Evaluating Use of Hydraulic Modified Sulfur Powder in Concrete Pavements: Laboratory Testing and Field Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydraulic-Modified Sulfur Powder
2.1.1. Synthesis Mechanism
2.1.2. Manufacturing Process
2.2. Materials
2.3. Basic Laboratory Tests
2.3.1. Microstructure Analysis
2.3.2. Mechanical Performance
2.3.3. Durability Performance
3. Field Study
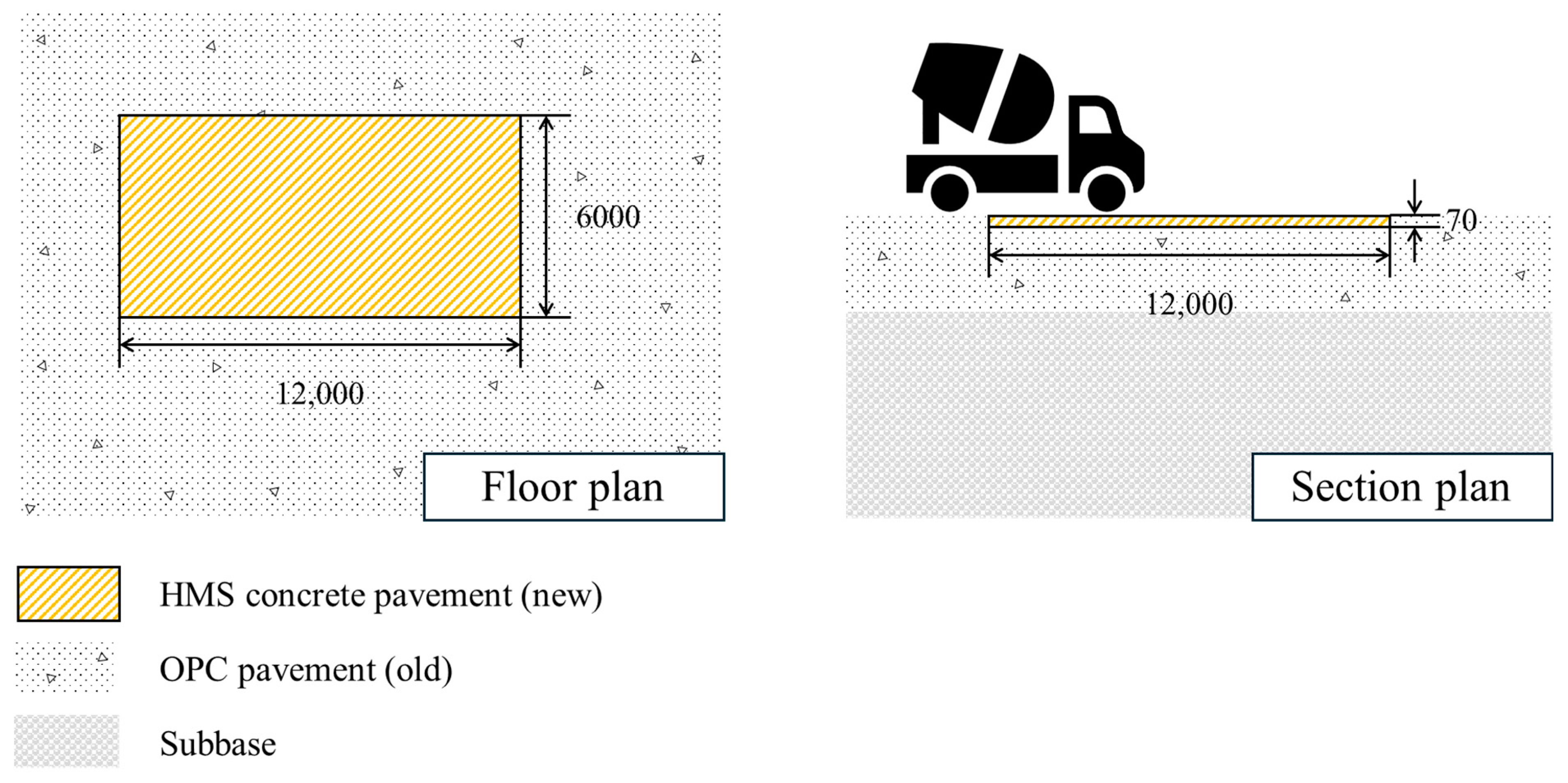
3.1. Overview of Field Study
3.2. Pavement Application
3.3. Field Testing
4. Results and Discussion
4.1. Basic Laboratory Tests
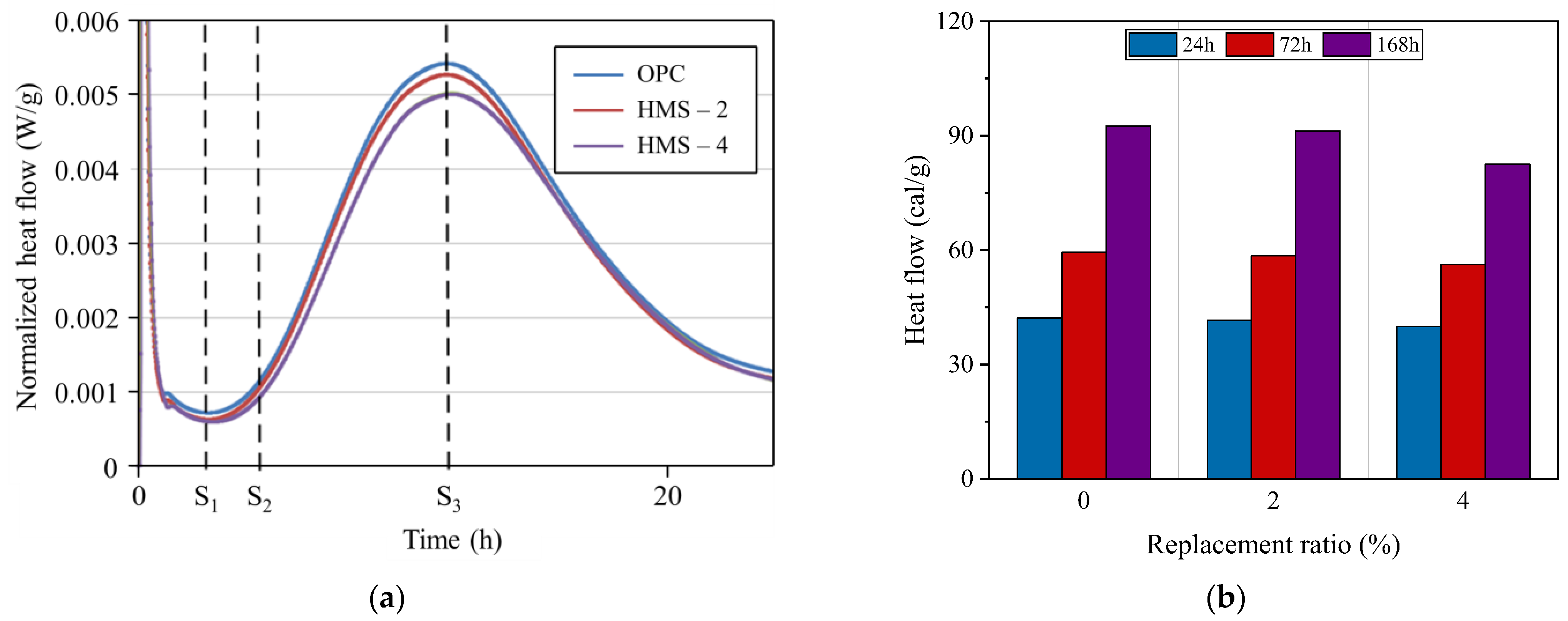
4.1.1. Microstructure Analysis
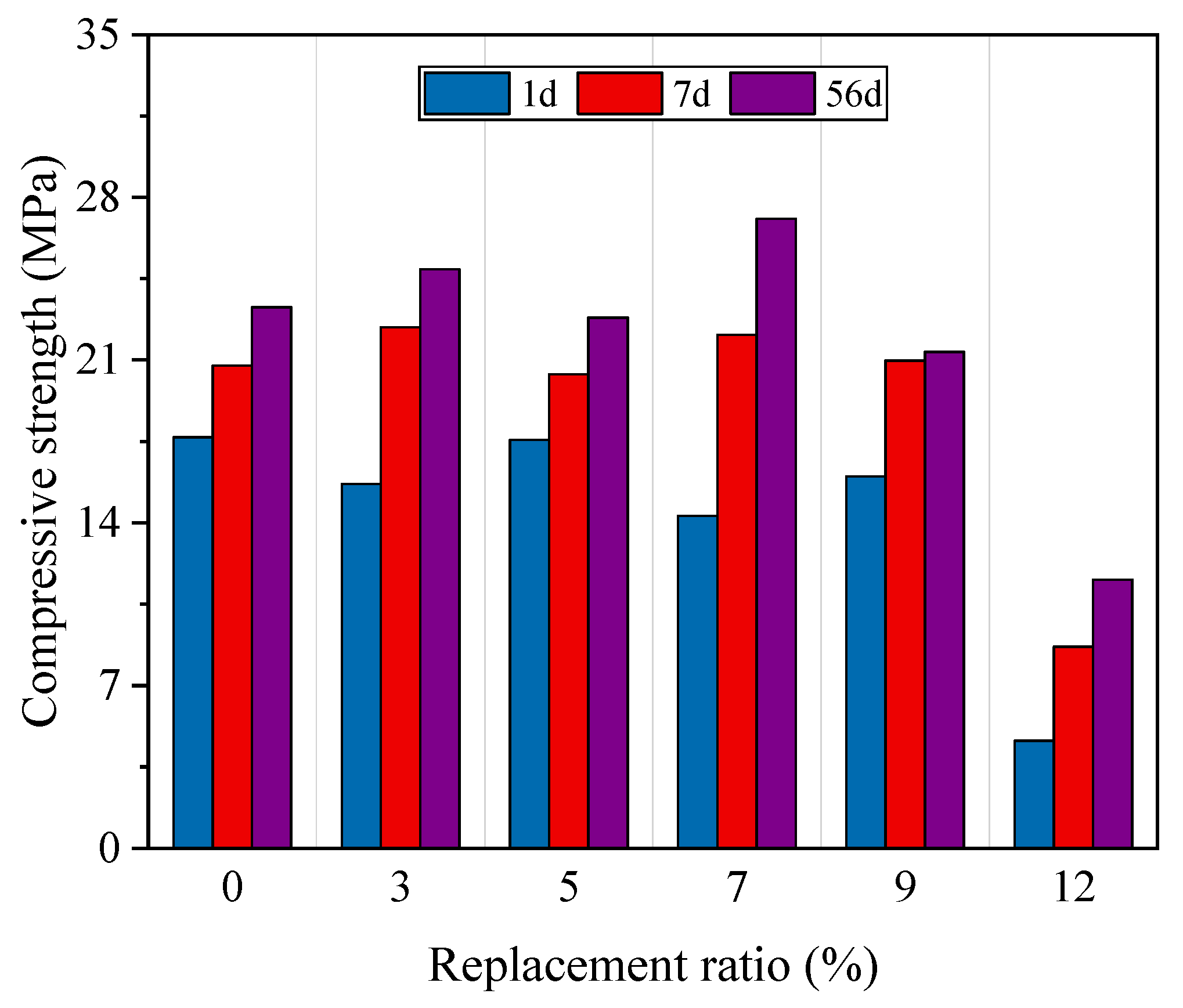
4.1.2. Mechanical Performance
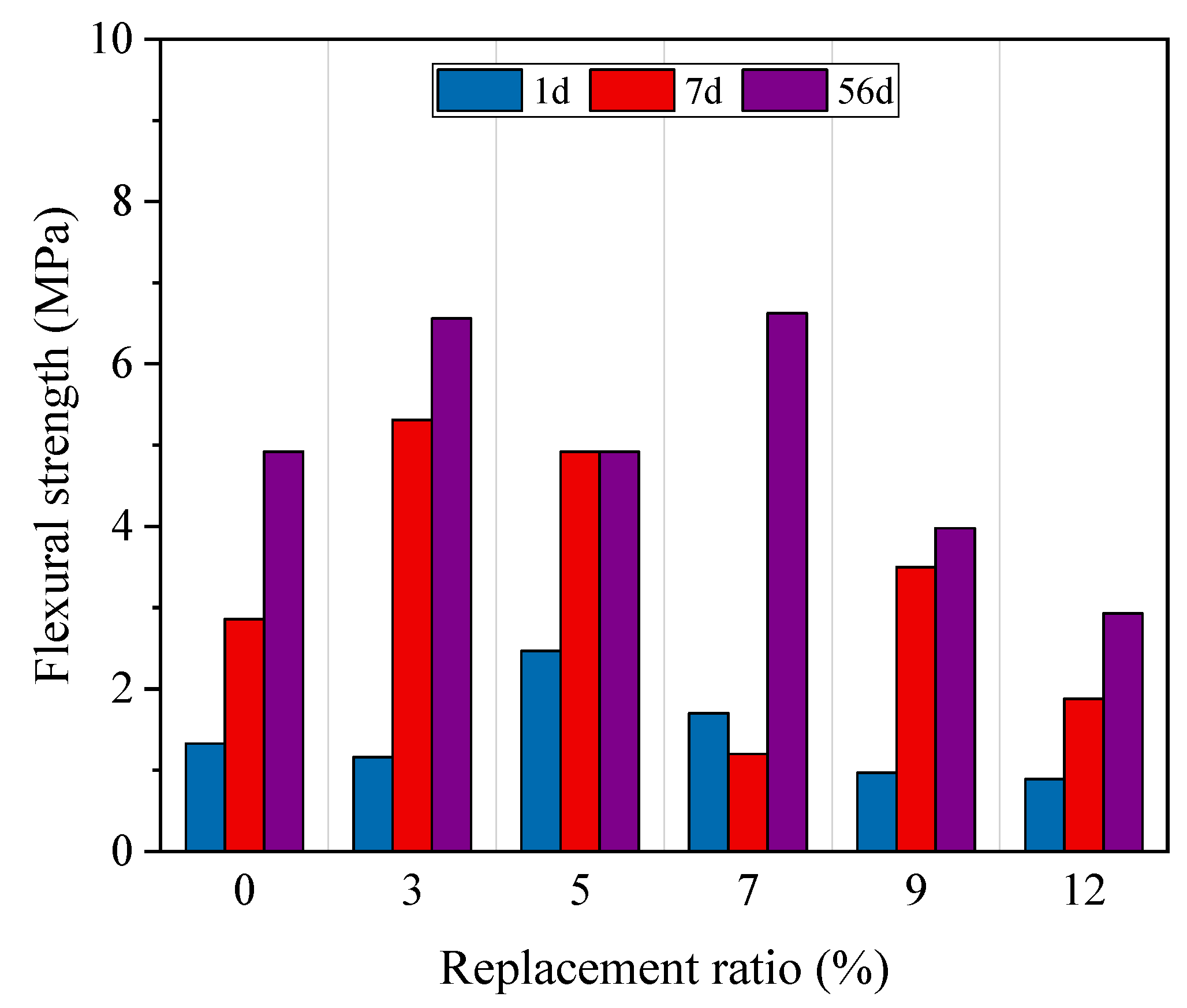
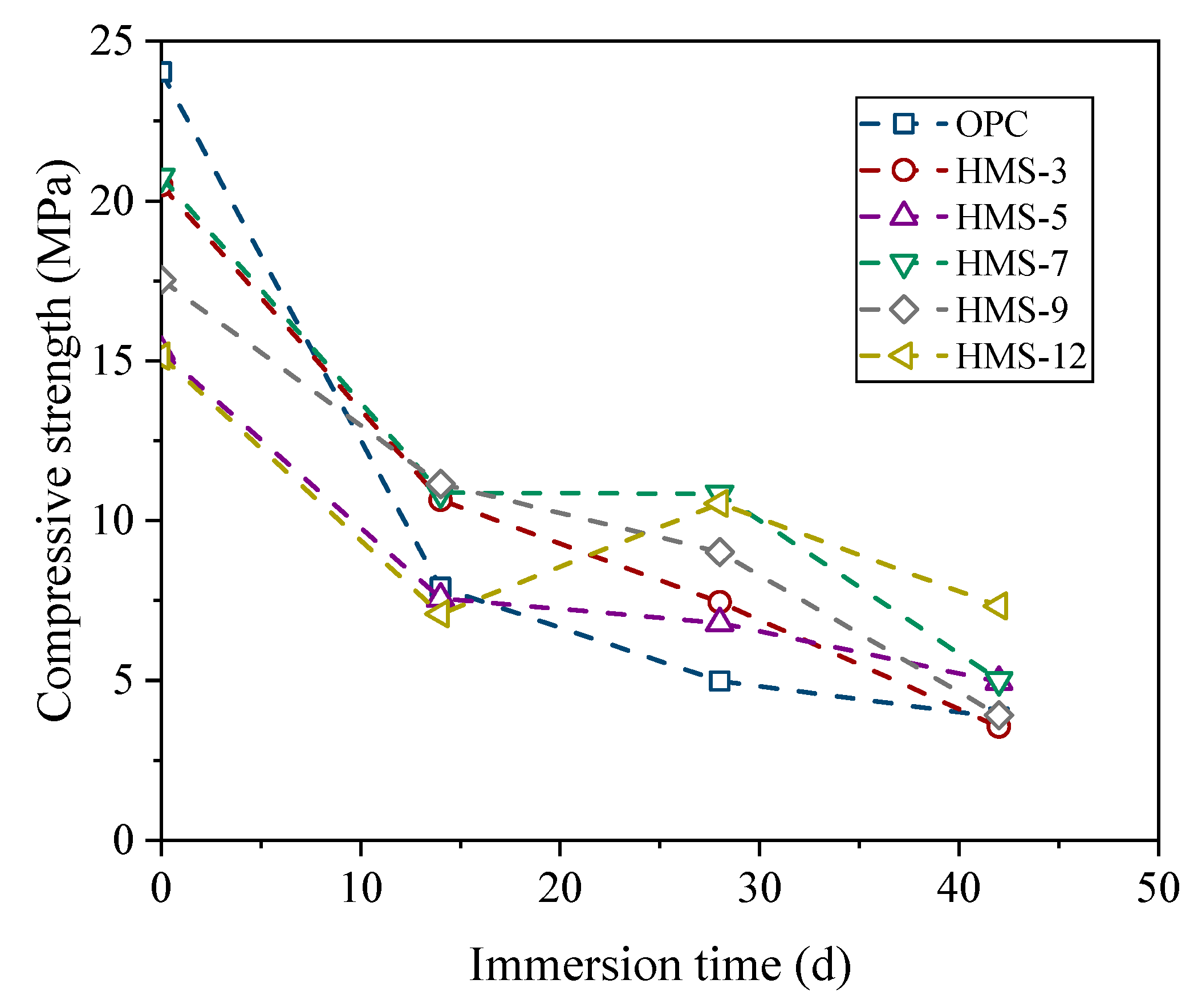
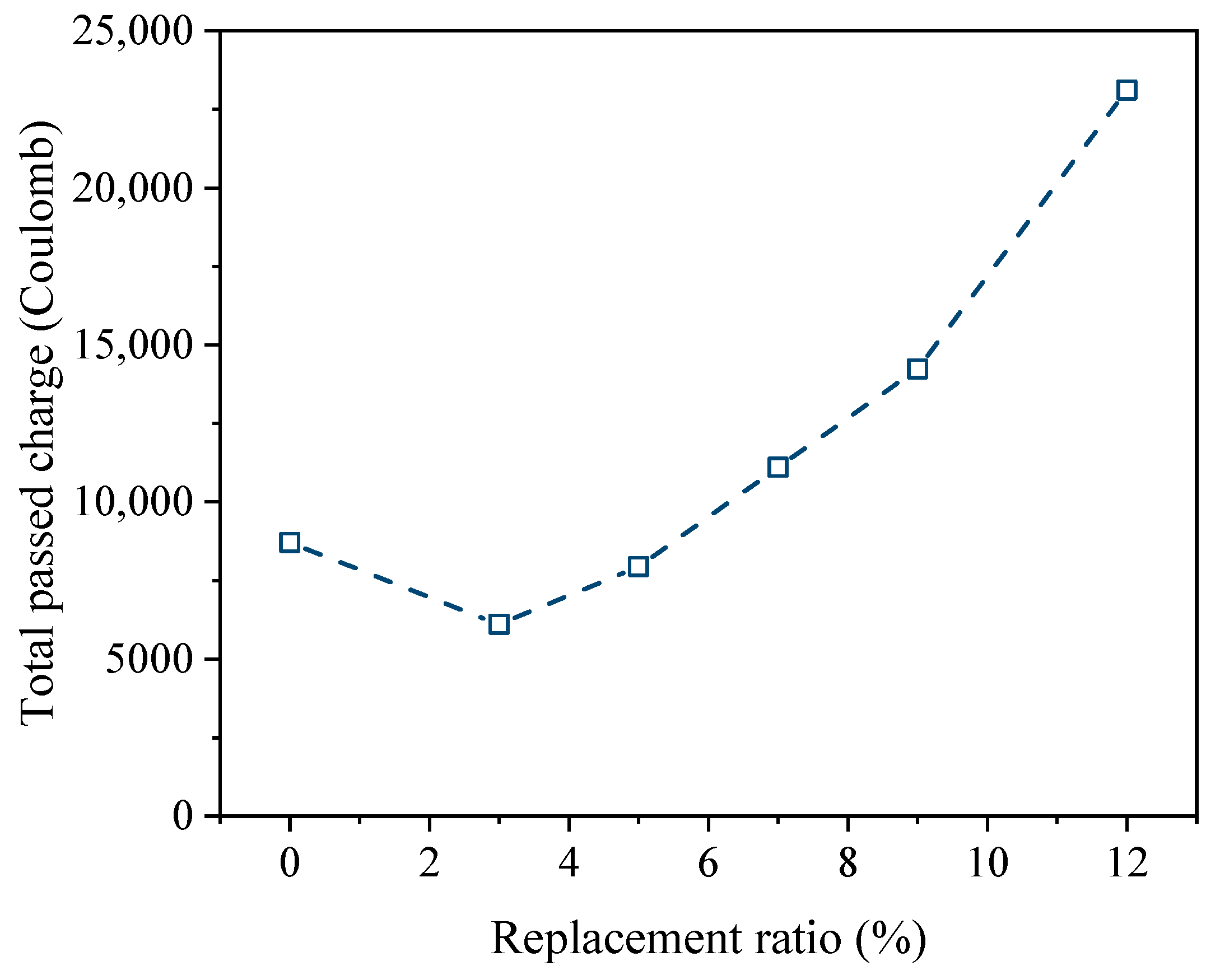
4.1.3. Durability Performance
4.2. Field Testing
| Age (d) | Test Standards | Acceptable Quality [35,44] | Results | |
|---|---|---|---|---|
| Air content (%) | - | KS F 2421 [48] | 3.5–6.5 | 4.0 |
| Slump (mm) | - | KS F 2402 [49] | 50–150 | 145 |
| Compressive strength (MPa) | 7 | KS F 2405 [30] | ≥21 | 30.6 ± 4.3 |
| Compressive strength (MPa) | 28 | KS F 2405 [30] | ≥21 | 42.0 ± 1.9 |
| Compressive strength (MPa) | 98 | KS F 2405 [30] | ≥21 | 63.7 ± 2.5 |
| Flexural strength (MPa) | 28 | KS F 2408 [50] | ≥3.15 | 6.0 ± 0.3 |
| Bond strength (MPa) | 28 | KS F 2762 [51] | ≥1.4 | 1.5 ± 0.1 |
4.3. Further Field Application Test
5. Conclusions
- Cement replacement with HMS altered the zeta potential and significantly reduced the heat liberation during hydration. In addition, SEM analysis confirmed that incorporating HMS positively affected material performance by promoting the agglomeration of rods into large rhombohedral shapes and enhancing the growth of the calcite crystal structure.
- The incorporation of HMS increased the compressive and flexural strengths. However, the mechanical performance decreased rapidly at a replacement ratio of 12%. In terms of durability, HMS was positively correlated with chemical resistance, but the chloride penetration test revealed increased vulnerability as the replacement ratio exceeded 9%. Our results indicate that maintaining an HMS cement replacement ratio of up to 9% ensures stable performance.
- A field study was conducted at a runway site in Chungju, Chungcheongbuk-do, Republic of Korea. The existing concrete pavement (6000 × 12,000 × 70 mm W × L × T) was removed, and HMS concrete was used for repaving. Under field conditions, the air content, slump, compressive strength, flexural strength, and bond strength met the quality criteria specified in Korean construction standards. After 3 months of monitoring, no significant abnormalities were observed.
- After evaluating the mechanical and durability properties of the field HMS concrete mixture, it was determined that all the quality criteria and evaluation indicators met acceptable standards. The comparative analysis between OPC and HMS concrete regarding sulfuric acid immersion penetration depth, compressive strength changes after sulfuric acid immersion, and chloride-ion penetration resistance revealed that HMS concrete outperformed OPC.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Worrell, E.; Reuter, M.A. Handbook of Recycling: State-of-the-Art for Practitioners, Analysts, and Scientists; Elsevier: Waltham, MA, USA, 2014. [Google Scholar]
- Ramachandra, T.; Shruthi, B. Spatial mapping of renewable energy potential. Renew. Sustain. Energy Rev. 2007, 11, 1460–1480. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Gupta, S.; Varjani, S.; Pandey, A.; Gnansounou, E.; You, S.; Ngo, H.H.; Wong, J.W. Trends in mitigation of industrial waste: Global health hazards, environmental implications and waste derived economy for environmental sustainability. Sci. Total Environ. 2022, 811, 152357. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, D.; Hashempour, M.; Heidari, A. Use of waste materials in concrete: A review. Pertanika J. Sci. Technol. 2018, 26, 499–522. [Google Scholar]
- Kanamarlapudi, L.; Jonalagadda, K.B.; Jagarapu, D.C.K.; Eluru, A. Different mineral admixtures in concrete: A review. SN Appl. Sci. 2020, 2, 760. [Google Scholar] [CrossRef]
- Chalee, W.; Sasakul, T.; Suwanmaneechot, P.; Jaturapitakkul, C. Utilization of rice husk–bark ash to improve the corrosion resistance of concrete under 5-year exposure in a marine environment. Cem. Concr. Compos. 2013, 37, 47–53. [Google Scholar] [CrossRef]
- Kim, B.-J.; Yi, C. Experimental study on the shrinkage properties and cracking potential of high strength concrete containing industrial by-products for nuclear power plant concrete. Nucl. Eng. Technol. 2017, 49, 224–233. [Google Scholar] [CrossRef]
- Mohamed, A.-M.O.; El-Gamal, M. Sulfur Concrete for the Construction Industry: A Sustainable Development Approach; J. Ross Publishing: Plantation, FL, USA, 2010. [Google Scholar]
- Gwon, S.; Ahn, E.; Shin, M. Self-healing of modified sulfur composites with calcium sulfoaluminate cement and superabsorbent polymer. Compos. Part B Eng. 2019, 162, 469–483. [Google Scholar] [CrossRef]
- Gulzar, M.A.; Rahim, A.; Ali, B.; Khan, A.H. An investigation on recycling potential of sulfur concrete. J. Build. Eng. 2021, 38, 102175. [Google Scholar] [CrossRef]
- Amanova, N.; Turaev, K.; Shadhar, M.H.; Tadjixodjayeva, U.; Jumaeva, Z.; Berdimurodov, E.; Eliboev, I.; Hosseini-Bandegharaei, A. Sulfur-based concrete: Modifications, advancements, and future prospects. Constr. Build. Mater. 2024, 435, 136765. [Google Scholar] [CrossRef]
- Shin, M.; Kim, K.; Gwon, S.-W.; Cha, S. Durability of sustainable sulfur concrete with fly ash and recycled aggregate against chemical and weathering environments. Constr. Build. Mater. 2014, 69, 167–176. [Google Scholar] [CrossRef]
- Rasheed, Q.; Sikandar, M.A.; Khan, M.H.; Akash, K.; Nasir, H.; Khan, B.J. Assessment of chloride-induced reinforcement corrosion protection of modified sulfur polymer composites. Constr. Build. Mater. 2021, 268, 121086. [Google Scholar] [CrossRef]
- Gwon, S.; Ahn, E.; Shin, M. Water permeability and rapid self-healing of sustainable sulfur composites using superabsorbent polymer and binary cement. Constr. Build. Mater. 2020, 265, 120306. [Google Scholar] [CrossRef]
- Dugarte, M.; Martinez-Arguelles, G.; Torres, J. Experimental evaluation of modified sulfur concrete for achieving sustainability in industry applications. Sustainability 2018, 11, 70. [Google Scholar] [CrossRef]
- El Gamal, M.M.; El-Dieb, A.S.; Mohamed, A.-M.O.; El Sawy, K.M. Performance of modified sulfur concrete exposed to actual sewerage environment with variable temperature, humidity and gases. J. Build. Eng. 2017, 11, 1–8. [Google Scholar] [CrossRef]
- Park, S.-B.; Kim, S.-C.; Kim, K.-H.; Hong, C.-H. Experimental study on development of artificial fishing reefs using environment-friendly sulfur concrete. J. Ocean Eng. Technol. 2007, 21, 58–64. [Google Scholar]
- Zheng, S.; Lu, X.; Zhao, J.; He, R.; Chen, H.; Geng, Y. Influence of industrial by-product sulfur powder on properties of cement-based composites for sustainable infrastructures. Constr. Build. Mater. 2023, 367, 130171. [Google Scholar] [CrossRef]
- Na, O.-J. A Study on the Mechanical Properties and Durability for the Concrete Using Modified Sulfur Mixture Liquid and Powder. Master’s Thesis, Sangmyung University, Seoul, Republic of Korea, 2014. [Google Scholar]
- Lee, J.-N.; Choe, M.-S.; Park, Y.-K.; Choe, K.-T.; Kim, C.-H. A Study on the Physicochemical Properties of Cement Concrete with Addition of Hydraulic Modified Sulfur That Can Be Used at Room Temperature; The Korean Society of Manufacturing Technology Engineers: Gangneung, Republic of Korea, 2022; p. 232. [Google Scholar]
- Lee, S.-H.; Hong, K.-N.; Park, J.-K.; Ko, J. Influence of aggregate coated with modified sulfur on the properties of cement concrete. Materials 2014, 7, 4739–4754. [Google Scholar] [CrossRef]
- Jung, W.K. Evaluation of Physical Properties of Concrete Using the Powder Type Low-Melting-Temperature Modified Sulfur. In Proceedings of the Korea Concrete Institute, Changwon, Republic of Korea, 2–4 May 2018; Volume 30, pp. 599–600. [Google Scholar]
- Huang, H.; Yuan, Y.; Zhang, W.; Zhu, L. Property assessment of high-performance concrete containing three types of fibers. Int. J. Concr. Struct. Mater. 2021, 15, 39. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Lei, Z.; Tong, L.; Sun, J.; Wang, Y.; Wang, X.; Jiang, X. Ternary cementless composite based on red mud, ultra-fine fly ash, and GGBS: Synergistic utilization and geopolymerization mechanism. Case Stud. Constr. Mater. 2023, 19, e02410. [Google Scholar] [CrossRef]
- KS F 2504; Standard Test Method for Density and Water Absorption Rate of Fine Aggregates. Korean Standards Association: Seoul, Republic of Korea, 2020.
- KS F 2503; Standard Test Method for Density and Water Absorption of Coarse Aggregates. Korean Standards Association: Seoul, Republic of Korea, 2019.
- KS F 2502; Standard Test Method for Sieve Analysis of Aggregates. Korean Standards Association: Seoul, Republic of Korea, 2019.
- KS F 4042; Polymer Modified Cement Mortar for Maintenance in Concrete Structure. Korean Standards Association: Seoul, Republic of Korea, 2012.
- ASTM C267; Standard Test Methods for Chemical Resistance of Mortars, Grouts, and Monolithic Surfacings and Polymer Concretes. American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 2020.
- KS F 2405; Test Method for Compressive Strength of Concrete. Korean Standards Association: Seoul, Republic of Korea, 2022.
- KS F 2596; Standard Test Method for Measuring Carbonation Depth of Concrete. Korean Standards Association: Seoul, Republic of Korea, 2019.
- KS F 2711; Standard Test Method for Resistance of Concrete to Chloride Ion Penetration by Electrical Conductance. Korean Standards Association: Seoul, Republic of Korea, 2017.
- KS F 2737; Standard Test Method for Penetration Depth of Chloride Ion in Concrete by Using Indicator. Korean Standards Association: Seoul, Republic of Korea, 2021.
- McCullough, B.F.; Fowler, D.W. Bonded Concrete Overlay (BCO) Project Selection, Design, and Construction; Center for Transportation Research, University of Texas: Austin, TX, USA, 1994. [Google Scholar]
- Korea Road Association, Bonded Concrete Overlay (KCS 44 99 10), Korean Construction Specification (KCS), Ministry of Land, Infrastructure and Transport (MOLIT), Seoul, Republic of Korea. 2023. Available online: https://kcsc.re.kr/StandardCode/Viewer/426 (accessed on 16 June 2024). (In Korean).
- Korea Expressway Corporation. Study on the Design and Long-Term Performance Evaluation of Bonded Concrete Overlay; Korea Expressway Corporation: Gimcheon, Gyeongsangbuk-do, Republic of Korea, 2013. [Google Scholar]
- Marchon, D.; Flatt, R.J. Mechanisms of cement hydration. In Science and Technology of Concrete Admixtures; Elsevier: Amsterdam, The Netherlands, 2016; pp. 129–145. [Google Scholar]
- Mehta, P.K.; Monteiro, P. Concrete: Microstructure, Properties, and Materials, 3rd ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Escalante-Garcıa, J.; Sharp, J. Effect of temperature on the hydration of the main clinker phases in Portland cements: Part II, blended cements. Cem. Concr. Res. 1998, 28, 1259–1274. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.; Song, C.; Choi, S. Effects of fineness and substitution rate of GGBFS on material characteristics of GGBFS-blended cement mortars: Hydration, non-evaporable water, pore structure, mechanical properties, self-desiccation, and autogenous shrinkage. J. Build. Eng. 2024, 92, 109741. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Ahn, J.W. A brief review of aragonite precipitated calcium carbonate (PCC) synthesis methods and its applications. Korean Chem. Eng. Res. 2017, 55, 443–455. [Google Scholar]
- Stepkowska, E.T. Hypothetical transformation of Ca(OH)2 into CaCO3 in solid-state reactions of portland cement. J. Therm. Anal. Calorim. 2005, 80, 727–733. [Google Scholar] [CrossRef]
- Sajid, H.U.; Kiran, R.; Bajwa, D.S. Effect of agro-derived corrosion inhibitors on the properties of Portland cement mortar. Constr. Build. Mater. 2021, 310, 125236. [Google Scholar] [CrossRef]
- Korea Concrete Institute (KCI), Normal Concrete (KCS 14 20 10), Korean Construction Specification (KCS), Ministry of Land, Infrastructure and Transport (MOLIT), Seoul, Republic of Korea. 2022. Available online: https://kcsc.re.kr/StandardCode/Viewer/426 (accessed on 16 June 2024). (In Korean).
- Franus, W.; Panek, R.; Wdowin, M. SEM investigation of microstructures in hydration products of portland cement. In Proceedings of the 2nd International Multidisciplinary Microscopy and Microanalysis Congress: Proceedings of InterM, Mugla, Turkey, 16–19 October 2014; pp. 105–112.
- Baur, I.; Keller, P.; Mavrocordatos, D.; Wehrli, B.; Johnson, C.A. Dissolution-precipitation behaviour of ettringite, monosulfate, and calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 341–348. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- KS F 2421; Standard Test Method for Air Content of Fresh Concrete by the Pressure Method (Air Receiver Method). Korean Standards Association: Seoul, Republic of Korea, 2016.
- KS F 2402; Test Method for Concrete Slump. Korean Standards Association: Seoul, Republic of Korea, 2022.
- KS F 2408; Standard Test Method for Flexural Strength of Concrete. Korean Standards Association: Seoul, Republic of Korea, 2016.
- KS F 2762; Standard Test Method for the Bond Strength of Concrete Repair and Overlay Materials by Pull-Off Method. Korean Standards Association: Seoul, Republic of Korea, 2016.
- ASTM C1202; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 2022.
- KS F 2438; Standard Test Method for Static Modulus of Elasticity and Poisson’s Ratio in Compression of Cylindrical Concrete Specimens. Korean Standards Association: Seoul, Republic of Korea, 2017.
- KS F 2424; Standard Test Method for Length Change of Mortar and Concrete. Korean Standards Association: Seoul, Republic of Korea, 2020.
- KS F 2609; Standard Test Method for the Water Absorption Coefficient of Building Materials. Korean Standards Association: Seoul, Republic of Korea, 2023.
- ASTM C779/C779M; Standard Test Methods for Abrasion Resistance of Horizontal Concrete Surfaces. American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 2019.
- KS F 2456; Test Method for Resistance of Concrete to Rapid Freezing and Thawing. Korean Standards Association: Seoul, Republic of Korea, 2013.
- KS F 2715; Standard Test Method for Water-Soluble Chloride in Mortar and Concrete. Korean Standards Association: Seoul, Republic of Korea, 2017.
- AASHTO T336; Standard Method of Test for Coefficient of Thermal Expansion of Hydraulic Cement Concrete. American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 2022.
- SS 13 72 44; Concrete Testing—Hardened Concrete—Scaling at Freezing. Swedish Institute for Standards (SIS): Stockholm, Sweden, 2005.
- AASHTO PP 34-99; Standard Practice for Estimating the Cracking Tendency of Concrete. American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 2005.
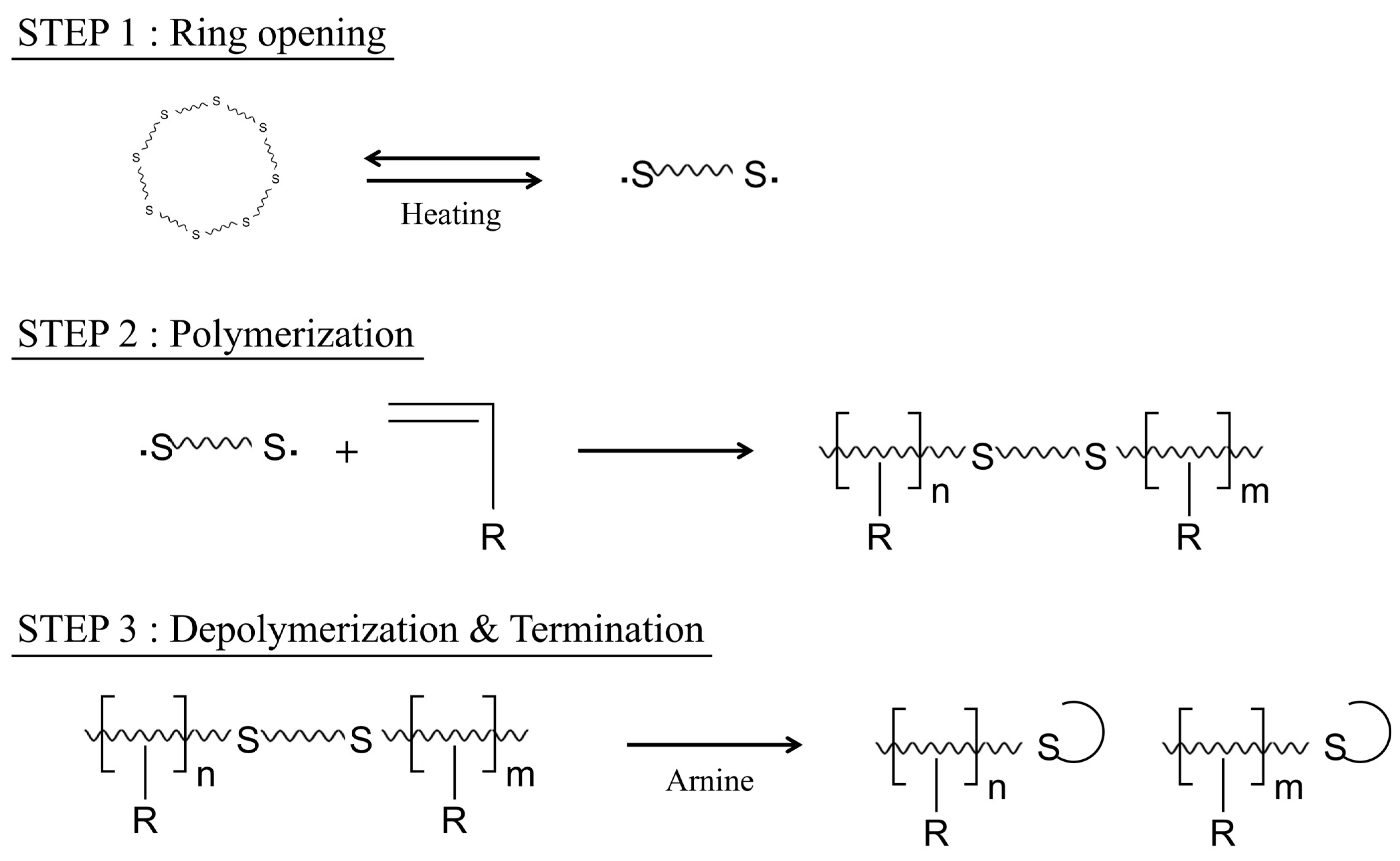













| Step | Action |
|---|---|
| 1 | Introduce powdered sulfur into a 500-mL temperature-controlled reactor. |
| 2 | Melt the sulfur and maintain the temperature at 130 °C. |
| 3 | Gradually add the DCPD monomer over approximately 30 min while stirring with an impeller. |
| 4 | Maintain the temperature at 130 °C and allow the reaction to proceed for 60 min. |
| 5 | When the mixture turns dark red, slowly add pyridine. |
| 6 | As the color deepens and the mixture becomes viscous, evaporate any unreacted pyridine. |
| 7 | Cool to room temperature. |
| Components | OPC | HMS |
|---|---|---|
| CaO | 62.79 | 0.094 |
| SiO2 | 21.74 | 3.11 |
| Al2O3 | 5.00 | 0.74 |
| Fe2O3 | 3.17 | 0.075 |
| MgO | 2.97 | 0.091 |
| SO3 | 1.67 | 25.66 |
| K2O | 1.36 | 0.032 |
| Na2O | 0.11 | 0.041 |
| SrO | - | 0.035 |
| P2O5 | - | 0.023 |
| TiO2 | - | 0.013 |
| LOI * | 1.19 | 70.08 |
| Coarse Aggregates | Fine Aggregates | ||||||
|---|---|---|---|---|---|---|---|
| Sieve Size (mm) | Passing Percentage (%) | Max (%) | Min (%) | Sieve Size (mm) | Passing Percentage (%) | Max (%) | Min (%) |
| 25 | 100 | 100 | 100 | 10 | 100 | 100 | 100 |
| 20 | 99 | 100 | 90 | 5 | 99 | 100 | 95 |
| 10 | 40 | 55 | 20 | 2.5 | 91 | 100 | 80 |
| 5 | - | 10 | - | 1.2 | 58 | 85 | 50 |
| 2.5 | - | 5 | - | 0.6 | 31 | 60 | 25 |
| PAN | - | - | - | 0.5 | 13 | 30 | 10 |
| 0.15 | 4 | 10 | 2 | ||||
| PAN | - | - | - | ||||
| Specimen ID | Water (kg/m3) | Cement (kg/m3) | Limestone (kg/m3) | HMS (kg/m3) | Sand (kg/m3) | Crushed Sand (kg/m3) | Stone Dust (kg/m3) | Chemical Admixture (kg/m3) |
|---|---|---|---|---|---|---|---|---|
| OPC | 569 | 1138 | 284 | - | 1051 | 3111 | 3177 | 8.5 |
| HMS-3 | 569 | 1137 | 242 | 43 | 1051 | 3111 | 3177 | 8.5 |
| HMS-5 | 569 | 1137 | 213 | 71 | 1051 | 3111 | 3177 | 8.5 |
| HMS-7 | 569 | 1137 | 185 | 99 | 1051 | 3111 | 3177 | 8.5 |
| HMS-9 | 569 | 1137 | 156 | 128 | 1051 | 3111 | 3177 | 8.5 |
| HMS-12 | 569 | 1137 | 114 | 171 | 1051 | 3111 | 3177 | 8.5 |
| Specimen ID | Water (kg/m3) | Cement (kg/m3) | Limestone (kg/m3) | HMS (kg/m3) | Sand (kg/m3) | Crushed Sand (kg/m3) | Stone Dust (kg/m3) | Chemical Admixture (kg/m3) |
|---|---|---|---|---|---|---|---|---|
| OPC | 347 | 694 | 174 | - | 641 | 1899 | 1939 | 5.21 |
| HMS-3 | 347 | 694 | 148 | 26 | 641 | 1899 | 1939 | 5.21 |
| HMS-5 | 347 | 694 | 130 | 43 | 641 | 1899 | 1939 | 5.21 |
| HMS-7 | 347 | 694 | 113 | 61 | 641 | 1899 | 1939 | 5.21 |
| HMS-9 | 347 | 694 | 95 | 78 | 641 | 1899 | 1939 | 5.21 |
| HMS-12 | 347 | 694 | 69 | 104 | 641 | 1899 | 1939 | 5.21 |
| Specimen ID | Water (kg/m3) | Cement (kg/m3) | Limestone (kg/m3) | HMS (kg/m3) | Sand (kg/m3) | Crushed Sand (kg/m3) | Stone Dust (kg/m3) | Chemical Admixture (kg/m3) |
|---|---|---|---|---|---|---|---|---|
| OPC | 436 | 873 | 218 | - | 806 | 2387 | 2437 | 6.55 |
| HMS-3 | 436 | 872 | 185 | 33 | 806 | 2387 | 2437 | 6.55 |
| HMS-5 | 436 | 872 | 164 | 55 | 806 | 2387 | 2437 | 6.55 |
| HMS-7 | 436 | 872 | 142 | 76 | 806 | 2387 | 2437 | 6.55 |
| HMS-9 | 436 | 872 | 120 | 98 | 806 | 2387 | 2437 | 6.55 |
| HMS-12 | 436 | 872 | 87 | 131 | 806 | 2387 | 2437 | 6.55 |
| Case | Width (m) | Length (m) | Area (m2) | Thickness (mm) | Volume (m3) |
|---|---|---|---|---|---|
| Test | 6.0 | 12.0 | 72.0 | 70 | 5.04 |
| Water (kg/m3) | Cement (kg/m3) | HMS (kg/m3) | Sand (kg/m3) | Gravel (kg/m3) | SP 1 (% 2) | AE 3 (% 4) |
|---|---|---|---|---|---|---|
| 152 | 368 | 32 | 911 | 849 | 1.4 | 1.0 |
| Age (d) | Test Standards | Acceptable Quality [35,44] | Results | |
|---|---|---|---|---|
| Compressive strength (MPa) | 3 | KS F 2405 [30] | ≥21 | 45.0 ± 0.4 |
| Compressive strength (MPa) | 28 | KS F 2405 [30] | ≥21 | 56.9 ± 2.9 |
| Flexural strength (MPa) | 28 | KS F 2408 [50] | ≥3.15 | 5.7 ± 0.2 |
| Bond strength (MPa]) | 3 | KS F 2762 [51] | ≥1.4 | 1.8 ± 0.1 |
| Static modulus of elasticity (MPa) | 28 | KS F 2438 [53] | 11,300–78,000 | 58,376 ± 1.933 |
| Length change (%) | 14 | KS F 2424 [54] | ≤0.15 | 0.0309 ± 0.0028 |
| Water absorption coefficient (kg/cm2) | 28 | KS F 2609 [55] | ≤0.18 | 0.001161 ± 0.000120 |
| Chemical resistance (%) | 28 | ASTM C 267 [29] | ≥75 | 84.1 ± 4.4 |
| Abrasion resistance (mm) | 28 | ASTM C 779—method B [56] | ≤2 | 0.31 ± 0.07 |
| Resistance to freezing and thawing (%) | 28 | KS F 2456 [57] | ≥80 | 98.1 ± 0.3 |
| Resistance to chloride-ion penetration (C) | 56 | KS F 2711 [32] | ≤1000 | 937 ± 8 |
| Chloride content (kg/m3) | 28 | KS F 2715 [58] | ≤0.3 | 0.020 ± 0.009 |
| Coefficient of thermal expansion (×10−6/°C) | 28 | AASHTO T 336 [59] | 4–20 | 10.86 |
| Scaling resistance (m56/m28) | 56 | SS 13 72 44 [60] | <2 | 1.93 ± 0.06 |
| Crack resistance (–) | 56 | AASHTO PP 34 99 [61] | No cracks occurred | No cracks occurred |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Choi, S. Evaluating Use of Hydraulic Modified Sulfur Powder in Concrete Pavements: Laboratory Testing and Field Application. Buildings 2024, 14, 2231. https://doi.org/10.3390/buildings14072231
Oh S, Choi S. Evaluating Use of Hydraulic Modified Sulfur Powder in Concrete Pavements: Laboratory Testing and Field Application. Buildings. 2024; 14(7):2231. https://doi.org/10.3390/buildings14072231
Chicago/Turabian StyleOh, Sangwoo, and Seongcheol Choi. 2024. "Evaluating Use of Hydraulic Modified Sulfur Powder in Concrete Pavements: Laboratory Testing and Field Application" Buildings 14, no. 7: 2231. https://doi.org/10.3390/buildings14072231
APA StyleOh, S., & Choi, S. (2024). Evaluating Use of Hydraulic Modified Sulfur Powder in Concrete Pavements: Laboratory Testing and Field Application. Buildings, 14(7), 2231. https://doi.org/10.3390/buildings14072231






