Abstract
Steel slag is a significant environmental liability generated by pyrometallurgical processes. Residue generation, such as granulated blast furnace slag and basic oxygen slag (BOF), is intrinsic in steel production. Blast furnace slag, generated in the carbothermal reduction of iron ore, is almost entirely used as a supplementary cement material in Portland cement. BOF slag, produced in the conversion of pig iron into steel in a basic oxygen converter, is still not consolidated or valued for reuse. This research proposes the reuse and valorization of BOF slag combined with blast furnace slag in clinker-free cement production. Cement formulations were produced with different slag and gypsum contents, ranging from 80 to 90% blast furnace slag, 10 to 20% gypsum, and 10 to 15% BOF slag. All formulations were evaluated for compressive strength at ages of 3, 7, 14, 28, 91, and 180 days of curing. At the initial ages, the cement formulations exhibited high resistance. On the 3rd day, the cement formulations reached up to 10 MPa, and on the 7th day, 40 MPa. At late ages, the best-performing formulation, ECO2, showed, after 28 days of hydration, a compressive strength greater than 50 MPa, and at 180 days, a compressive strength greater than 80 MPa. It was possible to understand that BOF slag acts in cement alkaline activation with pH increase, more or less actively due to the presence of lime, portlandite, and calcite.
1. Introduction
Despite the relevant and negative environmental impacts related to the cement production chain, its consumption continues to soar, especially in developing nations, such as China, which represents 51% of global production, and India, the second largest producer in the world, in addition to countries in Southeast Asia, Latin America, and Africa [1].
The growing concern about the environmental impacts caused by the production of Portland cement, combined with possible technological and economic gains, has led to many changes in the cement industry. However, these changes were insufficient to significantly reduce the generated environmental problems, mostly related to the predatory extraction of minerals, mainly limestone and clays, and the consequent need for limestone decarbonization and clay dehydroxylation. Both calcination processes stand out for their high emission of greenhouse gases, mostly CO2. In other words, the environmental problems are mainly related to the production of Portland clinker [1,2,3].
Alternative cement formulations using different materials and avoiding clinkerization to make the production chain less carbon-intensive are an important initiative to reduce emissions. Some technologies have been proposed without the conventional raw material use and with new technological routes. In some cases, the technologies start almost entirely from the reuse of waste, differing widely between them. Among these technologies, the production of alkali-activated cement, calcium aluminate cement (CAC), magnesium silicate cement (Novacem), and supersulfated cement (SSC) can be mentioned [1,4,5].
The production of these cements faces difficulties due to both the raw materials used and the understanding of their performance. Since the raw materials used may be of natural origin, as is the case with the use of bauxite (CAC) or magnesium silicate (Novacem), the limited supply of raw materials, either due to restricted availability in certain regions or due to competition with other production chains, can make the cement more expensive than Portland cement and limited to specific applications [4,5].
On the other hand, some formulations use high levels of industrial waste, as occurs in supersulfated cement. Its formulation uses up to 90% ground granulated blast furnace slag, 15% more than the maximum allowed by Brazilian standards [6] in the cement composition with the highest steel slag content (CPIII). The composition also includes 10–20% hydrated calcium sulfates but up to 5% of an alkaline activator, such as Portland cement or Portland clinker [4].
Given these formulations, the production of a clinker-free cement with low environmental impact and low greenhouse gas emissions from the exclusive use of steel waste, i.e., blast furnace slag (BFS) and basic oxygen furnace slag (BOF), combined with gypsum waste from construction and the ceramic industry, called eco-sustainable cement (ECO), was proposed. Eco-sustainable cement presents certain similarities to CSS regarding composition. However, it does not use either Portland clinker or Portland cement but uses BOF slag as an alkaline activator [7]. The mechanical and physical–chemical performance of ECO cement was similar to or superior to Brazilian blast furnace slag, Portland cement, and CSS [6,7,8].
In order to improve the mechanical performance of eco-sustainable cement in its final stages, this study was conducted. This study also aimed to test various cement formulations containing different amounts of ironmaking slags and gypsum waste, evaluate their mechanical performance, and understand the effect of raw materials on the cement hydration process, specifically in terms of chemical and mineralogical composition.
2. Materials and Methods
2.1. Materials
The raw materials used consisted of steelmaking slags coming from a blast furnace (blast furnace slag—BFS) and a Linz–Donawitz furnace (LD steel slag—BOF) obtained from Companhia Siderúrgica Nacional—CSN (Brazil)—and plaster residue from construction. As shown in Table 1, the same gypsum residue was used in the proposed formulations. However, three types of BOF slag and two types of blast furnace slag were used to evaluate the effect of different calcium oxides, aluminum, iron, and silicon levels in cement. After drying, the raw materials were fragmented to obtain a maximum particle size of 2.36 mm in diameter.

Table 1.
Raw materials used for cement production.
2.2. Methods
The conditions of the chemical and mineralogical analyses of BFS, BOFS, and gypsum waste carried out using X-ray fluorescence (XRF) and X-ray diffraction (XRD) techniques are presented in Table 2. Rietveld Refinement was used to obtain quantitative results. An internal standard of 10% rutile was added to quantify the amorphous phase.

Table 2.
Techniques and conditions for characterizing raw materials.
Ten cement formulations were proposed with different slag and gypsum contents, ranging from 80 to 90% BFS, 10 to 20% gypsum waste, and 10 to 15% BOF slag, as shown in Table 3. Due to the difference in the grindability of ironmaking slags, the cement grinding was carried out by fine grinding the BFS and GW mixture, with increasing replacements of (BFS + GW) with ground BOF slag to a Blaine fineness of 550 m2/Kg.

Table 3.
Composition of cementitious formulations.
The cementitious formulations were evaluated for chemical composition, as shown in Table 2 (XFR), and alkalinity of the cement pastes. The pastes were prepared with a water/cement ratio of 0.31, which was determined based on the normal consistency of cement. The alkalinity measurements were taken using a peagameter, Dgimed model DM2.
The cement mortar specimens prepared for compressive strength tests, according to [9], were subjected to curing in a humid chamber (relative humidity of (95 ± 5)% and room temperature of (24 ± 2) °C) until the test ages of 3, 7, 28, 91, and 180 days. Compressive strength tests were carried out on a Forney compression machine, model F-25EX-F-CPILOT. The compression machine’s working range of mechanical stress is from 20 to 1100 kN.
3. Results
3.1. Raw Material Characterization
The chemical composition of the BOF, BFS slags, and gypsum waste is shown in Table 4. The main oxides found for each raw material are different concerning their origin. BFS has high SiO2, CaO, and Al2O3 content, essential for hydration product formation, such as aluminates and hydrated calcium silicates. In BOF slag, the oxides CaO, Fe2O3, and SiO2 predominate, which help in the chemical activation of BFS and the formation of new hydration products, while the gypsum residue basically presents CaO and SO3, essential in the formation of ettringite.

Table 4.
Chemical composition of raw materials.
The gypsum waste from civil construction corresponds to the hydration residue of calcium sulfate hemihydrate. For this reason, its mineralogical composition corresponds mainly to gypsum; Table 5.

Table 5.
Mineralogical composition of the gypsum waste and BOF slag.
The mineralogical composition of the BOF slag used (Table 5) demonstrates that despite the different percentages found, the majority of phases in the three samples correspond to wustite (FeO), calcite (CaCO3), belite (β-2CaSiO2), brownmillerite, and magnetite. Portlandite was also identified in all slags. However, portlandite content was significant for BOF2 and BOF3 slags.
BOF slag exerts a relevant influence on the reactivity of the reactional environment by the alkaline activator function. In this sense, BOF3 presented the highest portlandite content and had the highest basicity (3.7). Ca(OH)2 has high solubility in water (1.6 g/L) [10], being easily solubilized and quickly raising the pH of the solution. On the other hand, BOF1 showed a high CaCO3 content. Calcite can also change the pH of the solution by slowly releasing hydroxyl ions due to its low solubility in water (1 mg/L), as shown in the following equations [11]:
CaCO3 → Ca2+ + CO32−
CO32− + H2O → HCO3− + OH−
HCO3− + H2O → H2CO3 + OH−
In this way, both phases (CaCO3 and Ca(OH)2) can affect the basicity of the medium, but, depending on solubility, with different speeds.
Another factor relevant to reactivity is the percentage of the amorphous phase, which, due to the slow cooling of BOF slags, usually has a low crystallization energy level retained in its atomic structure. For this reason, BOF slags tend to be difficult to solubilize, given the high stability of the crystalline phases. In this sense, BOF2 and BOF3 slags presented similar contents of around 10%, while BOF1 presented twice the glass phase in its atomic structure, which may indicate greater reactivity and binding capacity [12,13].
In terms of hydraulic behavior, the most reactive phase present in BOF, in the initial ages, corresponds to brownmillerite, which appeared prominently in BOF2 (19%), as it begins hydration in the first 24 h to form hydrated calcium ferroaluminates or hydroxy compounds. At the final ages, belite is the phase responsible for the formation of hydrated calcium silicates that corroborate the gain of mechanical resistance in the cement and was identified in a higher percentage in BOF3 (25%) [13].
3.2. Cement Chemical Composition and Alkalinity
The evolution of the hydration process and the number of products formed in ECO cement are closely dependent on the chemical composition of the cement. Also, the development of mechanical resistance is mainly related to the precipitation of hydration products such as ettringite and hydrated calcium silicates (C-S-H). Table 6 shows the chemical composition of the cement.

Table 6.
Chemical composition of cementitious formulations.
By the time the anhydrous grains of the cement mixture come into contact with water, the dissolution of the blast furnace slag begins, but very slowly, which makes its use unfeasible. This is because the dissolution of the slag occurs by hydroxylic attack, that is, by OH− ions. Thus, if the solution in contact with the grains is alkaline, dissolution will be faster as the solubility of the glassy structure of the silica that constitutes BFS is increased. In addition, the formation of hydrated silico-aluminous products on the surface of the slag grains is avoided, which would prevent the dissolution from continuing [14,15].
It is estimated that the initial pH of cement rich in blast furnace slag is around 11–12.5 and decreases as the hydration process develops [4,11]. The increase in pH in the solution is mainly related to the hydrated lime that makes up the BOF slag since calcium sulfate does not change the pH of the solution significantly.
This statement is confirmed by observing the alkalinity of the cements ECO3 802010 and 802015, which differ only in the BOF slag content, for which the alkalinity corresponds to 12.3 and 13.1, respectively. In addition to increasing the pH level, it is expected that lime introduces Ca2+ ions into the solution, causing the equilibrium of the solubility product to shift towards saturation, accelerating the hydration product precipitation. At the beginning of cement hydration, due to the high alkalinity, there are hydrates precipitated, such as ettringite, C-S-H, and C-A-H, ensuring the cement’s high resistance at early ages. On the other hand, excessive alkalinity, as observed in ECO3 802015, can create instability in the products formed and can lead to covering the surface of anhydrous grains with precipitated products, harming the development of resistance at later ages as a consequence of earlier product precipitation.
For calcium sulfate, the dissolved SO42− reacts with the aluminum released from BFS dissolution to form ettringite, one of the first products to be precipitated in this cement, which prevents the formation of low-permeability products on the surface of the slag particles. Therefore, the aluminum content is also a relevant factor for the initial strength gain.
The aluminum content in cement brings two relevant considerations: (i) the percentage of aluminum presented by the BFS, in which the higher the Al content, the higher the contribution to the increase in its dissolution speed; (ii) the aluminum content available in the mixture for the formation of ettringite.
Therefore, the dissolved aluminum content of the slag must be sufficient to react with the sulfate from the gypsum to appropriately form ettringite before the silicates. A low aluminum content and excess sulfate can impair the hydration of the BFS due to the rapid occurrence and growth of ettringite and monosulfate crystals on the surface of the BFS grain, causing its isolation and preventing hydration.
The inferences drawn from this study, together with the initial investigation for the ECO patent [7] and the relevant literature [4,5,12,13,14,15], have consistently demonstrated the points made in the previous observations and comments.
3.3. Compressive Strength of Cement Mortar Using ECO Formulations
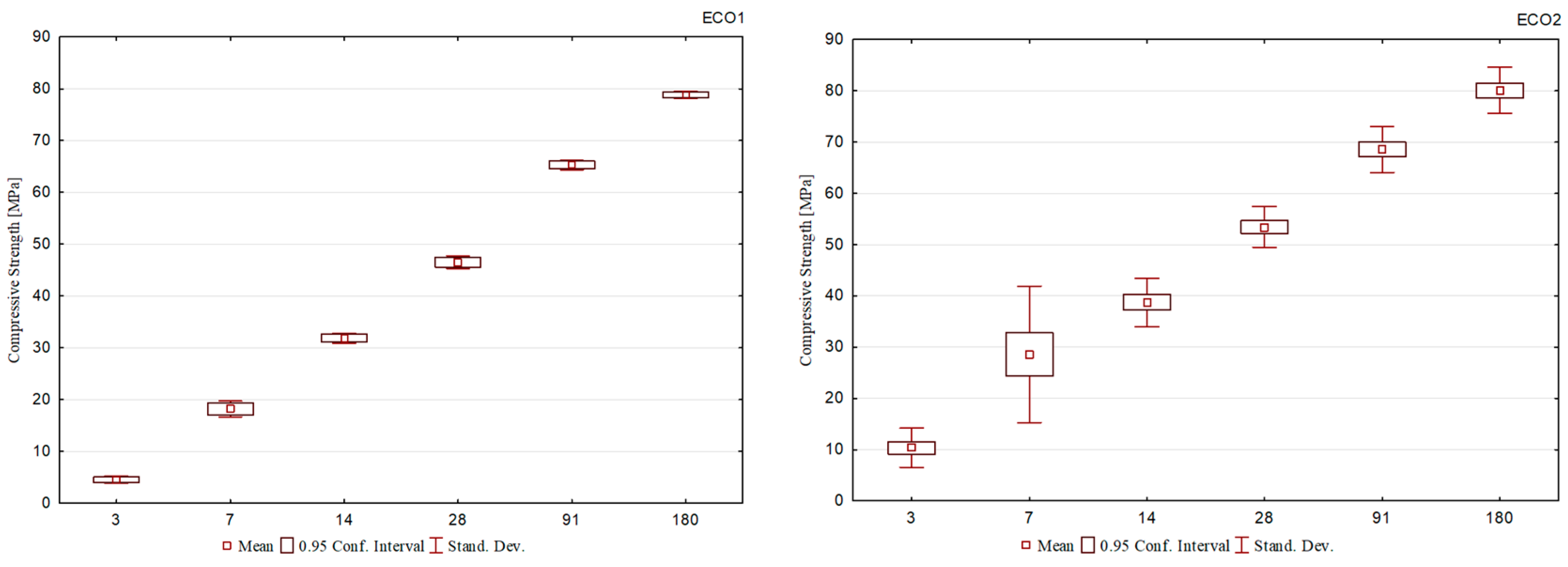
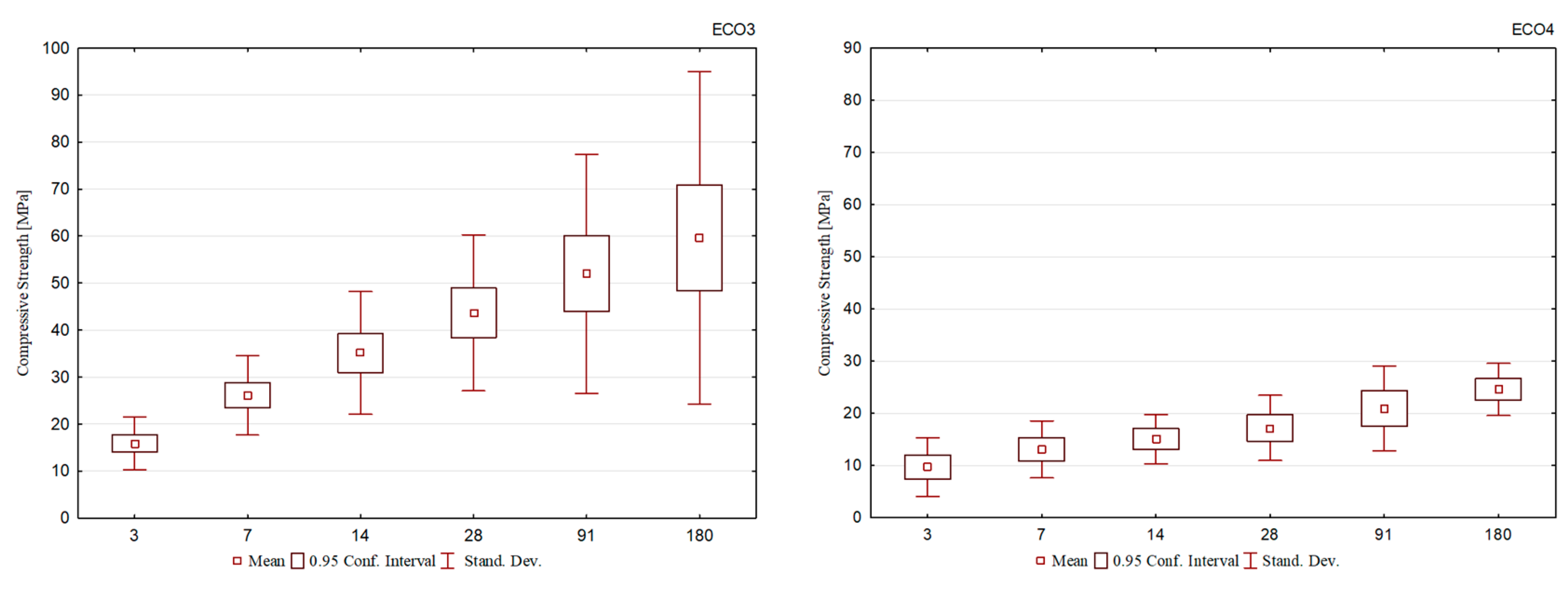
Compressive strength results for cement mortar specimens with ECO1, ECO2, ECO3, and ECO4 cement are shown in Figure 1, Figure 2, Figure 3 and Figure 4. Using analysis of variance (ANOVA), the effects of ‘type of cement’ and ‘hydration age’ were analyzed for all formulations. For all cement formulations, these effects are statistically significant variables to explain the different mechanical performances observed. In other words, within the same cement group, such as ECO2, the mechanical strength results of distinct formulations showed significant differences. The same was observed for ECO3 formulations.
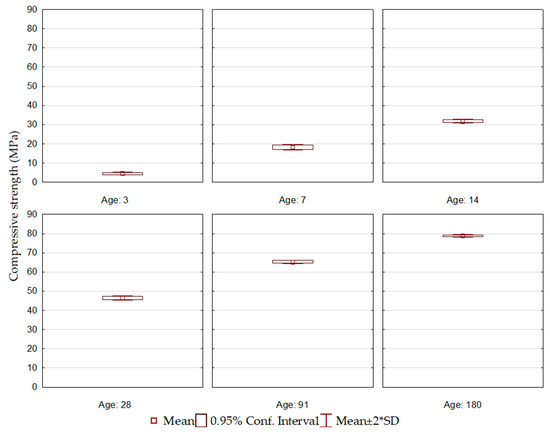
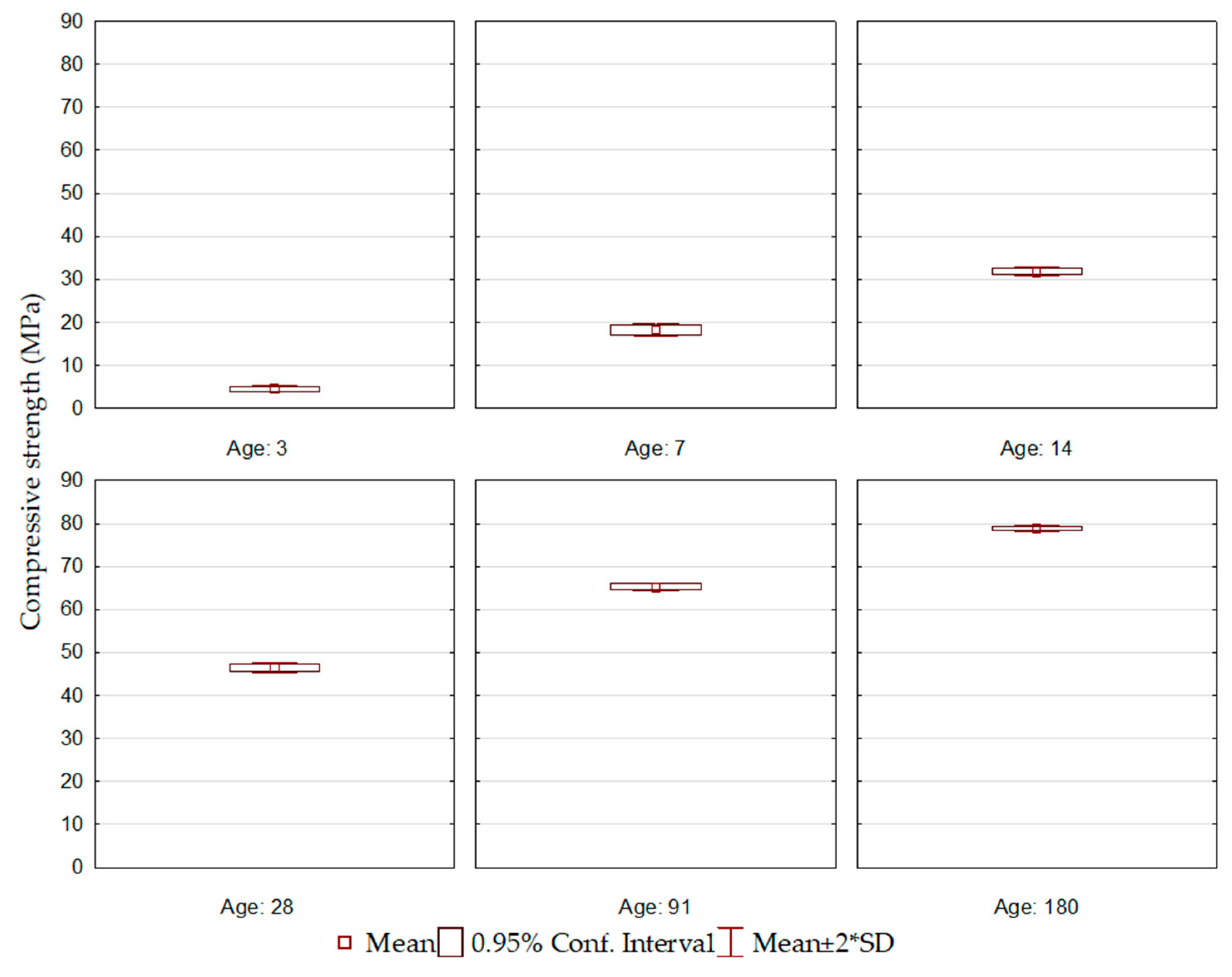
Figure 1.
ECO1 cement compressive strength.
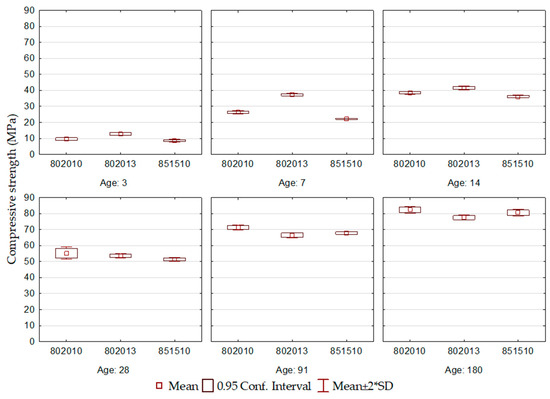
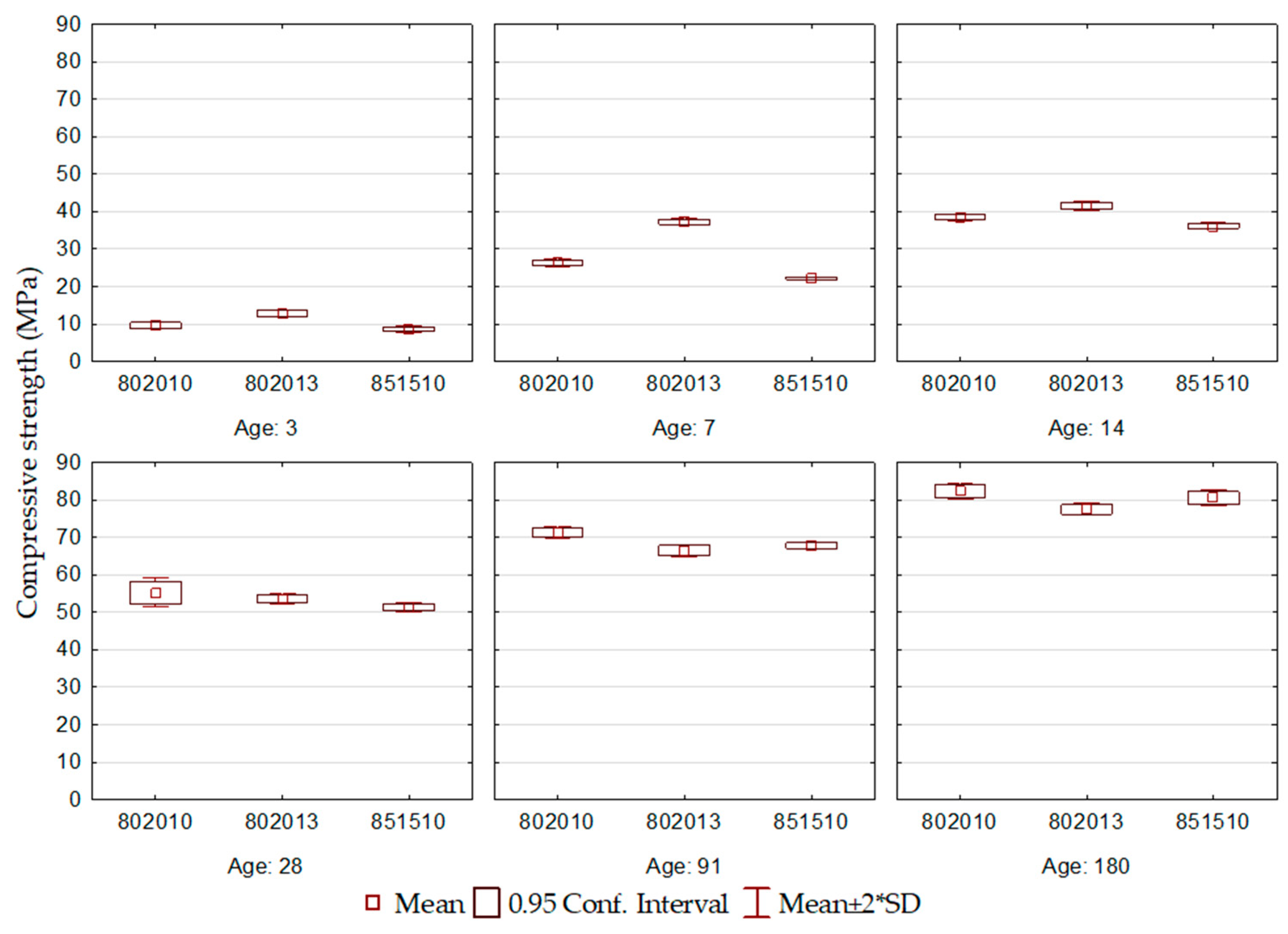
Figure 2.
ECO2 cement compressive strength.
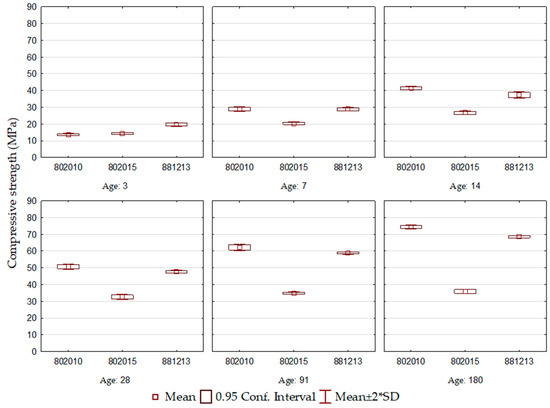
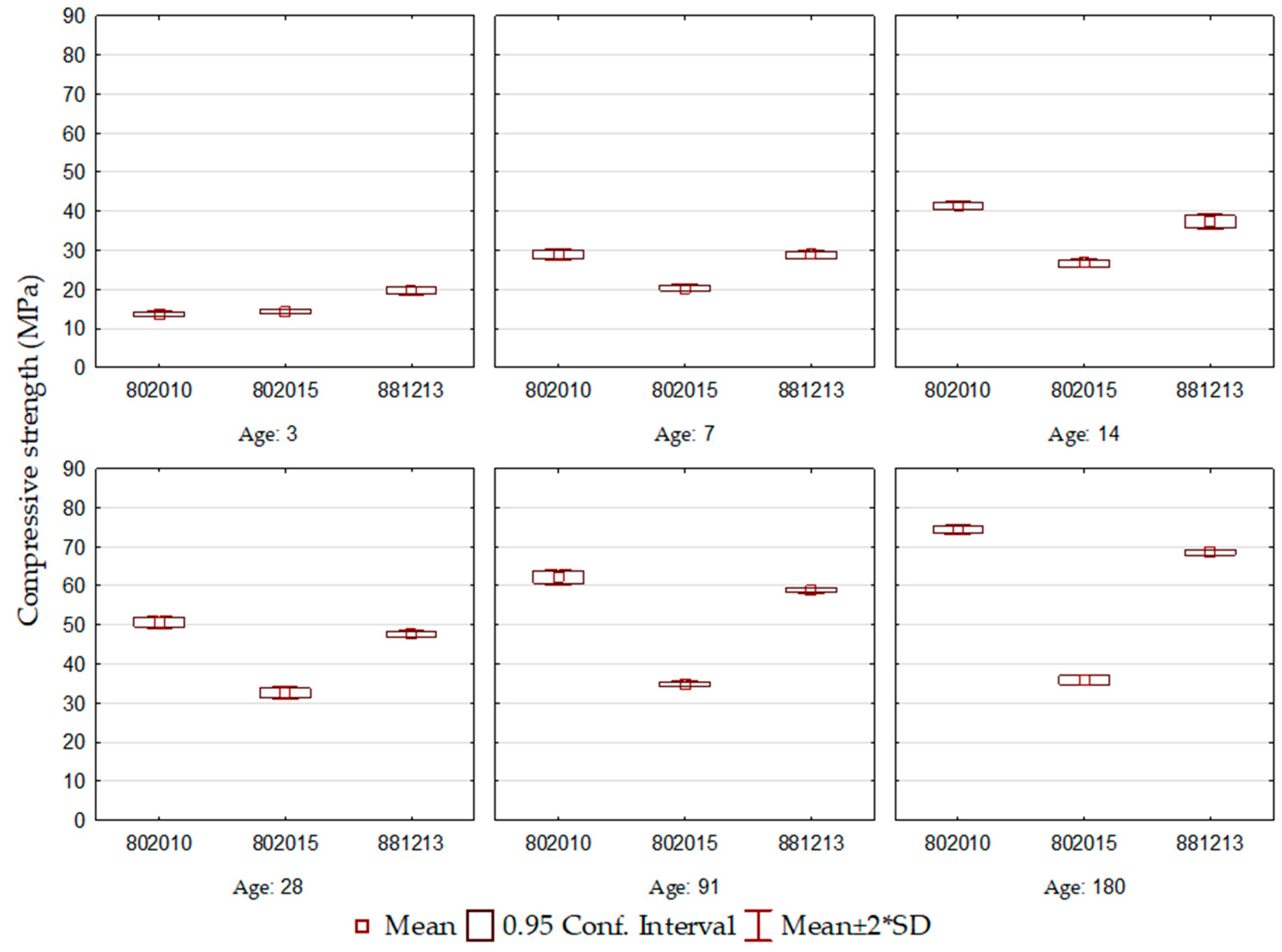
Figure 3.
ECO3 cement compressive strength.
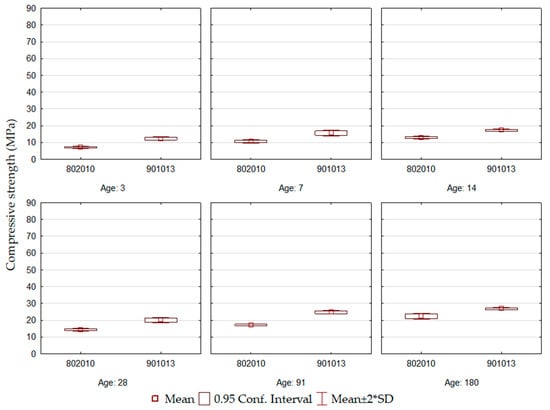
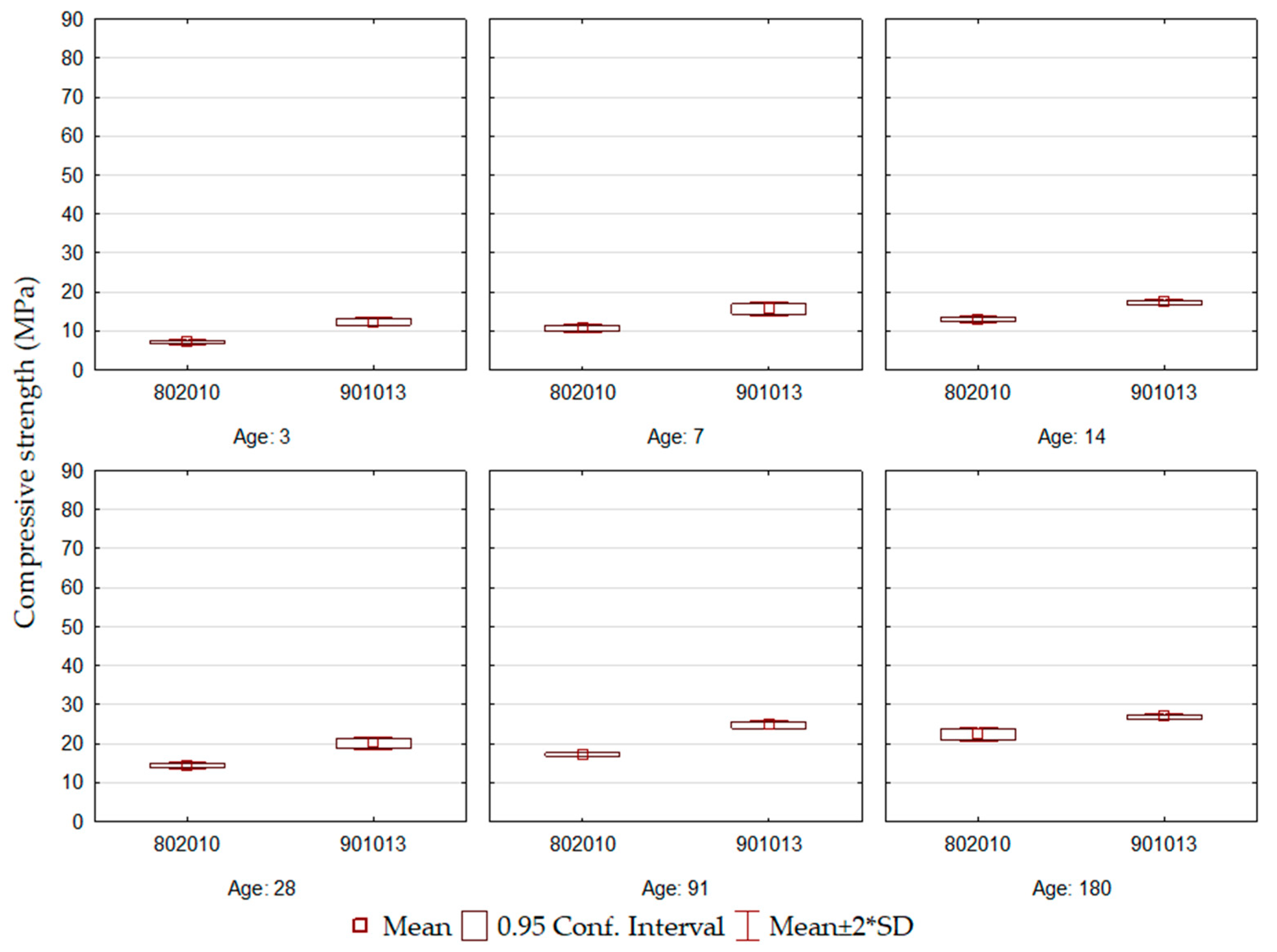
Figure 4.
ECO4 cement compressive strength.
Figure 1 presents the results of the compressive strength of ECO1 cement, produced based on the initial formulation of ECO cement [7]. The cement showed a gain in resistance over the ages. However, the resistance at the initial ages was lower than the Brazilian normative limits [6]. This behavior is attributed to the BOF slag content used in the formulation, the lowest among all ten proposed cement formulations, and the basicity of the BOF1 slag (2.8). It is understood that both factors may not have favored the beginning of the hydration process. On the other hand, due to the blast furnace slag used (BFS1) and the high aluminum content present in its composition, a resistance gain was observed, even if more slowly.
In the compressive strength results for the ECO2 cement, shown in Figure 2, it can be seen that the cement formulations showed a significant evolution in compressive strength over the ages.
At the initial ages, ECO2 cement exhibited high compressive strength. At 3 days, the compressive strength varied between 8 and 10 MPa, and at 7 days, from 20 to 40 MPa. At this last age (R7), the values approached the normative limits defined for most types of Portland cement at 28 days, as defined by [6]. The ECO2 cements were produced using BOF2, which presented high basicity (3.1) and the highest content of the brownmillerite phase, factors that may corroborate the development of initial resistance.
At early hydration ages, the ECO2-802013 formulation performed the best. It is noteworthy that this corresponds to the cement with the highest addition of BOF2 among the three formulations, which generated an environment of greater alkalinity, resulting in increased dissolution of blast furnace slag and accelerating the formation of hydration products.
At the later ages, the ECO2-802010 formulation demonstrated the best performance, with a compressive strength at 180 days greater than 80 MPa, corroborating the consistent strength growth throughout the hydration process. The ECO2-851510 formulation is attributed the worst performance due to the high BFS content and the reduction of gypsum, and consequently, the reduction in the contribution of calcium ions and sulfate ions, which compromised the availability of ions for the formation of hydration products.
For the ECO3 cement formulations, shown in Figure 3, the results demonstrate that the evolution of compressive strength over the ages did not occur uniformly for all cements. Compressive strength after 3 days of hydration reached values between 14 and 20 MPa, the highest among all the formulations studied. This behavior is understood through the basicity of the slag used, BOF3, which is the highest and equivalent to 3.7, responsible for accelerating the decomposition of blast furnace slag, given the alkalinity of the reaction environment. The best behavior is observed for the ECO3-802010 formulation, which maintains a constant evolution of compressive strength at all ages. It is seen that the ECO3-802015 cement had a compromised performance compared to the others, and this difference is more evident in the final ages. The mentioned formulation is composed of the highest BOF slag content, which allowed a rapid gain in resistance after the 3rd day, but from this age onwards, little evolution was observed (R7: 20.4 MPa to R180: 35.8 MPa).
The compressive strength results for the ECO4 formulation cements are shown in Figure 4 and demonstrate that they correspond to the lowest values observed among all formulations. Despite the development of initial resistance, there is no significant evolution over the ages, so between 3 and 180 days of curing, the difference was approximately 15 MPa. Among the formulations, the compound with the highest contents of BFS and BOF slag had slightly better results (ECO4-901013).
Figure 5 shows the average results for each group of cements. Observing the comparison of the evolution between them, it is seen that the cement mixtures of the ECO2 formulation, in addition to presenting a continuous gain in mechanical strength, showed less dispersion of the results, which demonstrates a more homogeneous behavior among them.
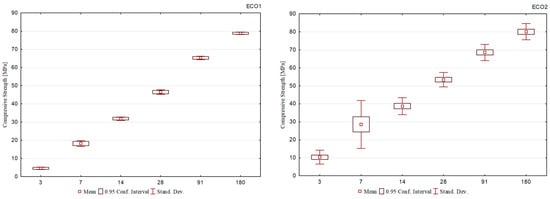
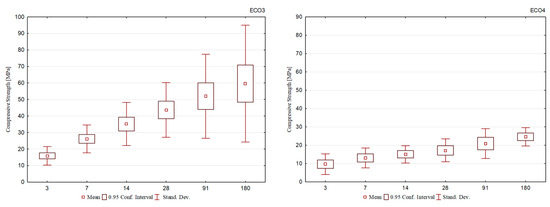
Figure 5.
Average compressive strength of all cement formulations.
The ECO2 cement formulations were produced using BOF2 slag, with high basicity, the highest brownmillerite content, and a relevant dicalcium silicate content, corroborating the development at the initial and final ages. In addition, the BFS1 slag was used, which presented the highest aluminum content in its composition, supporting its solubilization and ettringite formation.
4. Conclusions
In this study, four different eco-sustainable types of cement (ECO1, ECO2, ECO3, and ECO4) were produced using iron-making slags and gypsum waste, varying the raw material proportions, ranging from 80 to 90% blast furnace slag, 10 to 20% gypsum, and 10 to 15% BOF slag.
The strength of ECO cement depends on the precipitation of ettringite, C-S-H, and C-A-H. This ensures that the cement gains high mechanical strength at an early age due to the dissolution of BFS by hydroxyl attack. On the 3rd day, the cement formulations reached up to 10 MPa, and on the 7th day, 40 MPa. The contribution of OH− in the solution mainly comes from BOF slag, which provides an environment of high alkalinity from Ca(OH)2 and CaCO3 dissolution, as calcium sulfate does not have a significant effect on the pH level of the solution. BOF slag also takes part in the ECO cement hydration process through the hydration of brownmillerite.
Most ECO cement formulations showed a significantly high final strength, with the highest compressive strength values after 28 days of hydration, greater than 50 MPa, and at 180 days, greater than 80 MPa. This mechanical performance is believed to be influenced by the presence of BOF slag, which contains the belite phase commonly found in BOF slag and Portland cement. The belite phase is responsible for the gain in mechanical resistance after 28 days of curing due to its thermodynamic stability. During its hydration, C2S generates C-S-H and releases Ca(OH)2, which helps maintain an alkaline environment and allows the dissolution of BFS to continue progressing. However, excessive increases in alkalinity may favor the rapid dissolution of BFS and gain in initial strength, but they will also impede development in the final ages, due to covering the surface of anhydrous grains with precipitated products, harming the development of resistance at later ages.
This was observed in the ECO4 cement formulations. Despite the development of initial resistance, there is no significant evolution over the ages, so between 3 and 180 days of curing, the difference was approximately 15 MPa.
The ECO2 cement has demonstrated excellent mechanical performance in both the initial and final stages of the hydration process, with consistent strength growth throughout. This cement formulation was produced with BOF2 slag, which has a high basicity of 3.1, a significant brownmillerite content of 19%, and a relevant dicalcium silicate content of 21%, thus contributing to its development in the initial and final stages. Additionally, the BFS1 slag was used, which has the highest aluminum content (15%) in its composition, which supports its solubilization and ettringite formation.
5. Patents
Vernilli, F.; Oliveira, M. D. S.; Pereira, M. L. G.; Oliveira, L. M.; Zago, S.C. Cimento Ecossustentável sem clínquer à base de resíduos da siderurgia e da construção civil e uso do mesmo. 2022, Brasil. Número do registro: BR1020220227578, Instituição de registro: INPI—Instituto Nacional da Propriedade Industrial. Depósito: 8 November 2022.
Author Contributions
Conceptualization, S.C.Z., F.V., and M.D.; methodology, S.C.Z., F.V., and M.D.; validation, S.C.Z. and M.D.; formal analysis, M.D.; investigation, S.C.Z. and M.D.; resources, S.C.Z., F.V., and M.D.; data curation, S.C.Z.; writing—original draft preparation, S.C.Z.; writing—review and editing, S.C.Z., F.V., and M.D.; visualization, S.C.Z. and M.D.; supervision, F.V.; project administration, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IEA. Global Cement Production in the Net Zero Scenario, 2010–2030, IEA, Paris. IEA. Licence: CC BY 4.0. Available online: https://www.iea.org/data-and-statistics/charts/global-cement-production-in-the-net-zero-scenario-2010-2030-5260 (accessed on 20 January 2024).
- Capmas, A. Concreto e desenvolvimento sustentável. In Durabilidade do Concreto; Geraldo, C.I., Translator; Ibracon: São Paulo, Brazil, 2014; pp. 3–13. [Google Scholar]
- ROADMAP Tecnológico do Cimento: Potencial de Redução das Emissões de Carbono da Indústria do Cimento Brasileira Até 2050; SNIC: Rio de Janeiro, Brazil, 2019; 64p.
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- NBR 16697; Cimento Portland—Requisitos. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2018.
- Oliveira, M.D.S. Estudo da Obtenção de Cimento sem Clínquer a Partir de Misturas de Escórias Siderúrgicas. Master’s Thesis, Universidade de São Paulo, São Paulo, Brazil, 2021. [Google Scholar]
- EN 15743:2010; Supersulfated Cement—Composition, Specifications and Conformity Criteria. Comité Européen de Normalisation-CEN: Brussels, Belgium, 2010.
- Associação Brasileira de Normas Técnicas. NBR 7215. Cimento Portland—Determinação da Resistência à Compressão; ABNT: Rio de Janeiro, Brazil, 2019. [Google Scholar]
- Nonat, A. A Hidratação dos Cimentos; Alba Cincotto, M., Translator; Ibracon: São Paulo, Brazil, 2014. [Google Scholar]
- Baird, C.; Cann, N. Environmental Chemistry, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2012. [Google Scholar]
- Kaja, A.M.; Melzer, S.; Brouwers, H.J.H.; Yu, Q. On the optimization of BOF slag hydration kinetics. Cem. Concr. Compos. 2021, 124, 104262. [Google Scholar] [CrossRef]
- Zago, S.C.; Vernilli, F.; Cascudo, O. The Reuse of Basic Oxygen Furnace Slag as Concrete Aggregate to Achieve Sustainable Development: Characteristics and Limitations. Buildings 2023, 13, 1193. [Google Scholar] [CrossRef]
- Wu, Q.; Xue, Q.; Ye, Z. Research status of super sulfate cement. J. Clean. Prod. 2021, 294, 126228. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, W.; Li, Y.; Ba, H.; Li, N.; Ju, Y.; Zhao, B.; Jia, G.; Hu, W. The mechanism of hydration reaction of granulated blast furnace slag-steel slag-refining slag-desulfurization gypsum-based clinker-free cementitious materials. J. Build. Eng. 2021, 44, 103289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).