The Relationship between Molecular Structure and Foaming of Poly(ethylene glycol)—Poly(propylene glycol) Triblock Surfactants in Cementitious Materials
Abstract
:1. Introduction
1.1. Air Entrainment in Concrete
1.2. Chemical Structure of Triblock Copolymers
2. Experiments
2.1. Materials
2.2. Experimental Methods
2.2.1. Adsorption of Copolymer on Cement Particles
2.2.2. Paste Flowability
2.2.3. Foaming Measurements
Foam Index Test
Foam Volume
2.2.4. Physicochemical Measurements
2.2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.6. Micro-Computed Tomography (Micro-CT)
2.2.7. Gravimetric Water Sorption
2.2.8. Water Sorption Using Micro-CT
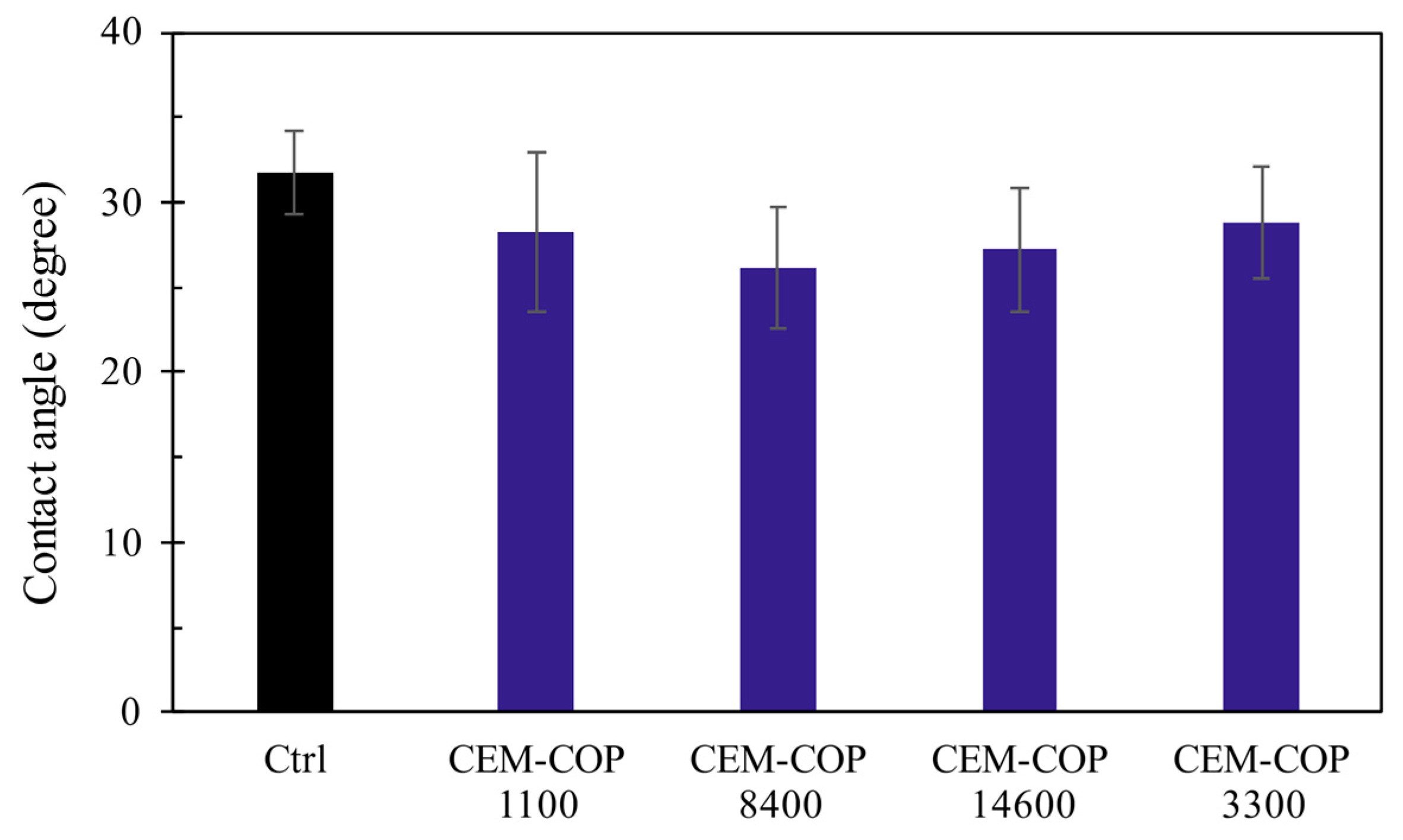
2.2.9. Contact Angle Measurements
2.2.10. Freeze-Thaw
3. Results and Discussion
3.1. Physicochemical Properties of PEG-PPG Triblock Copolymers
3.1.1. Hydrodynamic Size of Copolymers
3.1.2. FTIR in Solution
3.1.3. Equilibrium Surface Activity
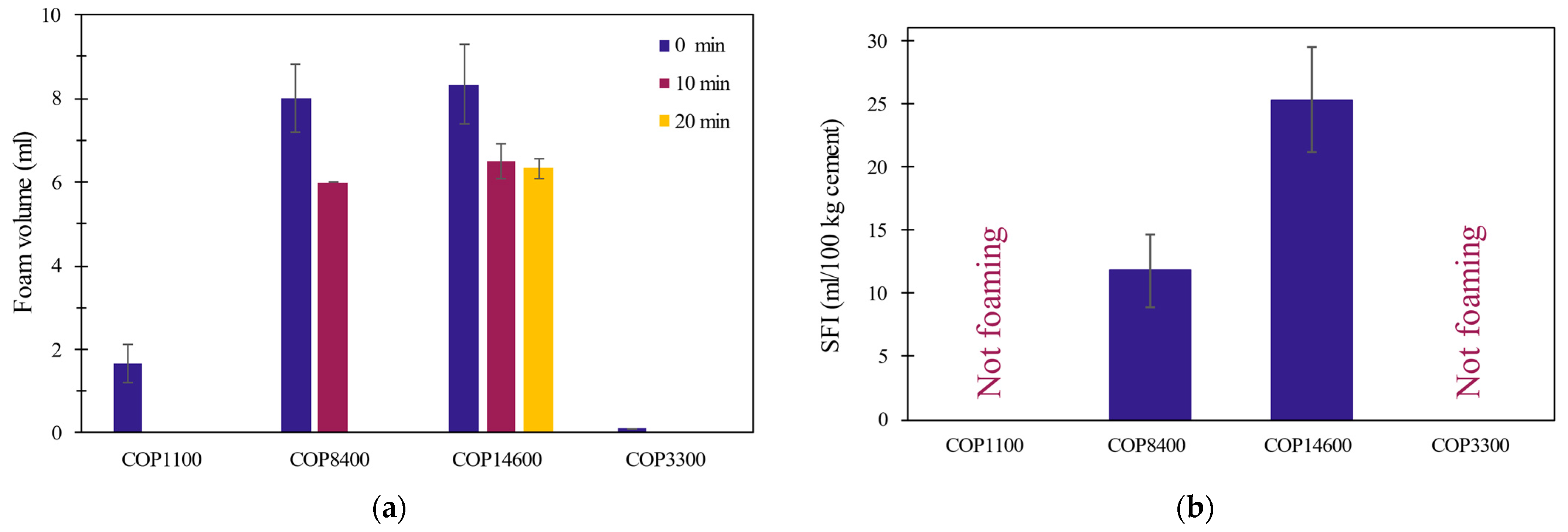
3.2. Foaming Properties in Cement Environment
3.3. Effect of PEG-PPG Triblock Copolymers on Cement Fresh Paste Properties
3.3.1. Adsorption on Cement Particles
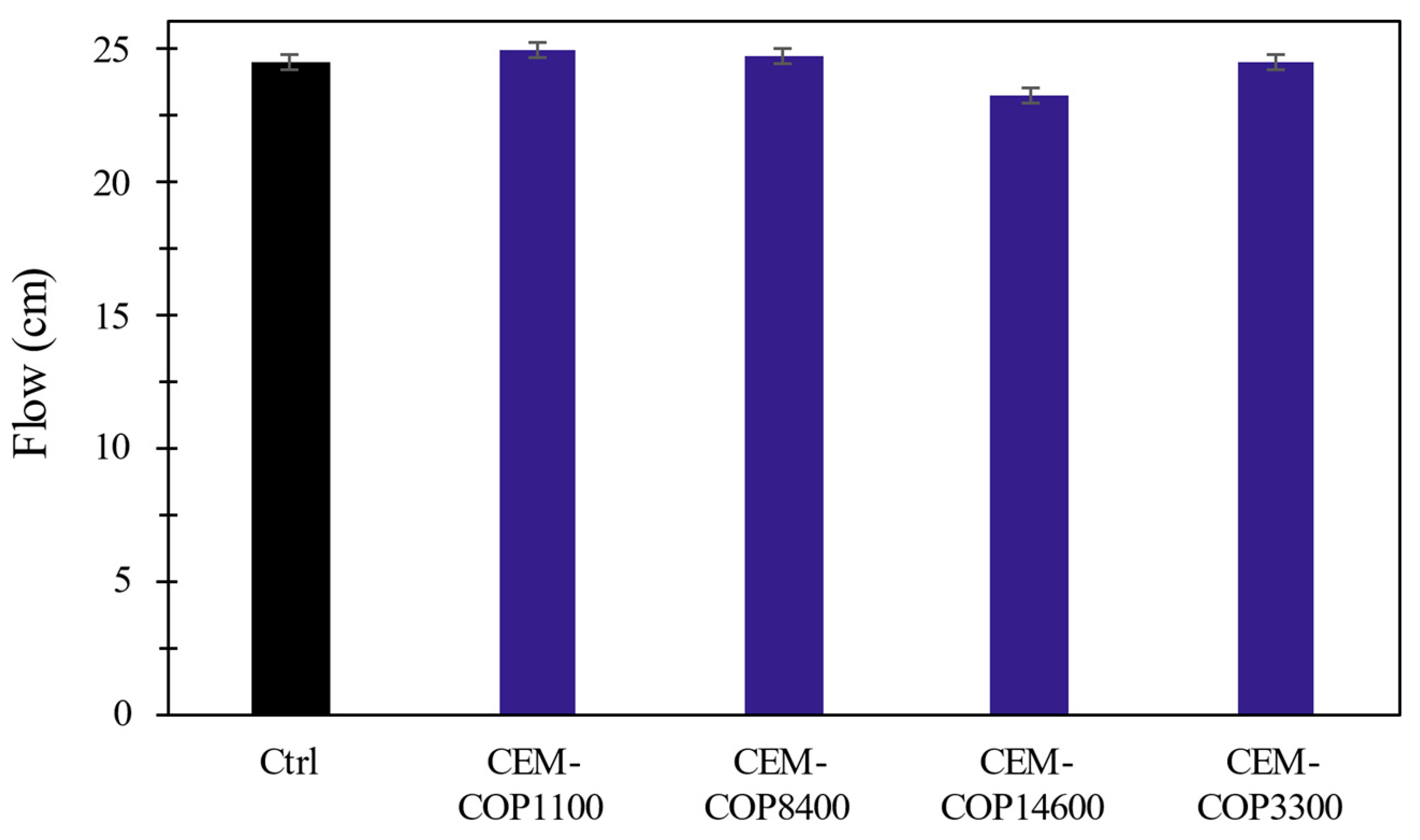
3.3.2. Effect on the Dispersion of Cement Paste
3.4. Effect of PEG-PPG Triblock Copolymers on Hardened Cement Paste Properties
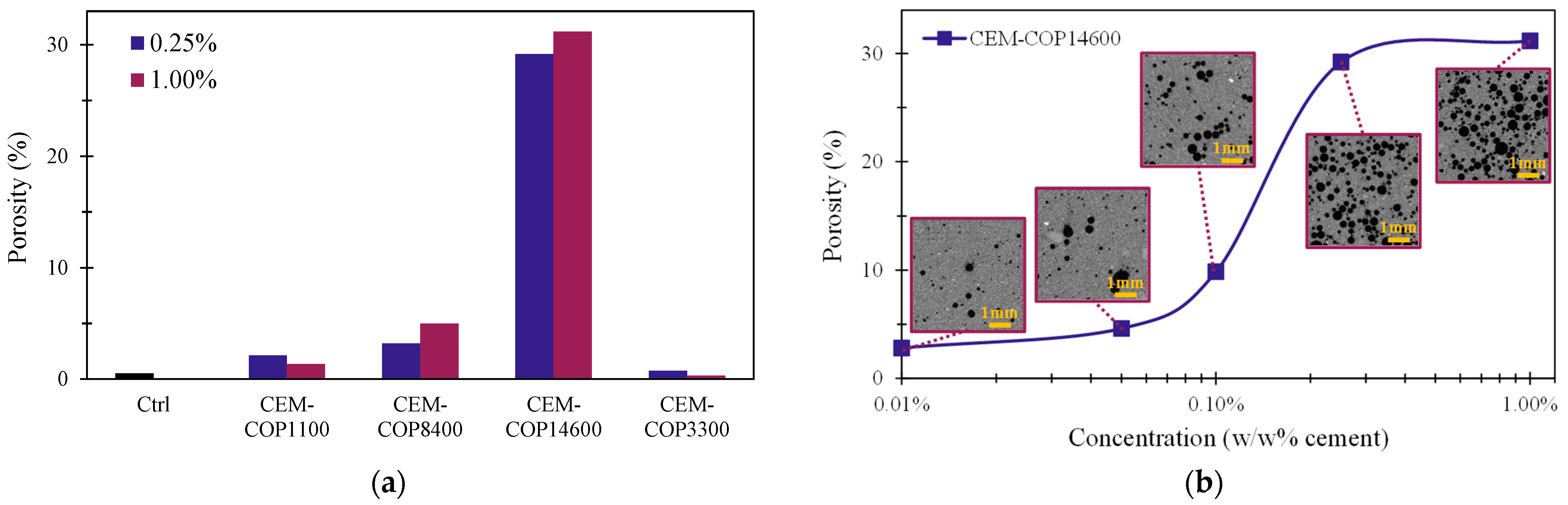
3.4.1. Void Structure
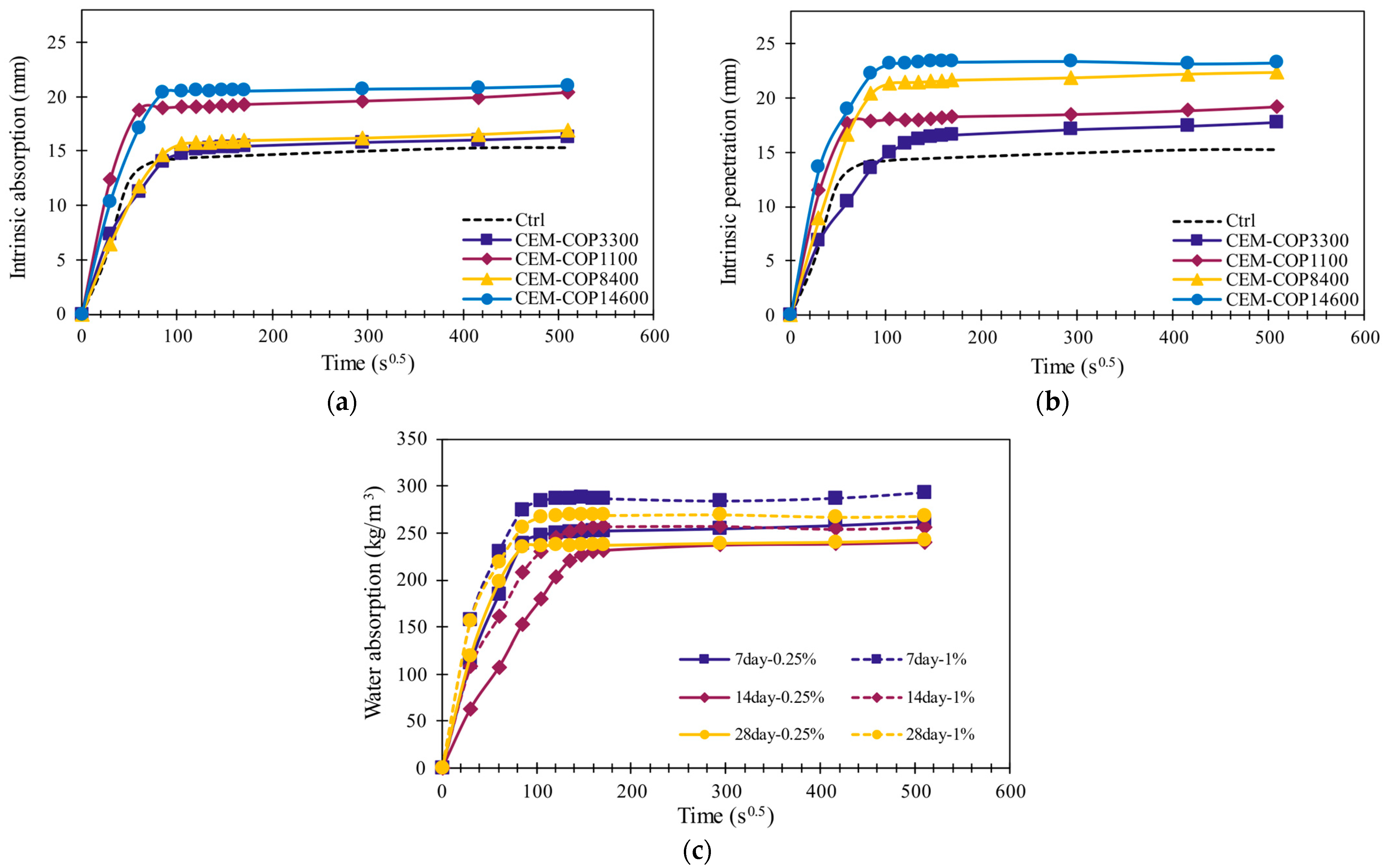
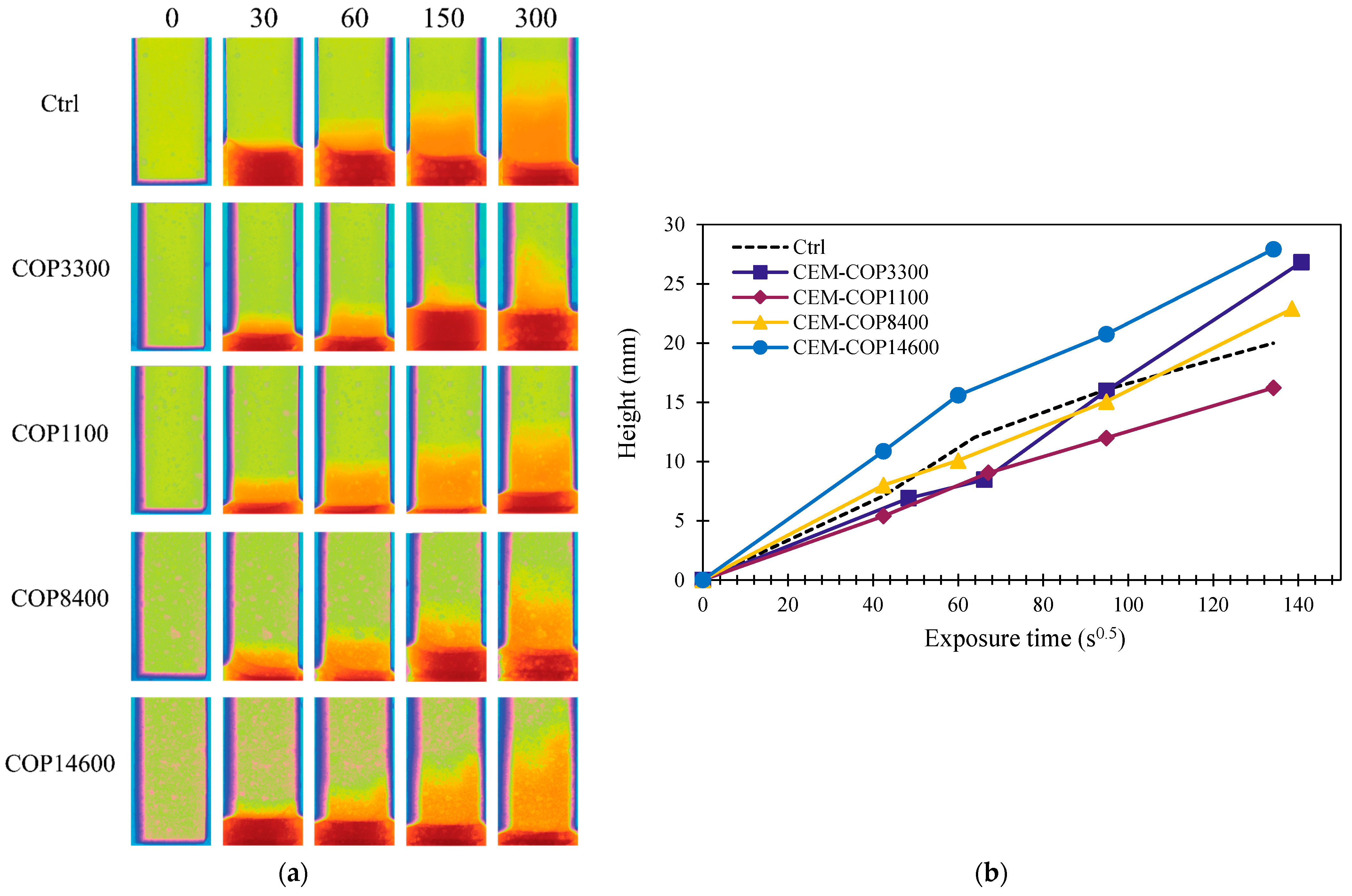
3.4.2. Water Sorption
Gravimetry
Micro-CT
3.4.3. Freeze-Thaw Damage
4. Conclusions
- The surface activity and foaming measurements showed that although all copolymers are capable of significantly reducing surface tension, only some of the copolymers (namely F-68 LF and F-108) produce stable foam. Interestingly, the copolymer with the lowest surface tension did not exhibit any foaming ability.
- Copolymers adsorb minimally to the cement particles, and as such, did not affect cement particle dispersion due to a lack of electrostatic repulsion.
- Certain copolymers including COP14600 can produce considerable amounts of air voids in the samples. The results showed that both foaming and air void volume showed a direct relationship with the HLB of the copolymers rather than their surface tension. Thus, HLB could be used as an indicator of the air-entraining performance of these copolymers in cement paste.
- The samples modified with the copolymers showed an increase in water sorption compared to the control sample. This is attributed to increased capillary porosity and increased surface hydrophilicity in the modified samples.
- Almost all samples experienced slightly increased water sorption that was linked to the change in microstructure and a lower hydrophobicity of the matrix. The results for water sorption were compared to micro-CT and freeze-thaw results, and a good correlation was observed between air-entrained properties of the microstructure and freeze-thaw resistance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhikary, S.K.; Ashish, D.K. Turning waste expanded polystyrene into lightweight aggregate: Towards sustainable construction industry. Sci. Total Environ. 2022, 837, 155852. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.K.; Ashish, D.K.; Rudžionis, Ž. Aerogel based thermal insulating cementitious composites: A review. Energy Build. 2021, 245, 111058. [Google Scholar] [CrossRef]
- Powers, T. A Working Hypothesis for Further Studies of Frost Resistance of Concrete, Concrete.Org. 1945. Available online: https://www.concrete.org/publications/internationalconcreteabstractsportal.aspx?m=details&i=8684&m=details&i=8684 (accessed on 7 December 2023).
- Powers, T.C.; Willis, T.F. The air requirement of frost resistant concrete. In Proceedings of the Twenty-Ninth Annual Meeting of the Highway Research Board, Washington, DC, USA, 13–16 December 1949; Volume 29. [Google Scholar]
- Powers, T.; Helmuth, R. Theory of volume changes in hardened portland-cement paste during freezing. In Proceedings of the Thirty-Second Annual Meeting of the Highway Research Board, Washington, DC, USA, 13–16 January 1953; Volume 32. [Google Scholar]
- Guo, J.; Sun, W.; Xu, Y.; Lin, W.; Jing, W. Damage Mechanism and Modeling of Concrete in Freeze–Thaw Cycles: A Review. Buildings 2022, 12, 1317. [Google Scholar] [CrossRef]
- Shah, H.A.; Yuan, Q.; Zuo, S. Air entrainment in fresh concrete and its effects on hardened concrete-a review. Constr. Build. Mater. 2020, 274, 121835. [Google Scholar] [CrossRef]
- Chatterji, S. Freezing of air-entrained cement-based materials and specific actions of air-entraining agents. Cem. Concr. Compos. 2003, 25, 759–765. [Google Scholar] [CrossRef]
- Masoule, M.S.T.; Baffoe, E.; Ghahremaninezhad, A. On the physicochemical properties and foaming characteristics of proteins in cement environment. Constr. Build. Mater. 2023, 366, 130204. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Kontogiannis, O.; Selianitis, D.; Perinelli, D.R.; Bonacucina, G.; Pippa, N.; Gazouli, M.; Pispas, S. Non-Ionic Surfactant Effects on Innate Pluronic 188 Behavior: Interactions, and Physicochemical and Biocompatibility Studies. Int. J. Mol. Sci. 2022, 23, 13814. [Google Scholar] [CrossRef]
- Devi, D.R.; Sandhya, P.; Hari, B.N.V. Poloxamer: A novel functional molecule for drug delivery and gene therapy. J. Pharm. Sci. Res. 2013, 5, 159. [Google Scholar]
- Kabanov, A.V.; Lemieux, P.; Vinogradov, S.; Alakhov, V. Pluronic® block copolymers: Novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002, 54, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Unosson, J.; Montufar, E.B.; Engqvist, H.; Ginebra, M.; Persson, C. Brushite foams—the effect of Tween® 80 and Pluronic® F-127 on foam porosity and mechanical properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, K.; Gür, B.; Şenol, O.; Aydın, M.R.; Gündoğdu, O. New bone cements with Pluronic®F127 for prophylaxis and treatment of periprosthetic joint infections. J. Mech. Behav. Biomed. Mater. 2021, 119, 104496. [Google Scholar] [CrossRef] [PubMed]
- Masoule, M.S.T.; Ghahremaninezhad, A. Effect of Poly(ethylene glycol)–Poly(propylene glycol) Triblock Copolymers on Autogenous Shrinkage and Properties of Cement Pastes. Buildings 2024, 14, 283. [Google Scholar] [CrossRef]
- Harris, N.J.; Hover, K.C.; Folliard, K.J.; Ley, M.T. The Use of the Foam Index Test to Predict Air-Entraining Admixture Dosage in Concrete Containing Fly Ash: Part II—Development of a Standard Test Method: Apparatus and Procedure. J. ASTM Int. 2008, 5, 1–15. [Google Scholar] [CrossRef]
- Corr, D.; Lebourgeois, J.; Monteiro, P.; Bastacky, S.; Gartner, E. Air void morphology in fresh cement pastes. Cem. Concr. Res. 2002, 32, 1025–1031. [Google Scholar] [CrossRef]
- Farnam, Y.; Washington, T.; Weiss, J. The Influence of Calcium Chloride Salt Solution on the Transport Properties of Cementitious Materials. Adv. Civ. Eng. 2015, 2015, 929864. [Google Scholar] [CrossRef]
- ASTM C1585; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. Available online: https://www.astm.org/c1585-20.html (accessed on 23 November 2023).
- Moradllo, M.K.; Hu, Q.; Ley, M.T. Using X-ray imaging to investigate in-situ ion diffusion in cementitious materials. Constr. Build. Mater. 2017, 136, 88–98. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Ley, M.T. Quantitative measurement of the influence of degree of saturation on ion penetration in cement paste by using X-ray imaging. Constr. Build. Mater. 2017, 141, 113–129. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Ley, M.T. Comparing ion diffusion in alternative cementitious materials in real time by using non-destructive X-ray imaging. Cem. Concr. Compos. 2017, 82, 67–79. [Google Scholar] [CrossRef]
- Zhou, Z.; Chu, B. Light-scattering study on the association behavior of triblock polymers of ethylene oxide and propylene oxide in aqueous solution. J. Colloid Interface Sci. 1988, 126, 171–180. [Google Scholar] [CrossRef]
- Nagarajan, R. Solubilization of hydrocarbons and resulting aggregate shape transitions in aqueous solutions of Pluronic® (PEO–PPO–PEO) block copolymers. Colloids Surf. B Biointerfaces 1999, 16, 55–72. [Google Scholar] [CrossRef]
- Sharma, R.K.; Shaikh, S.; Ray, D.; Aswal, V.K. Binary mixed micellar systems of PEO-PPO-PEO block copolymers for lamotrigine solubilization: A comparative study with hydrophobic and hydrophilic copolymer. J. Polym. Res. 2018, 25, 73. [Google Scholar] [CrossRef]
- Lal, S.; Datta, M. Organoclay Pluronic f68—Montmorillonite, as a Sustained Release Drug Delivery Vehicle for Propranolol Hydrochloride; European Chemical Bulletin: Budapest, Hungary, 2014. [Google Scholar]
- Dmitrenko, M.; Penkova, A.; Atta, R.; Zolotarev, A.; Plisko, T.; Mazur, A.; Solovyev, N.; Ermakov, S. The development and study of novel membrane materials based on polyphenylene isophthalamide—Pluronic F127 composite. Mater. Des. 2019, 165, 107596. [Google Scholar] [CrossRef]
- Elmowafy, M.; Alruwaili, N.K.; Shalaby, K.; Alharbi, K.S.; Altowayan, W.M.; Ahmad, N.; Zafar, A.; Elkomy, M. Long-Acting Paliperidone Parenteral Formulations Based on Polycaprolactone Nanoparticles; the Influence of Stabilizer and Chitosan on In Vitro Release, Protein Adsorption, and Cytotoxicity. Pharmaceutics 2020, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Tunstall, L.E.; Ley, M.T.; Scherer, G.W. Air entraining admixtures: Mechanisms, evaluations, and interactions. Cem. Concr. Res. 2021, 150, 106557. [Google Scholar] [CrossRef]
- Alexandridis, P.; Athanassiou, V.; Fukuda, S.; Hatton, T.A. Surface Activity of Poly(ethylene oxide)-block-Poly(propylene oxide)-block-Poly(ethylene oxide) Copolymers. Langmuir 1994, 10, 2604–2612. [Google Scholar] [CrossRef]
- Feneuil, B.; Pitois, O.; Roussel, N. Effect of surfactants on the yield stress of cement paste. Cem. Concr. Res. 2017, 100, 32–39. [Google Scholar] [CrossRef]
- Tiwari, S.; Namsani, S.; Singh, J.K. Effect of salt on the adsorption of ionic surfactants at the air-water interface. J. Mol. Liq. 2022, 360, 119498. [Google Scholar] [CrossRef]
- Jain, N.; Aswal, V.; Goyal, P.; Bahadur, P. Salt induced micellization and micelle structures of′ PEO/PPO/PEO block copolymers in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 85–94. [Google Scholar] [CrossRef]
- Lunagariya, J.; Kumar, N.S.; Asif, M.; Dhar, A.; Vekariya, R.L. Dependency of Anion and Chain Length of Imidazolium Based Ionic Liquid on Micellization of the Block Copolymer F127 in Aqueous Solution: An Experimental Deep Insight. Polymers 2017, 9, 285. [Google Scholar] [CrossRef]
- Pisal, S.; Wawde, G.M.; Bandivadekar, M.; Hajare, A.A.; Kadam, S. Vacuum foam drying for preservation of lasota virus: Screening of foaming agent and cycle optimization. Indian J. Biotechnol. 2006, 5, 491–497. [Google Scholar]
- BASF. Pluronic PE Types Technical Information. 1996. Available online: http://www.timing-ouhan.com/images/pluronicpetypes.pdf (accessed on 4 July 2024).
- Dodson, V.H. Concrete Admixtures; Springer Nature: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Federal Highway Administration. Fly Ash AEA Adsorption Capacity Estimation as Measured by Fluorescence or Foam Index. 2017. Available online: https://www.fhwa.dot.gov/publications/research/infrastructure/pavements/17118/index.cfm (accessed on 4 July 2024).
- Doulia, D.; Trägårdh, G.; Gekas, V. Interaction behaviour in ultrafiltration of nonionic surfactants Part II. Static adsorption below CMC. J. Membr. Sci. 1997, 123, 133–142. [Google Scholar] [CrossRef]
- Zhang, T.; Shang, S.; Yin, F.; Aishah, A.; Salmiah, A.; Ooi, T. Adsorptive behavior of surfactants on surface of Portland cement. Cem. Concr. Res. 2001, 31, 1009–1015. [Google Scholar] [CrossRef]
- Lange, A.; Plank, J. Contribution of non-adsorbing polymers to cement dispersion. Cem. Concr. Res. 2016, 79, 131–136. [Google Scholar] [CrossRef]
- Fang, Y.; Lin, Z.; Yan, D.; Zhang, X.; Ma, X.; Lai, J.; Liu, Y.; Chen, Z.; Wang, Z. Study on the Effect of Polycarboxylate Ether Molecular Structure on Slurry Dispersion, Adsorption, and Microstructure. Polymers 2023, 15, 2496. [Google Scholar] [CrossRef]
- Burgos-Montes, O.; Palacios, M.; Rivilla, P.; Puertas, F. Compatibility between superplasticizer admixtures and cements with mineral additions. Constr. Build. Mater. 2012, 31, 300–309. [Google Scholar] [CrossRef]
- Merlin, F.; Guitouni, H.; Mouhoubi, H.; Mariot, S.; Vallée, F.; Van Damme, H. Adsorption and heterocoagulation of nonionic surfactants and latex particles on cement hydrates. J. Colloid Interface Sci. 2005, 281, 1–10. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef]
- Qiao, M.; Chen, J.; Gao, N.; Shan, G.; Wu, J.; Zhu, B.; Ran, Q. Effects of Adsorption Group and Molecular Weight of Viscosity-Modifying Admixtures on the Properties of Cement Paste. J. Mater. Civ. Eng. 2022, 34, 04022148. [Google Scholar] [CrossRef]
- Shu, X.; Zhao, H.; Wang, X.; Zhang, Q.; Yang, Y.; Ran, Q.; Liu, J. Effect of hydrophobic units of polycarboxylate superplasticizer on the flow behavior of cement paste. J. Dispers. Sci. Technol. 2016, 38, 256–264. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Yang, Y.; Qi, S.; Ran, Q.; Liu, J. Synthesis, performance and working mechanism of a novel polyaryl ether superplasticizer for cement-based materials. J. Dispers. Sci. Technol. 2019, 41, 1–10. [Google Scholar] [CrossRef]
- Shi, L.; Huang, J.; Zeng, G.; Zhu, L.; Gu, Y.; Shi, Y.; Yi, K.; Li, X. Roles of surfactants in pressure-driven membrane separation processes: A review. Environ. Sci. Pollut. Res. 2019, 26, 30731–30754. [Google Scholar] [CrossRef]
- Qu, H.; Fu, C.; Yang, W.; Yang, Z.; Zhang, L. Preparation, application and water reducing mechanism of a novel fluorescent superplasticizer with improved flow retaining ability and clay tolerance. J. Dispers. Sci. Technol. 2018, 39, 1829–1839. [Google Scholar] [CrossRef]
- Choi, P.; Yeon, J.H.; Yun, K.-K. Air-void structure, strength, and permeability of wet-mix shotcrete before and after shotcreting operation: The influences of silica fume and air-entraining agent. Cem. Concr. Compos. 2016, 70, 69–77. [Google Scholar] [CrossRef]
- Kumar Mehta, P.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York, NY, USA, 2014; Available online: https://www.accessengineeringlibrary.com/content/book/9780071797870 (accessed on 4 July 2024).
- C457/C457M; Standard Test Method for Microscopical Determination of Parameters of the Air-Void System in Hardened Concrete, (n.d.). Available online: https://www.astm.org/c0457_c0457m-16.html (accessed on 4 July 2024).
- Micro Photonics. Structural Parameters Measured by SkyscanTM CT-Analyzer Software, n.d. Available online: https://www.microphotonics.com/wp-content/uploads/2016/01/CTAn_parameters.pdf (accessed on 4 July 2024).
- Zhuang, S.; Wang, Q.; Zhang, M. Water absorption behaviour of concrete: Novel experimental findings and model characterization. J. Build. Eng. 2022, 53, 104602. [Google Scholar] [CrossRef]
- Eriksson, D.; Gasch, T.; Ansell, A. A Hygro-Thermo-Mechanical Multiphase Model for Long-Term Water Absorption into Air-Entrained Concrete. Transp. Porous Media 2018, 127, 113–141. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Qiao, C.; Keys, M.; Hall, H.; Ley, M.T.; Reese, S.; Weiss, W.J. Quantifying Fluid Absorption in Air-Entrained Concrete Using Neutron Radiography. ACI Mater. J. 2019, 116, 213–226. [Google Scholar] [CrossRef]
- Li, W.; Pour-Ghaz, M.; Castro, J.; Weiss, J. Water Absorption and Critical Degree of Saturation Relating to Freeze-Thaw Damage in Concrete Pavement Joints. J. Mater. Civ. Eng. 2012, 24, 299–307. [Google Scholar] [CrossRef]
- Kearsley, E.P.; Wainwright, P.J. The effect of porosity on the strength of foamed concrete. Cem. Concr. Res. 2002, 32, 233–239. [Google Scholar] [CrossRef]
- Wong, H.; Pappas, A.; Zimmerman, R.; Buenfeld, N. Effect of entrained air voids on the microstructure and mass transport properties of concrete. Cem. Concr. Res. 2011, 41, 1067–1077. [Google Scholar] [CrossRef]
- Shen, C.; Zhu, Y.; Shi, W.; He, K.; Xiao, X.; Xu, X.; Shi, J.; Xu, G. Mechanically stable superhydrophobic surface on cement-based materials. Chem. Phys. 2020, 538, 110912. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, X.; Kumar, S.R.; Mark, J.E. Improved Hydrophilicity from Poly(ethylene glycol) in Amphiphilic Conetworks with Poly(dimethylsiloxane). Silicon 2009, 1, 173–181. [Google Scholar] [CrossRef]
- Yao, H.; Xie, Z.; Huang, C.; Yuan, Q.; Yu, Z. Recent progress of hydrophobic cement-based materials: Preparation, characterization and properties. Constr. Build. Mater. 2021, 299, 124255. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Sun, M.; He, Z.; Xu, H.; Tan, J. Mechanical and permeability properties of polymer-modified concrete using hydrophobic agent. J. Build. Eng. 2020, 31, 101337. [Google Scholar] [CrossRef]
- Qiao, C.; Moradllo, M.K.; Hall, H.; Ley, M.T.; Weiss, W.J. Electrical Resistivity and Formation Factor of Air-Entrained Concrete. ACI Mater. J. 2019, 116, 85–93. [Google Scholar] [CrossRef]
- Fagerlund, G. The international cooperative test of the critical degree of saturation method of assessing the freeze/thaw resistance of concrete. Matériaux Constr. 1977, 10, 231–253. [Google Scholar] [CrossRef]
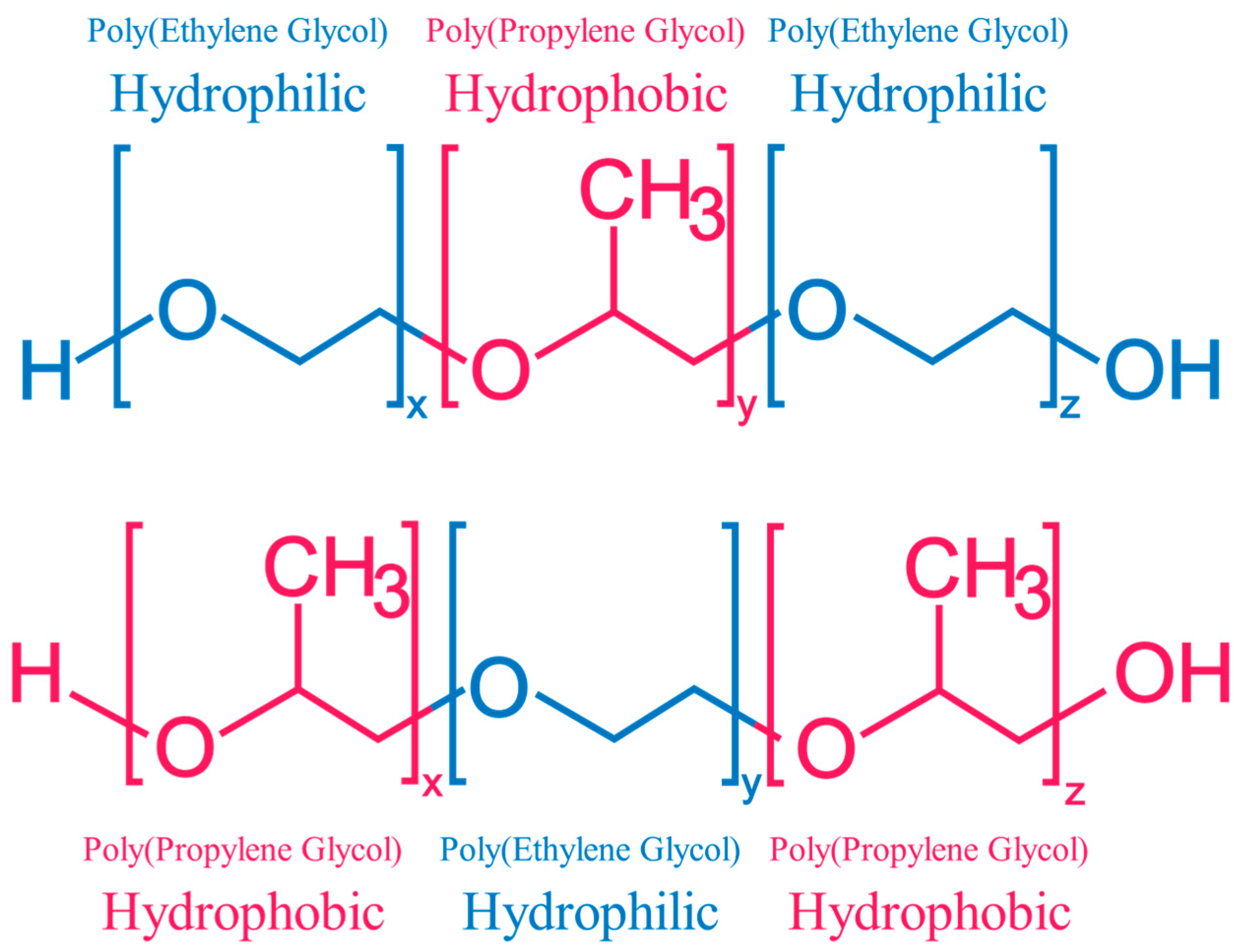

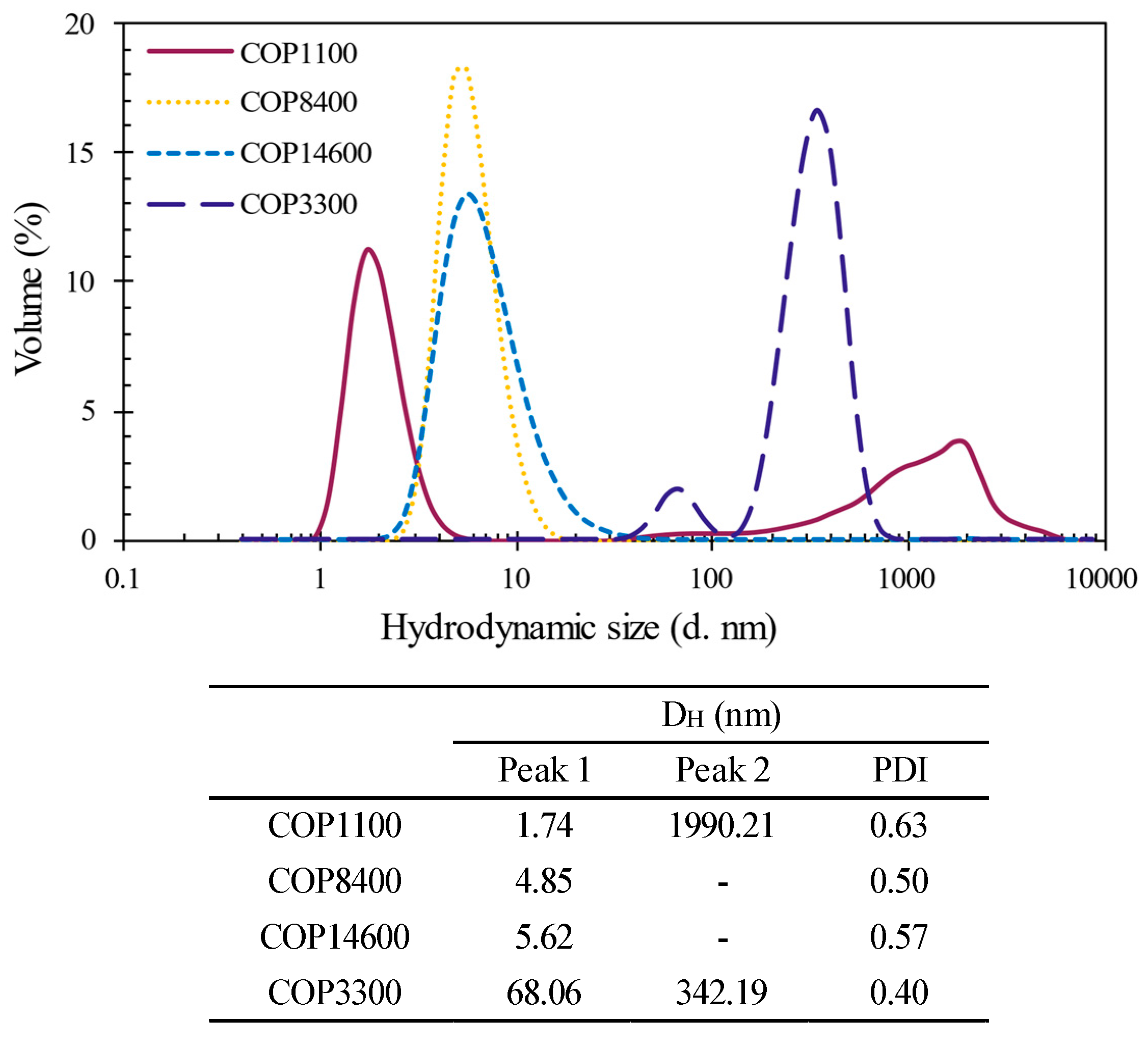
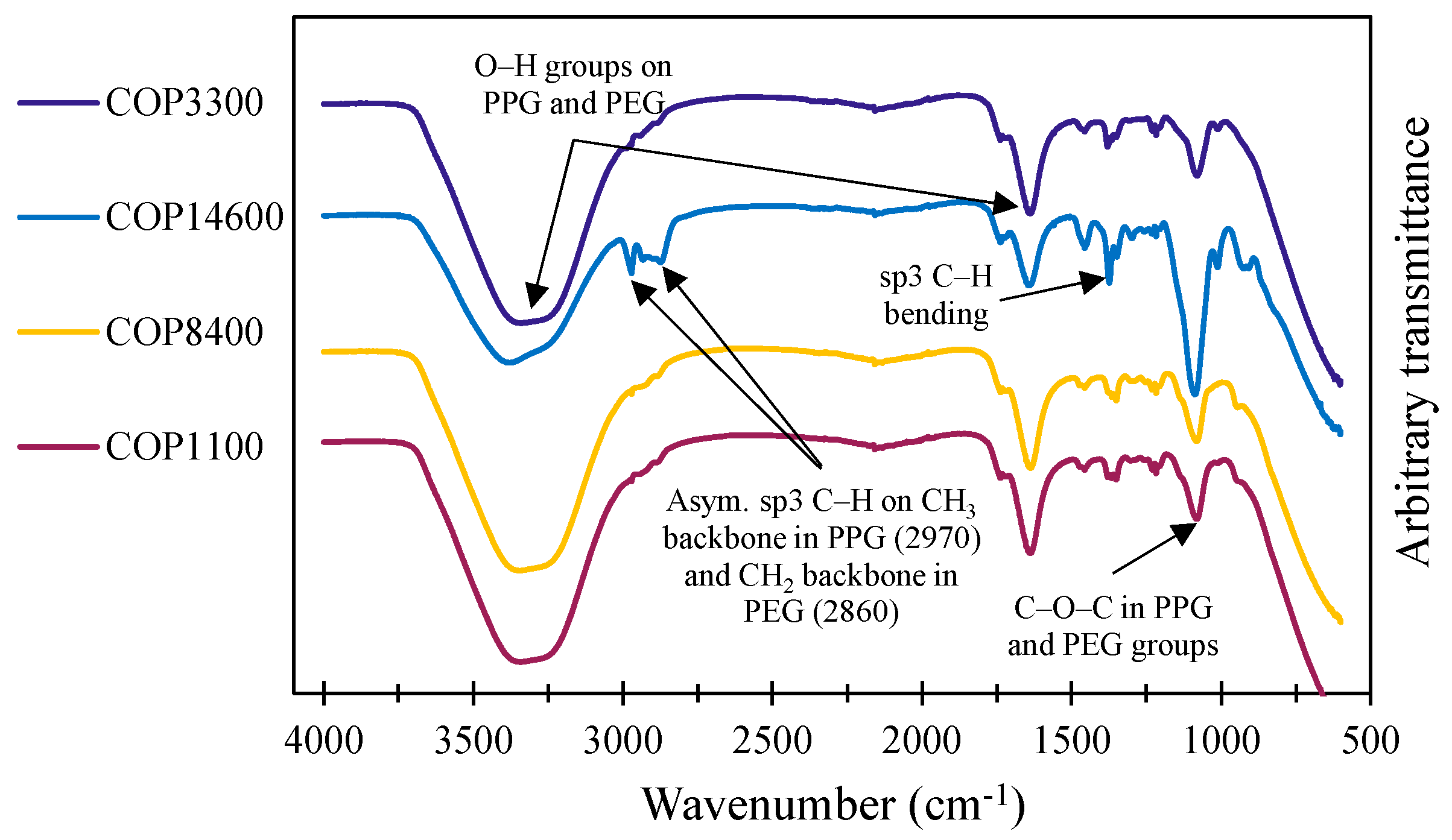
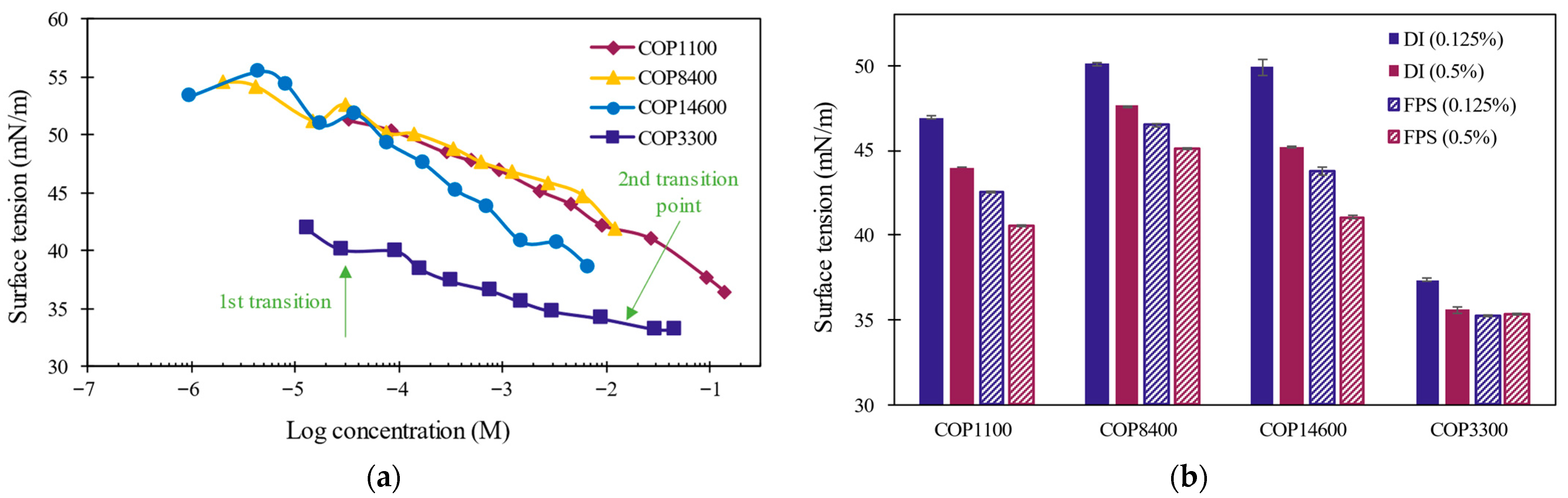


| Code | Pluronic® Identifier | Polymer Structure | x|z | y | Mn (Da) | PEG (%w) by 1H NMR | HLB | Form | Viscosity (cps) |
|---|---|---|---|---|---|---|---|---|---|
| COP1100 | L-31 | [PEG]x [PPG]y [PEG]z | 4 | 16 | 1100 | 10% | 5 | Liquid | 175 |
| COP8400 | F-68 LF | [PEG]x [PPG]y [PEG]z | 80 | 27 | 8400 | 80% | 29 | solid | 1000 |
| COP14600 | F-108 | [PEG]x [PPG]y [PEG]z | 141 | 44 | 14,600 | 82.5% | 27 | solid | 2800 |
| COP3300 | 31R1 | [PPG]x [PEG]y [PPG]z | 27 | 4 | 3300 | 10% | 1 | liquid | 660 |
| Composition | (%) |
|---|---|
| SiO2 | 20.6 |
| Al2O3 | 4.8 |
| Fe2O3 | 3.5 |
| CaO | 64 |
| MgO | 0.9 |
| Na2O | 0.1 |
| K2O | 0.3 |
| SO3 | 3.4 |
| LOI | 2.4 |
| Sample | Micro-CT Analysis | |||
|---|---|---|---|---|
| Copolymer | Conc. | Porosity (%) | Separation (mm) | Ave. Void Diameter (mm) |
| Ctrl | 0.00% | 0.51 | 0.54 | 0.11 |
| CEM-COP1100 | 0.25% | 2.15 | 0.83 | 0.28 |
| 1.00% | 1.38 | 1.46 | 0.42 | |
| CEM-COP8400 | 0.25% | 3.23 | 0.47 | 0.33 |
| 1.00% | 5.00 | 0.54 | 0.32 | |
| CEM-COP14600 | 0.01% | 2.80 | 0.53 | 0.29 |
| 0.05% | 4.60 | 0.50 | 0.29 | |
| 0.10% | 9.85 | 0.58 | 0.34 | |
| 0.25% | 29.20 | 0.28 | 0.32 | |
| 1.00% | 31.17 | 0.27 | 0.30 | |
| CEM-COP3300 | 0.25% | 0.77 | 1.27 | 0.49 |
| 1.00% | 0.33 | 1.23 | 0.42 | |
| Sample | Capillary Porosity (%) | |
|---|---|---|
| 0.25% | 1% | |
| Ctrl | 28.16 | 28.16 |
| CEM-COP1100 | 34.54 | 37.28 |
| CEM-COP8400 | 31.12 | 32.65 |
| CEM-COP14600 | 22.08 | 21.92 |
| CEM-COP3300 | 34.65 | 33.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tale Masoule, M.S.; Ghahremaninezhad, A. The Relationship between Molecular Structure and Foaming of Poly(ethylene glycol)—Poly(propylene glycol) Triblock Surfactants in Cementitious Materials. Buildings 2024, 14, 2100. https://doi.org/10.3390/buildings14072100
Tale Masoule MS, Ghahremaninezhad A. The Relationship between Molecular Structure and Foaming of Poly(ethylene glycol)—Poly(propylene glycol) Triblock Surfactants in Cementitious Materials. Buildings. 2024; 14(7):2100. https://doi.org/10.3390/buildings14072100
Chicago/Turabian StyleTale Masoule, Mohammad Sadegh, and Ali Ghahremaninezhad. 2024. "The Relationship between Molecular Structure and Foaming of Poly(ethylene glycol)—Poly(propylene glycol) Triblock Surfactants in Cementitious Materials" Buildings 14, no. 7: 2100. https://doi.org/10.3390/buildings14072100
APA StyleTale Masoule, M. S., & Ghahremaninezhad, A. (2024). The Relationship between Molecular Structure and Foaming of Poly(ethylene glycol)—Poly(propylene glycol) Triblock Surfactants in Cementitious Materials. Buildings, 14(7), 2100. https://doi.org/10.3390/buildings14072100







