Aluminate Long Afterglow Luminescent Materials in Road Marking Field Research Progress and Development: A Review
Abstract
:1. Introduction
2. The Concept of Long Afterglow Pavement Marking and the Current Status of Research
2.1. Definition of Long Afterglow Road Markings
2.2. Current Status of Domestic and International Research
2.2.1. Road Marking Paints
2.2.2. Energy Storage Luminescent Materials
3. Long Afterglow Line Marking Coating Type
3.1. Hot Melt Road Marking Coatings
3.2. Water-Based Marking Coatings
3.3. Two-Component Systems
3.4. Solvent-Based Systems
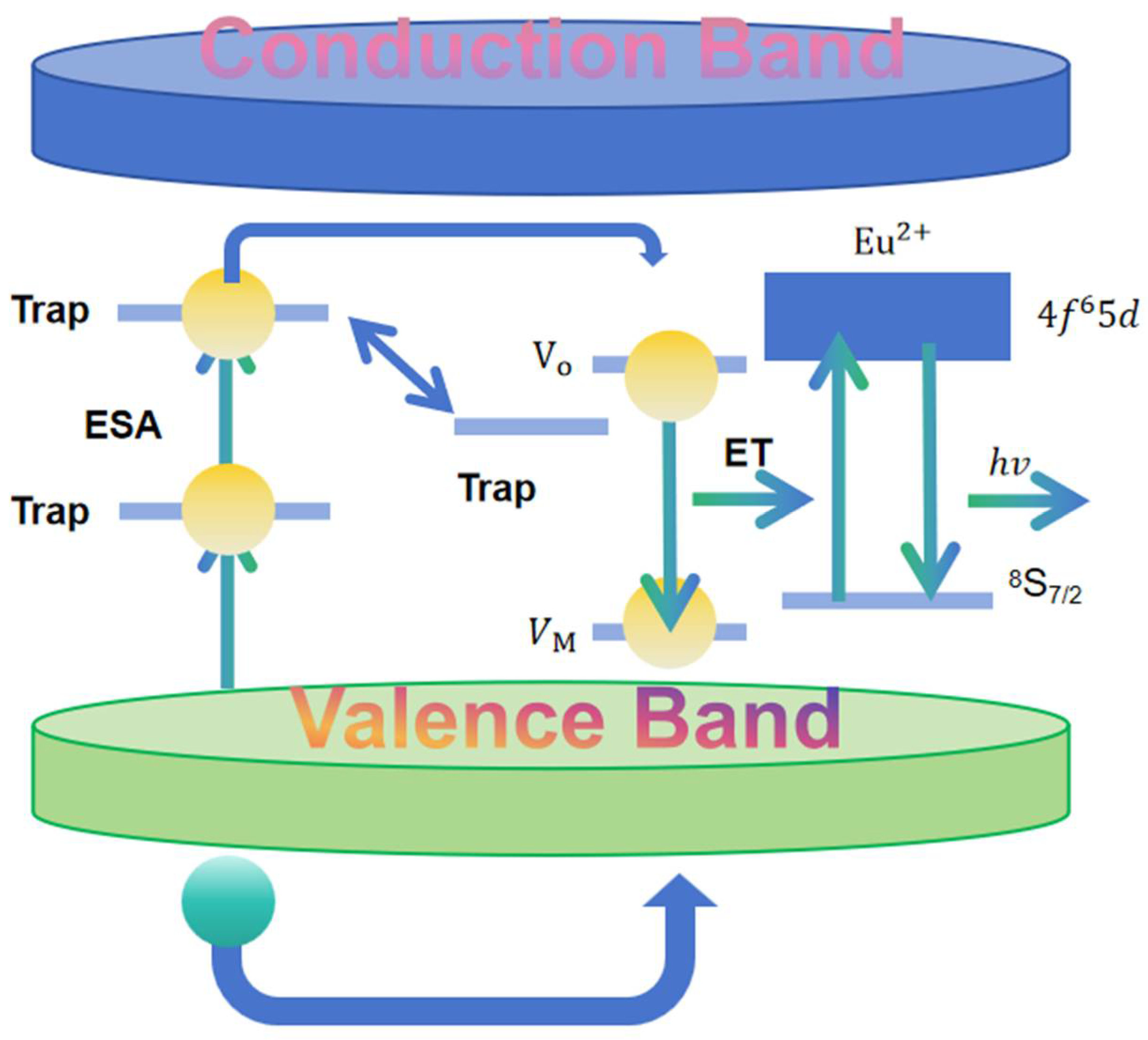
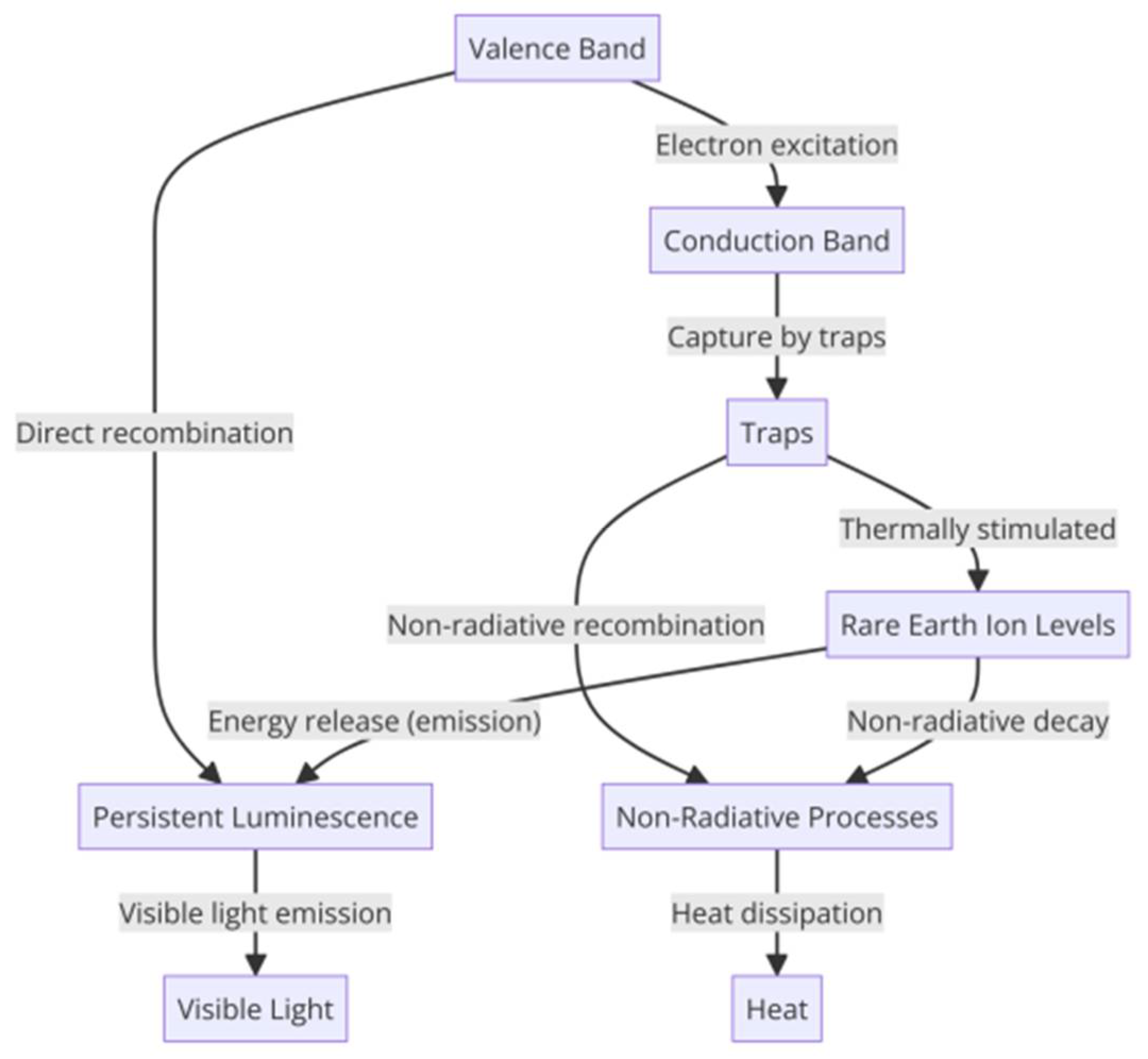
4. Luminescence Principle of Aluminate Materials
4.1. Electron Trap Model
4.2. Cavity Transfer Modeling
4.3. Energy Transfer Model
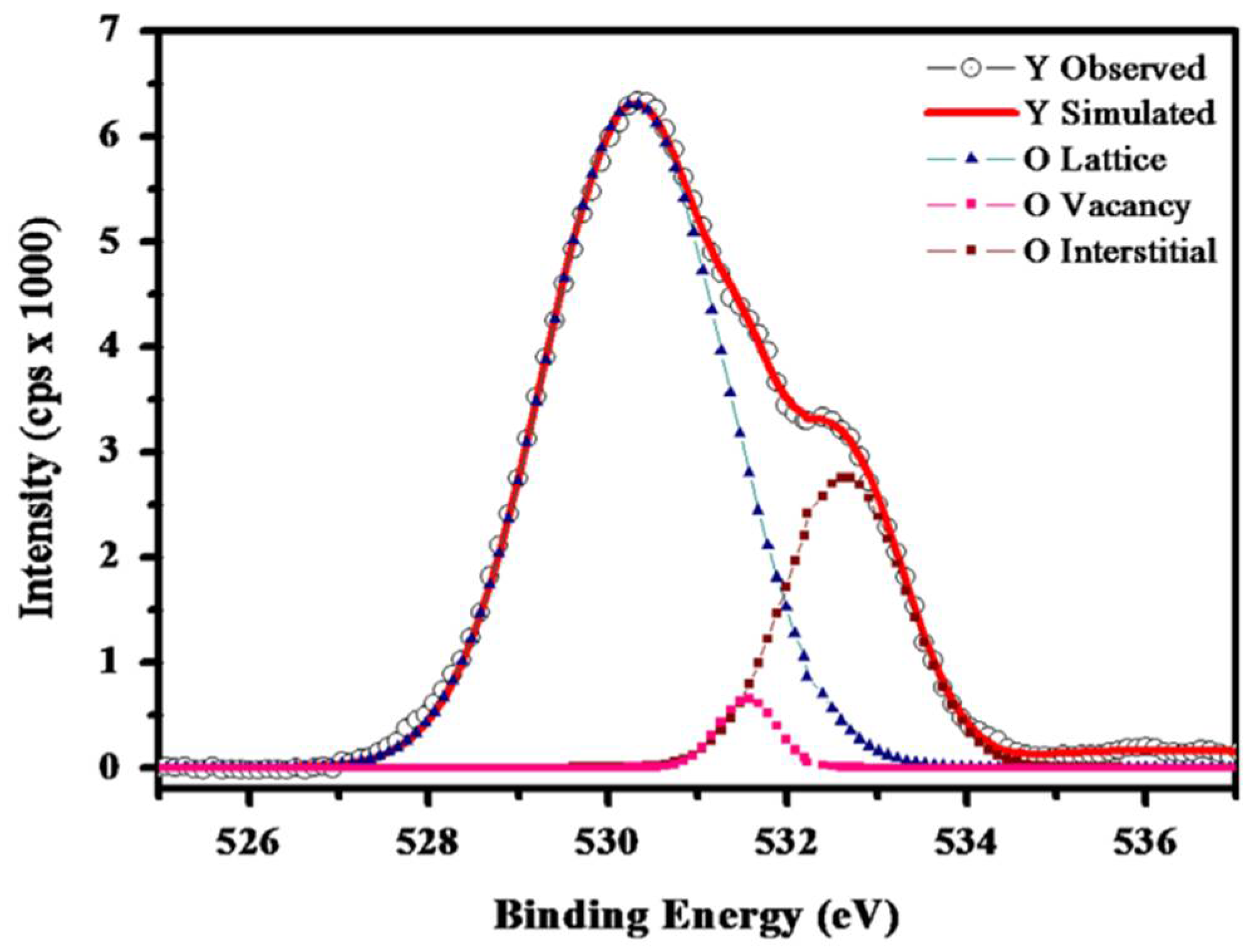
4.4. Oxygen Vacancy Model
5. Long-Lasting Phosphorescent Road Marking Paint Preparation Techniques
5.1. High-Temperature Solid-State Reaction Method
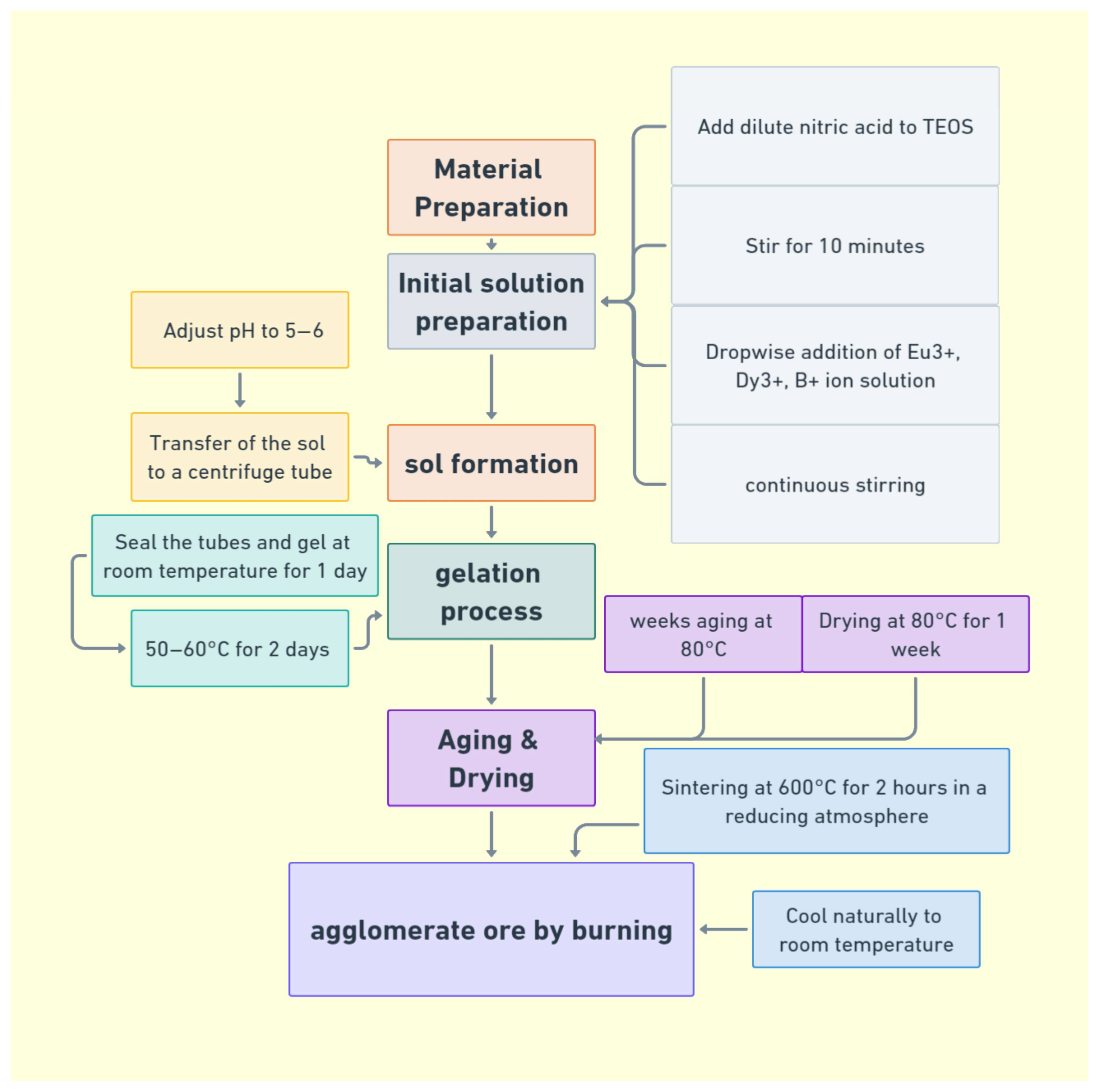
5.2. Sol–Gel Method
5.3. Hydrothermal Synthesis Method
5.4. Combustion Synthesis Method
5.5. Chemical Co-Precipitation Method
5.6. Particle Size and Milling Process
6. Aluminate Phosphor Durability Treatment Techniques
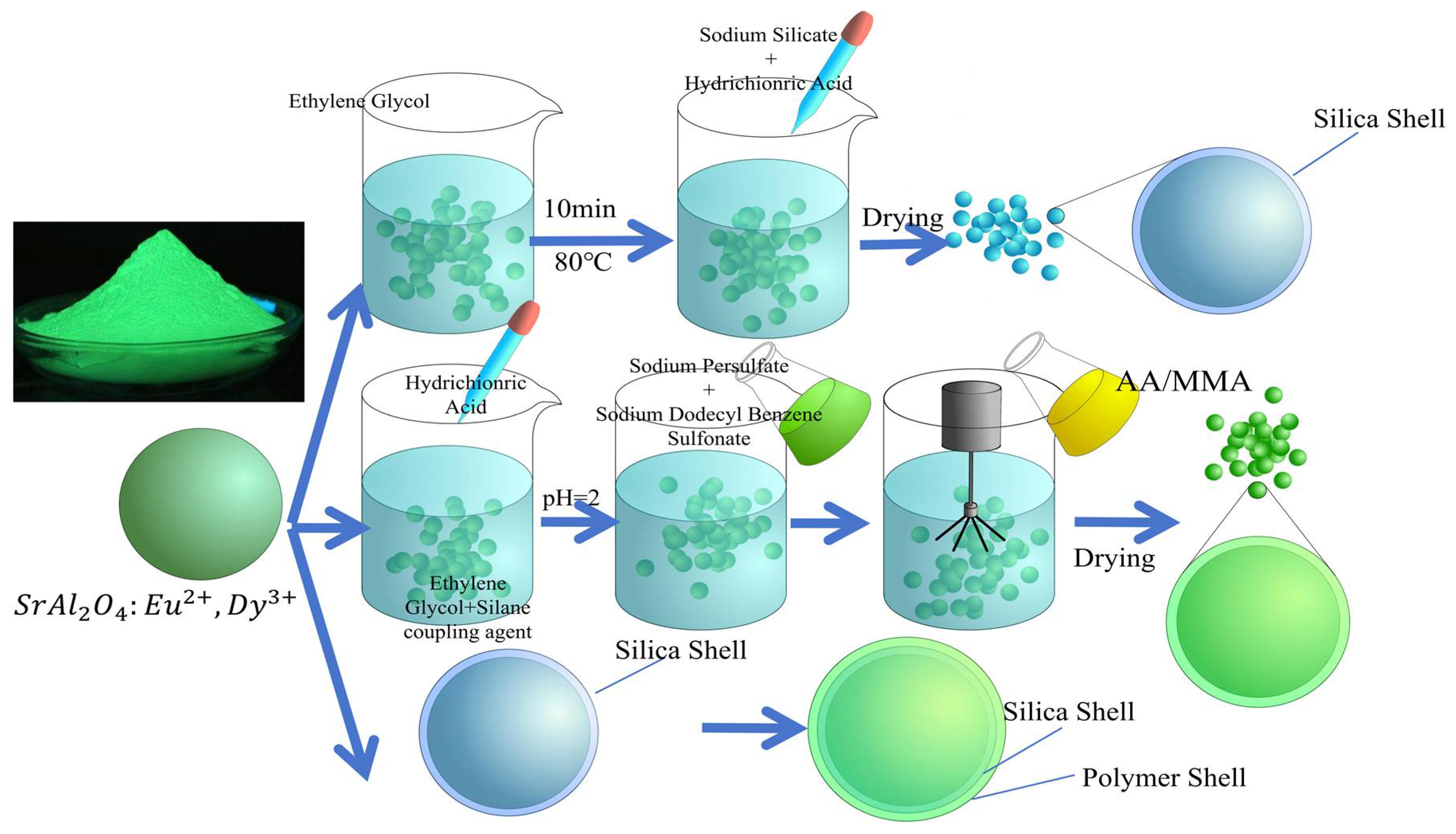
6.1. Inorganic Coating
6.2. Organic Coating
6.3. Organic–Inorganic Composite Coating
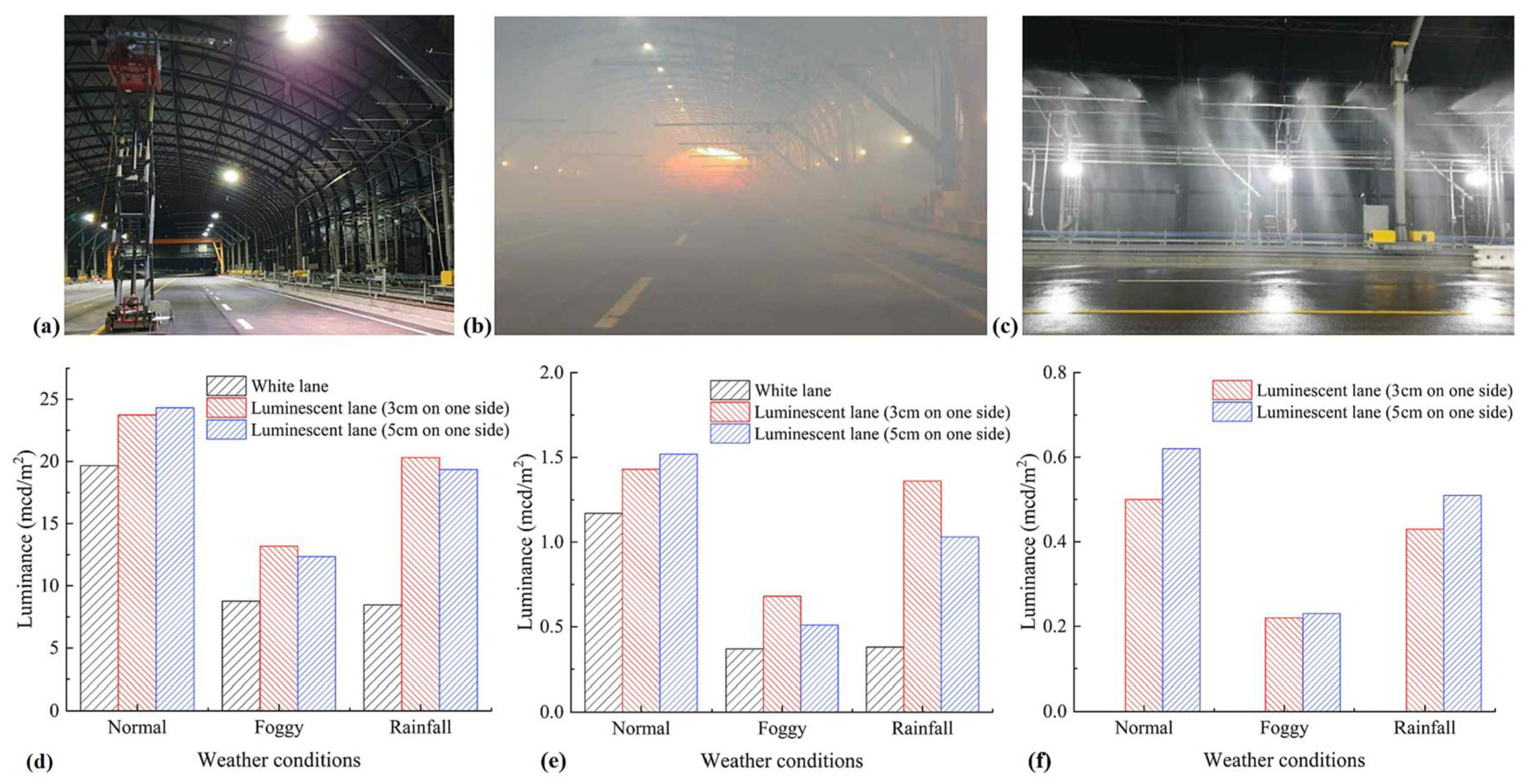
7. Long Afterglow Light-Emitting Labeling Line Performance Research
7.1. Water Resistance
7.2. Fluorescence Properties
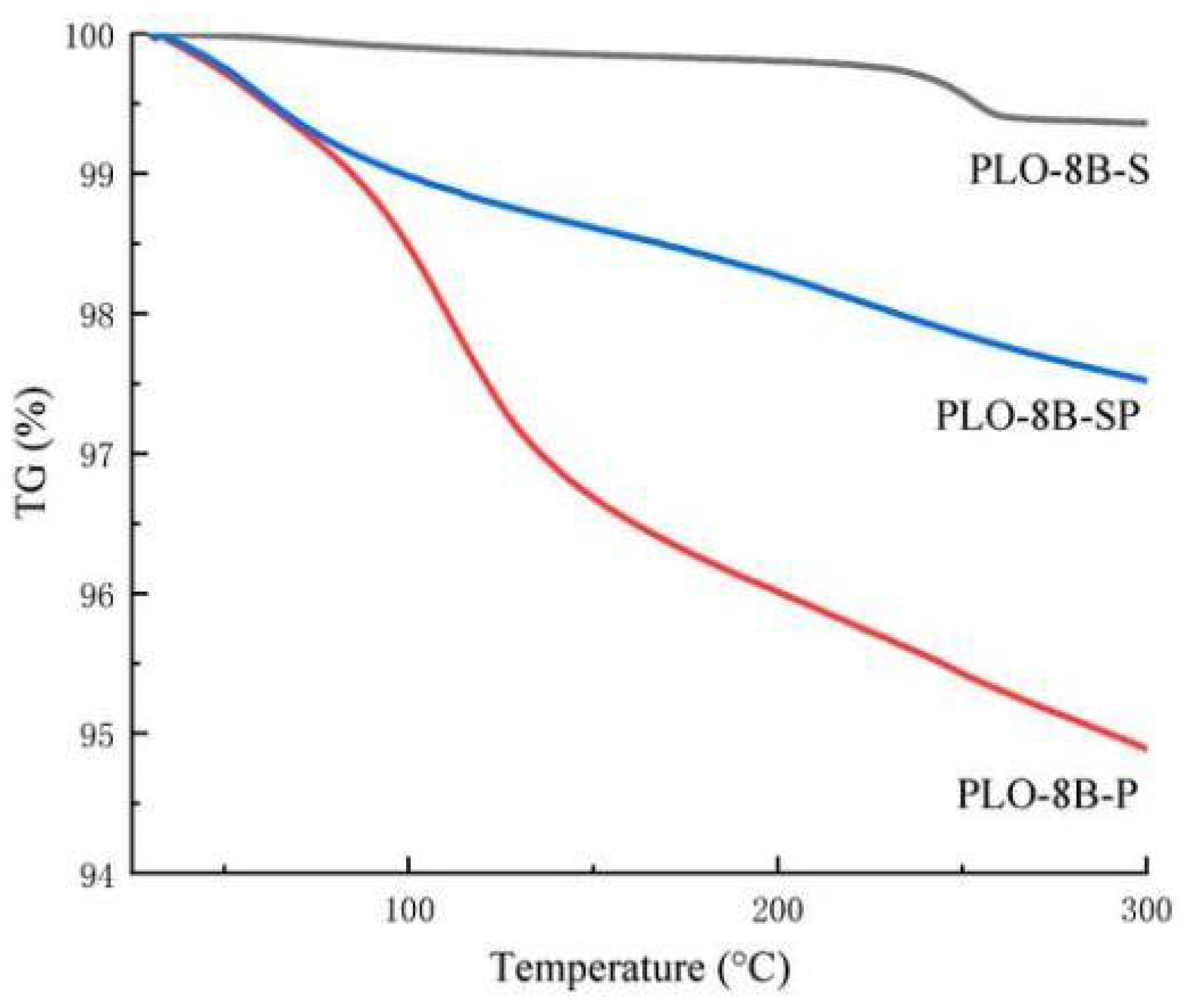
7.3. Thermal Stability
8. Research Direction and Development Prospect
8.1. Research Gaps
8.2. Research Direction and Development Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dwyer, C.; Himes, S. Investigation of Inlaid Pavement Marker Performance and Safety Effectiveness. Transp. Res. Rec. 2022, 1, 981–994. [Google Scholar] [CrossRef]
- Editorial Department, China Highway Journal. Review of academic research on Traffic Engineering in China, 2016. China J. Highw. Transp. 2016, 29, 1–161. (In Chinese) [Google Scholar] [CrossRef]
- Editorial Department, China Highway Journal. Review of academic research on Pavement Engineering in China, 2024. China J. Highw. Transp. 2024, 37, 1–81. [Google Scholar] [CrossRef]
- Li, R. The Analysis of Aircraft Operation Safety in Complex Airport; Nanjing University of Aeronautics and Astronautics: Nanjing, China, 2015. (In Chinese) [Google Scholar]
- Zheng, M.; Li, X.; Bai, Y.; Tang, S.; Li, P.; Zhu, Q. Sunlight-Activated Long Persistent Luminescent Coating for Smart Highways. Coatings 2023, 13, 1050. [Google Scholar] [CrossRef]
- He, R.; Liang, Y.-P.; Xie, R.-S.; Lu, X.; Geng, J. Research Progress in Application of Strontium Aluminate Long Afterglow Luminescent Materials in Road Markings. J. Change Univ. (Nat. Sci. Ed.) 2022, 42, 1–13. (In Chinese) [Google Scholar]
- Pashkevich, A.; Bartusiak, J.; Akowska, L.; Burghardt, T.E. Durable Waterborne Horizontal Road Markings for Improvement of Air Quality. Transp. Res. Procedia 2020, 45, 530–538. [Google Scholar] [CrossRef]
- Xie, R.-S.; Lu, X.-S.; Zhang, W.; He, R. Study on the Improvement Techniques of Road Marking Visibility. J. Wuhan Univ. Technol. (Transp. Sci. Eng.) 2023, 47, 317–322. (In Chinese) [Google Scholar]
- Lv, Y.-L. Research Progress on Long Afterglow Phosphors. Guangzhou Chem. Ind. 2017, 45, 6–7. (In Chinese) [Google Scholar]
- Mohajerani, A.; Bakaric, J.; Jeffrey, B.T. The Urban Heat Island Effect, Its Causes, and Mitigation, with Reference to the Thermal Properties of Asphalt Concrete. J. Environ. Manag. 2017, 197, 522–538. [Google Scholar] [CrossRef]
- Jinho, K.; Lee, S.Y.; Kim, T.H.; Han, K.-S.; Hwang, K.-T.; Cho, W.-S. Thermal Stability of SrAl2O4: Eu2+, Dy3+ with Long Afterglow Phosphorescence. J. Korean Ceram. Soc. 2014, 51, 618–622. [Google Scholar]
- Cheng, K.; Deng, J.-Y.; Zhang, J.-P.; Chang, J.X.; Zhang, L. Preparation and Performance of Modified Luminescent Powder for Long Afterglow Road Marking Paint. Coating Industry 2023. Available online: http://kns.cnki.net/kcms/detail/32.1154.TQ.20231221.0950.002.html (accessed on 28 April 2024). (In Chinese).
- Pan, P.; Li, Y.; Chen, Y.; Shu, S.; Hu, X.; Wang, N. Design and Performance Evaluation of the Epoxy-Based Self-Luminous Pavement Marking. Case Stud. Constr. Mater. 2023, 19, e02477. [Google Scholar] [CrossRef]
- Villa, C.; Brémond, R.; Eymond, F. Characterization of Luminescent Road Markings. Light. Res. Technol. 2023, 55, 459–473. [Google Scholar] [CrossRef]
- Li, F.J.; Cao, X.Y.; Liu, S.H.; He, L.L. Transition from Reflective to Energy-Storing Self-Illumination in Road Markings: A Review. Adv. Mater. Res. 2023, 1176, 63–76. [Google Scholar] [CrossRef]
- Babić, D.; Burghardt, T.E.; Babić, D. Application and Characteristics of Waterborne Road Marking Paint. Int. J. Traffic Transp. Eng. 2015, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Babić, D.; Burghardt, T.E.; Babić, D. Application of Waterborne Road Marking Paint in Croatia: Two Years of Road Exposure. In Proceedings of the International Conference on Traffic and Transport Engineering 2016, Lucerne, Switzerland, 6–10 July 2016. [Google Scholar]
- Fatemi, S.; Varkani, M.K.; Ranjbar, Z.; Bastani, S. Optimization of the Water-Based Road-Marking Paint by Experimental Design, Mixture Method. Prog. Org. Coat. 2006, 57, 337–344. [Google Scholar] [CrossRef]
- Cruz, M.; Klein, A.; Steiner, V. Sustainability Assessment of Road Marking Systems. Transp. Res. Procedia 2016, 14, 3334–3343. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Zareanshahraki, F.; Mannari, V. Enhancing Thermoplastic Road-Marking Paints Performance Using Sustainable Rosin Ester. Prog. Org. Coat. 2020, 140, 105493. [Google Scholar] [CrossRef]
- Taheri, M. Self-Cleaning Traffic Marking Paint. Surf. Interfaces 2017, 6, 100–108. [Google Scholar] [CrossRef]
- Dormidontova, T.V.; Filatova, A.V. Research of Influence of Quality of Materials on a Road Marking of Highways. Procedia Eng. 2016, 153, 167–173. [Google Scholar] [CrossRef]
- Burghardt, T.; Pashkevich, A.; Żakowska, L. Contribution of Solvents from Road Marking Paints to Tropospheric Ozone Formation. Bud. I Archit. 2016, 15, 49–54. [Google Scholar] [CrossRef]
- Naidu, V.; Bhaiswar, V. Review on Road Marking Paint Machine and Material. IOP Conf. Ser. Mater. Sci. Eng. 2020, 954, 012016. [Google Scholar] [CrossRef]
- Burghardt, T.E.; Pashkevich, A.; Żakowska, L. Influence of Volatile Organic Compounds Emissions from Road Marking Paints on Ground-Level Ozone Formation. Transp. Res. Procedia 2016, 14, 2110–2119. [Google Scholar] [CrossRef]
- Bi, Y.; Pei, J.; Chen, Z.; Zhang, L.; Li, R.; Hu, D. Preparation and Characterization of Luminescent Road-Marking Paint. Int. J. Pavement Res. Technol. 2021, 14, 123–131. [Google Scholar] [CrossRef]
- Hadizadeh, E.; Pazokifard, S.; Mirabedini, S.M. Optimizing Practical Properties of MMA-Based Cold Plastic Road Marking Paints Using Mixture Experimental Design. Prog. Org. Coat. 2020, 140, 105381. [Google Scholar] [CrossRef]
- Burghardt, T.E.; Pashkevich, A. Emissions of Volatile Organic Compounds from Road Marking Paints. Atmos. Environ. 2018, 194, 246–255. [Google Scholar] [CrossRef]
- Martínez, A.H.; López-Montero, T.; Miró, R.; Puig, R. Photoluminescent Applications for Urban Pavements. Sustainability 2023, 15, 15078. [Google Scholar] [CrossRef]
- Poulose, A.M.; Anis, A.; Shaikh, H.; Alhamidi, A. Strontium Aluminate-Based Long Afterglow PP Composites: Phosphorescence, Thermal, and Mechanical Characteristics. Polymers 2021, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lu, Z.; Zou, Y.; Zhu, Y.; Yu, J. Preparation and Performance Characterization of an Active Luminous Coating for Asphalt Pavement Marking. Coatings 2023, 13, 1108. [Google Scholar] [CrossRef]
- Lin, H.; Chen, F.; Zhang, H. Active Luminous Road Markings: A Comprehensive Review of Technologies, Materials, and Challenges. Constr. Build. Mater. 2023, 363, 129811. [Google Scholar] [CrossRef]
- Wong, W. Development and Applications of Long Afterglow Luminescent Materials. 2006. Available online: https://theses.lib.polyu.edu.hk/handle/200/1465 (accessed on 31 May 2024).
- Wang, W.; Sha, A.; Lu, Z.; Yuan, D.; Jiang, W.; Liu, Z. Cement Filled with Phosphorescent Materials for Pavement: Afterglow Decay Mechanism and Properties. Constr. Build. Mater. 2021, 284, 122798. [Google Scholar] [CrossRef]
- Shan, B.; Yang, X.; Cao, X.; Deng, M.; Tang, B. Preparation of High-Luminescent Materials and Application of Luminescent Coatings in Road Engineering. J. Mater. Civ. Eng. 2022, 34, 04022159. [Google Scholar] [CrossRef]
- Wu, S.; Pan, Z.; Chen, R.; Liu, X. Long Afterglow Phosphorescent Materials; Springer Nature: Dordrecht, The Netherlands, 2017. [Google Scholar]
- Fu, W.; Chen, Y.; Liu, K.; Qiu, B.; Teng, H.; Xu, Y. Nanoscale Aluminate-Based Afterglow Materials from Byproducts of Aluminum–Water-Based Hydrogen Production for Anticounterfeiting and Latent Fingerprint. ACS Nano Mater. 2023, 6, 22673–22683. [Google Scholar] [CrossRef]
- Mufhidin, A.; Rahmanita, S.A.; Rizalludin, R.; Asiyah, T.; Laelasari, L. Glow in the Dark Median Frontage and Pedestrian as a Guide for Drivers at Night. J. PenSil 2024, 13, 110–119. [Google Scholar] [CrossRef]
- Lyu, L.; Chen, Y.; Yu, L.; Li, R.; Zhang, L.; Pei, J. The Improvement of Moisture Resistance and Organic Compatibility of SrAl2O4: Eu2+, Dy3+ Persistent Phosphors Coated with Silica–Polymer Hybrid. Materials 2020, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Kodaimati, M.S.; Yan, D. Recent Advances in Persistent Luminescence Based on Molecular Hybrid Materials. Chem. Soc. Rev. 2021, 50, 5564–5589. [Google Scholar] [CrossRef] [PubMed]
- Nance, J.; Sparks, T.D. From Streetlights to Phosphors: A Review on the Visibility of Roadway Markings. Prog. Org. Coat. 2020, 148, 105749. [Google Scholar] [CrossRef]
- Lin, Z.; Kabe, R.; Nishimura, N.; Jinnai, K. Organic Long-Persistent Luminescence from a Flexible and Transparent Doped Polymer. Adv. Mater. 2018, 30, e1803713. [Google Scholar] [PubMed]
- Ibarhiam, S.F.; Alzahrani, S.O.; Snari, R.M.; Aldawsari, A.M.; Alhasani, M.; Saad, F.; El-Metwaly, N.M. Novel Multifunctional Acrylic Paint from Sugarcane Bagasse: Photoluminescence, Photochromism, Hydropho-bicity and Anticorrosion. Mater. Today Commun. 2022, 33, 104703. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Z.; Li, X.; Xiao, F. Performance, Environmental Impact and Cost Analysis of Marking Materials in Pavement Engineering, The-State-of-Art. J. Clean. Prod. 2021, 294, 126302. [Google Scholar] [CrossRef]
- Bacero, R.; To, D.; Arista, J.P.; Cruz, M.K.D. Evaluation of Strontium Aluminate in Traffic Paint Pavement Markings for Rural and Unilluminated Roads. J. East. Asia Soc. Transp. Stud. 2015, 11, 1726. [Google Scholar]
- Nance, J.; Sparks, T.D. Comparison of coatings for SrAl2O4:Eu2+,Dy3+ Powder in Waterborne Road Striping Paint under Wet Conditions. Prog. Org. Coat. 2020, 144, 105637. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Al-Nami, S.Y.; Alkhamis, K.; Al-Ahmed, Z.A.; Binyaseen, A.M.; Khalifa, M.E.; El-Metwaly, N.M. Simple Preparation of Long-Persistent Luminescent Paint with Superhydrophobic Anticorrosion Efficiency from Cellulose Nanocrystals and an Acrylic Emulsion. Ceram. Int. 2022, 48, 6363–6371. [Google Scholar] [CrossRef]
- Petrukhina, N.N.; Bezrukov, N.P.; Antonov, S.V. Preparation and Use of Materials for Color Road Pavement and Marking. Russ. J. Appl. Chem. 2021, 94, 265–283. [Google Scholar] [CrossRef]
- Yang, X.; Fan, Z.; He, Y.; Cui, K.; Liao, Z.; Hong, B.; Wang, D. Mechanical Properties, Luminescent Properties, and Durability of Solvent-Free Polyurethane-Based Phospho-rescent Road Markings on Asphalt Pavements. Constr. Build. Mater. 2024, 414, 135053. [Google Scholar] [CrossRef]
- Fiolić, M.; Babić, D.; Gates, T. Road Markings and Their Impact on Driver Behaviour and Road Safety: A Systematic Review of Current Findings. J. Adv. Transp. 2020, 2020, 7843743. [Google Scholar]
- Shaw, J.W.; Chitturi, M.V.; Noyce, D.A. Special-Color Pavement Marking for Highway Work Zones: Literature Review of International Practices. Transp. Res. Rec. J. Transp. Res. Board 2017, 2617, 78–86. [Google Scholar] [CrossRef]
- Van der Heggen, D.; Joos, J.J.; Feng, A. Persistent Luminescence in Strontium Aluminate: A Roadmap to a Brighter Future. Adv. Funct. Mater. 2022, 32, 8809. [Google Scholar] [CrossRef]
- Eeckhout, K.V.D.; Smet, P.F.; Poelman, D. Persistent Luminescence in Eu2+-Doped Compounds: A Review. Materials 2010, 3, 2536–2566. [Google Scholar] [CrossRef]
- Aitasalo, T.; Hölsä, J.; Jungner, H.; Lastusaari, M.; Niittykoski, J. Mechanisms of Persistent Luminescence in Eu2+, RE3+ Doped Alkaline Earth Aluminates. J. Lumin. 2001, 94–95, 59–63. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yamazaki, M.; Kawazoe, H.; Hosono, H. Long Lasting Phosphorescence and Photostimulated Luminescence in Tb-Ion-Activated Reduced Calcium Aluminate Glasses. J. Appl. Phys. 1999, 86, 3729–3733. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, R.; Tian, X.; Li, Y.; Du, B.; Nie, J.; Li, Z.; Chen, L.; Ren, J.; Qiu, J.; et al. Bio-Imaging with Persistent Phosphors: Coordination Geometry-Dependent Multi-Band Emission and Atypically Deep-Trap-Dominated NIR Persistent Luminescence from Chromium-Doped Aluminates (Advanced Optical Materials 7/2018). Adv. Opt. Mater. 2018, 6, 1870029. [Google Scholar] [CrossRef]
- Ueda, J.; Miyano, S.; Tanabe, S. Formation of Deep Electron Traps by Yb3+ Codoping Leads to Super-Long Persistent Luminescence in Ce3+-Doped Yttrium Aluminum Gallium Garnet Phosphors. ACS Appl. Mater. Interfaces 2018, 10, 20652–20660. [Google Scholar] [CrossRef]
- Zeng, P.; Wei, X.; Yin, M.; Chen, Y. Investigation of the Long Afterglow Mechanism in SrAl2O4: Eu2+/Dy3+ by Optically Stimulated Luminescence and Thermoluminescence. J. Lumin. 2018, 199, 400–406. [Google Scholar] [CrossRef]
- Liepina, V.; Millers, D.; Smits, K. Tunneling Luminescence in Long Lasting Afterglow of SrAl2O4: Eu2+, Dy3+. J. Lumin. 2017, 185, 151–154. [Google Scholar] [CrossRef]
- Brito, H.F.; Hölsä, J.; Laamanen, T.; Lastusaari, M.; Malkamäki, M.; Rodrigues, L.C.V. Persistent Luminescence Mechanisms: Human Imagination at Work. Opt. Mater. Express 2012, 2, 371–381. [Google Scholar] [CrossRef]
- Dorenbos, P. Charge transfer bands in optical materials and related defect level location. Opt. Mater. 2017, 69, 8–22. [Google Scholar] [CrossRef]
- Hölsä, J.; Jungner, H.; Lastusaari, M. Persistent Luminescence of Eu2+ Doped Alkaline Earth Aluminates, MAl2O4: Eu2+. J. Alloys Compd. 2001, 323, 326–330. [Google Scholar] [CrossRef]
- You, H.; Zhang, J.; Hong, G.; Zhang, H. Luminescent Properties of Mn2+ in Hexagonal Aluminates under Ultraviolet and Vacuum Ultraviolet Excitation. J. Phys. Chem. 2007, 111, 10657–10661. [Google Scholar] [CrossRef]
- Dorenbos, P. Mechanism of Persistent Luminescence in Eu2+ and Dy3+ Codoped Aluminate and Silicate Compounds. J. Electrochem. Soc. 2005, 152, H107–H110. [Google Scholar] [CrossRef]
- Aitasalo, T.; Hölsä, J.; Jungner, H. Thermoluminescence Study of Persistent Luminescence Materials: Eu2+- and R3+-Doped Calcium Aluminates, CaAl2O4:Eu2+,R3+. J. Phys. Chem. B 2006, 110, 4589–4598. [Google Scholar] [CrossRef]
- Si, J.H.; Kitaoka, K.; Qiu, J.R. Optically Encoded Second-Harmonic Generation in Germanosilicate Glass by a Femtosecond Laser. Opt. Lett. 1999, 24, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Stevels, A.; van der Does De Bye, J. Models for energy transfer and their application to aluminate phosphors. J. Lumin. 1979, 18–19, 809–815. [Google Scholar] [CrossRef]
- Li, J.; Li, J.-G.; Liu, S.; Li, X.; Sun, X.; Sakka, Y. Greatly enhanced Dy3+ emission via efficient energy transfer in gadolinium aluminate garnet (Gd3Al5O12) stabilized with Lu3+. J. Mater. Chem. C 2013, 1, 7614–7622. [Google Scholar] [CrossRef]
- Kutuk, S.; Bolat, S. Analysis of structural, electrical, and levitation properties of (RE: Nd, Sm, Gd) BCO-358 superconducting ceramics prepared by a modified MPMG technique. J. Mater. Sci. 2022, 57, 1198–1214. [Google Scholar] [CrossRef]
- Kutuk, S.; Bolat, S. Levitation force of (RE)BCO-358 bulk superconductors. AIP Conf. Proc. 2018, 2042, 020033. [Google Scholar] [CrossRef]
- Qiu, J.; Kojima, K.; Miura, K.; Mitsuyu, T.; Hirao, K. Infrared Femtosecond Laser Pulse–Induced Permanent Reduction of Eu3+ to Eu2+ in a Fluorozirconate Glass. Opt. Lett. 1999, 24, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Aitasalo, T.; Dereń, P.; Hölsä, J.; Krupa, J.-C.; Lastusaari, M.; Legendziewicz, J.; Niittykoski, J.; Stręk, W. Persistent Luminescence Phenomena in Materials Doped with Rare Earth Ions. J. Solid State Chem. 2003, 171, 114–122. [Google Scholar] [CrossRef]
- Clabau, F.; Rocquefelte, X.; Jobic, S.; Deniard, P.; Whangbo, M.-H.; Garcia, A.; Le Mercier, T. Mechanism of Phosphorescence Appropriate for the Long-Lasting Phosphors Eu2+-Doped SrAl2O4 with Codopants Dy3+ and B3+. Chem. Mater. 2005, 17, 3904–3912. [Google Scholar] [CrossRef]
- Arora, A.; Pandey, A. Probing the dominance of interstitial oxygen defects in ZnO. J. Alloys Compd. 2014, 587, 98–105. [Google Scholar]
- Li, X.; Zhou, Z.; Wang, Y.; Zhang, T.; Liu, J. Defects enhanced photoluminescence of Mn2+-doped ZrP2O7 blue. J. Alloys Compd. 2019, 798, 471–478. [Google Scholar]
- Zhao, L.; Chen, H. Oxygen vacancy-dependent humidity sensing performance induced. Mater. Today Chem. 2024, 23, 100581. [Google Scholar]
- Zhou, J.H.; Long, Z.W.; Wang, Q. Role of Oxygen Vacancies in Long Persistent Phosphor Ca2Ga2GeO7:Zn2+. J. Am. Ceram. Soc. 2018, 101, 2695–2700. [Google Scholar] [CrossRef]
- Khalid, A.H.; Kontis, K. Thermographic Phosphors for High Temperature Measurements: Principles, Current State of the Art and Recent Applications. Sensors 2008, 8, 5673–5744. [Google Scholar] [CrossRef]
- Wang, L.; Shang, Z.; Shi, M.; Cao, P.; Yang, B.; Zou, J. Preparing and Testing the Reliability of Long-Afterglow SrAl2O4: Eu2+, Dy3+ Phosphor Flexible Films for Temperature Sensing. RSC Adv. 2020, 10, 11418–11425. [Google Scholar] [CrossRef]
- Huang, A.; Wu, Y.; Pan, Z.; Wang, B.; Liang, X. Fabrication, characterization, and optimization of the composite long afterglow material Sr2MgSi2O7:Eu2+, Dy3+@ SrAl2O4:Eu2+, Dy3+. J. Sol. Gel. Sci. Technol. 2023, 105, 500–510. [Google Scholar] [CrossRef]
- Kutuk, S.; Bolat, S.; Terzioglu, C.; Altintas, S.P. An investigation of magnetoresistivity properties of an Y3Ba5Cu8Oy bulk superconductor. J. Alloys Compd. 2015, 650, 159–164. [Google Scholar] [CrossRef]
- Shi, M.; Lu, B.; Jin, Y.; Ge, M. Surface organic modification of SrAl2O4: Eu2+, Dy3+ via coupling agents to enhance hydrolysis resistance. J. Mater. Sci. Mater. Electron. 2021, 32, 20804–20816. [Google Scholar] [CrossRef]
- Du, J.; Feng, A.; Poelman, D. Temperature Dependency of Trap-Controlled Persistent Luminescence. Laser Photonics Rev. 2020, 14, 60. [Google Scholar] [CrossRef]
- Chiatti, C.; Fabiani, C.; Pisello, A.L. Long Persistent Luminescence: A Road Map Toward Promising Future Developments in Energy and Environmental Science. Annu. Rev. Mater. Res. 2021, 51, 409–433. [Google Scholar] [CrossRef]
- Jain, A.; Kumar, A.; Dhoble, S.J.; Peshwe, D.R. Persistent Luminescence: An Insight. Renew. Sustain. Energy Rev. 2016, 65, 135–153. [Google Scholar] [CrossRef]
- Leimane, M.; Krizmane, K.; Bite, I.; Grube, J.; Vitola, V. Sol–Gel Synthesis of Translucent and Persistent Luminescent SiO2@ SrAl2O4: Eu2+, Dy3+ B Materials. Materials 2023, 16, 4416. [Google Scholar] [CrossRef]
- Ju, Z.-H.; Zhang, S.-H.; Gao, X.-P.; Tang, X.-L.; Liu, W.-S. Reddish Orange Long Afterglow Phosphor Ca2SnO4:Sm3+ Prepared by Sol–Gel Method. J. Alloys Compd. 2011, 509, 8082–8087. [Google Scholar] [CrossRef]
- Jiang, L.; Chang, C.; Mao, D.; Zhang, B. A New Long Persistent Blue-Emitting Sr2ZnSi2O7:Eu2+, Dy3+ Prepared by Sol–Gel Method. Mater. Lett. 2004, 58, 1825–1829. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.K.; Kim, Y.J. Low Temperature Synthesis of Lu3Al5-xGaxO12:Ce3+,Cr3+ Powders Using a Sol-Gel Combustion Process and Its Persistent Luminescence Properties. Opt. Mater. 2020, 104, 109944. [Google Scholar] [CrossRef]
- Yang, Y. SrAl2O4: Eu2+, Dy3+ Preparation and Research of Long Afterglow Luminescent Powder and Long Afterglow Luminescent Glass; Wuhan University of Technology: Wuhan, China, 2023. (In Chinese) [Google Scholar]
- Hang, T.; Liu, Q.; Mao, D.; Chang, C. Long Lasting Behavior of Gd2O2S:Eu3+ Phosphor Synthesized by Hydrothermal Routine. Mater. Chem. Phys. 2008, 107, 142–147. [Google Scholar] [CrossRef]
- Puja, K.; Amba, M.; Anil, C.K.; Ambast, D.K.S. Structural and Optical Properties of Chromium Doped Zinc Gallate Long Persistent Phosphor Prepared by Surfactant Assisted Hydrothermal Method. J. Phys. Conf. Ser. 2021, 1913, 012039. [Google Scholar]
- Liu, H.; Hu, X.; Wang, J.; Liu, M.; Wei, W.; Yuan, Q. Direct Low-Temperature Synthesis of Ultralong Persistent Luminescence Nanobelts Based on a Biphasic Solution-Chemical Reaction. Chin. Chem. Lett. 2018, 29, 1641–1644. [Google Scholar] [CrossRef]
- Bonturim, E.; Merízio, G.L.; Reis, D.R.; Brito, H.F.; Rodrigues, L.C.V.; da Cunha Felinto, M.C.F. Persistent Luminescence of Inorganic Nanophosphors Prepared by Wet-Chemical Synthesis. J. Alloys Compd. 2018, 732, 705–715. [Google Scholar] [CrossRef]
- Cailing, J.; Jie, T.; Quan, Y. Defect Luminescence Based Persistent Phosphors—From Controlled Synthesis to Bioapplications. Chin. J. Chem. 2021, 39, 3188–3198. [Google Scholar]
- Mao, S.; Liu, Q.; Gu, M.; Mao, D.; Chang, C. Long Lasting Phosphorescence of Gd2O2S:Eu,Ti,Mg Nanorods via a Hydrothermal Routine. J. Alloys Compd. 2008, 465, 367–374. [Google Scholar] [CrossRef]
- Huang, P.; Liu, D.; Cui, C.E.; Wang, L.; Jiang, G. Synthesis and Luminescence Properties of Red Long-Lasting Phosphor Y2O2S:Eu3+, Zn2+, Ti4+ Nanotubes via Hydrothermal Method. Appl. Phys. A 2014, 116, 759–765. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, Q.; Li, Y.; Liu, Z.; Xu, J.; Zeng, H.; Wang, H. Hydrothermal Synthesis of Bi4Ge3O12: Eu3+ Phosphors with High Thermal Stability and Enhanced Photoluminescence Property. J. Alloys Compd. 2017, 693, 308–314. [Google Scholar] [CrossRef]
- Rojas-Hernandez, E.R.; Rubio-Marcos, F.; Rodriguez, Á.M.; Fernandez, J.F. Long Lasting Phosphors: SrAl2O4:Eu, Dy as the Most Studied Material. Renew. Sustain. Energy Rev. 2018, 81, 2759–2770. [Google Scholar] [CrossRef]
- Duan, C.J.; Wang, L.; Wu, X. (M,Ca)AlSiN3:Eu2+ (M=Sr, Mg) long persistent phosphors prepared by combustion synthesis and applications in displays and optical information storage. J. Lumin. 2022, 252, 119288. [Google Scholar]
- Pan, Y.X.; Dong, Y.; Tang, Z.T.; Ma, Y.X.; Zhang, J.; Zhang, X.D. Synthesis of CaAl2O4: Eu, Nd Long Persistent Phosphor by Combustion Processes and Its Optical Properties. Mater. Lett. 2007, 61, 3917–3920. [Google Scholar]
- Zhang, X.D.; Guo, C.F.; Liu, Y.F.; Ma, J.P. Synthesis of Nanocrystals of Long Persisting Phosphor by Modified Combustion Technique. J. Cryst. Growth 2004, 260, 523–529. [Google Scholar]
- Yao, W.; Zhang, J.; Wang, Y. Synthesis of Sr4Al14O25: Eu2+, Dy3+ Phosphor Nanometer Powders by Combustion Processes and Its Optical Properties. Mater. Sci. Eng. B 2006, 133, 48–54. [Google Scholar]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4: Eu2+, Dy3+. Acta Mater. 2007, 49, 689–692. [Google Scholar]
- Yerpude, A.N.; Dhoble, S.J. Combustion synthesis of rare earth activated and co-activated SrAl4O7 green long lasting phosphors. Optik 2016, 127, 4211–4216. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Wang, H.; Liu, Y. Synthesis of Yellow Persistent Phosphor Garnet by Mixed Fuel Solution Combustion Synthesis and Its Characteristic. J. Phys. Chem. Solids 2020, 141, 109408. [Google Scholar]
- Kwon, S.B.; Yoo, J.H.; Choi, S.H. Combustion Synthesis of Blue-Emitting Submicron CaAl4O7:Eu2+, Dy3+ Persistence Phosphor. Luminescence 2012, 27, 237–242. [Google Scholar]
- Li, Y.; Liu, H.; Dong, Y. Long Persistent Phosphors—From Fundamentals to Applications. Chem. Soc. Rev. 2016, 45, 2090–2136. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Z.; Zhao, Y.; Sun, S. Enhanced Luminescent Properties of Long-Persistent Sr2MgSi2O7: Eu2+, Dy3+ Phosphor Prepared by the Co-Precipitation Method. J. Lumin. 2008, 128, 157–164. [Google Scholar]
- Matsuzawa, T.; Murayama, Y.; Takeuchi, N. Luminescence of Long-Lasting CaAl2O4: Eu2+, Nd3+ Phosphor by Co-Precipitation Method. Mater. Chem. Phys. 2006, 99, 479–485. [Google Scholar]
- Chen, J.; Xu, H.; Wang, L.; Wu, X. Photoelectric Properties of Sr2MgSi2O7: Eu2+ Phosphors Produced by Co-Precipitation Method. J. Lumin. 2021, 233, 117810. [Google Scholar]
- Boiko, V.; Consentino, L.; Saladino, M.L.; Hreniak, D. Effect of Ce3+ Concentration on Persistent Luminescence of YAGG: Ce3+, Cr3+, Nd3+ Nanophosphors Obtained by the Co-Precipitation Method. Opt. Mater. 2020, 107, 109956. [Google Scholar] [CrossRef]
- Lu, H.; Chen, Z.; Yang, L. Influence of Calcination Temperature on the Microstructure and Photoluminescence of SrAl2O4: Eu2+, Dy3+ Phosphors. Materials 2019, 12, 1234. [Google Scholar]
- Kim, J.; Lee, S.; Park, H. High-Energy Milling Effects on the Structure and Luminescence of SrAl2O4: Eu2+, Dy3+ Phosphors. J. Lumin. 2018, 202, 335–342. [Google Scholar]
- Jiang, L.; Zhang, H.; Liu, Y. Preparation and Luminescent Properties of SrAl2O4: Eu2+, Dy3+ Phosphors by Spray Drying. J. Alloys Compd. 2021, 852, 156834. [Google Scholar]
- Zhang, T.; Li, X.; Wang, J. Effect of Sintering Temperature on the Luminescence Properties and Microstructure of SrAl2O4: Eu2+, Dy3+ Phosphors. Materials 2020, 13, 5678. [Google Scholar]
- Wu, Y.; Sikandar, M. Enhanced Water Resistance and Luminescent Properties of SrAl2O4: Eu2+, Dy3+ Phosphors via Silica Coating. Mater. Sci. Eng. B 2020, 260, 114620. [Google Scholar]
- Wu, Y.; Gan, J.; Wu, X. Study on the Silica-Polymer Hybrid Coated SrAl2O4: Eu2+, Dy3+ Phosphor as a Photoluminescence Pigment in a Waterborne UV Acrylic Coating. J. Mater. Res. Technol. 2021, 13, 1230–1242. [Google Scholar] [CrossRef]
- Sikandar, M.A.; Ahmad, W.; Khan, M.H.; Ali, F.; Waseem, M. Effect of Water Resistant SiO2 Coated SrAl2O4: Eu2+ Dy3+ Persistent Luminescence Phosphor on the Properties of Portland Cement Pastes. Constr. Build. Mater. 2019, 228, 116823. [Google Scholar] [CrossRef]
- Lü, X.; Zhong, M.; Shu, W.; Yu, Q.; Xiong, X.; Wang, R. Alumina Encapsulated SrAl2O4: Eu2+, Dy3+ Phosphors. Powder Technol. 2007, 177, 96206348. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, N.; Lu, X.; Ten Kate, M.; Valdesueiro, D. Performance Improvement by Alumina Coatings on Y3Al5O12: Ce3+ Phosphor Powder Deposited Using Atomic Layer Deposition in a Fluidized Bed Reactor. RSC Adv. 2016, 6, 76454–76462. [Google Scholar] [CrossRef]
- Chen, L.; Lin, C.-C.; Yeh, C.-W.; Liu, R.-S. Light Converting Inorganic Phosphors for White Light-Emitting Diodes. Materials 2010, 3, 2172–2195. [Google Scholar] [CrossRef]
- Wang, W.; Sha, A.; Li, X.; Zhang, F.; Jiang, W.; Yuan, D.; Liu, Z. Water Resistance and Luminescent Thermal Stability of SiO2 Coated Phosphor and Self-Luminous Cement-Based Materials: View from the Perspective of Hydration Balance. Constr. Build. Mater. 2021, 319, 126086. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, J.; Li, W.; Xu, L.; Guan, Q.; Liu, Y. Encapsulation of strontium aluminate phosphors to enhance water resistance and luminescence. Appl. Surf. Sci. 2009, 255, 7580–7585. [Google Scholar] [CrossRef]
- Anesh, M.P.; Gulrez, S.K.H.; Anis, A. Developments in Eu2+-Doped Strontium Aluminate and Polymer/Strontium Aluminate Composite. Adv. Polym. Technol. 2014, 33 (Suppl. S1), 21436. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, B.; Ren, B.; Tu, D.; Wang, Z. Smart Mechanoluminescent Phosphors: A Review of Strontium-Aluminate-Based Materials, Properties, and Their Advanced Application Technologies. Adv. Mater. 2023, 10, 2204925. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Lin, C.C.; Liu, R.S. Waterproof Alkyl Phosphate Coated Fluoride Phosphors for Optoelectronic Materials. Angew. Chem. 2015, 54, 10862–10866. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, D.A. Development of Color-Tunable Photoluminescent Polycarbonate Smart Window Immobilized with Silica-Coated lanthanide-Activated Strontium Aluminum Oxide Nanoparticles. Inorg. Chem. Commun. 2023, 150, 110473. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, Z.; Yan, D. Tuning Organic Room-Temperature Phosphorescence through the Confinement Effect of Inorganic Micro/Nanostructures. Small Struct. 2021, 2, 44. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Zhou, J.; Jin, K.; Fang, Q. Fluorinated and Thermo-Cross-Linked Polyhedral Oligomeric Silsesquioxanes: New Organic–Inorganic Hybrid Materials for High-Performance Dielectric Application. ACS Appl. Mater. Interfaces 2017, 9, 12782–12790. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; He, B.; Xiao, M.; Fang, Z.; Dai, K. Study on Properties and Mechanisms of Luminescent Cement-Based Pavement Materials with Super-Hydrophobic Function. Constr. Build. Mater. 2018, 165, 548–559. [Google Scholar] [CrossRef]
- Lin, Z.; Kabe, R.; Wang, K.; Adachi, C. Influence of Energy Gap between Charge-Transfer and Locally Excited States on Organic Long Persistence Luminescence. Nat. Commun. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Chiatti, C.; Fabiani, C.; Bondi, R.; Zampini, G.; Latterini, L.; Pisello, A.L. Controlled Combination of Phosphorescent and Fluorescent Materials to Exploit Energy-Saving Potential in the Built Environment. Energy 2023, 275, 127333. [Google Scholar] [CrossRef]
- Botterman, J.; Smet, P.F. Persistent Phosphor SrAl2O4:Eu,Dy in Outdoor Conditions: Saved by the Trap Distribution. Opt. Express 2015, 23, A868–A881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, X.; Zhang, Z.; Liu, Y. Mechanistic insights into the luminescence of aluminate long afterglow phosphors. J. Lumin. 2019, 206, 447–456. [Google Scholar]
- Liu, X.; Wang, J.; Chen, G. Stability and environmental impact of rare earth-doped aluminate phosphors. Mater. Sci. Eng. B 2020, 261, 114696. [Google Scholar]
- Wang, S.; Li, H.; Huang, X. Scalable synthesis of nanostructured aluminate phosphors for enhanced luminescence. Adv. Mater. 2018, 30, 1705913. [Google Scholar]
- Kim, D. Recent Developments in Lanthanide-Doped Alkaline Earth Aluminate Phosphors with Enhanced and Long-Persistent Luminescence. Nanomaterials 2021, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, B.; Qu, S.; Zheng, Y.; Li, X.; Zhu, M.; Luo, J.; Wang, C.; Wang, D.; Ren, Z.; et al. Tuning Mechanoluminescent Long-Afterglow Composites Toward Mechanical Energy Lighting. J. Mater. Sci. 2022, 57, 21378–21391. [Google Scholar] [CrossRef]
- Guo, R.; Liu, S. Design and Experiment of Self-Luminescent Asphalt-Based Pavement Materials. Constr. Build. Mater. 2022, 342, 127991. [Google Scholar] [CrossRef]
- Hölsä, J. Persistent Luminescence Beats the Afterglow: 400 Years of Persistent Luminescence. Electrochem. Soc. Interface 2009, 18, 42–45. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Kim, H. Durability of aluminate long afterglow materials under various climatic conditions. Constr. Build. Mater. 2017, 150, 598–604. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Pham, H.V. Performance of sunlight-activated long afterglow coatings in low sunlight regions. Sol. Energy 2021, 216, 560–570. [Google Scholar]







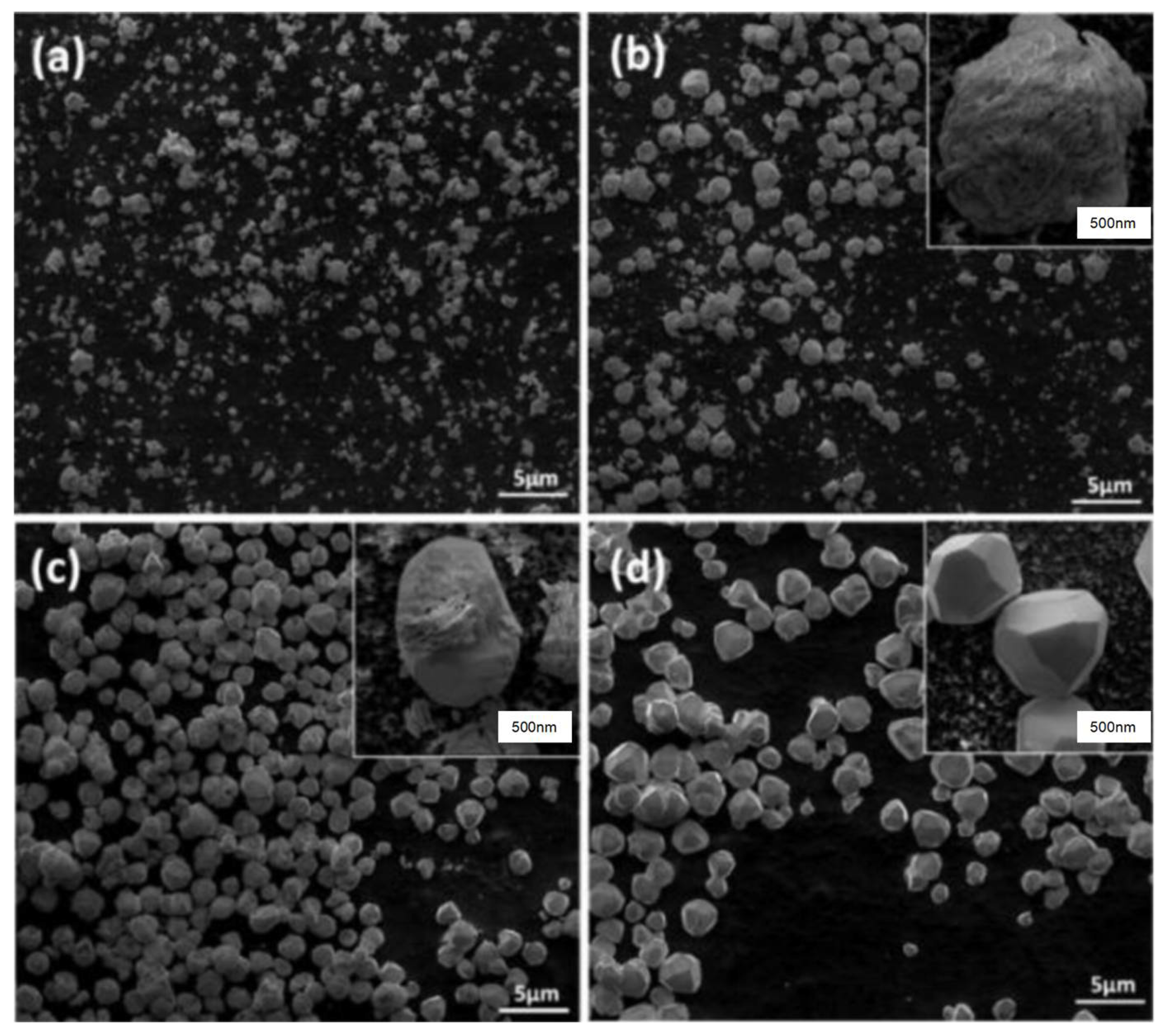
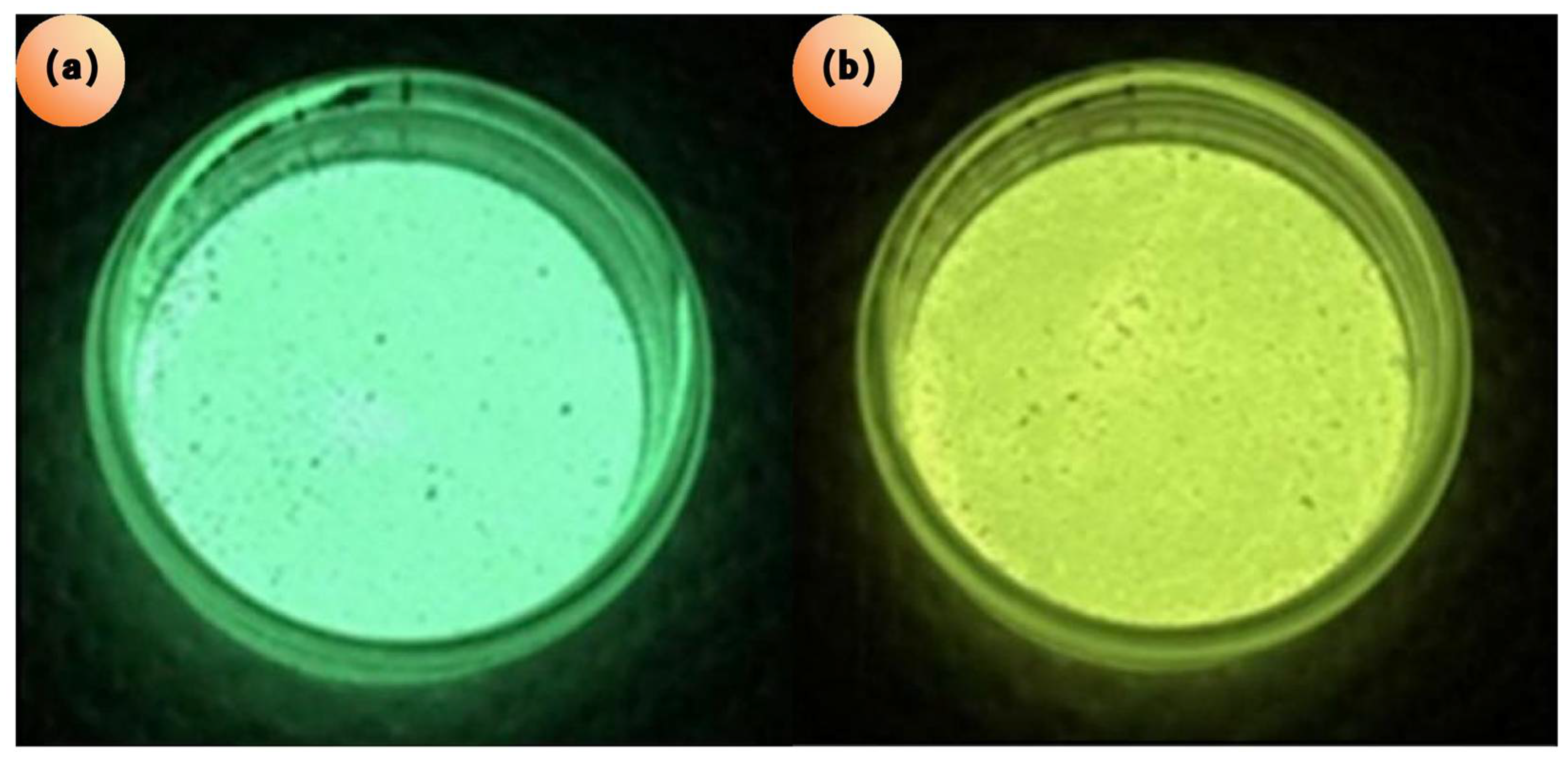


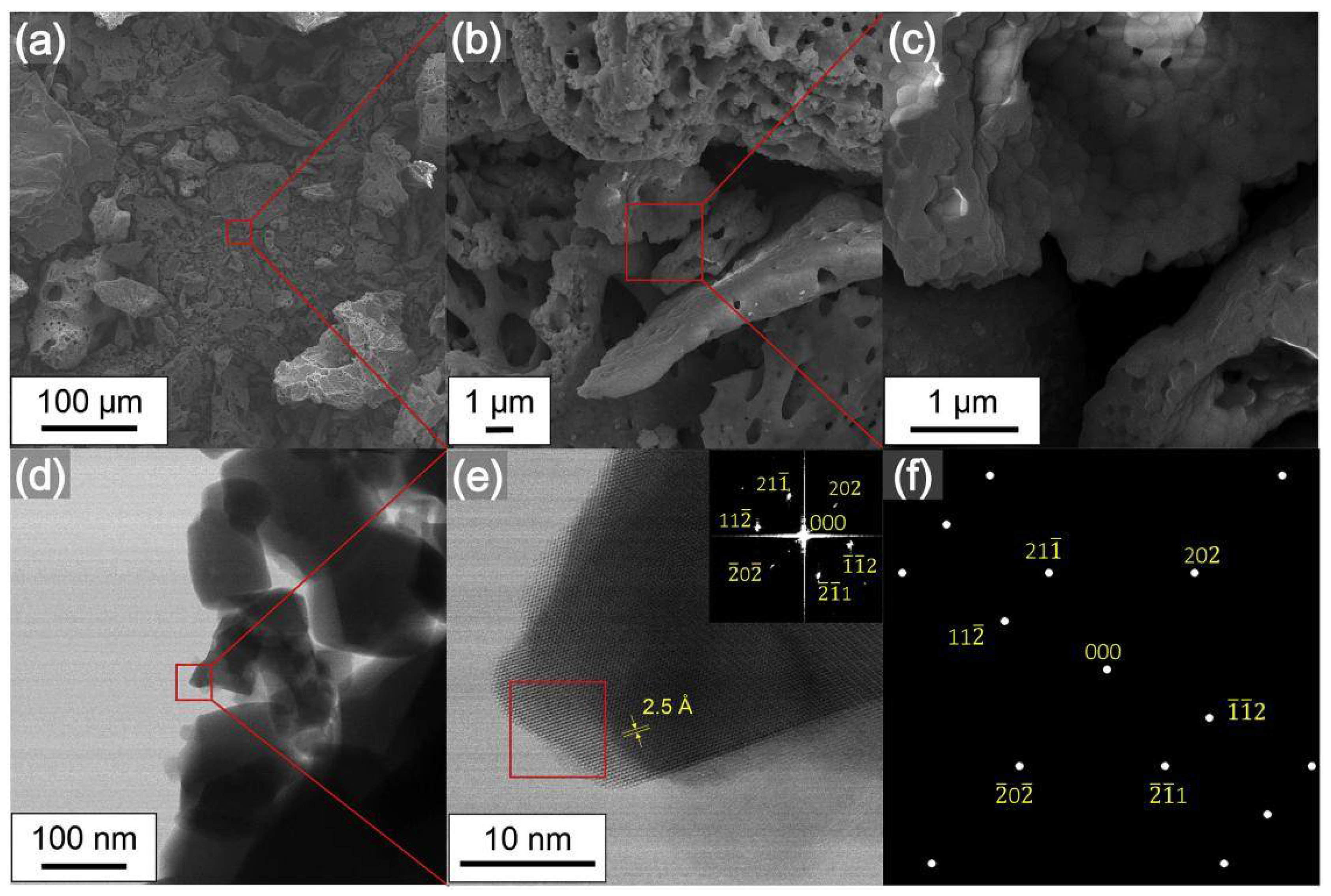

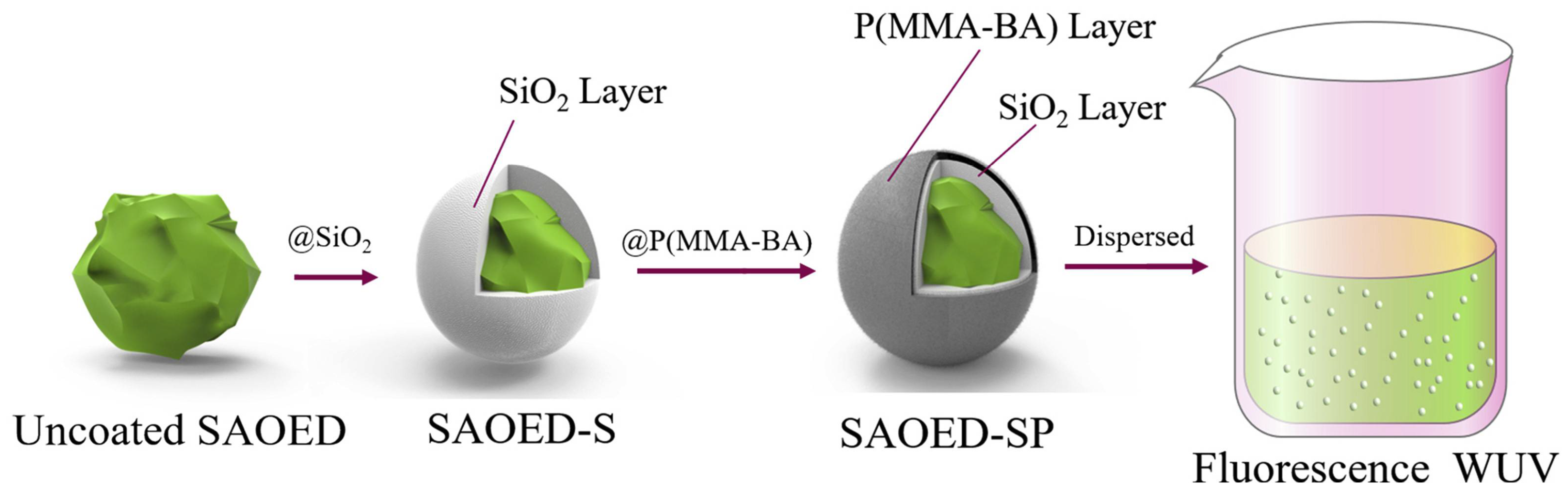



| Type | Main Characteristics | Advantages | Disadvantages | Durability | Construction Speed |
|---|---|---|---|---|---|
| Thermoplastic paint | Thermoplastic material needs heating to the liquid state | Strong adhesion, wear-resistant | The complex application requires specialized heating equipment | Very high | Slow |
| Water-based paint | Uses water as a diluent, eco-friendly | Safe, eco-friendly, non-toxic, easy to clean | Relatively poor weather resistance | Moderate | Fast |
| Two-component paint | Made from resin and hardener, cures chemically | Extremely strong adhesion, resistant to chemicals and mechanical wear | High application requirements, needs precise mixing | Very high | Moderate |
| Solvent-based paint | Uses organic solvents as a base, fast evaporation | Quick drying, easy to apply | Solvent evaporation may be harmful to the environment and health, flammable | High | Fast |
| Model | Mechanism Description | Process Details | Key Points |
|---|---|---|---|
| Electron Trap Model | Electrons are captured and released at defect or impurity sites within the material. Excitation by light moves electrons from the valence band to the conduction band, and some electrons are trapped by internal traps. | 1. Excitation by light 2. Electrons move to the conduction band 3. Electrons trapped by internal traps 4. Gradual release of electrons emits photons | 1. Depth and distribution of electron traps affect performance 2. Deeper traps extend luminescence duration 3. Co-doping forms deeper traps |
| Hole Transfer Model | Holes migrate through defect states to the electron capture center, where they complex with electrons to emit photons. | 1. Holes migrate through defect states 2. Holes complex with electrons at capture center 3. Photon emission occurs | 1. Optical properties influenced by material composition and processing conditions 2. Hole migration pathway optimized by doping level and thermal treatment 3. Enhanced optical properties for various applications |
| Energy Transfer Model | Energy is transferred non-radiatively from excited ions to surrounding activated ions. This enhances luminescence intensity and duration. | 1. Excited state energy transfer from Eu2+ to rare earth ions 2. Enhances luminescence performance through inter-ionic energy transfer | 1. Efficiency and path of energy transfer impact performance 2. Optimization involves selecting and concentrating dopant ions 3. Improves luminescence by fine-tuning dopant ions |
| Oxygen Vacancy Model | Oxygen vacancies act as significant electron traps. They capture electrons, which can later escape by absorbing energy at room temperature, returning to the excited state, and emitting photons. | 1. Oxygen vacancies capture electrons 2. Electrons escape by absorbing energy 3. Return to excited state and emit photons | 1. Proximity of vacancies to luminescent centers is crucial 2. Depth of defect levels affects electron escape and duration 3. Shallow or deep levels are not conducive to forming a long afterglow |
| Preparation Method | Characteristics | Advantages | Disadvantages | Typical Application Scenarios |
|---|---|---|---|---|
| High-Temperature Solid-State Reaction | Material synthesis by high-temperature heat treatment of precursors | Achieves good crystallinity at high temperatures | Requires high energy input, high equipment cost | Used for producing long afterglow luminescent materials |
| Sol–Gel Method | Material preparation at low temperatures through a chemical solution | Precise control of chemical composition and structure | Numerous intermediate stages, difficult to control | Used for preparing optoelectronic materials and coatings |
| Hydrothermal Synthesis | Material synthesis in a closed, high-pressure environment | Controllable particle size and shape, suitable for nanomaterial production | High equipment requirements, difficult to scale up | Used for producing nanoluminescent materials |
| Combustion Synthesis | Rapid material synthesis utilizing the exothermic nature of chemical reactions | Fast synthesis speed, relatively low energy consumption | May produce incompletely reacted materials, difficult to control | Rapid preparation of various luminescent materials |
| Chemical Co-precipitation Method | Material precipitation in solution by altering conditions | Can be conducted at room temperature, suitable for large-scale production | Requires precise control of reaction conditions, product purity may be low | Preparation of high-performance phosphorescent materials |
| Characteristic | Inorganic Coating | Organic Coating | Organic–Inorganic Composite Coating |
|---|---|---|---|
| Main Composition | Inorganic materials like SiO2 and Al2O3 | Organic polymers such as silicone, alkyl, and phosphate | Combination of organic polymers and inorganic materials |
| Protection | High resistance to environmental factors; protective layer against photobleaching | Improved water and organic solvent resistance | Enhanced mechanical strength, chemical stability, and optical properties |
| Optical Performance | May decrease luminescence due to refractive index mismatch | Potentially reduced luminescence efficiency due to light scattering and absorption | Maintains luminescence while enhancing room-temperature phosphorescence |
| Thermal Stability | Improved service life under high temperatures | Vulnerable to high temperatures, leading to degradation | Improved thermal stability |
| Water Resistance | Excellent optical stability and water resistance | Significantly improved water resistance | Superior water resistance combined with organic compatibility in aqueous environments |
| Chemical Stability | Good, but interaction with binders like cement can be problematic | Long-term chemical stability is a major challenge | Excellent chemical stability and environmental tolerance |
| Application Suitability | Suitable for harsh conditions but may affect luminescence efficiency | Suitable for various application scenarios, but may affect the light output | Broad application potential, including smart windows and security markings due to mechanoluminescent properties |
| Process Complexity | Process may affect luminescence efficiency | Simpler process compared to composites, but less stable | Complex preparation process requiring precise control over experimental conditions |
| Interfacial Compatibility | Issues with binder compatibility can arise | May introduce negative impacts due to cladding layer | Can introduce interfacial incompatibility problems leading to unstable material properties or reduced reliability |
| Long-term Reliability | Good, depending on the uniformity and thickness of the coating | Challenged by environmental factors like humidity and temperature | Higher long-term reliability through stable composite structure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Xie, Y.; Zhao, X.; He, Y.; Pei, J.; Xing, Y.; Wang, S.; Zhang, J. Aluminate Long Afterglow Luminescent Materials in Road Marking Field Research Progress and Development: A Review. Buildings 2024, 14, 2152. https://doi.org/10.3390/buildings14072152
Zhang F, Xie Y, Zhao X, He Y, Pei J, Xing Y, Wang S, Zhang J. Aluminate Long Afterglow Luminescent Materials in Road Marking Field Research Progress and Development: A Review. Buildings. 2024; 14(7):2152. https://doi.org/10.3390/buildings14072152
Chicago/Turabian StyleZhang, Fangzhi, Yue Xie, Xiaokang Zhao, Yinzhang He, Jianzhong Pei, Yuanhe Xing, Shaobo Wang, and Jiupeng Zhang. 2024. "Aluminate Long Afterglow Luminescent Materials in Road Marking Field Research Progress and Development: A Review" Buildings 14, no. 7: 2152. https://doi.org/10.3390/buildings14072152






