The Influence of the Aggressive Medium upon the Degradation of Concrete Structures: Numerical Model of Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
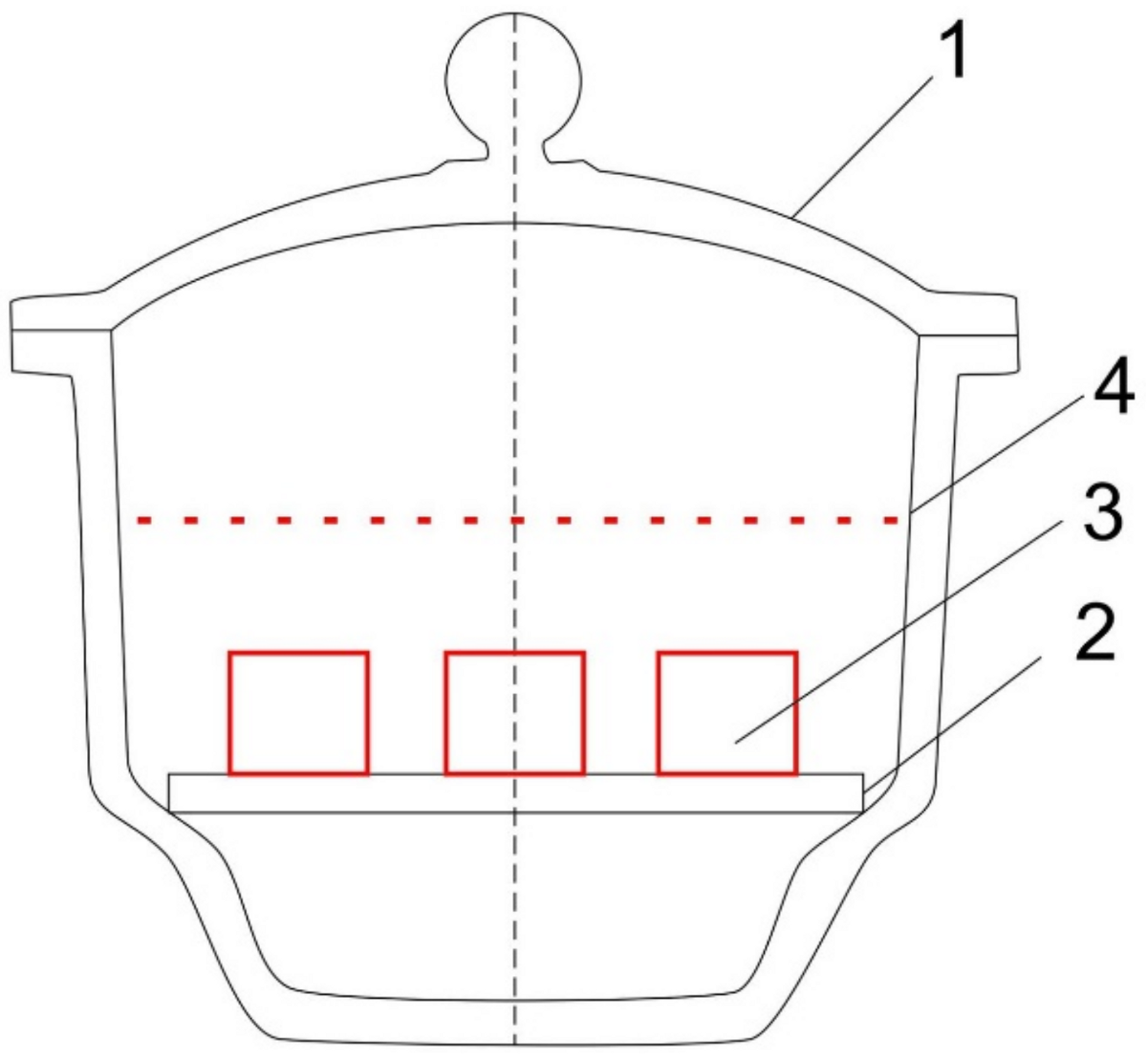
2.2. The Research Model
Material Destruction Model (Koroleva Model)
3. Results
3.1. Identification of Mechanical Properties
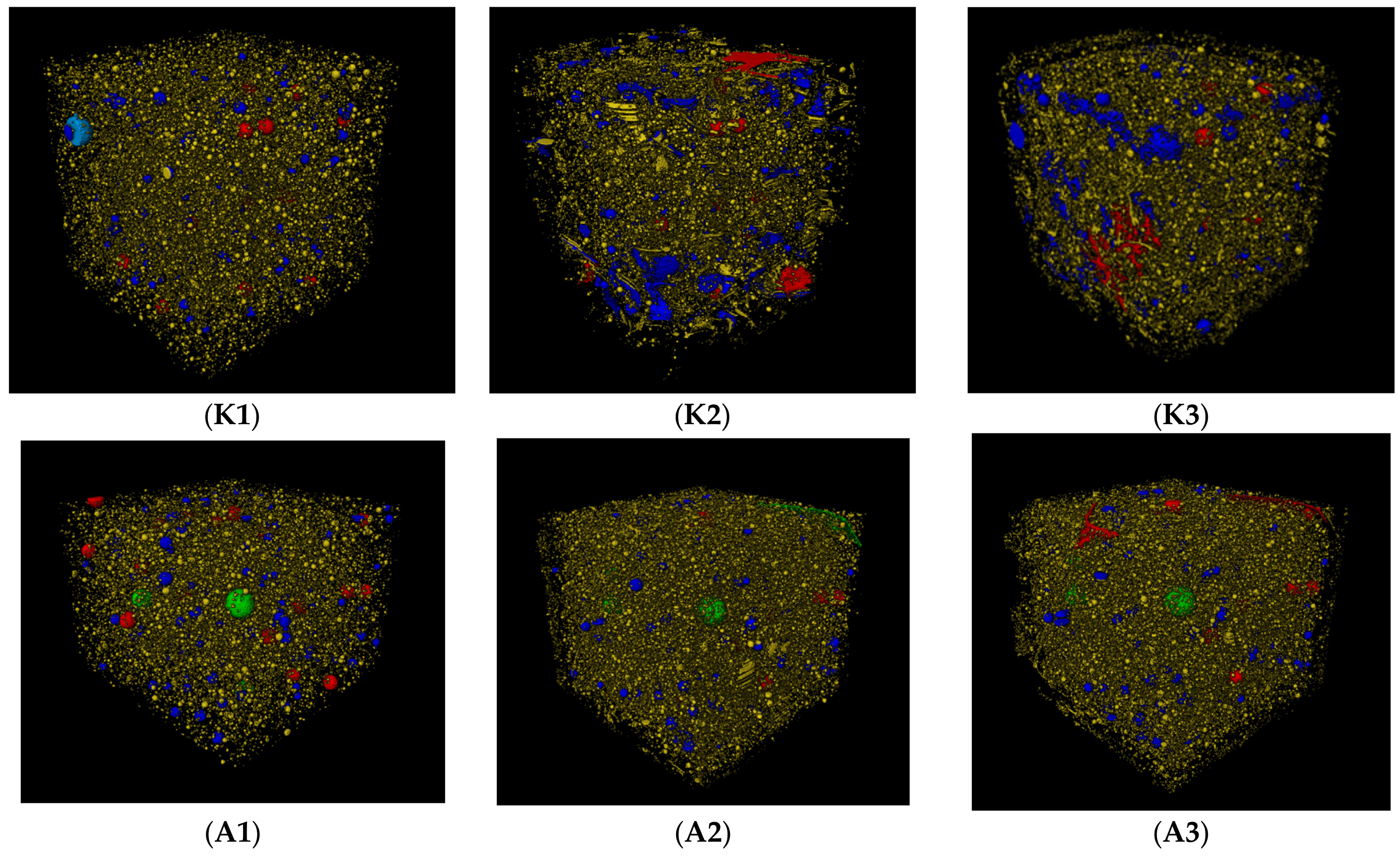
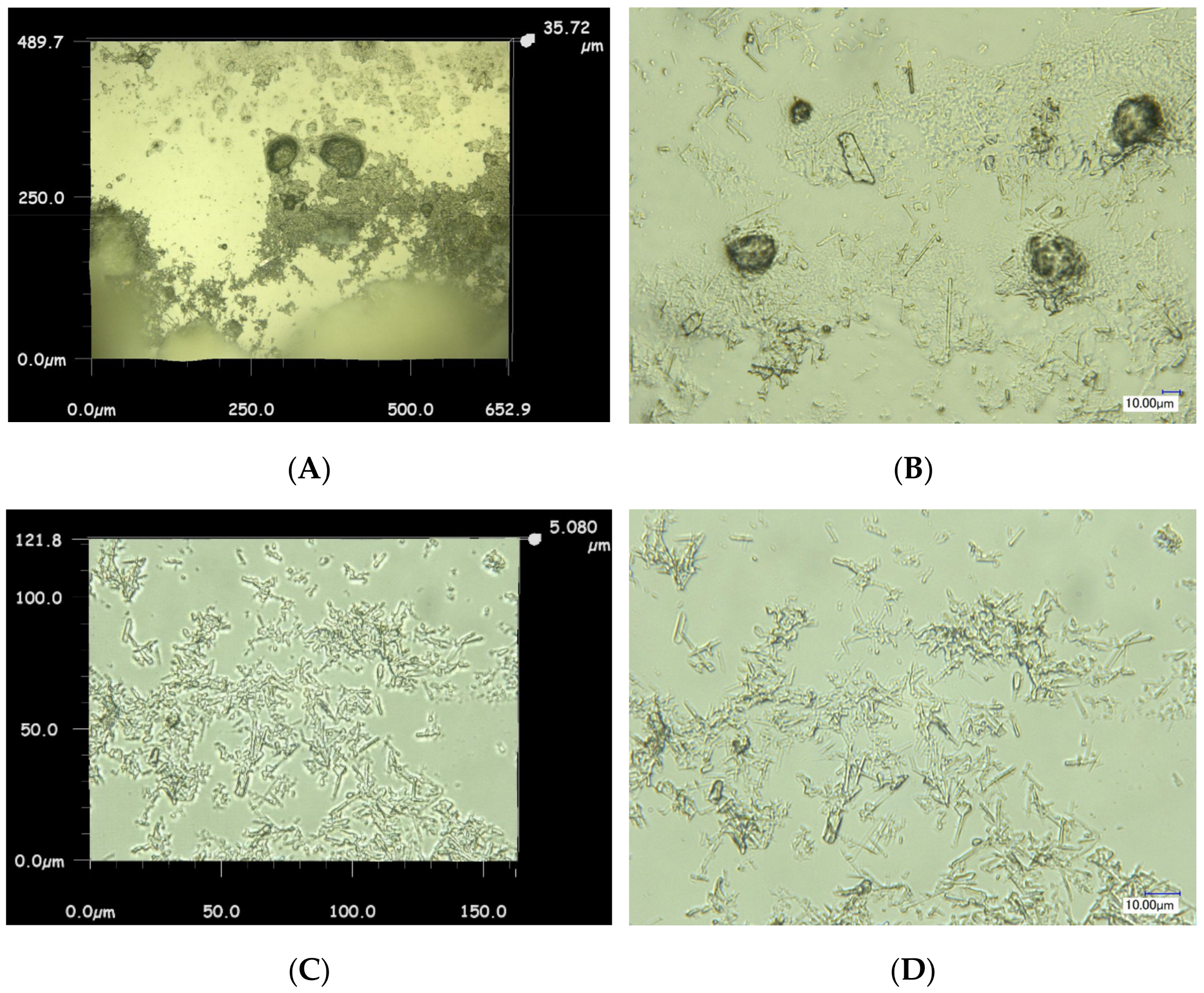
3.2. A study of the Impact of an Aggressive Medium on the Physical and Mechanical Properties of Cement Composites
3.3. The Degradation Model
3.4. Numerical Studies of Changes in the Compressive Strength of Cement Stone Aged in an Aggressive Medium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arum, C.; Olotuah, A. Making of strong and durable concrete. Emir. J. Eng. Res. 2006, 11, 25–31. [Google Scholar]
- Neville, A.M. Properties of Concrete; Longman Group Limited: Harlow, UK, 1995. [Google Scholar]
- Makhloufi, Z.; Kardi, E.H.; Bouhicha, M.; Benaissa, A.; Bennacer, R. The strength of limestone mortar with quaternary binder: Leaching effect by demineralized water. Constr. Build. Mater. 2012, 36, 171181. [Google Scholar] [CrossRef]
- Afroughsabet, V.; Biolzi, L.; Monteiro, P.J.M.; Gastaldi, M.M. Investigation of the mechanical and durability properties of sustainable high performance concrete based on calcium sulfoaluminate cement. J. Build. Eng. 2021, 43, 102656. [Google Scholar] [CrossRef]
- Witkowska-Dobrev, J.; Szlachetka, O.; Dohojda, M.; Wiśniewski, K. Effect of Acetic Acid on Compressive Strength and Geometric Texture of the Surface of C20/25 Class Concrete. Sustainability 2021, 13, 5136. [Google Scholar] [CrossRef]
- Hubáček, A.; Hela, R. Concrete in the Environment of Agricultural Buildings. Solid State Phenom. 2021, 322, 38–40. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, L.; Walkley, B.; Black, J.R.; San Nicolas, R. Degradation resistance of different cementitious materials to phosphoric acid attack at early stage. Cem. Concr. Res. 2022, 151, 106606. [Google Scholar] [CrossRef]
- Kumar, N.V.S. Effet des solutions d’acide sulfurique et chlorhydrique sur le béton de poussière de roche concassée. Mater. Today Proc. 2021, 46, 509–513. [Google Scholar] [CrossRef]
- Youssari, F.Z.; Taleb, O.; Benosman, A.S. Towards understanding the behavior of fiber-reinforced concrete in aggressive environments: Acid attacks and leaching. Constr. Build. Mater. 2023, 368, 130444. [Google Scholar] [CrossRef]
- Jiang, C.; Jiang, L.; Tang, X.; Gong, J.; Chua, H. Impact of calcium leaching on mechanical and physical behaviors of high belite cement pastes. Constr. Build. Mater. 2021, 286, 122983. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, L.; Zhang, Y.; Pu, Q.; Xu, Y. Predicting the calcium leaching behavior of cement pastes in aggressive environments. Constr. Build. Mater. 2011, 243, 88–96. [Google Scholar] [CrossRef]
- Omrane, M.; Mouli, M.; Benosman, A.S.; Senhadji, Y. Deterioration of Mortar Composites in Acidic Environment. Adv. Mater. Res. 2015, 1064, 3–8. [Google Scholar] [CrossRef]
- Jebli, M.; Jamin, F.; Pelissou, C.; Malachanne, E.; Garcia-Diaz, E.; El Youssoufia, M.S. Leaching effect on mechanical properties of cement-aggregate interface. Cem. Concr. Compos. 2018, 87, 10–19. [Google Scholar] [CrossRef]
- Babaahmadi, A.; Tang, L.; Abbas, Z.; Martensson, P. Physical and mechanical properties of cementitious specimens exposed to an electrochemically derived accelerated leaching of calcium. Int. J. Concr. Struct. Mater. 2015, 9, 295–306. [Google Scholar] [CrossRef]
- Kamali, S.; Gérard, B.; Moranville, M. Modelling the leaching kinetics of cement-based materials influence of materials and environment. Cem. Concr. Compos. 2003, 25, 451–458. [Google Scholar] [CrossRef]
- Cai, X.; He, Z.; Shao, Y.; Sun, H. Macro- and micro-characteristics of cement binders containing high volume fly ash subject to electrochemical accelerated leaching. Constr. Build. Mater. 2016, 116, 25–35. [Google Scholar] [CrossRef]
- Allsopp, D.; Seal, K.J.; Gaylarde, C.C. Introduction to Biodeterioration, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004; 252p. [Google Scholar]
- Yakovleva, G.; Sagadeev, E.; Stroganov, V.; Kozlova, O.; Okunev, R.; Ilinskaya, O. Metabolic activity of micromycetes affecting urban concrete constructions. Sci. World J. 2018, 2018, 8360287. [Google Scholar] [CrossRef] [PubMed]
- Samson, E.; Marchand, J. Modeling the effect of temperature on ionic transport in cementitious materials. Cem. Concr. Res. 2007, 37, 455–468. [Google Scholar] [CrossRef]
- Samson, E.; Marchand, J.; Snyder, K.A. Calculation of ionic diffusion coefficients on the basis of migration test results. Mater. Struct. 2003, 36, 156–165. [Google Scholar] [CrossRef]
- Bastidas-Arteaga, E.; Sánchez-Silva, M.; Chateauneuf, A.; Silva, M.R. Coupled reliability model of biodeterioration, chloride ingress and cracking for reinforced concrete structures. Struct. Saf. 2008, 30, 110–129. [Google Scholar] [CrossRef]
- Biondini, F.; Bontempi, F.; Frangopol, D.M.; Malerba, P.G. Cellular automata approach to durability analysis of concrete structures in aggressive environments. J. Struct. Eng. 2004, 130, 1724–1737. [Google Scholar] [CrossRef]
- Frangopol, D.M.; Lin, K.-Y.; Estes, A.C. Reliability of reinforced concrete girders under corrosion attack. J. Struct. Eng. 1997, 123, 286–297. [Google Scholar] [CrossRef]
- Tu, X.; Wu, Y. Numerical analysis on corrosion and mechanical performance of shear stud connector in concrete. Constr. Build. Mater. 2023, 363, 129816. [Google Scholar] [CrossRef]
- Thoft-Christensen, P. Stochastic Modelling of the Diffusion Coefficient for Concrete; Department of Building Technology and Structural Engineering, Aalborg University: Aalborg, Denmark, 2002. [Google Scholar]
- Ozbolt, J.; Balabanic, G.; Kuster, M. 3D numerical modelling of steel corrosion in concrete structures. Corros. Sci. 2011, 53, 4166–4177. [Google Scholar] [CrossRef]
- Liberati, E.A.; Nogueira, C.G.; Leonel, E.D.; Chateauneuf, A. Nonlinear formulation based on FEM, Mazars damage criterion and Fick’s law applied to failure assessment of reinforced concrete structures subjected to chloride ingress and reinforcements corrosion. Eng. Fail. Anal. 2014, 46, 247–268. [Google Scholar] [CrossRef]
- Kalinkin, A.; Krzhizhanovskaya, M.; Gurevich, B.; Kalinkina, E.; Tyukavkina, V. Hydration of mechanically activated blended cements studied by in situ X-ray diffraction. Inorg. Mater. 2015, 51, 828–833. [Google Scholar] [CrossRef]
- Burmeister, C.; Titscher, L.; Breitung-Faes, S.; Kwade, A. Dry grinding in planetary ball mills: Evaluation of a stressing model. Adv. Powder Technol. 2018, 29, 191–201. [Google Scholar] [CrossRef]
- Vdovin, E.; Stroganov, V.; Amel’chenko, M.; Kraus, E.; Fazleev, I. Biostability of Road Cement-Mineral Materials Reinforced with Dispersed Fibre. In International Scientific Conference on Socio-Technical Construction and Civil Engineering; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2021; Volume 169, pp. 289–296. [Google Scholar] [CrossRef]
- Stroganov, V.; Sagadeev, E.; Ibragimov, R.; Potapova, L. Mechanical activation effect on the biostability of modified cement compositions. Constr. Build. Mater. 2020, 246, 118506. [Google Scholar] [CrossRef]
- Kara, C.; Kutuk, S.; Kutuk-Sert, T. Improvement of the durability of concrete by substitution of raw ground colemanite. Case Stud. Constr. Mater. 2023, 19, e02393. [Google Scholar] [CrossRef]
- Kutuk, S. Morphology, Crystal Structure and Thermal Properties of Nano-Sized Amorphous Colemanite Synthesis. Arab. J. Sci. Eng. 2024. [Google Scholar] [CrossRef]
- Ruslan, I.; Ruslan, B.; Evgenij, K. The effect of metal and polypropylene fiber on technological and physical mechanical properties of activated cement compositions. Case Stud. Constr. Mater. 2022, 16, e00882. [Google Scholar] [CrossRef]
- Strokin, K.; Novikov, D.; Konovalova, V. Forecasting the durability of reinforced concrete under conditions of microbiological corrosion. E3S Web Conf. 2021, 274, 04003. [Google Scholar] [CrossRef]
- Stroganov, V.F.; Sagadeev, E.V.; Potapova, L.I.; Kukoleva, D.A. A comprehensive study of the processes of biodamage of mineral building materials. Izvestiya KGASU 2011, 4, 274–281. [Google Scholar]
- Galitsyn, V.P.; Shkurenko, S.I.; Slastnov, A.E.; Pakhomov, P.M. Correlation of Breaking Elongation and Linear Density of a Fiber Obtained from Ultrarhigh-Molecular-Weight Polyethylene Gel with Its Strength Indicators. Fibre Chem. 2023, 55, 25–30. [Google Scholar] [CrossRef]
- Svintsov, A.P.; Nikolenko, Y.V.; Fediuk, R.S. Aggressive effect of vegetable oils and organic fatty acids on cement-sand mortar and concrete. Constr. Build. Mater. 2022, 329, 127037. [Google Scholar] [CrossRef]
- Rossetti, A.; Ikumi, T.; Segura, I.; Irassar, E.F. Sulfate performance of blended cements (limestone and illite calcined clay) exposed to aggressive environment after casting. Cem. Concr. Res. 2021, 147, 106495. [Google Scholar] [CrossRef]
- Possenti, E.; Conti, C.; Gatta, G.D.; Marinoni, N.; Merlini, M.; Realini, M.; Vaughan, G.B.; Colombo, C. Synchrotron X-ray diffraction computed tomography to non-destructively study inorganic treatments for stone conservation. iScience 2022, 25, 105112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Li, M.-C.; Qi, M.; Ge, S.; Dadda, A. Micro-macro investigation on bio-cemented sand under different grouting saturation: An effective enhancement method. Geomech. Energy Environ. 2024, 37, 100530. [Google Scholar] [CrossRef]
- Ghassemi, P.; Toufigh, V. Durability of epoxy polymer and ordinary cement concrete in aggressive environments. Constr. Build. Mater. 2020, 234, 117887. [Google Scholar] [CrossRef]
- Kutuk-Sert, T.; Ozturk, M.; Kutuk, S. Digital image processing of warm mix asphalt enriched with nanocolemanite and nanoulexite minerals. Constr. Build. Mater. 2023, 399, 132542. [Google Scholar] [CrossRef]
- Ibragimov, R.A.; Korolev, E.V.; Deberdeev, T.R.; Leksin, V.V. Efficient complex activation of Portland cement through processing it in the vortex layer machine. Struct. Concr. 2019, 20, 851–859. [Google Scholar] [CrossRef]
- Yuan, H.; Dangla, P.; Chatellier, P.; Chaussadent, T. Degradation modeling of concrete submitted to biogenic acid attack. Cem. Concr. Res. 2015, 70, 29–38. [Google Scholar] [CrossRef]
- Bao, H.; Xu, G.; Wang, Q.; Peng, Y.; Liu, J. Study on the deterioration mechanism of cement-based materials in acid water containing aggressive carbon dioxide. Constr. Build. Mater. 2020, 243, 118233. [Google Scholar] [CrossRef]






| Inorganic Ions, mg/L | |||
|---|---|---|---|
| Ca2+ | NO3− | S2− | CO32− |
| 640 | 60 | 1000 | 700 |
| Organic acids, mg/L | |||
| Acetic | Citric | Malic | |
| 1980 | 16,020 | 400 | |
| Time, Hour | Control Sample | Activated | ||
|---|---|---|---|---|
| Water Absorption by Weight, % | Water Weight, g | Water Absorption by Weight, % | Water Weight, g | |
| 0 | 0 | 0.00 | 0 | 0.00 |
| 0.25 | 3.2 | 16.61 | 2.7 | 14.50 |
| 1 | 4 | 20.76 | 3.4 | 18.26 |
| 3 | 5 | 25.95 | 3.9 | 20.94 |
| 6 | 5.7 | 29.58 | 4.1 | 22.02 |
| 12 | 6.1 | 31.66 | 4.2 | 22.55 |
| 24 | 6.15 | 31.92 | 4.3 | 23.09 |
| 36 | 6.2 | 32.18 | 4.4 | 23.63 |
| 48 | 6.25 | 32.44 | 4.5 | 24.17 |
| Type of Sample | Total Pore Volume, mm3: | ||
|---|---|---|---|
| Prior to the Tests | After Fourteen Days of Tests | After Twenty-Eight Days of Tests | |
| control sample | 84.7 | 82.7 | 72.4 |
| sample obtained via activation | 70.7 | 39.2 | 35.2 |
| Composition | Compressive Strength, MPa after Exposure Throughout the Day: | |||
|---|---|---|---|---|
| 0 | 7 | 14 | 28 | |
| control sample | 42.5 100% | 34.8 82% | 29.8 70% | 26.8 63% |
| sample obtained via activation | 54.8 100% | 54.8 91% | 47.1 86% | 43.7 80% |
| Name of the Composition | Kinetic and Energy Parameters | ||||
|---|---|---|---|---|---|
| n | kd | ΔS, J/mol·K | BE, J/mol | U, J/mol | |
| control sample | 4.74 | 3.78 × 10−8 | −147.82 | 51,765.86 | 41,636.37 |
| sample obtained via activation | 7.91 | 1.83 × 10−14 | −263.3 | 11,5373.39 | 77,053.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruslan, I.; Farid, S.; Rashit, K.; Evgeny, K. The Influence of the Aggressive Medium upon the Degradation of Concrete Structures: Numerical Model of Research. Buildings 2024, 14, 1762. https://doi.org/10.3390/buildings14061762
Ruslan I, Farid S, Rashit K, Evgeny K. The Influence of the Aggressive Medium upon the Degradation of Concrete Structures: Numerical Model of Research. Buildings. 2024; 14(6):1762. https://doi.org/10.3390/buildings14061762
Chicago/Turabian StyleRuslan, Ibragimov, Shakirzyanov Farid, Kayumov Rashit, and Korolev Evgeny. 2024. "The Influence of the Aggressive Medium upon the Degradation of Concrete Structures: Numerical Model of Research" Buildings 14, no. 6: 1762. https://doi.org/10.3390/buildings14061762





