Investigation on Properties of Pervious Concrete Containing Co-Sintering Lightweight Aggregate from Dredged Sediment and Rice Husks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. RSM Design
2.3. Characterization of LWA
2.4. Preparation of Pervious Concrete
2.5. Characteristics of Pervious Concrete
2.5.1. Compressive Strength of Pervious Concrete
2.5.2. Dynamic Adsorption of Pervious Concrete
3. Results and Discussion
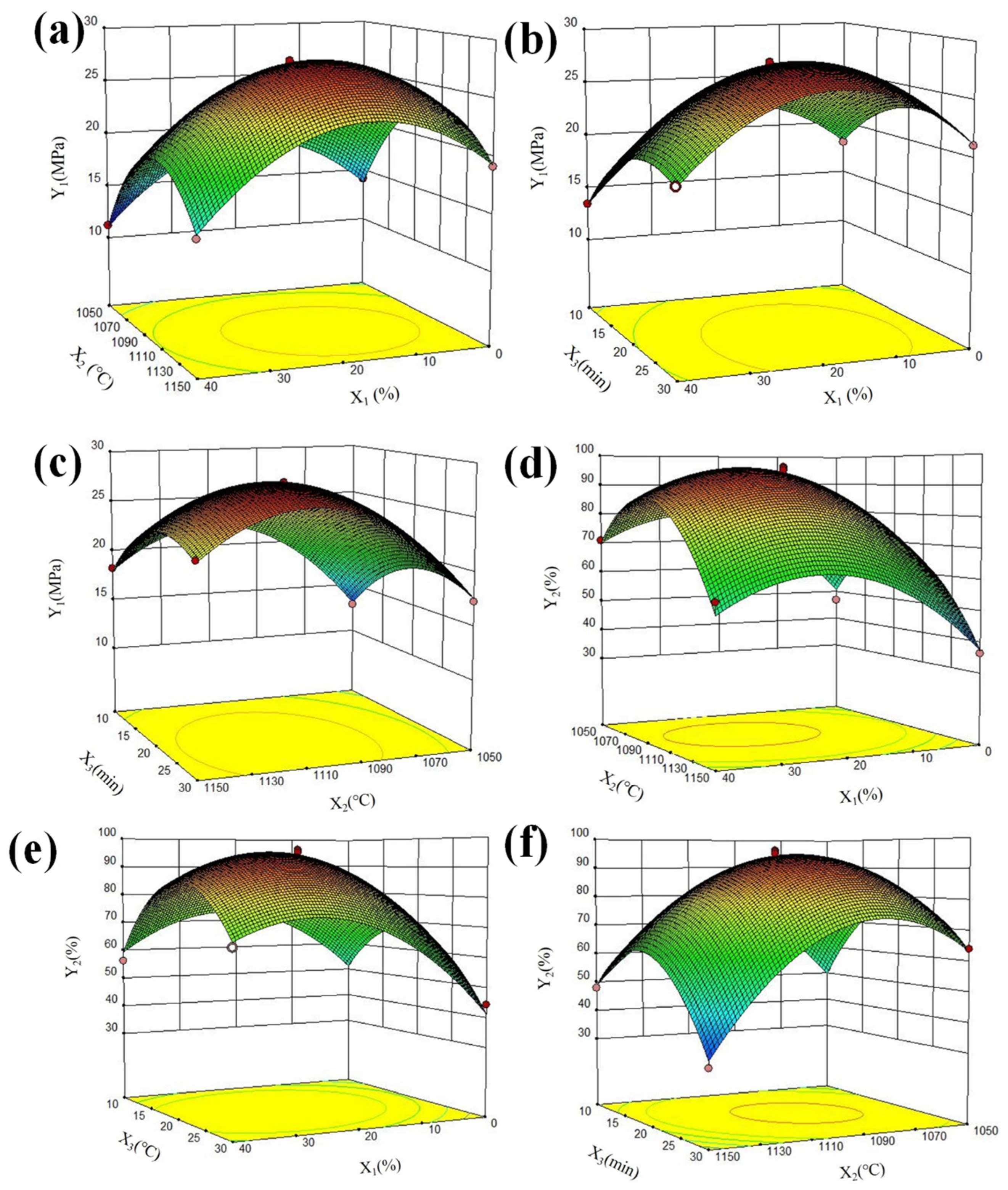
3.1. RSM Analysis
3.2. Process Optimization and Characterization of LWA
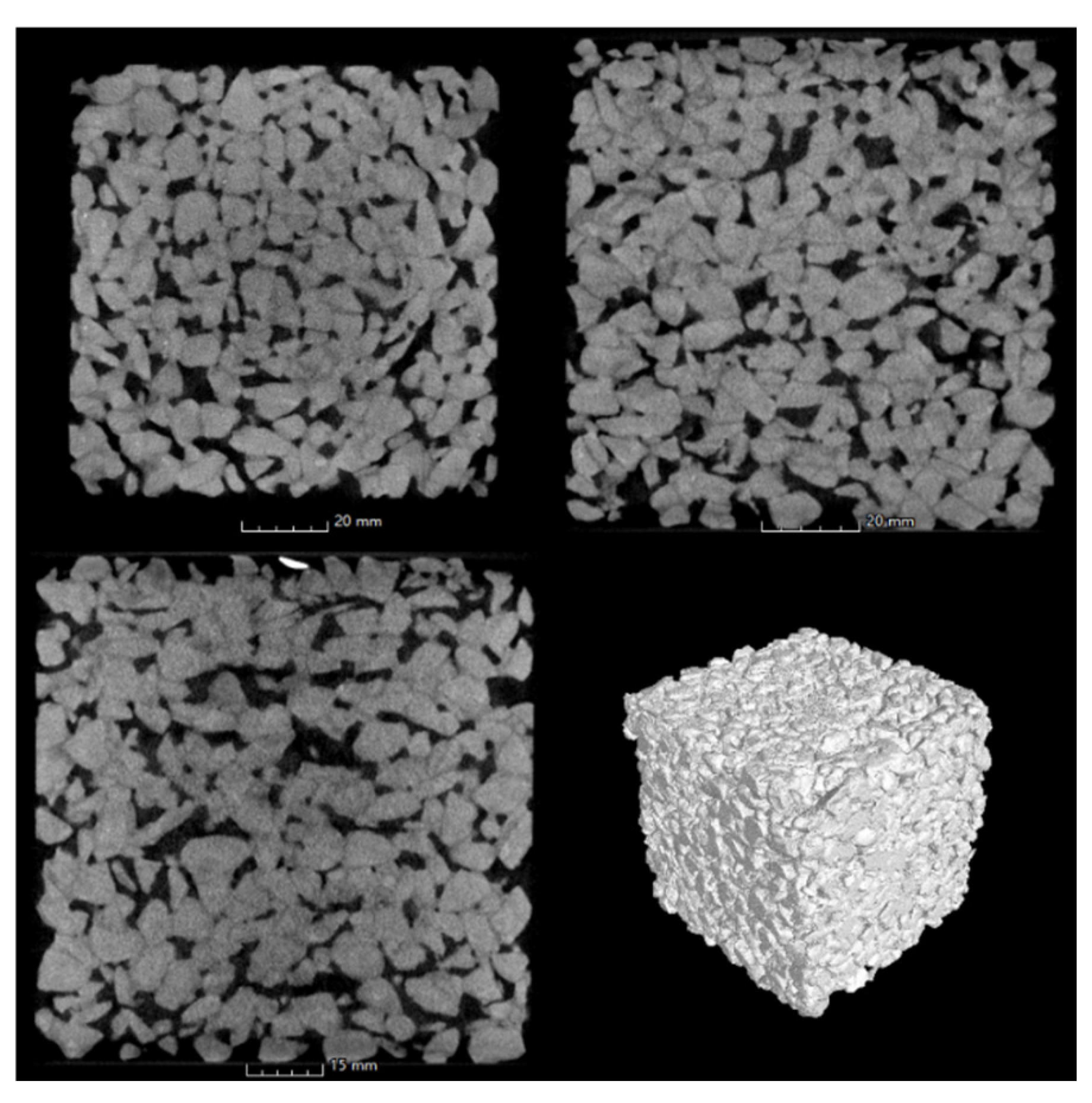
3.3. Performances of Pervious Concrete
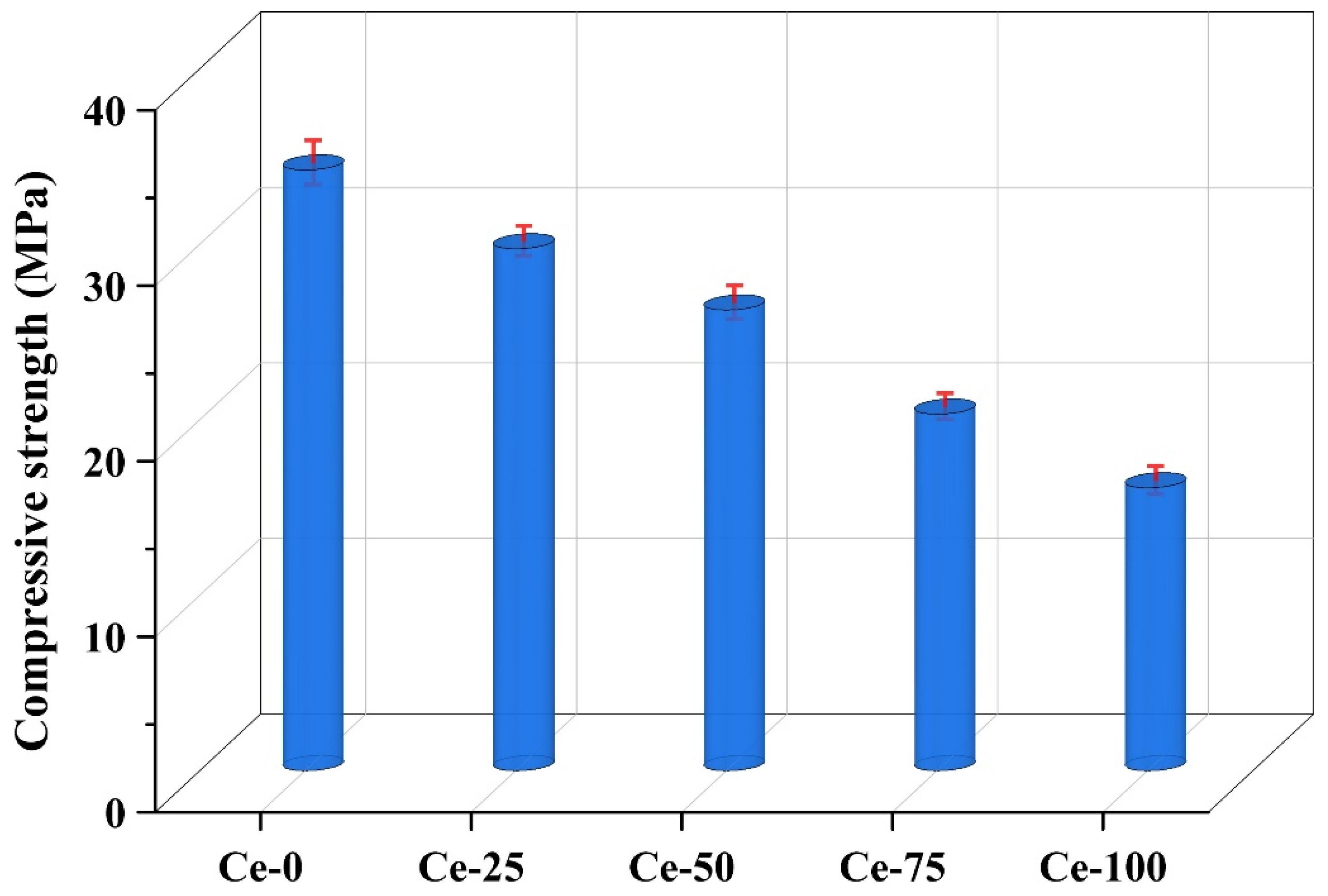
3.3.1. Mechanical Property of Pervious Concrete
3.3.2. Removal Capacity to Pb of Pervious Concrete
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Xie, Q.; Chen, S.; Xing, M.; Guan, T.; Wu, D. Inactivation of phosphorus in the sediment of the Lake Taihu by lanthanum modified zeolite using laboratory studies. Environ. Pollut. 2019, 247, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bravo, A.G.; Lagerkvist, A.; Bertilsson, S.; Sjöblom, R.; Kumpiene, J. Sources and remediation techniques for mercury contaminated soil. Environ. Int. 2015, 74, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.L. Distribution, Toxic Potential, and Influence of Land Use on Conventional and Emerging Contaminants in Urban Stormwater Pond Sediments. Arch. Environ. Contam. Toxicol. 2019, 76, 265–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Gao, G.; Fu, B.; Ye, Z.; Shen, Q. Exploring responses of lake area to river regulation and implications for lake restoration in arid regions. Ecol. Eng. 2019, 128, 18–26. [Google Scholar] [CrossRef]
- Perelo, L.W. Review: In situ and bioremediation of organic pollutants in aquatic sediments. J. Hazard. Mater. 2010, 177, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, P.; Yin, H. In situ control of internal nutrient loading and fluxes in the confluence area of an eutrophic lake with combined P inactivation agents and modified zeolite. Sci. Total Environ. 2021, 775, 145745. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, W.; Li, L.; Zhang, M.; Wang, D. Environmental impact and optimization of lake dredged-sludge treatment and disposal technologies based on life cycle assessment (LCA) analysis. Sci. Total Environ. 2021, 787, 147703. [Google Scholar] [CrossRef]
- Chaturvedi, E.; Maiti, M.; Laik, S.; Mandal, A. Mineralogical and structural characterization of the sediments of Krishna Godavari and Mahanadi Basin and their influences on hydrate formation kinetics. Adv. Powder Technol. 2021, 32, 1247–1263. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Xu, X.; Xu, J.; Song, S.; Yang, W.; Han, J. Effects of different soil amendments on dredged sediment improvement and impact assessment on reed planting. Ecol. Eng. 2024, 206, 107306. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Bai, Y.; Xu, G. Effects of sintering temperature on the characteristics of lightweight aggregate made from sewage sludge and river sediment. J. Alloys Compd. 2018, 748, 522–527. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, Y.; Sheng, L. Preparation of ceramsite from municipal sludge and its application in water treatment: A review. J. Environ. Manag. 2021, 287, 112374. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Wang, Y.; Yang, F.; Ma, Z. Preparation of CFB fly ash/sewage sludge ceramsite and the morphological transformation and release properties of sulfur. Constr. Build. Mater. 2023, 373, 130864. [Google Scholar] [CrossRef]
- Lim, Y.C.; Lin, S.-K.; Ju, Y.-R.; Wu, C.-H.; Lin, Y.-L.; Chen, C.-W.; Dong, C.-D. Reutilization of dredged harbor sediment and steel slag by sintering as lightweight aggregate. Process Saf. Environ. Prot. 2019, 126, 287–296. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Qin, J.; Liu, Y.; Qu, Y.; Liu, J.; Cao, R.; Zhang, Y. Mechanical properties of sintered ceramsite from iron ore tailings affected by two-region structure. Constr. Build. Mater. 2020, 240, 117919. [Google Scholar] [CrossRef]
- Wu, H.; Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Liang, S.; Lv, J.; Lu, S.; Wu, W.; Wu, S. Intensified organics and nitrogen removal in the intermittent-aerated constructed wetland using a novel sludge-ceramsite as substrate. Bioresour. Technol. 2016, 210, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, L.; Ma, X.; Mei, R.; Li, C.; Sun, Z. The formation of porous light ceramsite using Yellow River sediment and its application in concrete masonry production. Case Stud. Constr. Mater. 2022, 17, e01340. [Google Scholar] [CrossRef]
- Chen, H.-J.; Yang, M.-D.; Tang, C.-W.; Wang, S.-Y. Producing synthetic lightweight aggregates from reservoir sediments. Constr. Build. Mater. 2012, 28, 387–394. [Google Scholar] [CrossRef]
- Wang, L.; Shao, Y.; Zhao, Z.; Chen, S.; Shao, X. Optimized utilization studies of dredging sediment for making water treatment ceramsite based on an extreme vertex design. J. Water Process Eng. 2020, 38, 101603. [Google Scholar] [CrossRef]
- Wei, Y.-L.; Kuo, P.-J.; Yin, Y.-Z.; Huang, Y.-T.; Chung, T.-H.; Xie, X.-Q. Co-sintering oyster shell with hazardous steel fly ash and harbor sediment into construction materials. Constr. Build. Mater. 2018, 172, 224–232. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, M.; Muslim, H.; Hou, T.; Bian, B.; Yang, Z.; Yang, W.; Zhang, L. Co-utilization of lake sediment and blue-green algae for porous lightweight aggregate (ceramsite) production. Chemosphere 2022, 287, 132145. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Rong, H.; Zhou, X.; Chen, F.; Li, X.; Wang, T.; Hou, H. Adsorption mechanism of lead ions on porous ceramsite prepared by co-combustion ash of sewage sludge and biomass. Sci. Total Environ. 2020, 702, 135017. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J. Hazard. Mater. 2006, 134, 257–267. [Google Scholar] [CrossRef]
- Lu, M.; Wang, R.; Xue, Y.; Ren, L.; Chen, S.; Liu, J.; Mei, M.; Wang, T.; Li, J. Eco-friendly ceramsite from dredged sediment/biomass for Pb(II) removal: Process optimization and adsorption mechanistic insights. J. Environ. Chem. Eng. 2022, 10, 108939. [Google Scholar] [CrossRef]
- Mymrin, V.; Scremim, C.B.; Stella, J.C.; Pan, R.C.Y.; Avanci, M.A.; Bosco, J.C.; Rolim, P. Environmentally clean materials from contaminated marine dredged sludge, wood ashes and lime production wastes. J. Clean. Prod. 2021, 307, 127074. [Google Scholar] [CrossRef]
- Wang, T.; Cai, C.; Xue, Y.; Xiao, Y.; Chen, S.; Liu, J.; Mei, M.; Li, J. Regulation of ash slagging behavior for sewage sludge by rice husk addition: Focusing on control mechanisms. J. Clean. Prod. 2021, 284, 124677. [Google Scholar] [CrossRef]
- Lima, G.T.d.S.; Rocha, J.C.; Cheriaf, M. Investigation of the properties of pervious concrete with a recycled aggregate designed with a new combination of admixture. Constr. Build. Mater. 2022, 340, 127710. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Fawzy, A.M. Experimental investigation on permeability indices and strength of modified pervious concrete with recycled concrete aggregate. Constr. Build. Mater. 2018, 193, 105–127. [Google Scholar] [CrossRef]
- Zhong, R.; Leng, Z.; Poon, C.-s. Research and application of pervious concrete as a sustainable pavement material: A state-of-the-art and state-of-the-practice review. Constr. Build. Mater. 2018, 183, 544–553. [Google Scholar] [CrossRef]
- Lyu, Q.; Dai, P.; Chen, A. Sandwich-structured porous concrete manufactured by mortar-extrusion and aggregate-bed 3D printing. Constr. Build. Mater. 2023, 392, 131909. [Google Scholar] [CrossRef]
- Wijeyawardana, P.; Nanayakkara, N.; Gunasekara, C.; Karunarathna, A.; Law, D.; Pramanik, B.K. Improvement of heavy metal removal from urban runoff using modified pervious concrete. Sci. Total Environ. 2022, 815, 152936. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Z.; Behfarnia, K.; Faghihian, H.; Soltaninia, S.; Behravan, A.; Ahmadi, S. Application of pervious alkali-activated slag concrete to adsorb runoff contaminants. Constr. Build. Mater. 2023, 375, 130998. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Harvey, J.T.; Liang, X.; Xie, N.; Jia, M. Purification effect on runoff pollution of porous concrete with nano-TiO2 photocatalytic coating. Transp. Res. Part D Transp. Environ. 2021, 101, 103101. [Google Scholar] [CrossRef]
- Quan, J.; Li, X.; Liang, S.; Hu, G.; Li, X.; Yu, W.; Yuan, S.; Duan, H.; Hu, J.; Hou, H.; et al. Enhancing phosphorus removal by novel porous concrete fabricated with alkali-activated aggregate derived from industrial solid wastes. Resour. Conserv. Recycl. 2024, 204, 107520. [Google Scholar] [CrossRef]
- Holmes, R.R.; Hart, M.L.; Kevern, J.T. Heavy metal removal capacity of individual components of permeable reactive concrete. J. Contam. Hydrol. 2017, 196, 52–61. [Google Scholar] [CrossRef]
- Marathe, S.; Sadowski, Ł.; Shree, N. Geopolymer and alkali-activated permeable concrete pavements: Bibliometrics and systematic current state of the art review, applications, and perspectives. Constr. Build. Mater. 2024, 421, 135586. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Chen, J.P. Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA. Water Res. 2016, 101, 564–573. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.-s.; Chen, Z.; Xue, Q.; Sun, Q.; Zhou, Y.; Chen, X.; Liu, L.; Poon, C.S. Production of lightweight aggregate ceramsite from red mud and municipal solid waste incineration bottom ash: Mechanism and optimization. Constr. Build. Mater. 2021, 287, 122993. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standards for Surface Water. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Riley, C.M. Relation of Chemical Properties to the Bloating of Clays. J. Am. Ceram. Soc. 1951, 34, 121–128. [Google Scholar] [CrossRef]
- T/CSTM 00548-2022; Light Aggregate with High Strength Prepared from Solid Waste. Chinese Society for Testing & Materials, Zhongguancun: Beijing, China, 2022.
- GB/T50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2019.
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Long, Y.; Pu, K.; Yang, Y.; Huang, H.; Fang, H.; Shen, D.; Geng, H.; Ruan, J.; Gu, F. Preparation of High-strength ceramsite from municipal solid waste incineration fly ash and clay based on CaO-SiO2-Al2O3 system. Constr. Build. Mater. 2023, 368, 130492. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Harun, Z.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A novel green ceramic hollow fiber membrane (CHFM) derived from rice husk ash as combined adsorbent-separator for efficient heavy metals removal. Ceram. Int. 2017, 43, 4716–4720. [Google Scholar] [CrossRef]
- Wu, X.; Gu, F.; Su, C.; Wang, W.; Pu, K.; Shen, D.; Long, Y. Preparing high-strength ceramsite from ferronickel slag and municipal solid waste incineration fly ash. Ceram. Int. 2022, 48, 34265–34272. [Google Scholar] [CrossRef]
- Zou, J.L.; Xu, G.R.; Li, G.B. Ceramsite obtained from water and wastewater sludge and its characteristics affected by Fe2O3, CaO, and MgO. J. Hazard. Mater. 2009, 165, 995–1001. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Xie, S.; Pan, L.; Li, C.; You, F.; Wang, Y. Immobilization of heavy metals in ceramsite produced from sewage sludge biochar. Sci. Total Environ. 2018, 628–629, 131–140. [Google Scholar] [CrossRef]
- Raif Boğa, A.; Ferdi Şenol, A. The effect of waste marble and basalt aggregates on the fresh and hardened properties of high strength self-compacting concrete. Constr. Build. Mater. 2023, 363, 129715. [Google Scholar] [CrossRef]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Chen, S.; Ruan, S.; Zeng, Q.; Liu, Y.; Zhang, M.; Tian, Y.; Yan, D. Pore structure of geopolymer materials and its correlations to engineering properties: A review. Constr. Build. Mater. 2022, 328, 127064. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, S.; Bu, R.; Cai, X.; Sun, X. Purification of runoff pollution using porous asphalt concrete incorporating zeolite powder. Constr. Build. Mater. 2024, 411, 134740. [Google Scholar] [CrossRef]



| DS | RH | ||
|---|---|---|---|
| Proximate analysis | Ash | 88.21 | 14.17 |
| Volatile Matter | 7.91 | 71.41 | |
| Fixed carbon | 3.88 | 14.42 | |
| Ultimate analysis | C | 3.04 | 41.68 |
| H | 1.31 | 5.76 | |
| O | 5.68 | 37.68 | |
| N | 1.76 | 0.71 | |
| Ash analysis | Al2O3 | 17.33 | 1.32 |
| SiO2 | 60.78 | 86.94 | |
| Fe2O3 | 3.02 | 2.24 | |
| Na2O | 4.71 | 0.12 | |
| K2O | 2.31 | 3.62 | |
| CaO | 5.02 | 1.55 | |
| P2O5 | 0.78 | 0.28 | |
| MgO | 1.22 | 0.93 | |
| Trace element leaching analysis | Cd | 0.21 | N.d. |
| Cd | 0.58 | 0.044 | |
| Cr | 0.52 | 0.005 | |
| Cu | 1.01 | 0.044 | |
| Zn | 1.44 | 1.074 |
| Items | Optimized Direction | Parameters | Predicted Value | ||
|---|---|---|---|---|---|
| Min | Max | Optimum | |||
| Level | X1 (%) | Set value | 0 | 50 | 21 |
| X2 (°C) | Set value | 1050 | 1150 | 1100 | |
| X3 (min) | Set value | 10 | 30 | 21 | |
| Response values | Y1 (MPa) | Max | 11.19 | 40 | 27.57 |
| Y2 (%) | Max | 31.06 | 100 | 94.00 | |
| Indexes | SDC-H | Limit Value a |
|---|---|---|
| Bulk density (kg/m3) | 1182 | ≤1200 |
| Cylinder compressive strength (MPa) | 27.63 | ≥12.5 |
| Water absorption (%) | 7.6 | ≤10 |
| Softening coefficient | 0.87 | ≥0.8 |
| Sturdiness (%) | 3.15 | ≤8 |
| Pb (mg/L) | N.d. | <0.3 |
| Cd (mg/L) | N.d. | <0.03 |
| Cr (mg/L) | 0.027 | <0.2 |
| Cu (mg/L) | 0.094 | <1.0 |
| Zn (mg/L) | 0.022 | <1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, H.; Yue, K.; He, Y.; Hu, Z.; Wang, R.; Huang, S.; Zhou, X.; Wang, T. Investigation on Properties of Pervious Concrete Containing Co-Sintering Lightweight Aggregate from Dredged Sediment and Rice Husks. Buildings 2024, 14, 2276. https://doi.org/10.3390/buildings14082276
Rong H, Yue K, He Y, Hu Z, Wang R, Huang S, Zhou X, Wang T. Investigation on Properties of Pervious Concrete Containing Co-Sintering Lightweight Aggregate from Dredged Sediment and Rice Husks. Buildings. 2024; 14(8):2276. https://doi.org/10.3390/buildings14082276
Chicago/Turabian StyleRong, Hao, Kedong Yue, Yuting He, Zhen Hu, Rui Wang, Shuangshuang Huang, Xian Zhou, and Teng Wang. 2024. "Investigation on Properties of Pervious Concrete Containing Co-Sintering Lightweight Aggregate from Dredged Sediment and Rice Husks" Buildings 14, no. 8: 2276. https://doi.org/10.3390/buildings14082276





