2)
- Determination of Setting Time
- (3)
- Compressive Strength

2.4. Materials Characterization
2.5. Experimental Techniques
3. Results and Discussion
3.1. Properties
3.1.1. Effect of Carbide Slag to Blast Furnace Slag Ratio on Properties of CAB Pastes
3.1.2. Effect of NaOH on Properties of CAB Pastes
3.1.3. Effect of Curing Conditions on Properties of CAB Pastes
3.2. Reaction Products
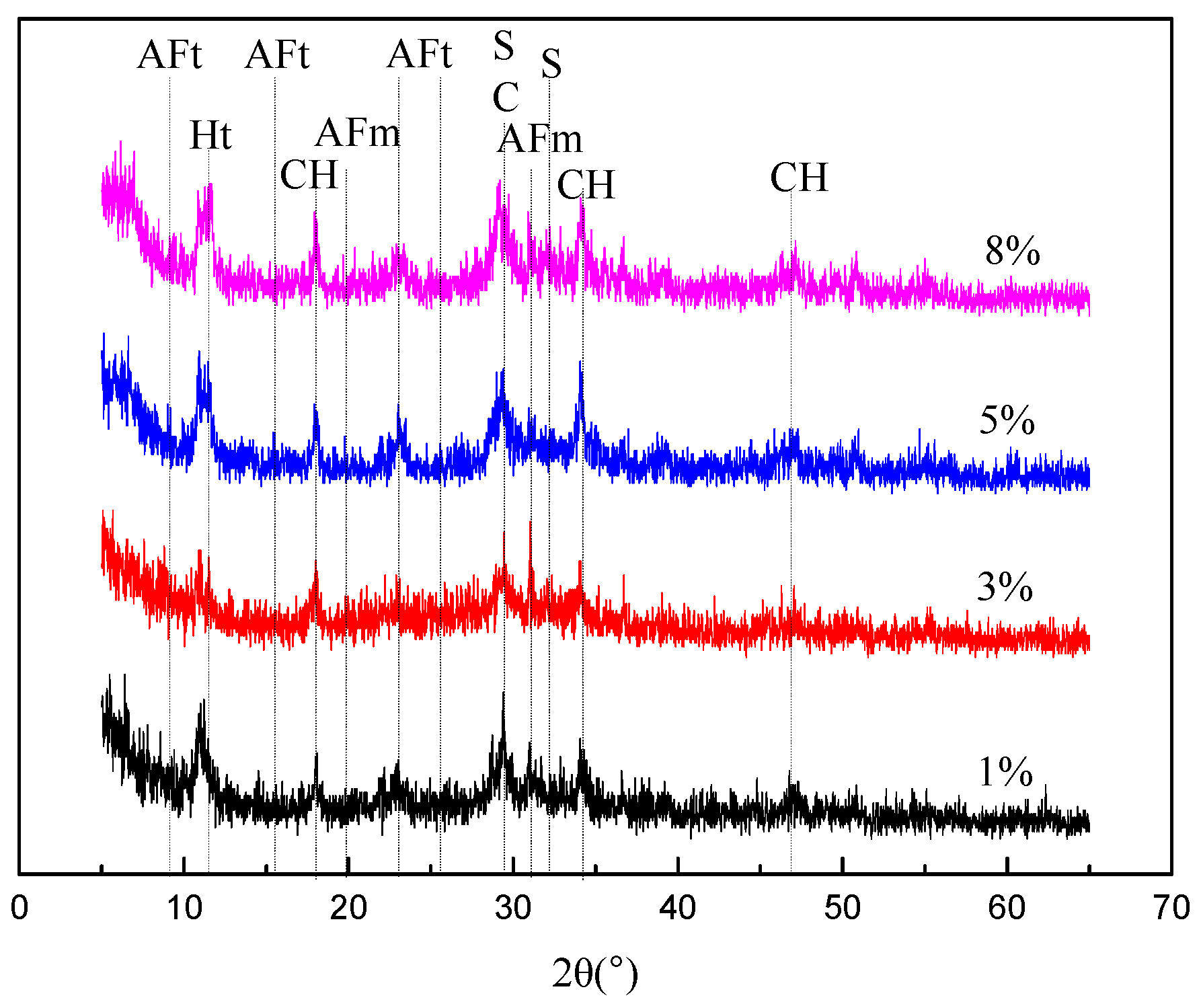
3.2.1. XRD Findings
3.2.2. DSC/TG Analysis
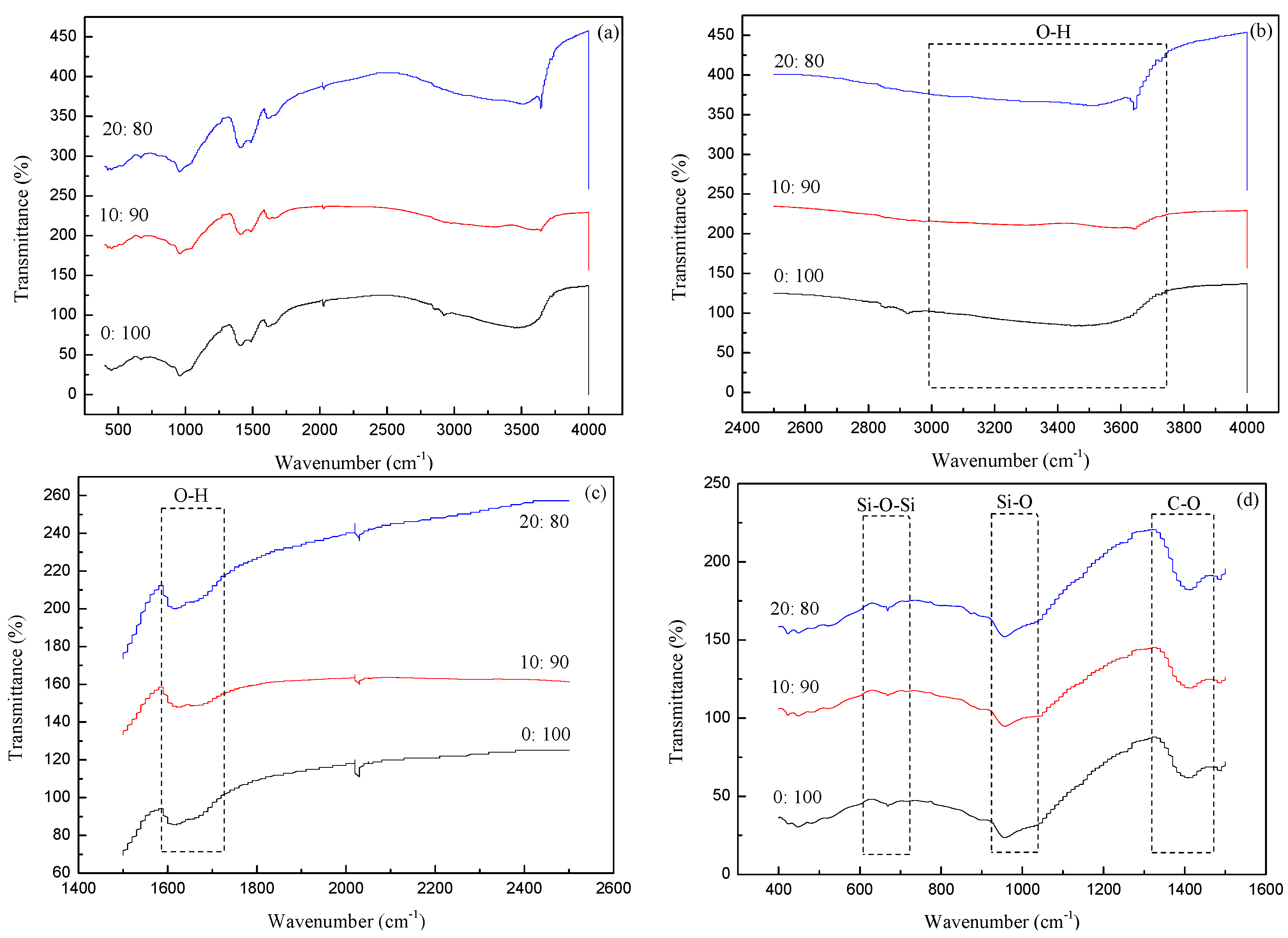
3.2.3. FTIR Analysis
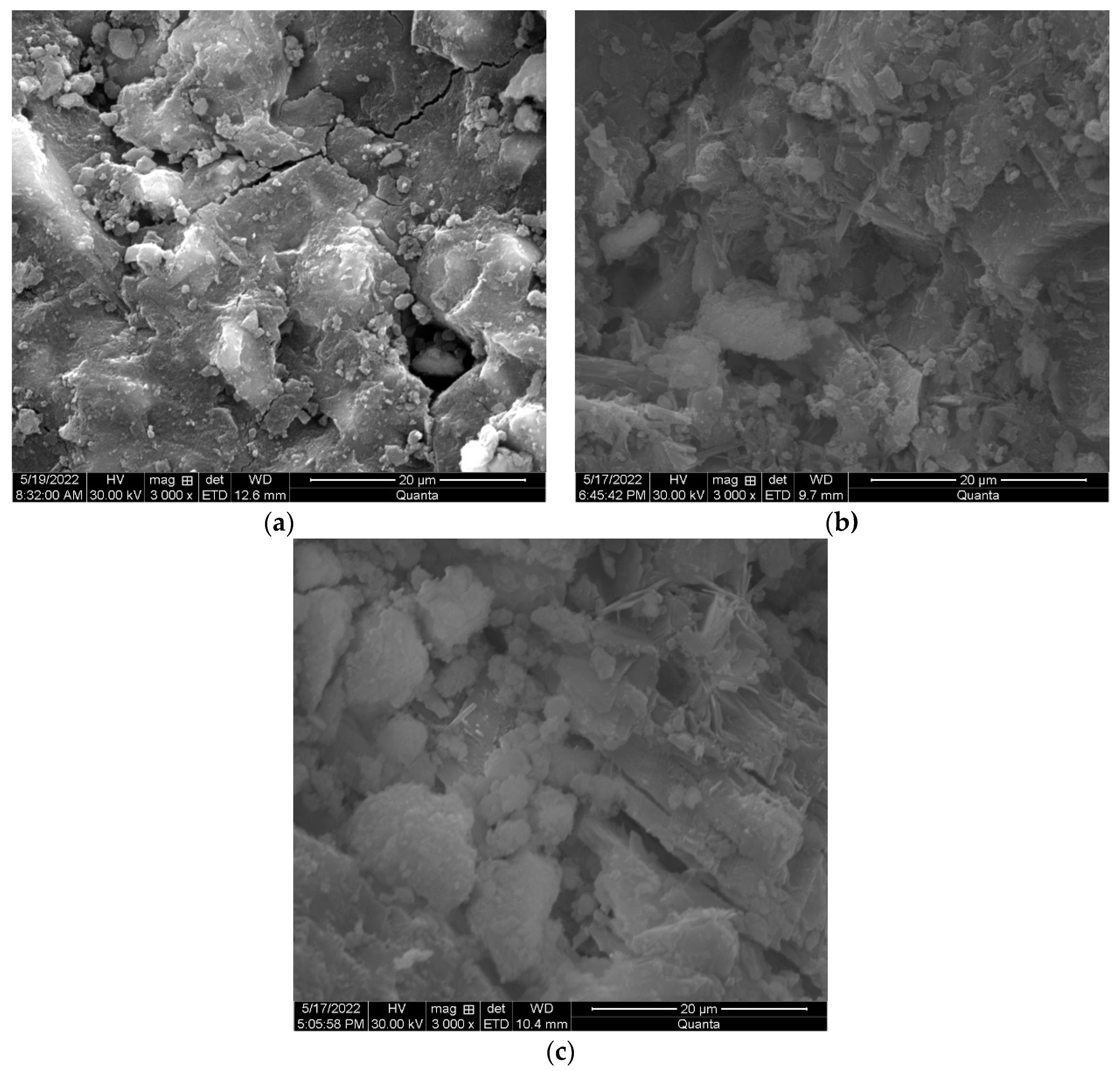
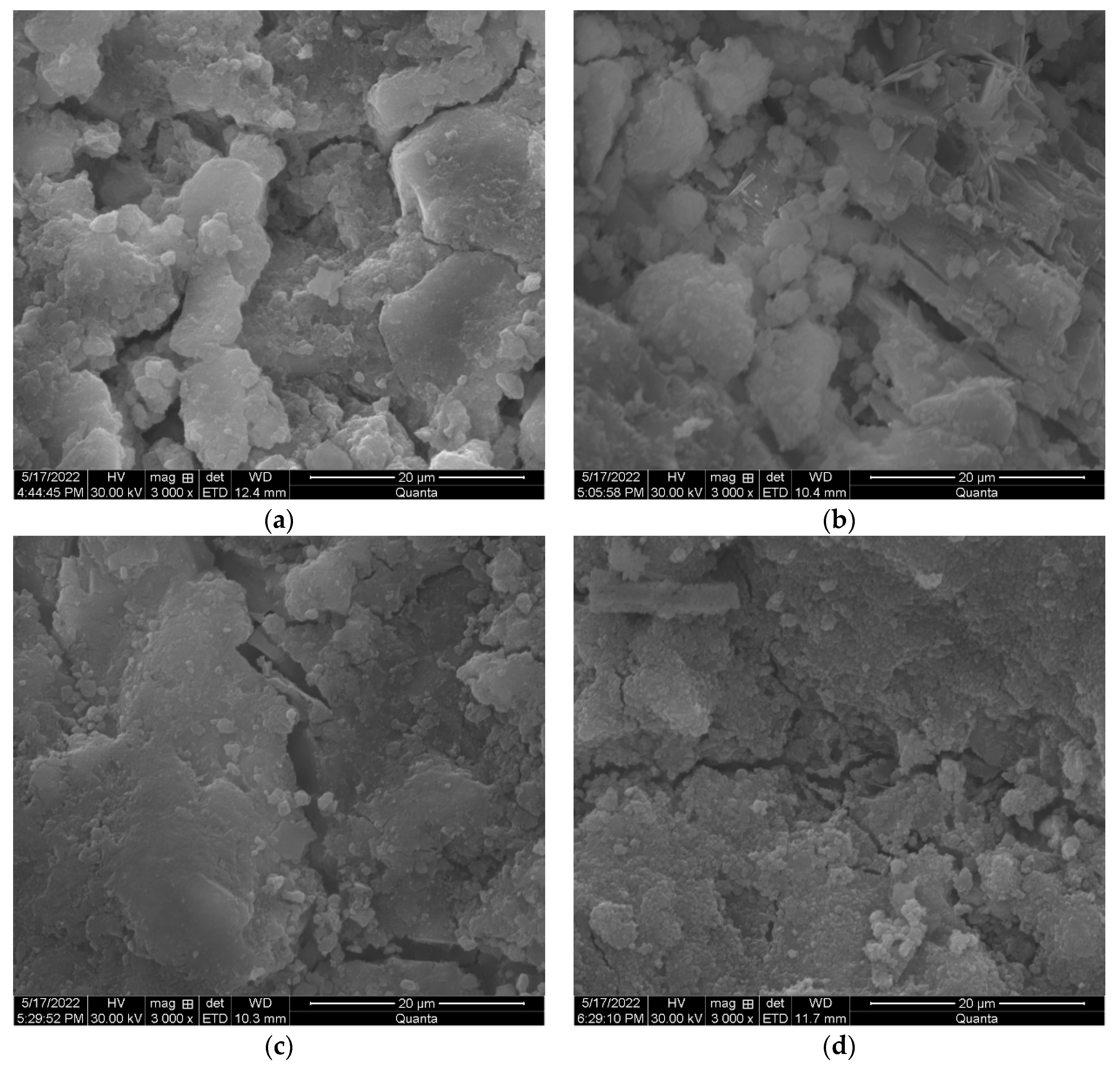
3.3. Microstructure Development
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, X.; Yao, J.; Zheng, R. Forecast of Energy Consumption and Carbon Emissions in China’s Building Sector to 2060. Energies 2022, 15, 4950. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Peng, S.; Li, C.; Wei, L. Tracing CO2 emissions of China’s construction sector. J. Clean. Prod. 2020, 275, 124165. [Google Scholar] [CrossRef]
- Cheng, M.; Lu, Y.; Zhu, H.; Xiao, J. Measuring CO2 emissions performance of China’s construction industry: A global Malmquist index analysis. Environ. Impact Assess. Rev. 2022, 92, 106673. [Google Scholar] [CrossRef]
- Hu, S. The cement industry has great achievements in the national “double carbon” strategy. China Cem. 2022, 4, 32–33. [Google Scholar]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Olukotun, N.; Sam, A.R.M.; Lim, N.H.A.S.; Abdulkareem, M.; Mallum, I.; Adebisi, O. Mechanical Properties of Tin Slag Mortar. Recycling 2021, 6, 42. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, Y.; Bai, H.; Kang, S.; Zhang, T.; Song, S. Eco-friendly geopolymer prepared from solid wastes: A critical review. Chemosphere 2021, 267, 128900. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Alameda, A.; Mañosa, J.; Giro-Paloma, J.; Formosa, J.; Chimenos, J.M. Alkali-Activated Binders Using Bottom Ash from Waste-to-Energy Plants and Aluminium Recycling Waste. Appl. Sci. 2021, 11, 3840. [Google Scholar] [CrossRef]
- Benhamouda, A.; Castro-Gomes, J.; Pereira-De-Oliveira, L. Rheology, Mechanical Properties and Porosity of Ternary Alkali-Activated Binders Based on Mining Mud Waste with Waste Glass and Metakaolin. CivilEng 2021, 2, 236–253. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, J.; Liao, Y. Infuence of ground granulated blast furnace slag on the early hydration and microstructure of alkali activated converter steel slag binder. J. Therm. Anal. Calorim. 2022, 147, 243–252. [Google Scholar] [CrossRef]
- Huang, L.; Ye, H. Factors Affecting Kinetics and Gel Composition of Alkali–Silica Reaction in Alkali-Activated Slag Mortars. Int. J. Civ. Eng. 2021, 19, 453–462. [Google Scholar] [CrossRef]
- Li, P.; Tang, J.; Bai, Y.; Chen, X.; Chen, J. Experimental study on the pH for activating ground granulated blast-furnace slag activity at different temperatures. Sadhana 2019, 44, 213. [Google Scholar] [CrossRef]
- Hruby, P.; Bilek, V.; Topolar, L.; Kalina, L.; Iliushchenko, V.; Koplik, J.; Masilko, J.; Soukal, F. Resistance of alkali-activated blast furnace slag to acids. J. Physics Conf. Ser. 2021, 2341, 012002. [Google Scholar] [CrossRef]
- Usherov-Marshak, A.; Vaičiukynienė, D.; Krivenko, P.; Bumanis, G. Calorimetric Studies of Alkali-Activated Blast-Furnace Slag Cements at Early Hydration Processes in the Temperature Range of 20–80 °C. Materials 2021, 14, 5872. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tan, H.; Zhang, J.; Deng, X.; Kong, X.; Chen, P.; Jian, S.; He, X.; Yang, J. Enhancement in compressive strength of carbide slag activated ground granulated blast furnace slag by introducing CaCl2 and NaCl. Constr. Build. Mater. 2023, 385, 131071. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, D.; Ji, W.; Zhao, X. Experiment on the Properties of Soda Residue-Activated Ground Granulated Blast Furnace Slag Mortars with Different Activators. Materials 2022, 15, 3578. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Simon, B.; Laurent, F.; Patapy, C. Experimental evidence for the acceleration of slag hydration in blended cements by the addition of CaCl2. Cem. Concr. Res. 2022, 149, 106558. [Google Scholar]
- Karam, R.; Paris, M.; Deneele, D.; Wattez, T.; Cyr, M.; Bulteel, D. Effect of sediment incorporation on the reactivity of alkali-activated GGBFS systems. Mater. Struct. 2021, 54, 18. [Google Scholar] [CrossRef]
- Klimenko, N.N.; Mikhailenko, N.Y.; Delitsin, L.M.; Sigaev, V.N. Effect of Particle Size of Quartz Sand Filler on Microstructure and Strength of Alkali-Activated Slag-Based Materials. Arab. J. Sci. Eng. 2021, 46, 4337–4352. [Google Scholar] [CrossRef]
- Bilici, S.; Kabay, N.; Miyan, N.; Omur, T.; Ozkan, H. Effect of washing aggregate sludge waste on the properties of alkali-activated blast furnace slag. J. Build. Eng. 2023, 63, 105527. [Google Scholar] [CrossRef]
- Occhicone, A.; Vukčević, M.; Bosković, I.; Ferone, C. Red Mud-Blast Furnace Slag-Based Alkali-Activated Materials. Sustainability 2021, 13, 11298. [Google Scholar] [CrossRef]
- Niu, H.; Helser, J.; Corfe, I.J.; Kuva, J.; Butcher, A.R.; Cappuyns, V.; Kinnunen, P.; Illikainen, M. Incorporation of bioleached sulfidic mine tailings in one-part alkali-activated blast furnace slag mortar. Constr. Build. Mater. 2022, 333, 127195. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, T.; Cao, L.; Kan, L.; Wu, M. Influence of steel slag on the properties of alkali-activated fly ash and blast-furnace slag based fiber reinforced composites. Cem. Concr. Compos. 2021, 116, 103875. [Google Scholar] [CrossRef]
- Feng, S.; Zhu, J.; Wang, R.; Qu, Z.; Song, L.; Wang, H. The Influence of CaO and MgO on the Mechanical Properties of Alkali-Activated Blast Furnace Slag Powder. Materials 2022, 15, 6128. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Q.; Lu, J. The effects of cations and concentration on reaction mechanism of alkali-activated blast furnace ferronickel slag. Compos. Part B Eng. 2022, 236, 109825. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, C.; Sang, Z.; Li, D. Mechanical Properties and Microstructure of Alkali-Activated Soda Residue-Blast Furnace Slag Composite Binder. Sustainability 2022, 14, 11751. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, Z.; Qi, X.; Wang, W.; Guo, S. Alkali-activated materials without commercial activators: A review. J. Mater. Sci. 2024, 59, 3780–3808. [Google Scholar] [CrossRef]
- Najimi, M.; Ghafoori, N.; Sharbaf, M. Alkali-activated natural pozzolan/slag binders: Limitations and remediation. Mag. Concr. Res. 2019, 72, 919–935. [Google Scholar] [CrossRef]
- Thomas, S.E.; Ramaswamy, K.; Thomas, B.S. A review on rheological behaviour of alkali activated materials and the influence of composition factors. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Kong, Y.; Kurumisawa, K. Fresh properties and characteristic testing methods for alkali-activated materials: A review. J. Build. Eng. 2023, 75, 106830. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Huang, Z. New insights into the early reaction of NaOH-activated slag in the presence of CaSO4. Compos. Part B 2020, 198, 108207. [Google Scholar] [CrossRef]
- Kahlouche, R.; Badaoui, A. Mechanical Performance and Durability of Mortar Based on Slag Cement and NaOH-Activated Slag. Mater. Sci. Forum 2022, 1078, 179–188. [Google Scholar] [CrossRef]
- Adesanya, E.; Perumal, P.; Luukkonen, T.; Yliniemi, J.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Opportunities to improve sustainability of alkali-activated materials: A review of side-stream based activators. J. Clean. Prod. 2021, 286, 125558. [Google Scholar] [CrossRef]
- Omur, T.; Kabay, N.; Miyan, N.; Özkan, H.; Özkan, Ç. The effect of alkaline activators and sand ratio on the physico-mechanical properties of blast furnace slag based mortars. J. Build. Eng. 2022, 58, 104998. [Google Scholar] [CrossRef]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Carter, K.; Ziehl, P. Energy and CO2 emission assessments of alkali-activated concrete and Ordinary Portland Cement concrete: A comparative analysis of different grades of concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Komkova, A.; Habert, G. Environmental impact assessment of alkali-activated materials: Examining impacts of variability in constituent production processes and transportation. Constr. Build. Mater. 2023, 363, 129032. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Zhong, M.; Wang, Z.; Qian, G.; Liu, J.; Gong, X. Relationship between pore structure and hydration activity of CaO from carbide slag. Chin. J. Chem. Eng. 2019, 27, 2771–2782. [Google Scholar] [CrossRef]
- Zhang, T.; Chu, G.; Lyu, J.; Cao, Y.; Xu, W.; Ma, K.; Song, L.; Yue, H.; Liang, B. CO2 mineralization of carbide slag for the production of light calcium carbonates. Chin. J. Chem. Eng. 2022, 43, 86–98. [Google Scholar] [CrossRef]
- Seo, J.; Park, S.; Yoon, H.N.; Jang, J.G.; Kim, S.H.; Lee, H.K. Utilization of Calcium Carbide Residue Using Granulated Blast Furnace Slag. Materials 2019, 12, 3511. [Google Scholar] [CrossRef] [PubMed]
- Kalina, L.; Bílek, V.; Hrubý, P.; Iliushchenko, V.; Kalina, M.; Smilek, J. On the action mechanism of lignosulfonate plasticizer in alkali-activated slag-based system. Cem. Concr. Res. 2022, 157, 106822. [Google Scholar] [CrossRef]
- GB/T 8077-2012; Methods for Testing Uniformity of Concrete Admixture. China Building Materials Federation: Beijing, China, 2012.
- GB/T 1346-2011; Test Method for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. China Building Materials Federation: Beijing, China, 2011.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). China Building Materials Federation: Beijing, China, 2021.
- Yang, J.; Zhang, Q.; He, X.; Su, Y.; Zeng, J.; Xiong, L.; Zeng, L.; Yu, X.; Tan, H. Low-carbon wet-ground fly ash geopolymer activated by single calcium carbide slag. Constr. Build. Mater. 2022, 353, 129084. [Google Scholar] [CrossRef]
- Zheng, H. The Preparation and Property Research of One-Part Alkali-Activated Slag; Guangxi University: Nanning, China, 2021. [Google Scholar]
- Rashad, A.M.; Morsi, W.M.; Khafaga, S.A. Effect of limestone powder on mechanical strength, durability and drying shrinkage of alkali activated slag pastes. Innov. Infrastruct. Solut. 2021, 6, 127. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z. Effect of Na2O concentration and water/binder ratio on carbonation of alkali-activated slag/fly ash cements. Constr. Build. Mater. 2020, 269, 121258. [Google Scholar] [CrossRef]
- Gijbels, K.; Pontikes, Y.; Samyn, P.; Schreurs, S.; Schroeyers, W. Effect of NaOH content on hydration, mineralogy, porosity and strength in alkali/sulfate-activated binders from ground granulated blast furnace slag and phosphogypsum-ScienceDirect. Cem. Concr. Res. 2020, 132, 106054. [Google Scholar] [CrossRef]
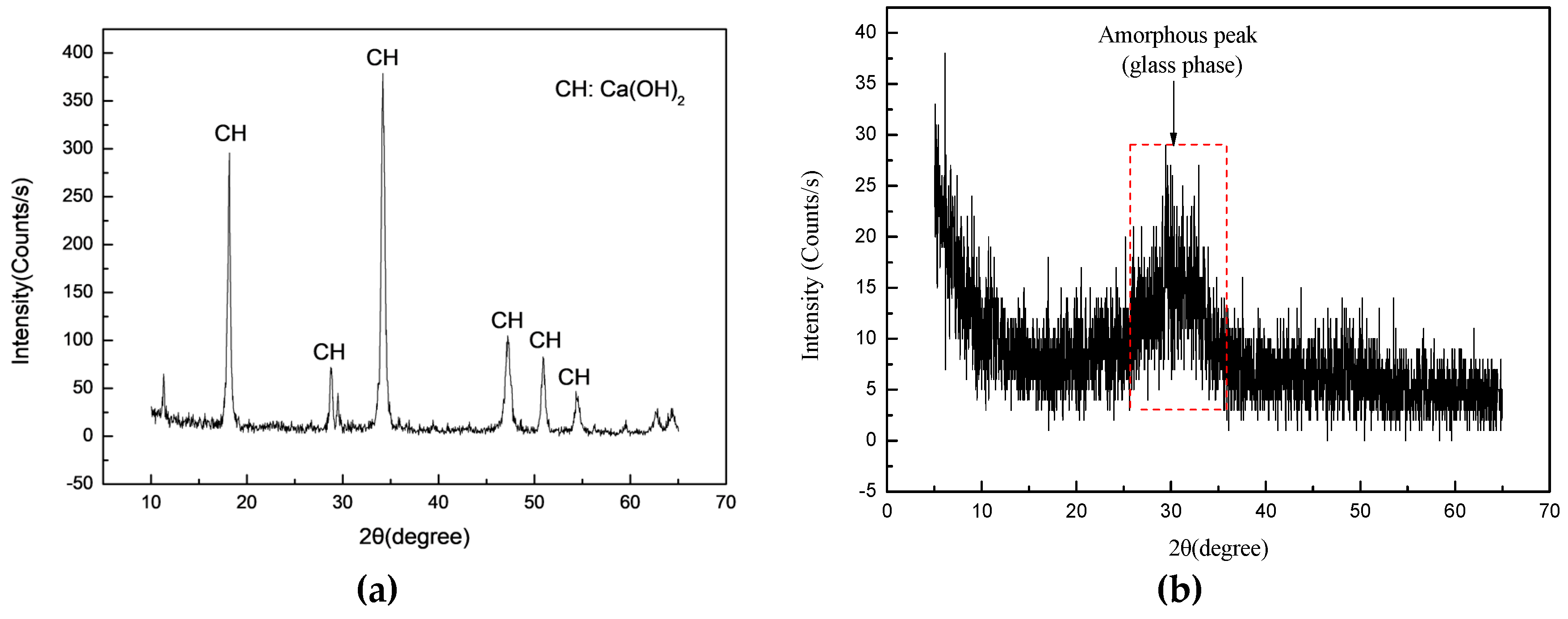
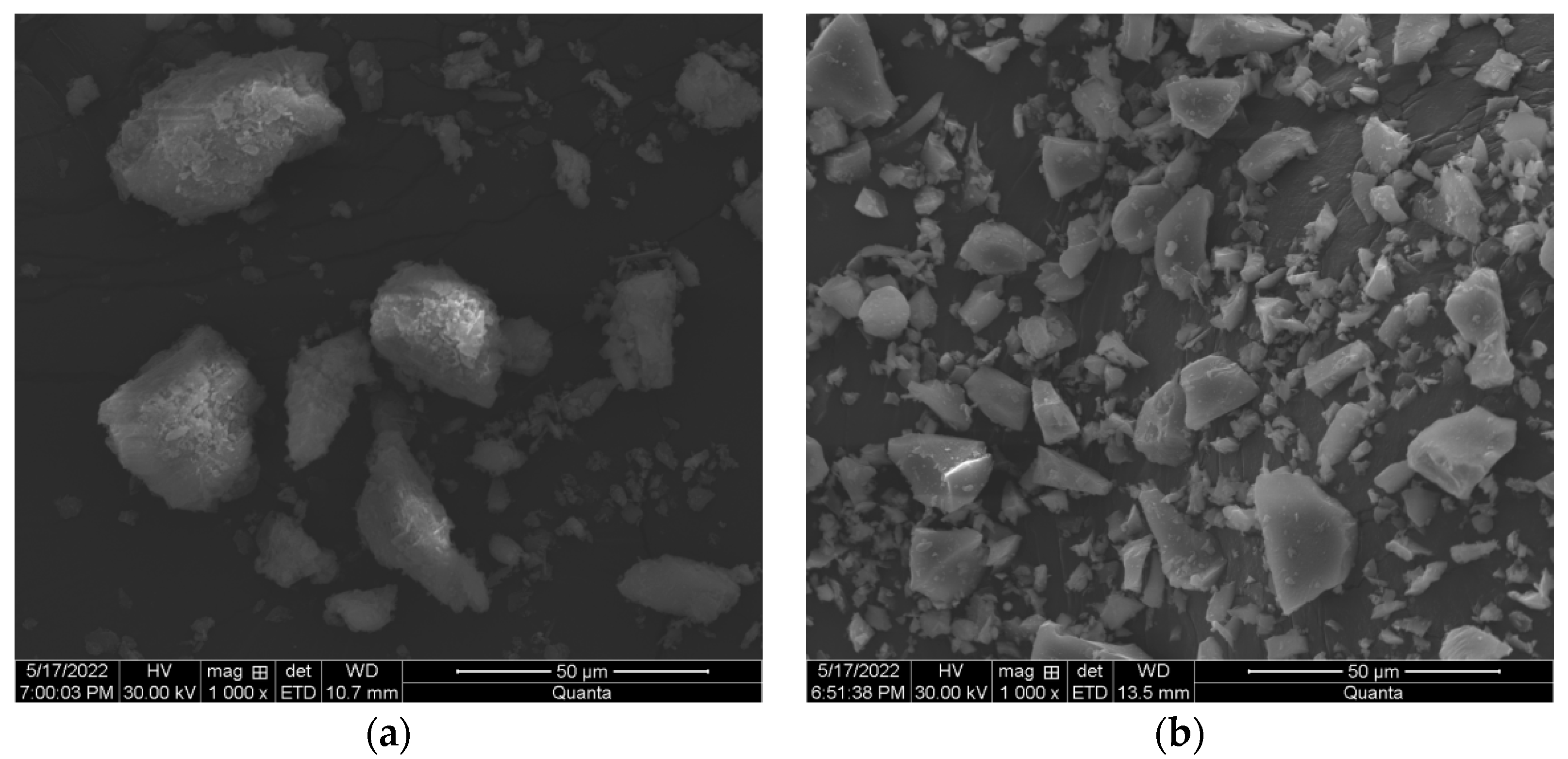
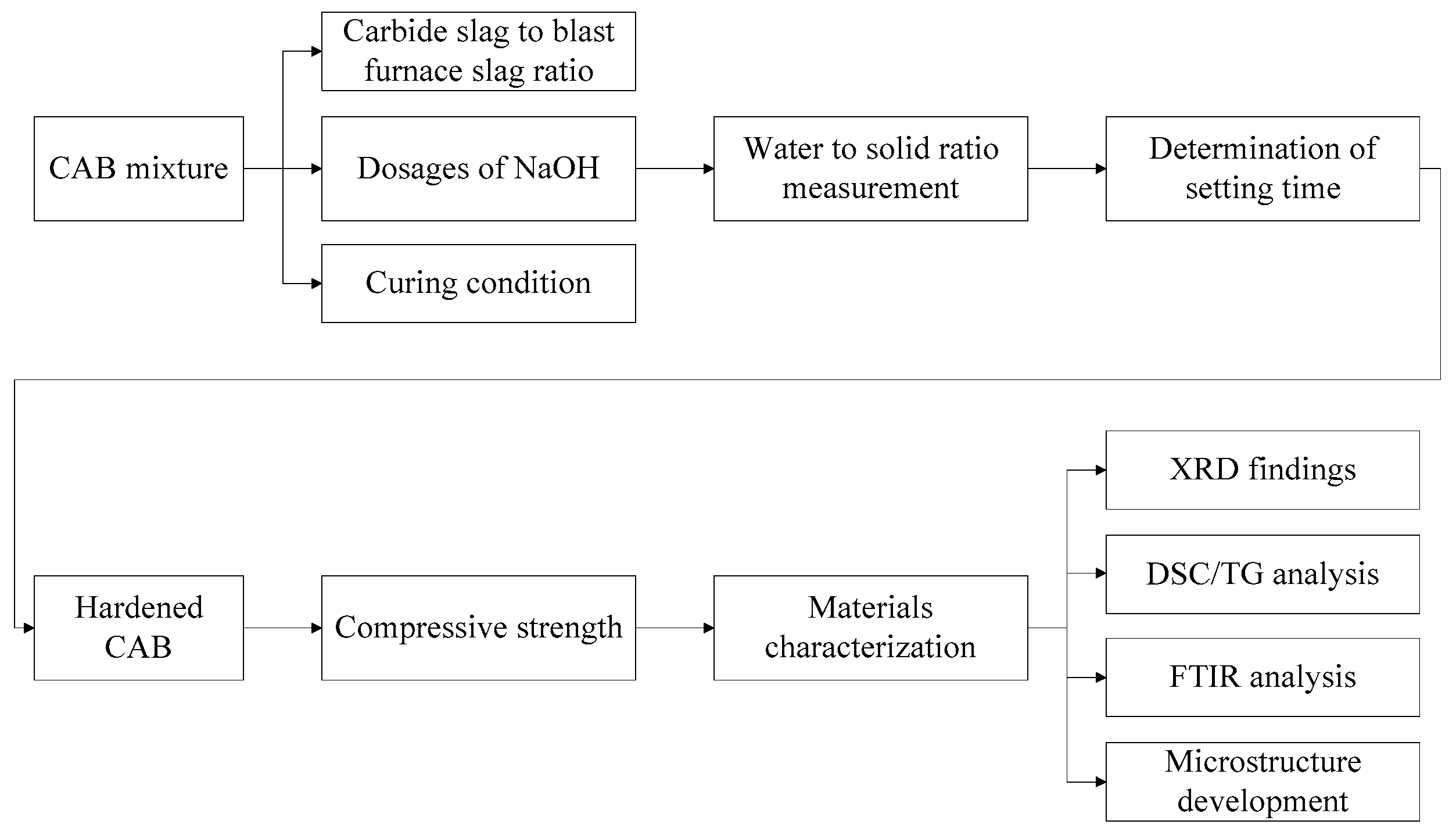



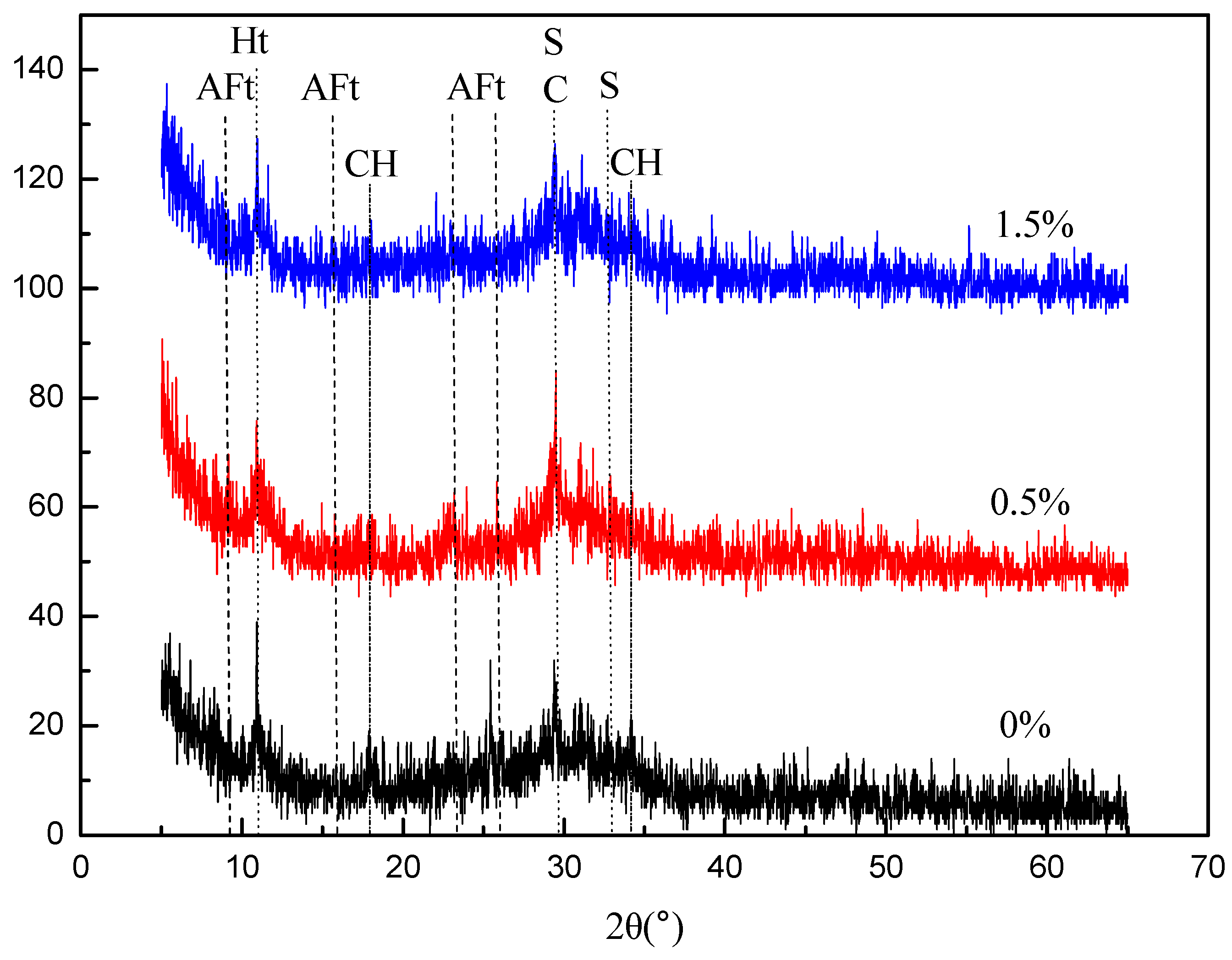



| Carbide Slag | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | CaO | TiO2 | V2O5 | Fe2O3 | SrO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Composition | 0.74 | 0.34 | 2.21 | 4.23 | 0.08 | 0.78 | 0.93 | 0.01 | 90.29 | 0.05 | 0.02 | 0.24 | 0.08 |
| Blast Furnace Slag | Na2O | MgO | Al2O3 | SiO2 | SO3 | Cl | K2O | CaO | TiO2 | MnO | Fe2O3 | SrO | Y2O3 | ZrO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Composition | 0.48 | 9.01 | 15.62 | 27.24 | 2.73 | 0.07 | 0.42 | 42.21 | 1.22 | 0.47 | 0.40 | 0.07 | 0.01 | 0.06 |
| Mix | Blast Furnace Slag | Carbide Slag | Sodium Hydroxide | Curing Conditions |
|---|---|---|---|---|
| M1 | 100% | 0% | 3% | Standard curing |
| M2 | 95% | 5% | 3% | Standard curing |
| M3 | 90% | 10% | 3% | Standard curing |
| M4 | 85% | 15% | 3% | Standard curing |
| M5 | 80% | 20% | 3% | Standard curing |
| M6 | 70% | 30% | 3% | Standard curing |
| Mix | Blast Furnace Slag | Carbide Slag | Sodium Hydroxide | Curing Conditions |
|---|---|---|---|---|
| M7 | 80% | 20% | 1% | Standard curing |
| M8 | 80% | 20% | 2% | Standard curing |
| M9 | 80% | 20% | 3% | Standard curing |
| M10 | 80% | 20% | 4% | Standard curing |
| M11 | 80% | 20% | 5% | Standard curing |
| M12 | 80% | 20% | 6% | Standard curing |
| M13 | 80% | 20% | 8% | Standard curing |
| M14 | 80% | 20% | 10% | Standard curing |
| Mix | Blast Furnace Slag | Carbide Slag | Sodium Hydroxide | Curing Conditions |
|---|---|---|---|---|
| M15 | 90% | 10% | 3% | Standard curing |
| M16 | 90% | 10% | 3% | Water curing |
| M17 | 90% | 10% | 3% | Steam curing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, K.; Sun, N.; Wang, J.; Xue, K.; Xu, L.; Ren, Y.; Yan, Z.; Sima, T. Compressive Strength and Microstructure of Carbide Slag and Alkali-Activated Blast Furnace Slag Pastes in China. Buildings 2024, 14, 1681. https://doi.org/10.3390/buildings14061681
Li Z, Xu K, Sun N, Wang J, Xue K, Xu L, Ren Y, Yan Z, Sima T. Compressive Strength and Microstructure of Carbide Slag and Alkali-Activated Blast Furnace Slag Pastes in China. Buildings. 2024; 14(6):1681. https://doi.org/10.3390/buildings14061681
Chicago/Turabian StyleLi, Zhixin, Kaidong Xu, Nan Sun, Jina Wang, Kaiwang Xue, Longyun Xu, Yi Ren, Zhenzhou Yan, and Tongbao Sima. 2024. "Compressive Strength and Microstructure of Carbide Slag and Alkali-Activated Blast Furnace Slag Pastes in China" Buildings 14, no. 6: 1681. https://doi.org/10.3390/buildings14061681
APA StyleLi, Z., Xu, K., Sun, N., Wang, J., Xue, K., Xu, L., Ren, Y., Yan, Z., & Sima, T. (2024). Compressive Strength and Microstructure of Carbide Slag and Alkali-Activated Blast Furnace Slag Pastes in China. Buildings, 14(6), 1681. https://doi.org/10.3390/buildings14061681






