Design and Verification of Experimental Device for Pressurized Bubbling Absorption and Transport Characteristics in Vacuum Environment
Abstract
:1. Introduction
2. Experimental Apparatus
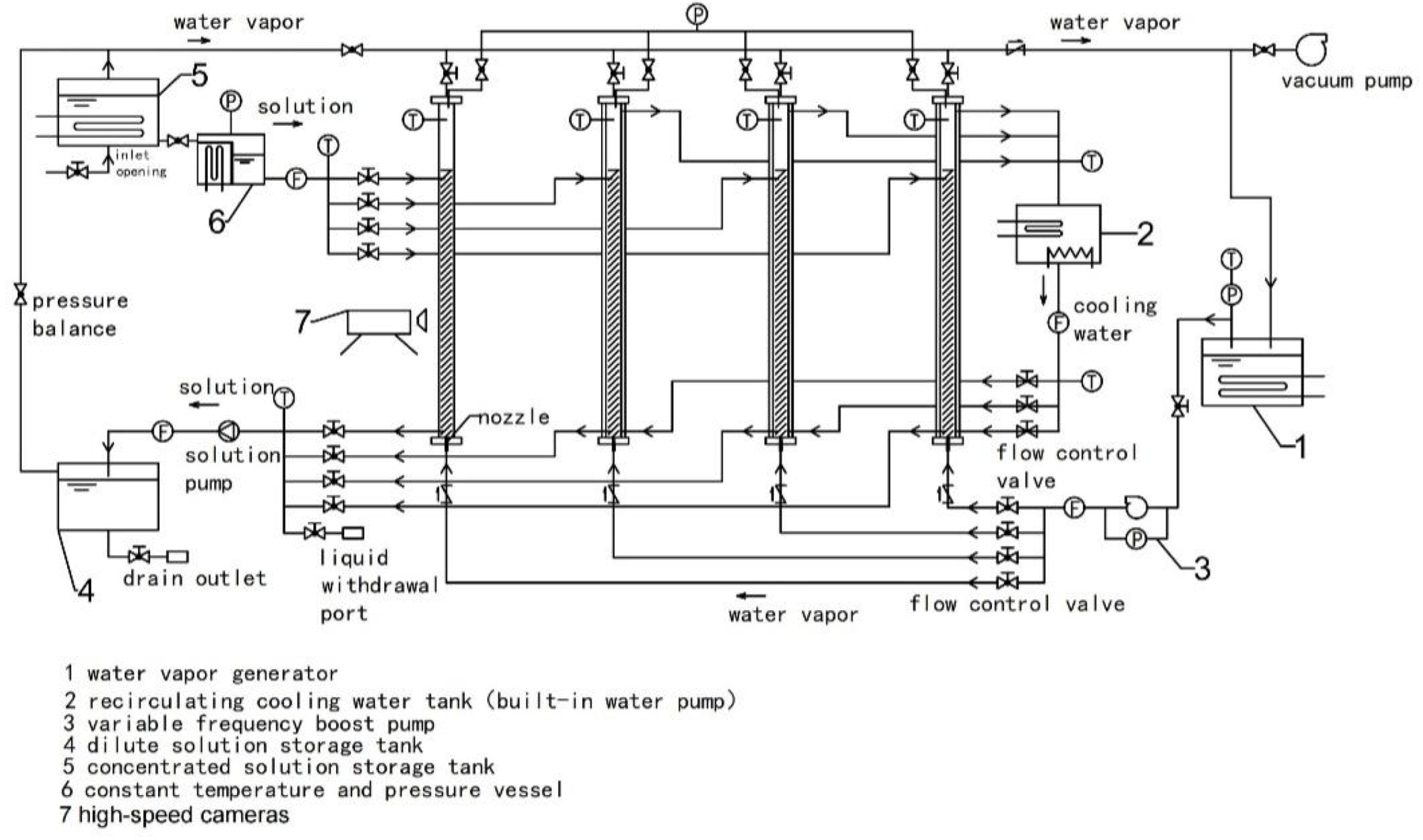
2.1. System Structure
- (1)
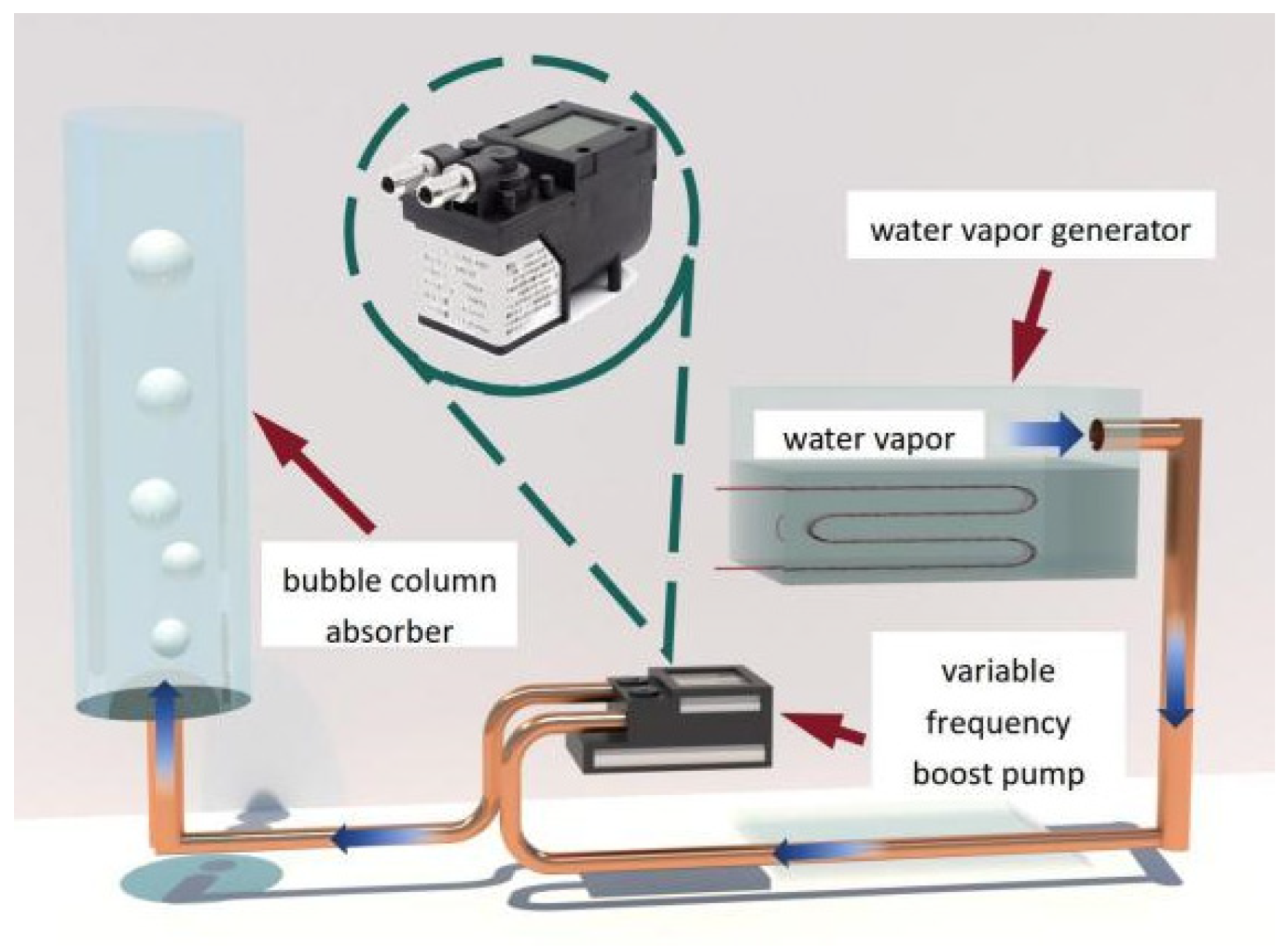
- The steam pressurization system primarily consists of a variable frequency booster pump, vacuum pump, steam generator, flow control valve, and check valve. The system is utilized to provide the necessary steam for the experiment while ensuring a suitable flow rate. To overcome the resistance caused by hydraulic head and flow, the system employs a variable frequency booster pump to generate the required pressure for water vapor, thereby ensuring the generation and upward movement of bubbles, as depicted in Figure 3. The steam generator incorporates an electric heating function, enabling the heating, boiling, and vaporization of distilled water under low pressure, producing water vapor. The check valve serves as a precaution against liquid backflow and helps maintain pressure equilibrium within the system.
- (2)
- The solution system comprises several components: a concentrated solution storage tank, a dilute solution storage tank, a solution pump, a constant temperature and pressure tank, a flow control valve, and pipelines. These components are utilized for storing, generating, and circulating concentrated and dilute solutions, while ensuring the smooth absorption of the solution into the bubble absorber at a specific temperature. The lower part of the concentrated solution storage tank is equipped with an inlet to draw in the solution using the vacuum pressure of the system. The outlet is connected to the constant temperature and pressure tank, and a steam system is linked to the pipeline above. The constant temperature and pressure tank maintain a steady temperature and the pressure of the water vapor entering the bubble absorber.
- (3)
- The bubble absorber encompasses a visual bubble absorber, consisting of a cylindrical glass jacket and a high-speed camera, as well as a visual bubble absorber, constructed with a cylindrical double-layer copper jacket and a temperature sensor. The visual bubble absorber aids in determining the flow characteristics of bubbles during solution absorption, while the non-visual bubble absorber helps in assessing the heat and mass transfer properties. Both absorbers are connected to a sampling port for convenient testing of solution concentration.
- (4)
- The cooling water system comprises a constant temperature circulation water bath, a flow control valve, valves, and pipelines. The constant temperature circulation water bath supplies cooling water to the bubble absorber and acts as a consistent heat source for the concentrated solution storage tank.
- (5)
- The experimental data testing and analysis system incorporates various instruments, including thermocouples, pressure sensors, flow meters, density analyzers, high-speed cameras, data acquisition devices, and computers. The material parameters of the experimental setup are listed in Table 1, while the instrument parameters used are outlined in Table 2.
2.2. System Operation
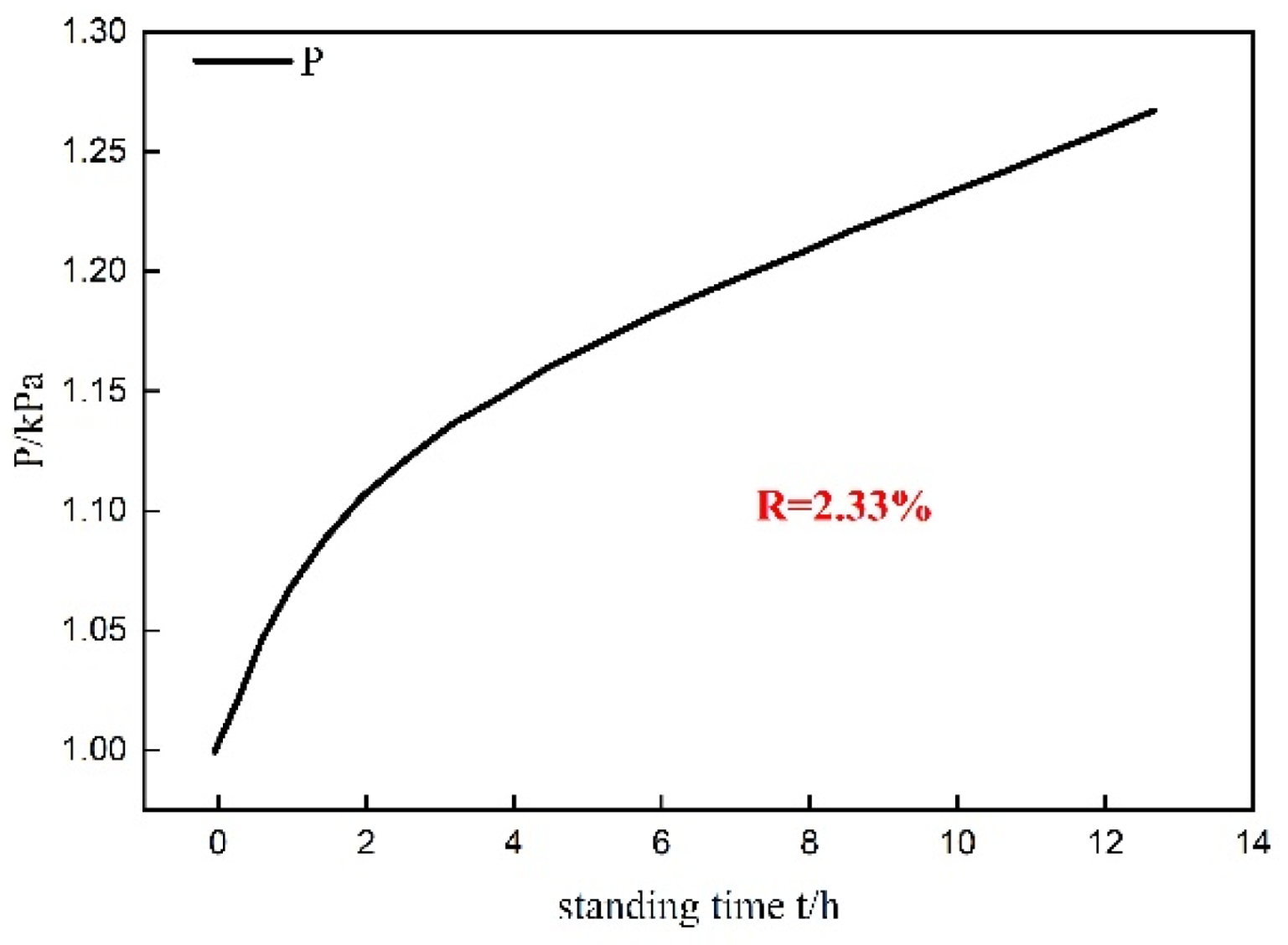
3. System Reliability
4. System Accuracy Validation
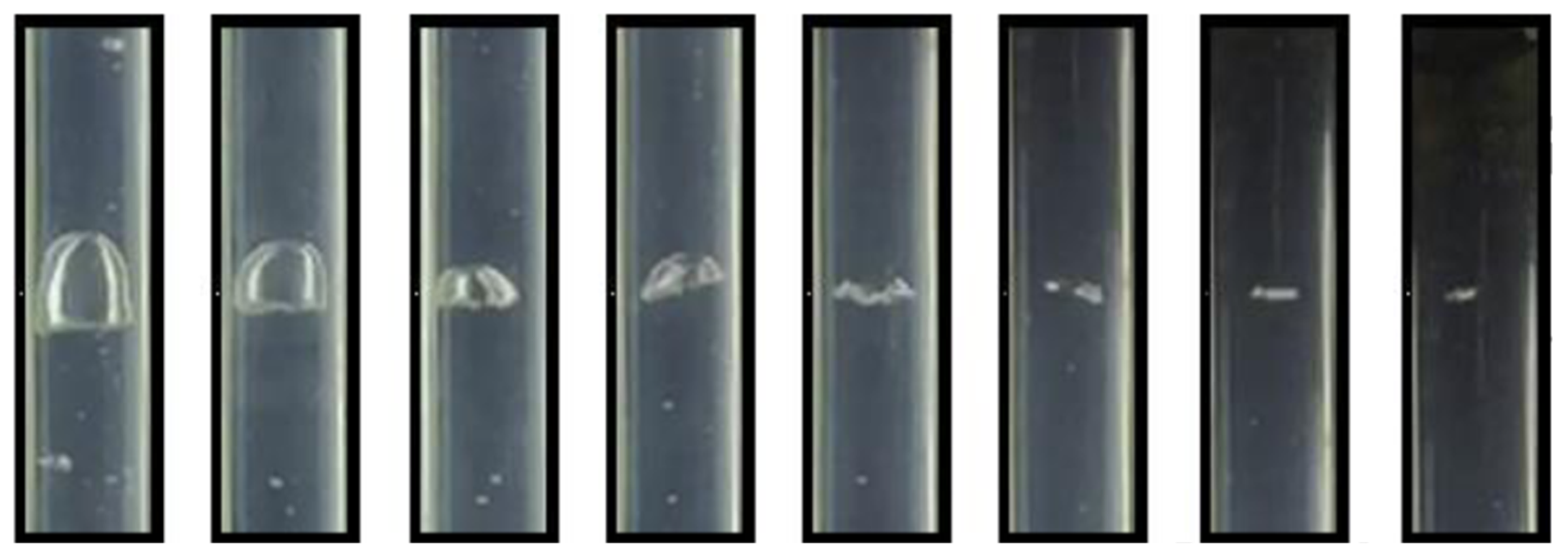
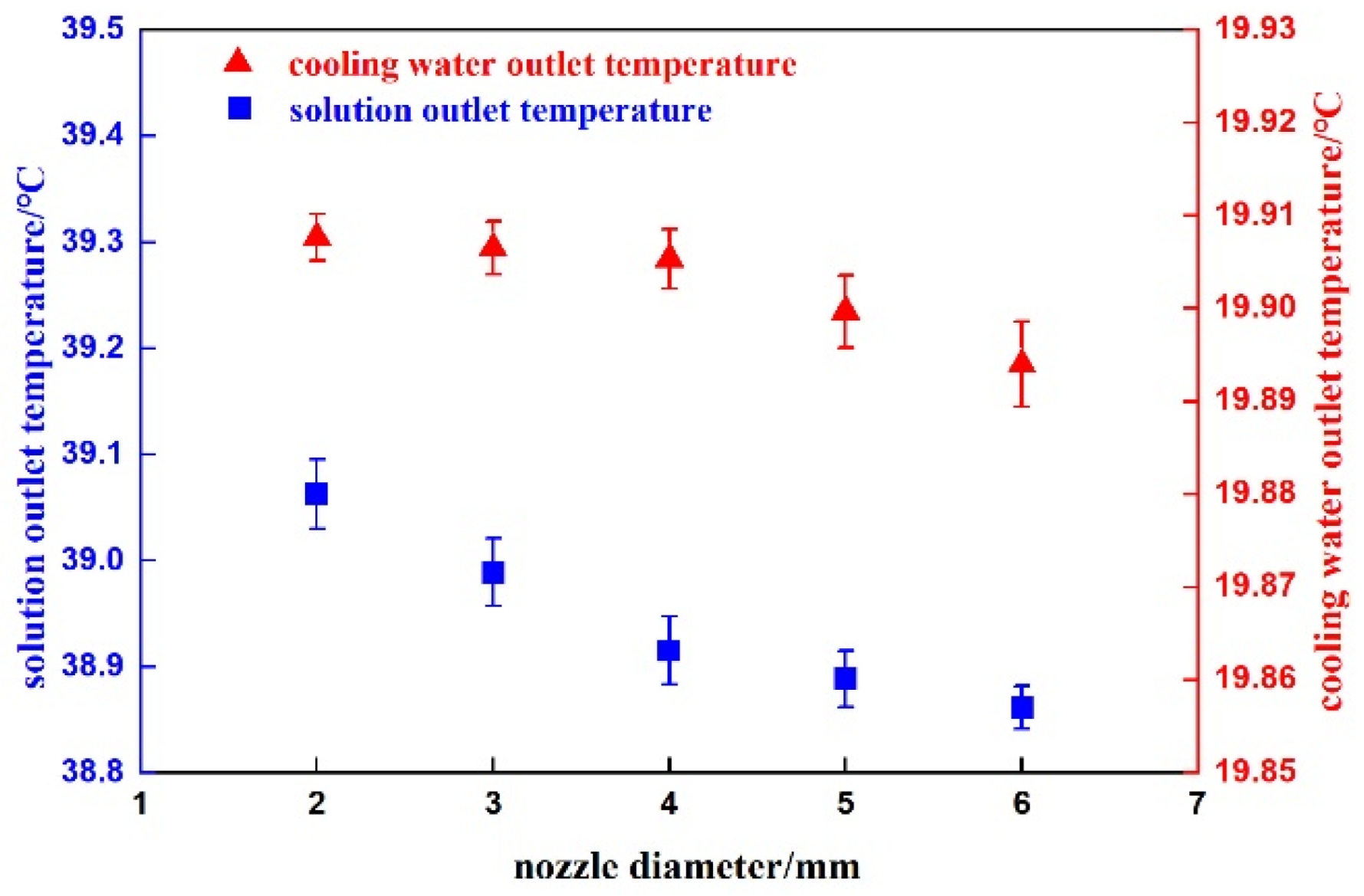
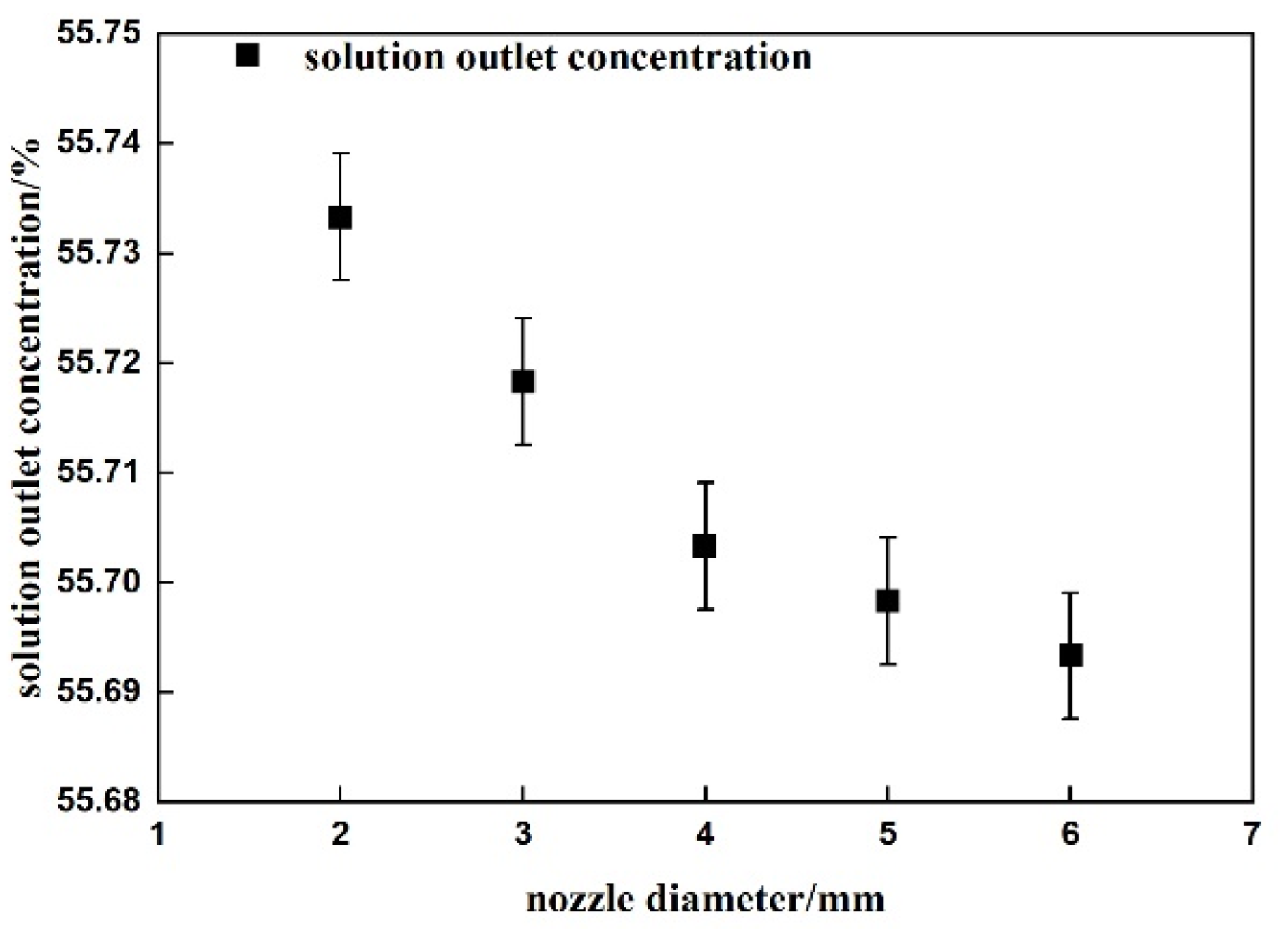
4.1. Bubble Flow Verification
4.2. Heat Transfer Verification
4.3. Energy Conservation Verification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Inayat, M.; Järvinen, M. Steam Gasification of Polyethylene Terephthalate (PET) with CaO in a Bubbling Fluidized Bed Gasifier for Enriching H2 in Syngas with Response Surface Methodology (RSM). Appl. Energy 2023, 348, 121536. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Romero, R. Performance of Different Experimental Absorber Designs in Absorption Heat Pump Cycle Technologies: A Review. Energies 2014, 7, 751–766. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, S.; Wu, X. Numerical Simulation and Experiment for R124-DMAC Bubble Absorption Process in a Vertical Tubular Absorber. Int. J. Therm. Sci. 2019, 138, 124–133. [Google Scholar] [CrossRef]
- Yang, L.; Du, K.; Zhang, X. Influence Factors on Thermal Conductivity of Ammonia-Water Nanofluids. J. Cent. South Univ. Technol. 2012, 19, 1622–1628. [Google Scholar] [CrossRef]
- Kemoun, A.; Cheng Ong, B.; Gupta, P.; Al-Dahhan, M.H.; Dudukovic, M.P. Gas Holdup in Bubble Columns at Elevated Pressure via Computed Tomography. Int. J. Multiph. Flow 2001, 27, 929–946. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Xu, S.; Wang, W. Effect of Vibration Parameters on the Bubble Absorption Characteristics of Working Fluids R134a–DMAC in a Vertical Tube. Int. J. Refrig. 2019, 99, 234–242. [Google Scholar] [CrossRef]
- Kang, Y.T.; Nagano, T.; Kashiwagi, T. Visualization of Bubble Behavior and Bubble Diameter Correlation for NH3–H2O Bubble Absorption. Int. J. Refrig. 2002, 25, 127–135. [Google Scholar] [CrossRef]
- Kim, H.Y.; Saha, B.B.; Koyama, S. Development of a Slug Flow Absorber Working with Ammonia–Water Mixture: Part II—Data Reduction Model for Local Heat and Mass Transfer Characterization. Int. J. Refrig. 2003, 26, 698–706. [Google Scholar] [CrossRef]
- Li, L.; Kang, Y.T. Effects of Bubble Coalescence and Breakup on CO2 Absorption Performance in Nanoabsorbents. J. CO2 Util. 2020, 39, 101170. [Google Scholar] [CrossRef]
- Li, L.; Kang, Y.T. Bubble Behaviors and CO2 Absorption Characteristics in Nanoabsorbents. J. CO2 Util. 2019, 33, 488–499. [Google Scholar] [CrossRef]
- Roy, S. Catalytic Wet Air Oxidation of Oxalic Acid Using Platinum Catalysts in Bubble Column Reactor: A Review. J. Eng. Sci. Technol. Rev. 2010, 3, 95–107. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, M.; Hu, J.; Wu, X.; Wang, W. Visual Experimental Research on Bubble Absorption in a Vertical Tube with R124–DMAC Working Pair. Exp. Therm. Fluid Sci. 2016, 74, 1–10. [Google Scholar] [CrossRef]
- Kang, Y.T.; Nagano, T.; Kashiwagi, T. Mass Transfer Correlation of NH3–H2O Bubble Absorption. Int. J. Refrig. 2002, 25, 878–886. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, S.; Wu, X.; Wang, W. Heat and Mass Transfer Characteristics of R124-DMAC Bubble Absorption in a Vertical Tube Absorber. Exp. Therm. Fluid Sci. 2017, 81, 466–474. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, S.; Wu, X.; Hu, J.; Wang, W. Visual Experimental Research on the Effect of Nozzle Orifice Structure on R124–DMAC Absorption Process in a Vertical Bubble Tube. Int. J. Refrig. 2016, 68, 107–117. [Google Scholar] [CrossRef]
- Kim, H.Y.; Saha, B.B.; Koyama, S. Development of a Slug Flow Absorber Working with Ammonia-Water Mixture: Part I—Flow Characterization and Experimental Investigation. Int. J. Refrig. 2003, 26, 508–515. [Google Scholar] [CrossRef]
- Kim, J.-K.; Jung, J.Y.; Kang, Y.T. The Effect of Nano-Particles on the Bubble Absorption Performance in a Binary Nanofluid. Int. J. Refrig. 2006, 29, 22–29. [Google Scholar] [CrossRef]
- Wu, W.-D.; Liu, G.; Chen, S.-X.; Zhang, H. Nanoferrofluid Addition Enhances Ammonia/Water Bubble Absorption in an External Magnetic Field. Energy Build. 2013, 57, 268–277. [Google Scholar] [CrossRef]
- Donnellan, P.; Cronin, K.; Lee, W.; Duggan, S.; Byrne, E. Absorption of Steam Bubbles in Lithium Bromide Solution. Chem. Eng. Sci. 2014, 119, 10–21. [Google Scholar] [CrossRef]
- Ayou, D.S.; Bruno, J.C.; Coronas, A. Integration of a Mechanical and Thermal Compressor Booster in Combined Absorption Power and Refrigeration Cycles. Energy 2017, 135, 327–341. [Google Scholar] [CrossRef]
- Xie, G.; Wu, Q.; Fa, X.; Zhang, L.; Bansal, P. A Novel Lithium Bromide Absorption Chiller with Enhanced Absorption Pressure. Appl. Therm. Eng. 2012, 38, 1–6. [Google Scholar] [CrossRef]
- Khalili, S.; Garousi Farshi, L. Design and Performance Evaluation of a Double Ejector Boosted Multi-Pressure Level Absorption Cycle for Refrigeration. Sustain. Energy Technol. Assess. 2020, 42, 100836. [Google Scholar] [CrossRef]
- Lee, J.B.; Derome, D.; Carmeliet, J. Drop Impact on Natural Porous Stones. J. Colloid Interface Sci. 2016, 469, 147–156. [Google Scholar] [CrossRef]
- Kabir, H.; Garg, N. Rapid Prediction of Cementitious Initial Sorptivity via Surface Wettability. NPJ Mater. Degrad. 2023, 7, 52. [Google Scholar] [CrossRef]




| Instrument | Model Number | Range | Precision | Remark |
|---|---|---|---|---|
| Solution pump | AQG-24-silicone tube | 480 mL/min | 2% | -- |
| Variable frequency booster pump | V60L-JJ-24V | 18 L/min | -- | ΔP ≥ −88 kPa |
| Vacuum pump | ROAIRVAC9.0 | 4 L | -- | -- |
| Thermal insulation material | Polyethylene insulation material | -- | -- | -- |
| Bubble absorber | Visualization | Material: glass; nozzle diameter: 4 mm; pipe diameter: 30 mm; pipe length: 1.2 m | ||
| Non-visualization | Material: copper; nozzle diameter: 2 mm, 4 mm, 6 mm; inner pipe diameter: 30 mm; outer pipe diameter: 40 mm; pipe length: 1.2 m | |||
| Instrument | Model Number | Range | Precision | Remark |
|---|---|---|---|---|
| Solution flowmeter | NKGF02F1L1 | 0.6~6 L/H | ±0.5%FS | -- |
| Gas flowmeter | MF5000 | 0~50 L/min | ±1.5%FS | -- |
| Cooling water flowmeter | NKGF02F1L1 | 0.6~6 L/H | ±0.5%FS | -- |
| Pressure sensor | PT-8303B | 0~150 kPa | ±0.5%FS | -- |
| Thermoelectric couple | SWT-KPS-NI600-T1-20-2000 | −200~300 °C | ±0.5 °C | -- |
| Densitometer | KEM WBA-505 | 0~3 g/cm3 | ±0.00005 g/cm3 | -- |
| High speed camera | Phantom Ametek V211 | -- | -- | -- |
| Data acquisition instrument | Keysight 34901A | -- | -- | -- |
| Experimental Conditions Parameters | Numerical Value | |
|---|---|---|
| Liquor | Inlet temperature | 35~40 °C |
| Inlet flow rate | 10~50 L/h | |
| Cooling water | Inlet temperature | 20 °C |
| Inlet flow rate | 0.0143 L/h | |
| Other | Nozzle diameter | 2~6 mm |
| Steam inlet velocity | 2 m/s | |
| Pressure ratio | 1.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Wang, G.; Li, J.; Cui, X.; Xiong, Y.; Lü, X.; Zhang, X. Design and Verification of Experimental Device for Pressurized Bubbling Absorption and Transport Characteristics in Vacuum Environment. Buildings 2024, 14, 1685. https://doi.org/10.3390/buildings14061685
Gao J, Wang G, Li J, Cui X, Xiong Y, Lü X, Zhang X. Design and Verification of Experimental Device for Pressurized Bubbling Absorption and Transport Characteristics in Vacuum Environment. Buildings. 2024; 14(6):1685. https://doi.org/10.3390/buildings14061685
Chicago/Turabian StyleGao, Jialiang, Gang Wang, Jitong Li, Xiaoyan Cui, Yaxuan Xiong, Xiaoshu Lü, and Xuejing Zhang. 2024. "Design and Verification of Experimental Device for Pressurized Bubbling Absorption and Transport Characteristics in Vacuum Environment" Buildings 14, no. 6: 1685. https://doi.org/10.3390/buildings14061685







