Abstract
In order to study the effects of soda residue content, particle size, moisture content, and curing age on the unconfined compressive strength (UCS) of soda residue cement lime soil (SRCLS), a 4-factor, 4-level orthogonal experimental design was employed in this study. Different conditions of SRCLS UCS and their impacts were tested and analyzed. The internal microstructure and hydration products of SRCLS were studied using SEM and XRD to explore the strengthening mechanism of SR in SRCLS. The results indicate that as the soda residue content gradually increased, SRCLS UCS initially increased and then decreased, with a maximum increase of up to 67%. With increasing soda residue particle size and moisture content, the UCS of SRCLS gradually decreased. The optimized mix ratio was determined to be soda residue:cement:lime:soil = 3%:3%:6%:100%, with the soda residue dried naturally and an ideal particle size of 0.15 mm. The factors influencing the unconfined compressive strength (UCS) of SRCLS, in order of importance, are curing age, soda residue content, moisture content, and particle size of SR. Among these, curing age and soda residue content have a significant impact on the UCS. An adequate amount of SR can act as a fine aggregate filler, replace lime, promote cement hydration, and enhance chloride ion binding. This improves the grading of SRCLS materials and facilitates the formation of cementitious products from AFm, AFt, and Friedel’s salt, resulting in denser and stronger SRCLS materials. The research findings provide a reference for the mix design of SRCLS and the large-scale utilization of waste soda residue.
1. Introduction
More than 60% of China’s pure soda is produced by the ammonia-soda method, generating a considerable amount of solid waste soda residue during the alkali production process [1]. According to statistics, approximately 10 m3 of wastewater and 300–600 kg of solid soda residue are discharged for every ton of pure soda produced [2]. With over 50 alkali production enterprises nationwide, the annual discharge of SR exceeds 10 million tons [3]. The conventional treatment method for solid waste soda residue involves direct surface discharge and accumulation. This practice allows harmful components to seep into the ground through rainfall, consequently contaminating soil and groundwater, causing soil salinization [4] and polluting the ecological environment. Scholars both domestically and internationally have explored various methods for treating soda residue and utilizing it as a resource. These methods include the preparation of adsorbents [5], cementitious materials [6,7], fired bricks [8], the improvement of acidic soil [9,10], and engineering fillers [11]. These efforts have yielded numerous beneficial results. However, due to the low strength and high Cl- content of soda residue [12,13], engineering projects are prone to alkali efflorescence reactions and steel corrosion, making it challenging to simultaneously address the requirements of load-bearing capacity, environmental protection, and large-scale disposal in soda residue utilization projects. Currently, the comprehensive utilization rate of soda residue is less than 15%, significantly falling short of the national target, which aims for a solid waste comprehensive utilization rate of at least 55% [14,15].
Lime and cement are commonly used soil stabilizers in subgrade and pavement engineering [16], capable of enhancing the strength of engineered soil mixes, facilitating rapid soil consolidation, and improving road-use performance. With an annual increase in highway mileage exceeding 150,000 km in China, the demand for cement and lime raw materials is substantial [17]. However, cement and lime production are characterized by high energy consumption and severe pollution. Statistics reveal that producing 1 ton of cement requires 1.3 tons of limestone, 0.3 tons of clay, and emits 0.825 tons of CO2. In contrast, producing 1 ton of quicklime consumes 1.7 tons of limestone, 0.4 tons of coal [18,19], and emits 1.5 tons of CO2 [20]. With the increasing focus on carbon emissions, some regions have begun imposing explicit restrictions on the production of lime and cement [21]. Research has demonstrated that soda residue contains certain mineral components such as CaCO3 and Ca(OH)2 [22], which may induce cement hydration and exert a binding effect between soil particles, thus potentially substituting for some lime and cement in stabilizing subgrade soil [23]. Subgrade, or the base layer of roads, strength requirements are relatively low, with no steel reinforcement, thereby eliminating potential corrosion risks. Consequently, the road sector has become a key focus area for the reuse of solid waste soda residue due to its substantial potential for waste absorption [24]. Zhang [25] formulated soda residue, cement, and soft soil in specific proportions to create SRS. Through experimentation, it was demonstrated that the optimal SRS unconfined compressive strength (UCS) was achieved with a ratio of 30% soda residue, 12% cement, and 70% soil. Guo showed that an appropriate amount of soda residue and lime could enhance the strength and water stability of shield tunneling soil, with the prepared soda residue-modified shield tunneling soil serving as an excellent roadbed material [26]. Li et al. [27] utilized soda residue to partially replace lime in preparing SRS and investigated the strength mechanism of SRS, confirming that a soda residue content of 3% led to a significant increase of 34.6% in the SRS 7 day UCS. Xu Jingkuo [28] optimized the mix ratio of SRCLS through experiments and found that the highest strength was achieved with a ratio of 0% soda residue, 2% cement, 3% lime, and 65% soil. This optimized mix was successfully applied in the construction of roadbed filling projects on Central Avenue in the Binhai New Area of Tianjin.
In summary, numerous scholars have conducted extensive research on the preparation of soda residue composite soil for road use through combining soda residue with cement and lime. However, most of the research outcomes have focused on the impact of soda residue content and its influence on material strength, as well as mix design. Surveys indicate that soda residue from the ammonia-soda process has a moisture content exceeding 90% when it leaves the factory, resulting in a viscous state that cannot be directly added and mixed [29]. Therefore, preprocessing of the material particle size and moisture content is required. The moisture content and particle size of soda residue are crucial parameters that must be addressed in the engineering design and application of SRCLS. Currently, there is a lack of research on the design of soda residue particle size and moisture content as well as their impact on material strength.
Consideration of the soda residue content, moisture content, particle size, and curing period may affect the mechanical properties of SRCLS specimens, compaction, adequate reflection between materials, and cementitious material formation. To systematically analyze the influence characteristics of soda residue content, particle size, moisture content, and curing age on the strength of SRCLS, this paper designed an orthogonal experimental scheme, analyzed the impact of soda residue content, particle size, moisture content, and curing age factors on the strength of SRCLS, and optimized the material mix ratio. Additionally, using SEM electron microscopy scanning and XRD experiments, we analyzed the microstructure of SRCLS and its hydration products, exploring the mechanism of soda residue enhancing the strength of SRCLS. This study aims to provide references for the large-scale utilization and engineering design of solid waste soda residue in the field of road construction.
2. Materials and Methods
2.1. Materials
The composition of SRCLS material includes soda residue, lime, cement, soil, and water. The main materials are shown in Figure 1.

Figure 1.
Main composition of SRCLS: (a) soda residue; (b) lime; (c) cement; (d) soil.
2.1.1. Soda Residue
The experimental SR was obtained from a Huai’an alkali factory. Freshly produced SR is a grayish-white sticky paste with a pungent odor and strong corrosiveness. The moisture content of the fresh SR was as high as 105.8%, with a pH value of 9.2, indicating alkalinity. The moisture content of the air-dried SR was 31.45%, with an UCS of only 0.20 MPa, indicating low strength. The basic physical and mechanical parameters and chemical components of the SR are shown in Table 1.

Table 1.
SR physical and mechanical parameters and chemical composition.
The microstructure of the fresh SR and air-dried SR is illustrated in Figure 2. The inner pores of the SR were well-developed, forming agglomerated structures with internal pore development for both the fresh SR and air-dried SR. These structures consisted of small agglomerates formed by individual particle-point contacts, which then interacted to create larger agglomerates, resulting in a loose structure. The agglomerates of fresh SR were relatively large, approximately 60~100 μm in size, and were primarily composed of granular calcite [30]. In contrast, the agglomerates of the air-dried SR generally had sizes smaller than 20 μm, containing some columnar CaSO4 internally. This phenomenon was attributed to the decrease in moisture content, which weakens particle cohesion. Consequently, the distinctive particle characteristics of the air-dried SR became more pronounced.
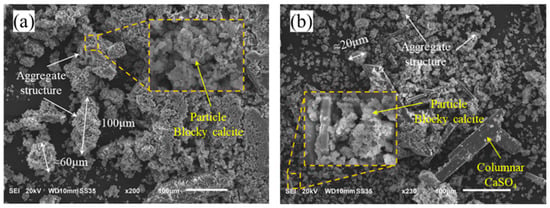
Figure 2.
SEM micrographs of SR: (a) fresh SR; (b) air-dried SR.
2.1.2. Soil
The test soil was collected from the experimental section of Wenyuan Road in Huai’an. The natural moisture content of the soil was 3.25%, with a dry density of 1.68 g/cm3, and a 7-day unconfined compressive strength (UCS) of 0.41 MPa. The liquid limit and plastic limit were 39.1% and 19.0%, respectively, resulting in a plasticity index of 20.1, indicating a low liquid limit clay. XRD and SEM analyses were employed to examine the composition and internal structure of the soil, with the results depicted in Figure 3.

Figure 3.
Internal structure of the soil mass: (a) 2000×; (b) 4000×.
The main components of the soil were SiO2 and CaCO3, with a relatively dense internal structure and small pores. Soil particles were predominantly in layered and flaky forms, with mutual interlocking to form a cohesive structure. Particle sizes were concentrated in the range of 20 to 40 μm.
2.1.3. Lime
The lime used in the experiment was produced by the Huihui Industrial Company and primarily consists of CaO (47%) and Ca(OH)2 (23%), with a CaCO3 content of approximately 10%. Table 2 shows the mineral composition of lime.

Table 2.
Mineral composition of lime.
2.1.4. Cement
The cement employed was a P.O.32.5 cement, with an apparent density of 3.1 g/cm3 and a residue on the 80 μm sieve of 6.4%. The mineral composition of the cement is shown in Table 3 The contents of C3S, C2S, C3A, and C4AF were 48.5%, 26.2%, 11.1%, and 12.3%, respectively.

Table 3.
Mineral composition of cement.
2.2. Orthogonal Experimental Design
The experiment was conducted against the backdrop of the Wenyuan Road renovation project in Huai’an City. The original pavement base material was cement–lime–soil, with a design mix ratio of cement:lime:soil = 3%:9%:100%; the optimum moisture content was 18.8%. In the experiment, SR was used to replace a portion of lime as a cementitious material to prepare SRCLS. Due to the high soluble salt content in the SR, it will have a certain impact on the strength of the mixture, therefore it is necessary to study the SR content as a factor so that the mixture can meet the road strength requirements while adding SR. The SR obtained from the factory had a high moisture content and a viscous consistency, making it unsuitable for direct use. This required drying and crushing treatment to control both the moisture content and particle size. This will incur a certain cost, and the lower the moisture content, the smaller it can be broken, so the higher its cost. At the same time, when the particle size of SR is different, the contact area between the SR and the soil will be different, and the efficiency of the reaction in the mixture will also be different. Therefore, the particle size of SR and the moisture content of SR were selected as one of the variables. Because the mixture contains cement, the curing age is very important. Therefore, the experiment employed an orthogonal design method to analyze the interactive effects of different factors on the UCS of SRCLS including the SR content (A), SR particle size (B), SR moisture content (C), and curing age (D). A 4-factor and 4-level orthogonal experimental design scheme was devised utilizing the L16(44) orthogonal experimental table, comprising a total of 16 groups. The specific factors and their levels are outlined in Table 4.

Table 4.
Orthogonal factor level table.
2.3. Specimen Preparation
After natural air drying, the test soil was crushed and passed through a standard sieve with a 2.36 mm opening. The SR was then processed to meet the specified moisture content and particle size. Following the designed mass ratio, the SR, cement, lime, and soil were uniformly mixed with water. The additives were added in the following sequence: lime, SR, cement, and water, with thorough mixing after each addition. Ultra-pure water filtered by a UPTC-10 water purifier was used as the experimental water. For the 16 groups of specimens, the designed moisture content was 18.8%. Prior to compaction, the mixed material was sealed in plastic bags and immersed in water for 24 h. The standard specimens for unconfined compressive strength (UCS) testing were cylindrical, with a diameter of 50 mm and a height of 50 mm. Curing conditions were maintained at a temperature of 20 ± 1 °C and a humidity of 95%. Specimens at different ages were immersed in water for 24 h at the end of their respective curing periods.
2.4. Testing Methods
2.4.1. Unconfined Compressive Strength (UCS) Tests
The mechanical property of the SRCLS specimen tested was the UCS. The UCS tests were carried out according to the national standard for geotechnical testing methods (GB/T50123-2019) [31]. A WAW-1000 testing machine was used as the experimental apparatus. The specimens were cured for different lengths of time (1 day, 7 days, 14 days, and 28 days) before testing. Based on previous studies, the loading rate was set to 1.0 kN/s. UCS tests were completed on at least three specimens within each group to quantify their strength. Prior to the UCS test, the SRCLS specimens, which had been immersed in water for 24 h, were removed and their surface excess was absorbed using a soft cloth. The specimens were then placed on the compression machine for the strength test. The maximum pressure P (N) at which the specimens failed was recorded. Three specimens were prepared for each group, and the average UCS value Rc of each group was calculated.
2.4.2. Microstructure Analyses
To better characterize the microstructure of the SRCLS and identify the dependency of the UCS on the microstructure, a series of SEM observations was carried out through a field emission-scanning electron microscope. It is worth noting that prior to the SEM analysis, the samples were immersed in anhydrous alcohol for 24 h to suspend the slag hydration and then dried at 50 °C to a constant weight [32].
2.4.3. X-ray Diffraction (XRD) Tests
To explore the strength mechanism of SRCLS, the specimens from the UCS test were crushed and then dried in an oven. After drying, the samples were sieved through a 1 mm standard sieve, mixed uniformly, and then sampled for XRD testing. The XRD testing was conducted using a Cu target under a tube voltage of 200 KV and tube current of 400 mA. The diffraction angle 2θ scanning range was set to 10–70° with a scanning rate of 1 (°)/min [32]. The research and testing process is shown in Figure 4.
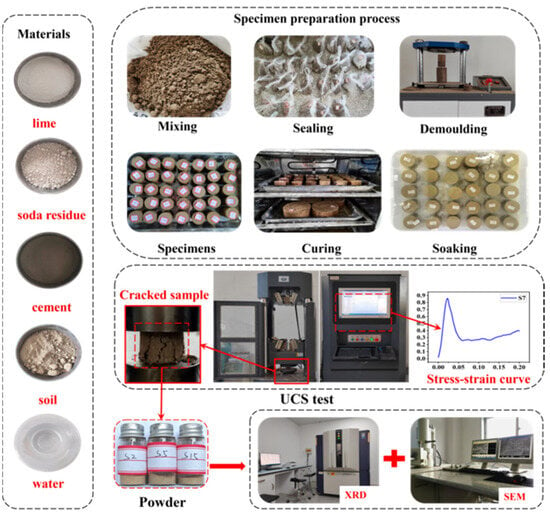
Figure 4.
Experimental procedure.
3. Results and Discussion
3.1. Results of Orthogonal Experiments
The orthogonal experimental results for the SRCLS UCS are presented in Table 5.

Table 5.
Test results for the SRCLS UCS.
Among groups S1 to S16 of SRCLS materials, the highest UCS value was 0.867 MPa (S7), and the lowest was 0.407 MPa (S16). Compared to the control group, the strength increase reached up to 67%. Referring to the JTG/T F20-2015 standard [33], the 7-day UCS of SRCLS should exceed 0.5 MPa. Based on the 7-day UCS reference, groups S2, S5, S12, and S15, with three sets of SRCLS in the 7-day curing period, could meet the strength requirements for secondary road sub-base layers.
3.2. Analysis of Factors Affecting UCS of SRCLS
The analysis of variance method [34] was employed to examine the influence of different levels of SR content, particle size, moisture content, and curing age on the UCS of SRCLS. By calculating the total response values at different levels of each factor (where j denotes the factor, i denotes the level) and the mean value (where represents the total response value corresponding to factor i at level j and denotes the mean value of ), the results of the SRCLS UCS range analysis are shown in Table 6.

Table 6.
Range analysis results of the orthogonal experiments.
3.2.1. Effect of SR Content
The results presented in Figure 5 indicate that as the SR content gradually increased, the SRCLS UCS response value initially increased and then decreased. When the SR content was 3%, the SRCLS response value was the highest. However, as the SR content continued to increase, the SRCLS response value decreased. The experiment confirms that an appropriate amount of SR can effectively improve the strength of SRCLS. However, when applying it in engineering, it is crucial to enforce strict control of the SR content to avoid excessive SR content leading to a decrease in material strength.
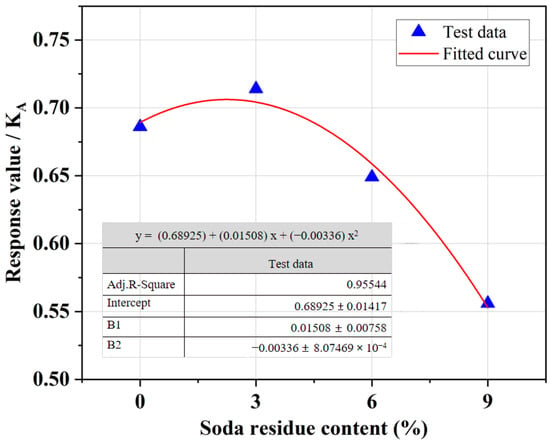
Figure 5.
Effect of SR content on the UCS of SRCLS.
3.2.2. Effect of Particle Size
Figure 6 demonstrates that as the particle size of SR increases, the UCS of SRCLS gradually decreases. The analysis suggests that smaller particle sizes of SR lead to a larger specific surface area, which facilitates more thorough contact between the SR, lime, cement, and soil. This favors better mixing and promotes the cementation reactions, thereby generating more cementitious materials, enhancing material densification and strength. However, smaller SR particle sizes entail higher crushing costs. Hence, when applying SRCLS in engineering projects, it is unnecessary to excessively pursue finer SR particle sizes. Instead, controlling the SR particle size appropriately while ensuring the strength of the SRCLS is sufficient.
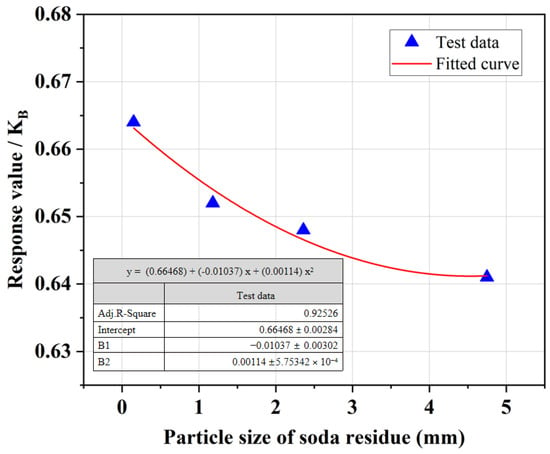
Figure 6.
Effect of particle size on the UCS of SRCLS.
3.2.3. Effect of Moisture Content
Figure 7 demonstrates that as the moisture content of SR increased, the UCS of SRCLS gradually decreased. Lower moisture content in the SR led to the formation of hydration products through the combination of salts in the SR and free water in other mixture components, resulting in denser bonding between the SR and soil matrix, thus enhancing strength. Typically, the initial moisture content of the freshly produced SR was 105.8%, while that of the air-dried SR was 31.45%. Therefore, drying the SR would increase the production costs and complexity. Therefore, in engineering applications, air-dried SR can be used to ensure the strength of SRCLS.
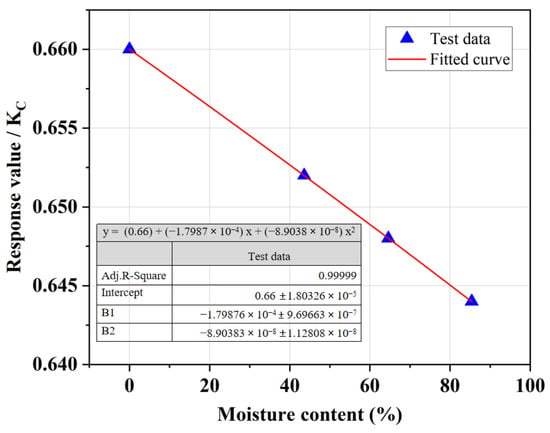
Figure 7.
Effect of moisture content on the UCS of SRCLS.
3.2.4. Effect of Curing Period
Figure 8 shows that as the curing period increased, the UCS of SRCLS gradually improved. With the extended curing time, the crystallization of lime, carbonation reactions, and hydration products of cement increased, leading to a progressive enhancement in material strength. Adequate curing can effectively improve the strength of SRCLS. However, longer curing times also imply longer waiting times for construction at upper layers of the pavement.
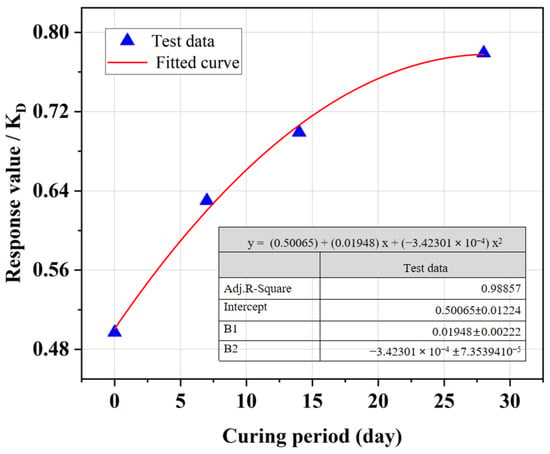
Figure 8.
Effect of curing period on the UCS of SRCLS.
For the SRCLS UCS, the optimal material proportion is SR:cement:lime:soil = 3%:3%:6%:100%. Natural air-dried SR is suitable for use, with particle sizes preferably controlled at the 0.15 mm level.
3.3. Sensitivity Analysis of Influencing Factors
- (1)
- Range analysis
The range analysis method of the orthogonal experiment was employed, and the range Rm corresponding to the factor level was calculated based on the value, Rm = Max(, , ……, ) − Min(, , ……, ). The magnitude of R values was used to determine the primary and secondary relationships of the factors. The sensitivity range analysis of each factor to the UCS of SRCLS is shown in Figure 9.
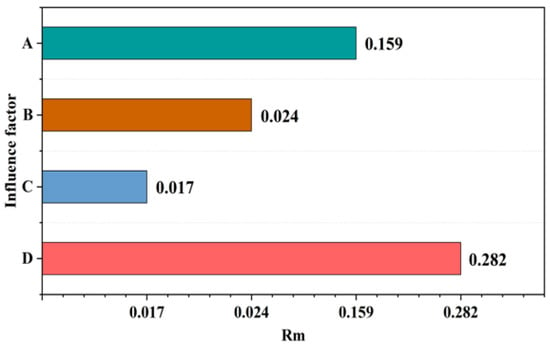
Figure 9.
SRCLS UCS range analysis results: (A) the SR content, (B) SR particle size, (C) SR moisture content, and (D) curing age.
The range values corresponding to the four influencing factors of SR content, SR particle size, SR moisture content, and curing age were 0.159, 0.024, 0.017, and 0.282, respectively. The order of importance of these factors on the UCS value of SRCLS materials is indicated as follows: Rm(D) > Rm(A) > Rm(B) > Rm(C).
- (2)
- Variance analysis
Due to the presence of errors in the actual experimental process, range analysis could not determine the specific reasons for the fluctuations in experimental indicators or assess the significance of influencing factors. Therefore, variance analysis was conducted based on the experimental results [35], and the results are presented in Table 7.

Table 7.
Results of the orthogonal experimental variance analysis.
Due to the absence of a blank factor in the orthogonal table, the factor with the smallest sum of squares of deviations, SR particle size B, was chosen as the error term. The analysis revealed that the significance indicator p-values of SR content A and curing age D were both less than 0.05. This indicates that the curing age and SR content significantly affect the UCS of SRCLS materials, while the particle size and moisture content of SR have relatively minor and insignificant effects. The factors influencing the UCS value of SRCLS materials are ranked in the following order: FD > FA > FB > FC. Therefore, the primary and secondary sequence of factors affecting the UCS value of SRCLS materials are as follows: curing age, SR content, SR particle size, and SR moisture content. The sensitivity analysis results of factors are consistent with the range analysis results.
4. Microstructure and Strengthening Mechanism
4.1. Influence of SR Content on UCS Patterns
Based on the significance analysis of influencing factors, the effects of the SR particle size and moisture content on the unconfined compressive strength (UCS) of SRCLS were relatively weak. Therefore, four groups, S2, S5, S12, and S15, with a 7-day curing period, were selected for studying the influence of the SR content on SRCLS UCS. The relationship between the material UCS and SR content was plotted, as shown in Figure 10.
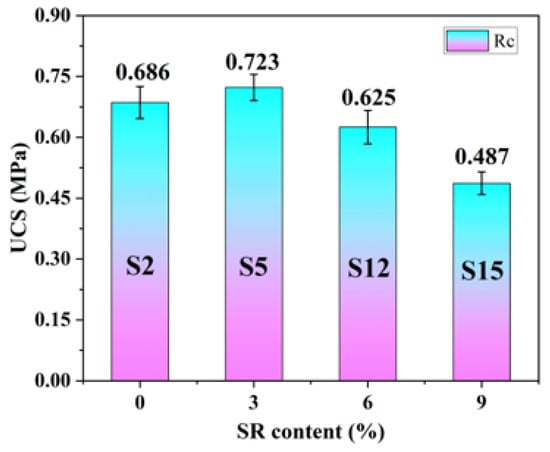
Figure 10.
Material strength characteristic curves.
Analysis of Figure 8 revealed that with the increase in SR content from 0% to 9%, the 7-day UCS of SRCLS materials initially increased and then decreased. At a 3% SR content (S5), the UCS value of the material was the highest, increasing from 0.686 MPa to 0.723 MPa compared to the control group (S2), representing a 5.4% increase. However, when the SR content reached 9% (S15), the UCS of SRCLS materials decreased significantly by 29% compared to the S2 group. This indicates that adding an appropriate amount of SR can enhance the strength of cement–lime–soil materials, while excessive content leads to a significant reduction in strength.
4.2. Microstructure of SRCLS
Specimens from groups S2, S5, and S15 representing no SR addition, appropriate SR addition, and excessive SR addition, respectively, were selected for observation and SEM electron microscopy scanning. The local real shots and microstructures of SRCLS specimens with different SR contents are shown in Figure 11 and Figure 12.

Figure 11.
In-situ images of SRCLS specimens: (a) SR = 0%; (b) SR = 3%, and (c) SR = 9%.

Figure 12.
Microstructure of SRCLS: (a) SR = 0%; (b) SR = 3%, and (c) SR = 9%.
Figure 11 illustrates significant differences in the compactness of SRCLS specimens with different SR contents. The structure of specimens from group S2 (without SR) and group S5 (with a 3% SR content) appeared denser. As the SR content increased to 9%, Figure 11c shows that specimens from group S15 contained unreacted SR, indicating that some SR did not participate in the reaction, which resulted in a looser structure of the specimens.
Figure 12 depicts that when no SR was added (group S2), the internal soil particles and large aggregates of SRCLS were evident, with numerous small pores forming a relatively compact overall structure. When the SR content was 9% (group S15), the large aggregates decreased, and there were larger voids between the particles and small aggregates, indicating weaker internal bonding. The internal structure of group S5 with a 3% SR content was the densest, with no apparent internal pores compared to groups S2 and S15. Figure 8 also confirms that compared to group S2, the UCS of SRCLS in group S5 was moderately increased (by 5.4%), while the UCS reduction in group S15 reached 29%. There was a positive correlation between the internal microstructure and macroscopic mechanical properties of the material. The analysis suggests that SR promotes the hydration reaction of cement–lime–soil materials. Both existing and newly generated cementitious materials collectively fill the internal pores of the material, effectively enhancing the strength of the SRCLS by creating a denser internal structure.
4.3. Strengthening Mechanism
Figure 13 illustrates the XRD test results of SRCLS specimens with different SR contents at a 7-day curing age. To present the hydration products of SRCLS clearly, the XRD graph provides the test results for 2 theta ranging from 10° to 70°. UCS tests indicate that adding the appropriate amount of SR can enhance the strength of cement–lime–soil (CLS) material, while an excessive content leads to a reduction in strength. The analysis suggests several mechanisms by which SR enhances the strength of CLS:
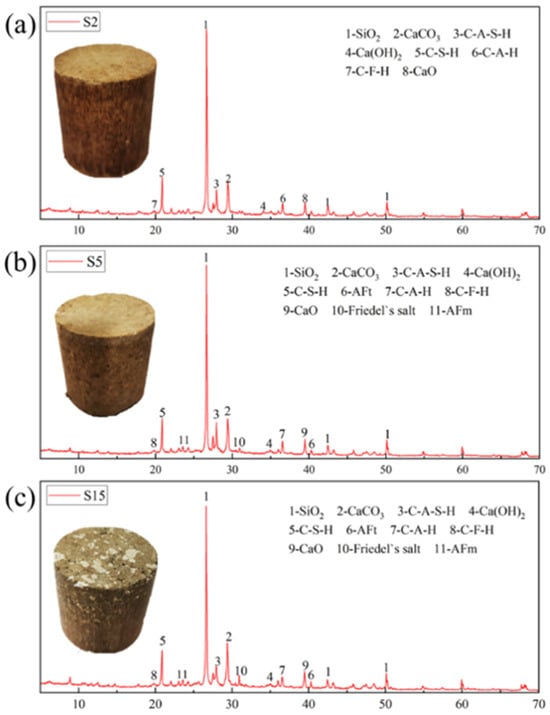
Figure 13.
Component analysis of SRCLS: (a) SR = 0%; (b) SR = 3%, and (c) SR = 9%.
- (1)
- Fine aggregate filling effect: The high content of CaCO3 in SR allows SR particles to act as fine aggregates in the system, improving the gradation of the system, reducing inter-particle voids, and enhancing internal compactness, thus serving as fillers during strength formation [36].
- (2)
- Partial lime substitution effect: The main component of lime is Ca(OH)2, which reacts with active substances in soil. Similarly, SR contains a significant amount of Ca(OH)2 and has an alkaline nature. Its addition, it increases the alkaline environment of the mixture, further promoting the leaching of active silicon and aluminum ions from the soil [37]. Active SiO2 and Al2O3 in the soil react with Ca(OH)2 in soda residue to produce calcium silicate hydrate (C-S-H) and calcium aluminate hydrate (C-A-H) that partially substitute for lime, as represented by Equations (1) to (2). These cementitious substances bind soil particles together, forming larger aggregates, filling particle voids, thus improving the density and strength of the cement–lime–soil.
- (3)
- Promotion of cement hydration: Compared to the S2 group without SR, XRD tests of the S5 and S15 groups with added SR revealed the presence of AFt and AFm hydration products in SRCLS. C3A in cement hydrated the fastest, reacting with water to form 4CaO·Al2O3·13H2O (C4AH13) [38] in pure water. The SR contained a small amount of CaSO4·2H2O. In the SRCLS system, the hydration product of C3A is ettringite (AFt) [39]. The reaction equations are given by Equations (3) and (4).When CaSO4·2H2O is exhausted in the system, AFm is formed from the reaction between C4AH13 and AFt, as shown in Equation (5). Both AFt and AFm possess stronger cementitious properties than C-S(A)-H, wrapping around soil particle surfaces, enhancing internal compactness, and increasing the material strength.
- (4)
- Chloride ion binding: XRD tests of the S5 and S15 groups revealed the presence of Friedel’s salt in SRCLS. This was attributed to the dissolution of CaCl2 and NaCl from SR, which then combines with the unhydrated C3A and AFt in the system. The former combination involves Cl− directly reacting with C3A to form hydrated calcium chloride containing chlorine (Friedel’s salt) [40], while the latter involves Cl− substituting for the sulfate ions of AFt to form Friedel’s salt [41]. These reactions are represented by Equations (6)–(8).
The chemical composition of soda residue is quite complex. In summary, the components in soda residue including CaCO3, Ca(OH)2, CaCl2, and NaCl are key factors enhancing the strength of SRCLS. They respectively contribute to fine aggregate filling, the lime substitution effect, the promotion of cement hydration, and binding with chloride ions. This improves the gradation of SRCLS materials and facilitates the formation of cementitious products from AFm, AFt, and Friedel’s salt during strength development, resulting in denser and stronger SRCLS materials.
5. Conclusions
This study designed a 4-factor, 4-level orthogonal experiment to investigate the effects of SR content, SR particle size, moisture content, and curing age on the UCS of SRCLS. The influence patterns and sensitivity of different factors on strength were studied through range analysis and variance analysis. The mechanism by which SR enhances the strength of SRCLS was discussed, and the following conclusions were drawn:
- (1)
- With the gradual increase in SR content within the range of 0% to 9%, SRCLS UCS initially increased and then decreased. As the SR particle size or SRCLS moisture content increased gradually, the SRCLS UCS gradually decreased, while an increase in curing age led to a gradual increase in SRCLS UCS.
- (2)
- Appropriate SR content effectively enhanced the UCS of SRCLS, while excessive SR content decreased the material’s UCS value. The optimal mix ratio for SRCLS was SR:cement:lime:soil = 3%:3%:6%:100%. Naturally-dried SR is suitable for use as raw material, with a recommended particle size controlled at 0.15 mm.
- (3)
- Range analysis and variance analysis of the orthogonal experiment indicate that the factors affecting the UCS of SRCLS, in order of importance, are curing age, SR content, particle size, and SR moisture content. Among these, curing age and SR content are relatively more significant factors affecting the UCS.
- (4)
- An adequate SR content can serve as a fine aggregate filler, a substitute for lime, promote cement hydration, and combine with chloride ions. This improves the grading of SRCLS and promoting the formation of cementitious products from AFm, AFt, and Friedel’s salt, ultimately enhancing the strength of SRCLS.
- (5)
- The paper also identified several areas for improvement and further in-depth exploration including different types of soils, lime, and cement dosages as well as SRCLS moisture content.
Author Contributions
Conceptualization, W.C.; Methodology, W.Y.; Software, W.Y.; Validation, X.W., Q.X., G.W., J.C. and S.Z.; Writing—original draft, W.C.; Writing—review & editing, W.Y. and G.W.; Supervision, X.W. and Q.X.; Funding acquisition, W.C. and Q.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 51904110) and Huaiyin Institute of Technology Postgraduate Science and Technology Innovation Project (No. HGYK202426).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Liu, Y.; Zhou, B. Synthesis and characterization of fly ash geopolymer paste for goaf backfill: Reuse of soda residue. J. Clean. Prod. 2020, 260, 121045. [Google Scholar] [CrossRef]
- Sun, S.; Zheng, Q.; Tang, J.; Zhang, G.; Zhou, L.; Shang, W. Experimental research on expansive soil improved by soda residue. Chin. J. Geotech. Eng. 2012, 33, 1608–1612. [Google Scholar]
- He, J.; Wang, X.-Q.; Su, Y.; Li, Z.-X.; Shi, X.-K. Shear Strength of Stabilized Clay Treated with Soda Residue and Ground Granulated Blast Furnace Slag. J. Mater. Civ. Eng. 2019, 31, 06018029. [Google Scholar] [CrossRef]
- Yang, Y.B.; Pu, Y.Q.; Yan, W.J.; Guo, W.; Wang, H.C. Microstructure and Chloride Ion Dissolution Characteristics of Soda Residue. J. South China Univ. Technol. (Nat. Sci. Ed.) 2017, 45, 82–89. [Google Scholar]
- Yan, Y.; Chen, C.; Li, Q.; Sun, X.; Wang, L. Arsenate removal from groundwater by modified alkaline residue. Desalination Water Treat. 2016, 57, 20401–20410. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Q.; Liu, Q.; Lv, X.J. Preparation and hydration characteristics of soda residue-slagbased cementitious materials. J. China Univ. Min. Technol. 2022, 51, 802–811. [Google Scholar] [CrossRef]
- Xu, D.; Ni, W.; Wang, Q.; Xu, C.; Jiang, Y. Preparation of clinker-free concrete by using soda residue composite cementitious material. J. Harbin Inst. Technol. 2020, 52, 151–160. [Google Scholar]
- Zhao, X.; Liu, C.; Wang, L.; Zuo, L.; Zhu, Q.; Ma, W. Physical and mechanical properties and micro characteristics of fly ash-based geopolymers incorporating soda residue. Cem. Concr. Compos. 2019, 98, 125–136. [Google Scholar] [CrossRef]
- Kasikowski, T.; Buczkowski, R.; Cichosz, M. Utilisation of synthetic soda-ash industry by-products. Int. J. Prod. Econ. 2008, 112, 971–984. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Wang, Z. Preparation of Pavement Brick from Soda Residue. J. Ceram. 2014, 35, 629–633. [Google Scholar] [CrossRef]
- Zhao, X.H.; Liu, C.Y.; Wang, W.J.; Zhu, N. Experimental Research on Physical and Mechanical Properties of Soda Residue Mixing Soils Used for Filling Embankment. Bull. Chin. Ceram. Soc. 2017, 36, 1406–1411+1423. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Z.; Yan, L.; Cao, P. Study on test methods and geotechnical properties of soda residue. Chin. J. Geotech. Eng. 2007, 29, 1211–1214. [Google Scholar]
- Yu, H.; Hu, L.; Xu, Q. Consolidation Mechanism of Chloride Ion in Soda Residue from Ammonia Soda Process Method. J. Wuhan Univ. Technol. (Mater. Sci.) 2023, 38, 616–622. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Zhou, B. Performance Optimization and Characterization of Soda Residue-Fly Ash Geopolymer Paste for Goaf Backfill: Beta-Hemihydrate Gypsum Alternative to Sodium Silicate. Materials 2020, 13, 5604. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, Y.; Jiang, L.; Zhang, C.; Wang, Q. Characteristics of ammonia-soda residue and its reuse in magnesium oxychloride cement pastes. Constr. Build. Mater. 2021, 300, 123981. [Google Scholar] [CrossRef]
- Jia, S.H. Experimental Study on the Solidification Mechanism and Mechanical Properties of Lime Cement Composite Soil. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2011. [Google Scholar]
- Chen, Y.C. Evaluating greenhouse gas emissions and energy recovery from municipal and industrial solid waste using waste-to-energy technology. J. Clean. Prod. 2018, 192, 262–269. [Google Scholar] [CrossRef]
- Shen, W.; Liu, Y.; Yan, B.; Wang, J.; He, P.; Zhou, C.; Huo, X.; Zhang, W.; Xu, G.; Xu, G. Cement industry of China: Driving force, environment impact and sustainable development. Renew. Sustain. Energy Rev. 2017, 75, 618–628. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Shen, W. Preparation and Application of Eco-Pavement Base Materials from Industrial Solid Waste. Master’s Thesis, China Building Materials Industry Press, Beijing, China, 2021. [Google Scholar]
- Wang, X.; Li, Y.; He, L.; Zhang, Z.; Wang, G.; Jin, L.; Jia, M. Analysis of Carbon Dioxide and Atmospheric Pollutant Emissions from China’s Cement Industry from 2011 to 2022; China Environmental Monitoring: Beijing, China, 2024; pp. 1–11. [Google Scholar]
- Guo, W.; Zhang, Z.; Xu, Z.; Zhang, J.; Bai, Y.; Zhao, Q.; Qiu, Y. Mechanical properties and compressive constitutive relation of solid waste-based concrete activated by soda residue-carbide slag. Constr. Build. Mater. 2022, 333, 127352. [Google Scholar] [CrossRef]
- Yan, C.; Song, X.; Zhu, P.; Sun, Y.; Li, Y.; Zhang, J. Experimental Study on Strength Characteristics of High-Moisture Alkali Slag. Chin. J. Geotech. Eng. 2007, 29, 6. [Google Scholar]
- Zhu, S.; Tang, Y.; Xu, Q.; Zhang, K.; Li, H.; Zhu, Z.; Yin, W. Mechanical Properties Test and Enhancement Mechanism of Lime Soil Modified by High Content Soda Residue for Road Use. Coatings 2022, 12, 1539. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, X.; Jiang, D.; Xu, S.; Zong, Z. Experimental Study on Unconfined Compressive Strength and Water Stability of Alkali Slag Soil. Highway 2020, 65, 212–218. [Google Scholar]
- Guo, Q.; Li, B.; Ding, J.; Chen, J.; Sun, S.; Ma, Y. Experimental Study on Road Performance of Industrial Waste Slag Improved Mud Shield Tunneling Slag Soil [J/OL]. J. Civ. Environ. Eng. 2024, 1–11. [Google Scholar]
- Li, H.; Zhu, S.; Yin, W.; Zhu, Z.; Zhang, K.; Bai, X.; Liu, D.; Tang, Y. Study on Strength Test and Application of Lime Soil in Pavement Base Modified by Soda Residue. Adv. Civ. Eng. 2022, 2022, 4887647. [Google Scholar]
- Xu, J. Research on Alkali Slag Subgrade Treatment. Ph.D. Thesis, Tianjin University, Tianjin, China, 2009. [Google Scholar]
- Yin, W.; Zhang, K.; Ouyang, S.; Bai, X.; Sun, W.; Zhao, J. Experimental Study on Gangue Backfilling Materials Improved by Soda Residue and Field Measurement of Surface Subsidence. Front. Earth Sci. 2021, 9, 747675. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, Z.; Zhao, G.; Liang, T. Experimental study on shear strength characteristics of mud construction waste lightweight soil. Mar. Georesources Geotechnol. 2022, 40, 1184–1192. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Standard for Geotechnical Testing Method. China Planning Press: Beijing, China, 2019.
- Liu, Q.; Sun, J.; Lu, C. Modern Testing and Analysis Methods for Materials; Tsinghua University Press: Beijing, China, September 2014. [Google Scholar]
- JTG/T F20-2015; Technical Guidelines for Construction of Highway Roadbases. China Communications Press: Beijing, China, 2015.
- Yahye, M.A.; Liu, L.; Honglin, W.U.; Sun, Y.; Sun, H.; Ma, J.; Zhang, L. Experimental research on mechanical properties of Fiber-Reinforced Polyurethane Elastic Concrete (FRPEC). Constr. Build. Mater. 2022, 328, 126929. [Google Scholar]
- Zhang, L. Experimental Study on Strength of Composite Cemented Soil Based on Orthogonal Test. Eng. Constr. Des. 2021, 21, 99–101. [Google Scholar]
- Mehdipour, I.; Khayat, H.K. Elucidating how particle packing controls rheology and strength development of dense cementitious suspensions. Cem. Concr. Compos. 2019, 104, 103413. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, S.; Yang, X. Study on Mechanism of Volcanic Ash Modified Coarse Grained Sulfate Saline Soil Roadbed Fillers and Its Action Mechanism. Chin. J. Geotech. Eng. 2019, 41, 588–594. [Google Scholar]
- Li, L.; Xie, Y.; Feng, Z.; Zhu, C. Cement Hydration Mechanism and Its Research Methods. Concrete 2011, 6, 76–80. [Google Scholar]
- Xu, Z.; Zhang, M.; Xu, H. Study on Hydration Mechanism of Lime-Gypsum-Fly Ash Cement Slurry. J. Environ. Eng. 2009, 3, 1879–1884. [Google Scholar]
- Cheng, Y.; Huang, X. Experimental Study on the Influence of Chloride Salt on the Strength of Alkali Activated Slag Paste. J. Beijing Univ. Aeronaut. Astronaut. 2015, 41, 693–700. [Google Scholar]
- Du, Z.; Chen, S.; Yin, D.; Yao, D.; Zhang, Z. Experimental Study on Stability of Filling Body in Chloride Salt Erosion Environment. J. China Univ. Min. Technol. 2021, 50, 532–538+547. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).