Effect of Microwave Pretreatment on the Properties and Microstructure of Low-Concentration Carbon Dioxide Early Cured Cement-Based Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation and Curing of Specimens
2.3. Methods
2.3.1. Moisture Content Test
2.3.2. Compressive Strength Test
2.3.3. Carbonation Depth Test
2.3.4. CO2 Absorptivity Test
2.3.5. Thermogravimetric Analysis Test (TGA)
2.3.6. X-ray Diffraction Test (XRD)
2.3.7. Scanning Electron Microscope Test (SEM)
2.3.8. Mercury Intrusion Porosimetry Test (MIP)
3. Results and Discussion
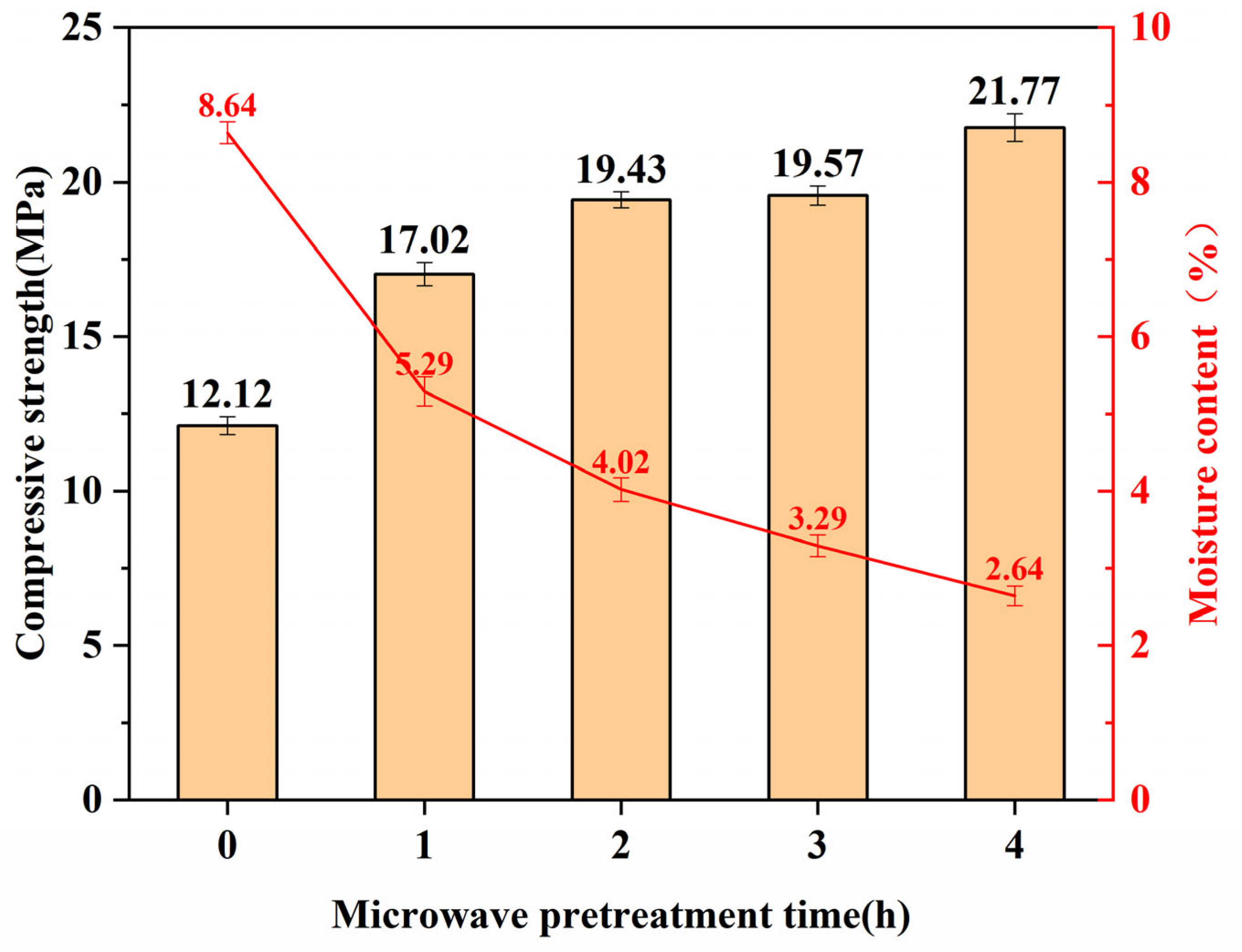
3.1. The Influence of Moisture Content
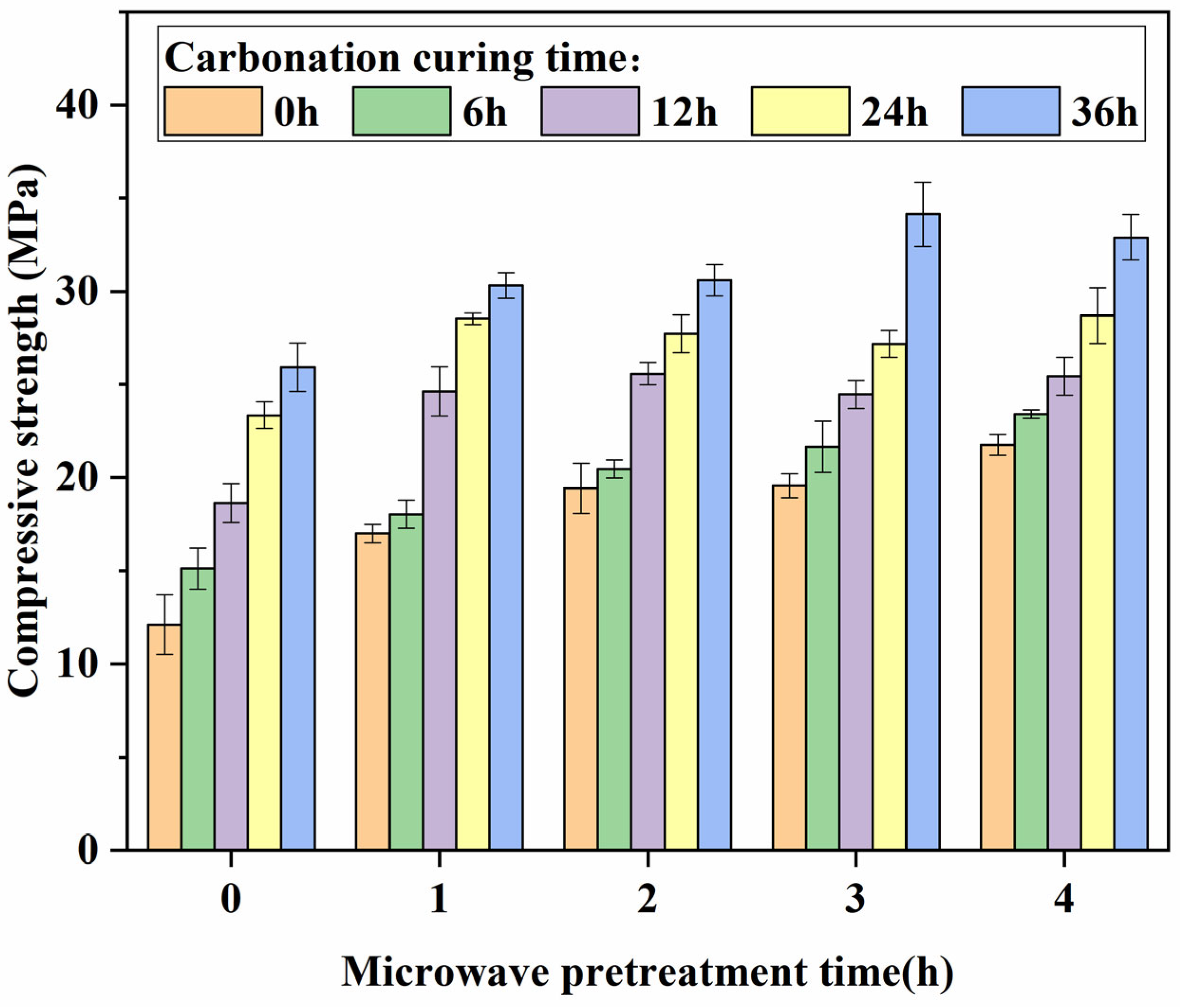
3.2. The Influence of Compressive Strength
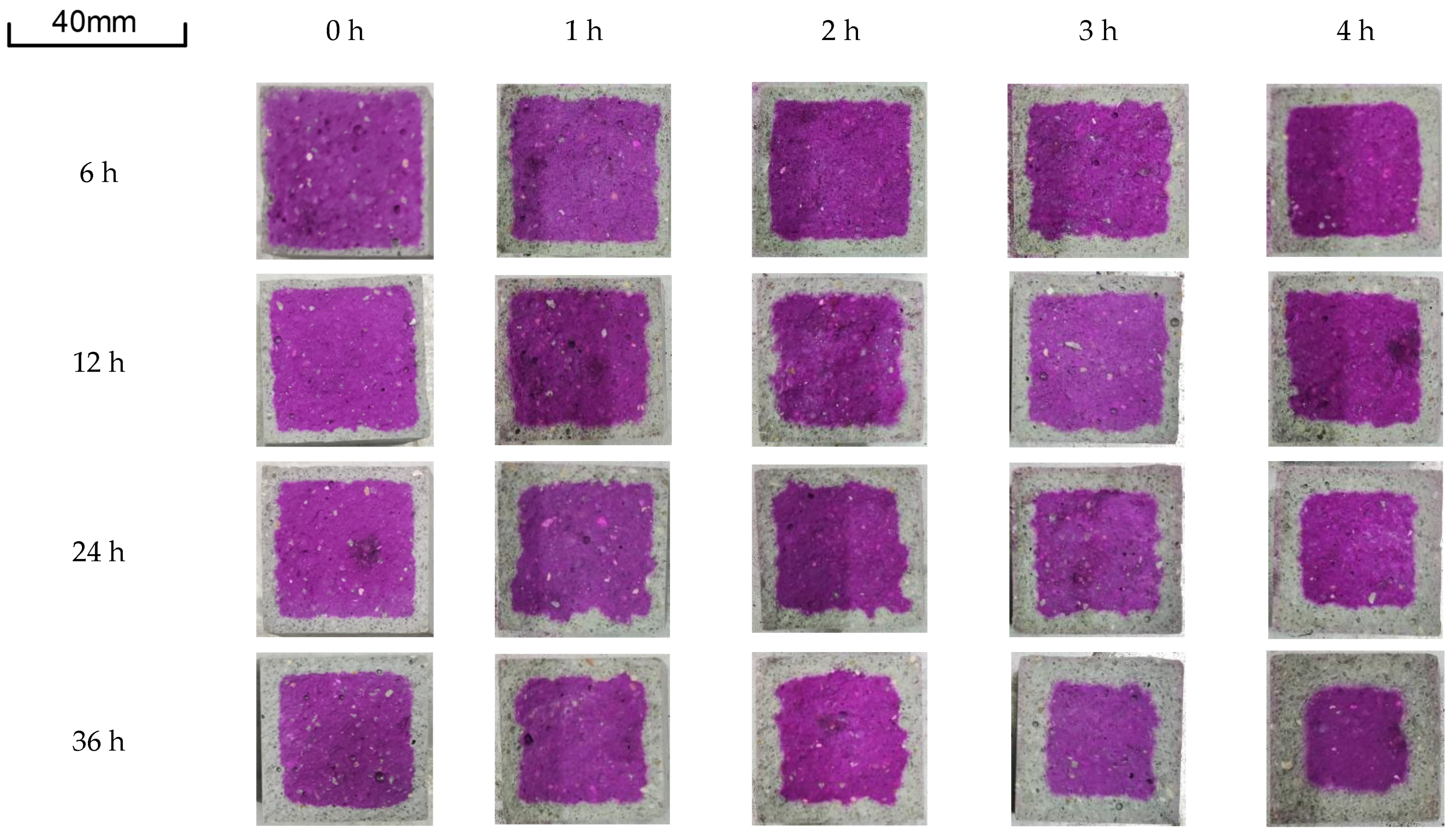
3.3. Carbonation Depth Analysis
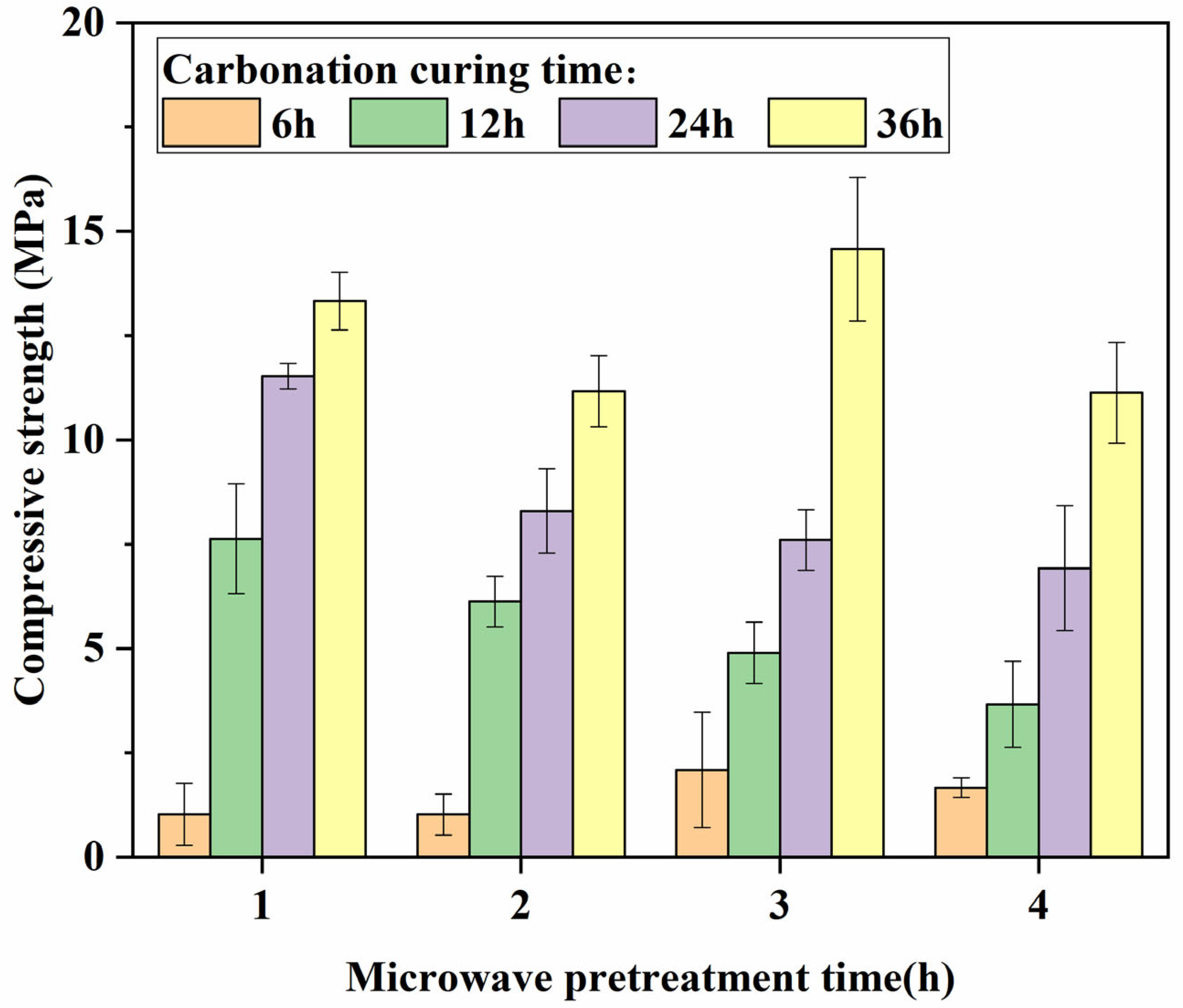
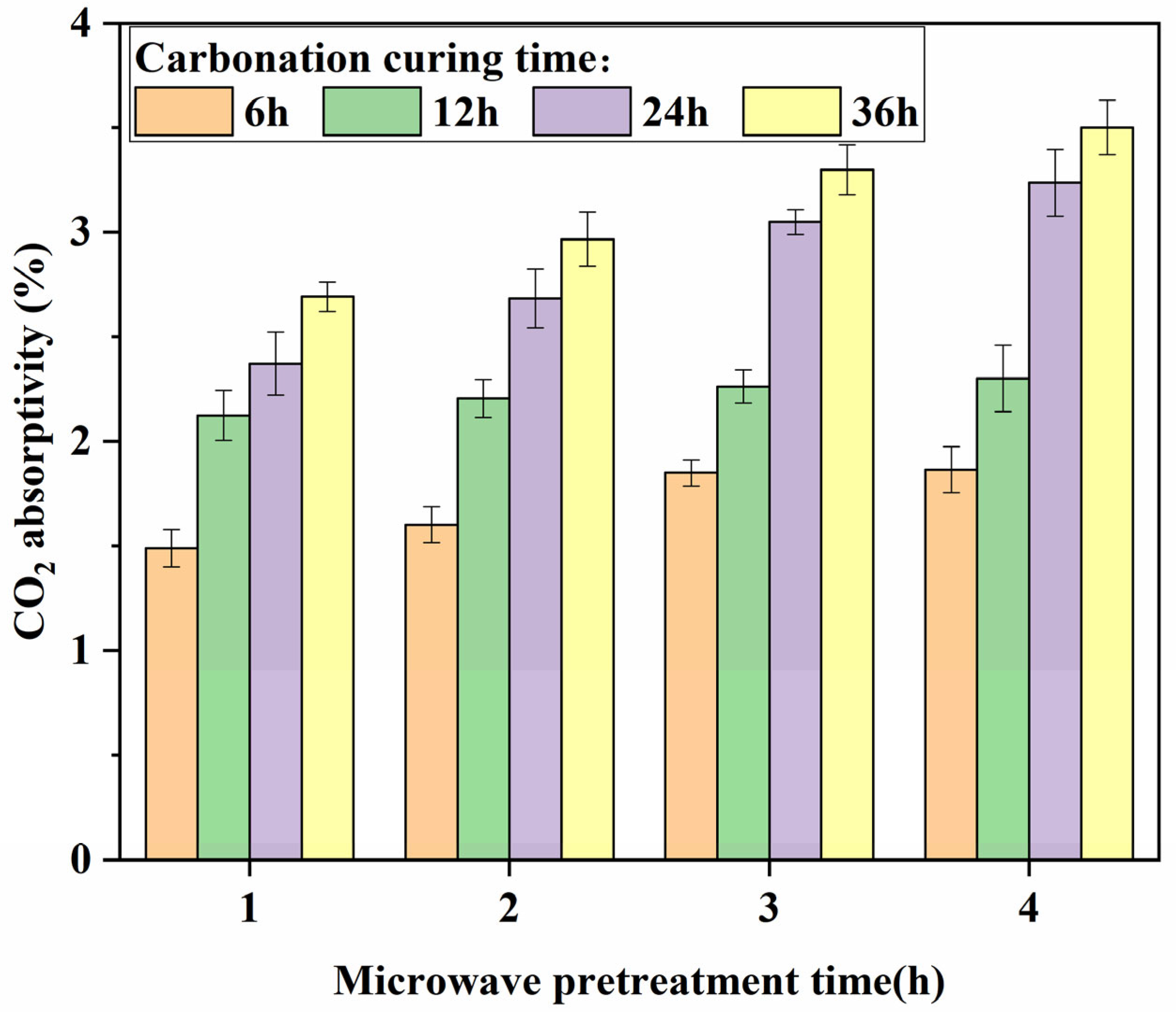
3.4. CO2 Absorptivity Analysis
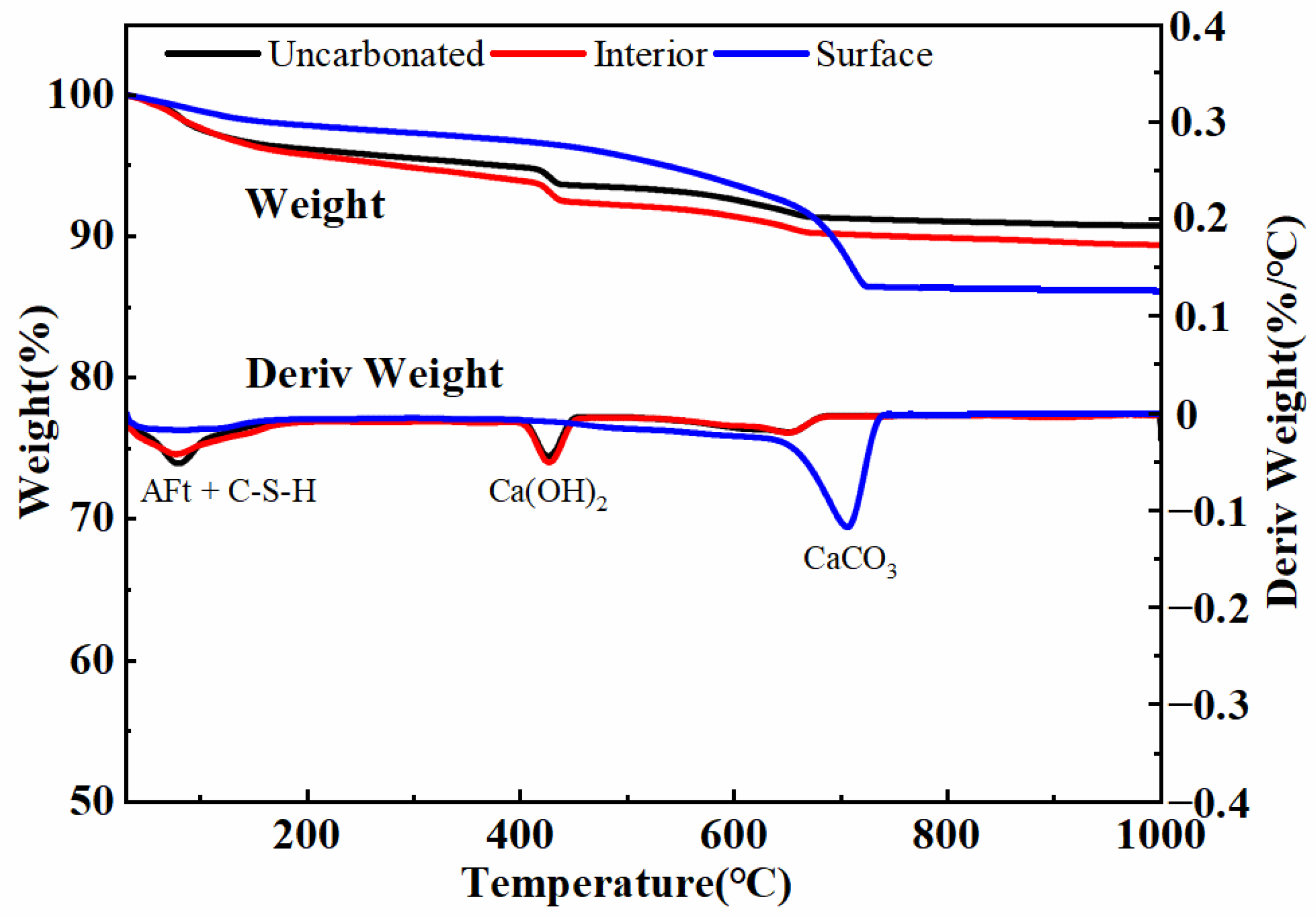
3.5. Thermogravimetric Analysis (TGA)
3.6. X-ray Diffraction Analysis (XRD)
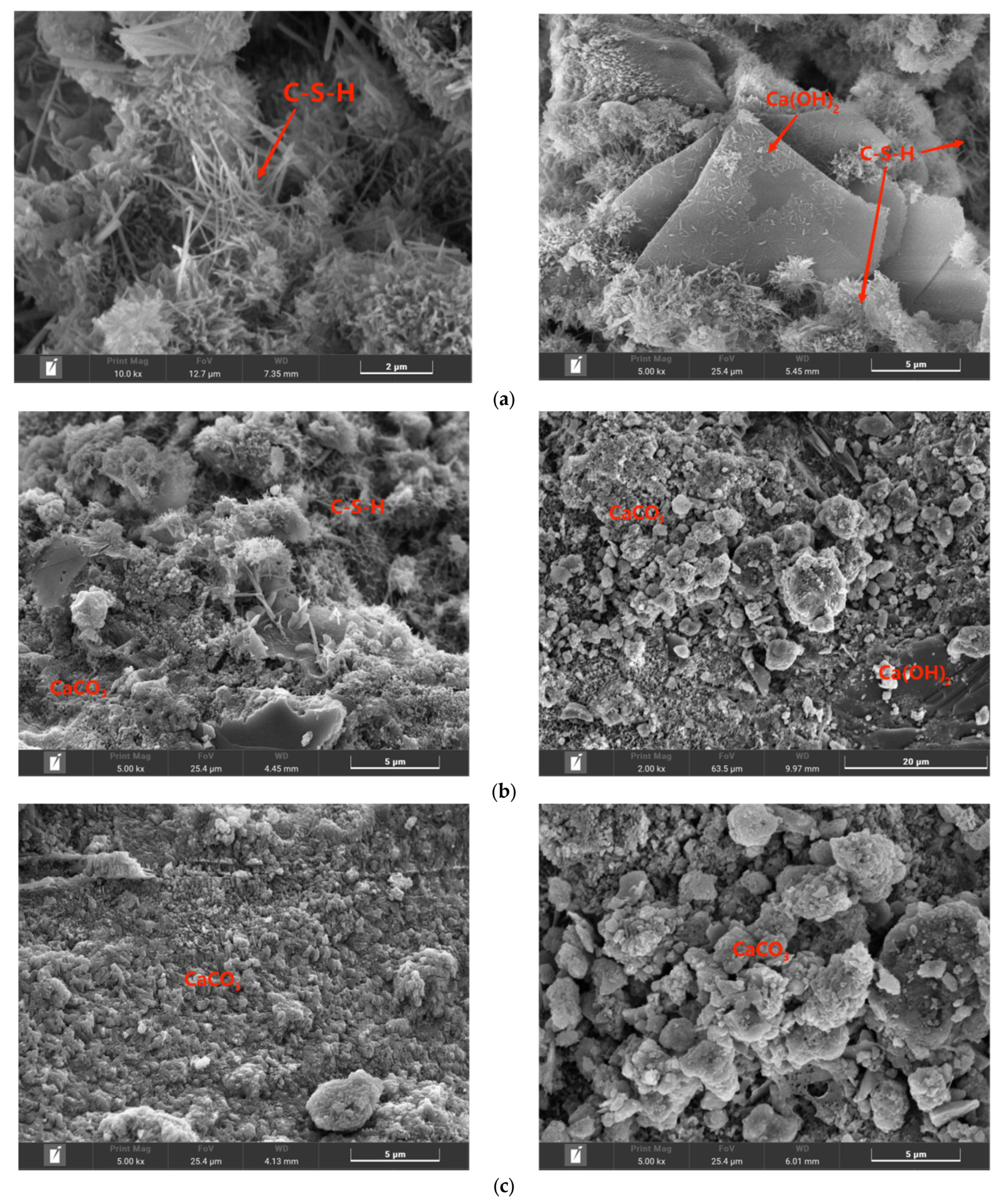
3.7. Scanning Electron Microscope Analysis
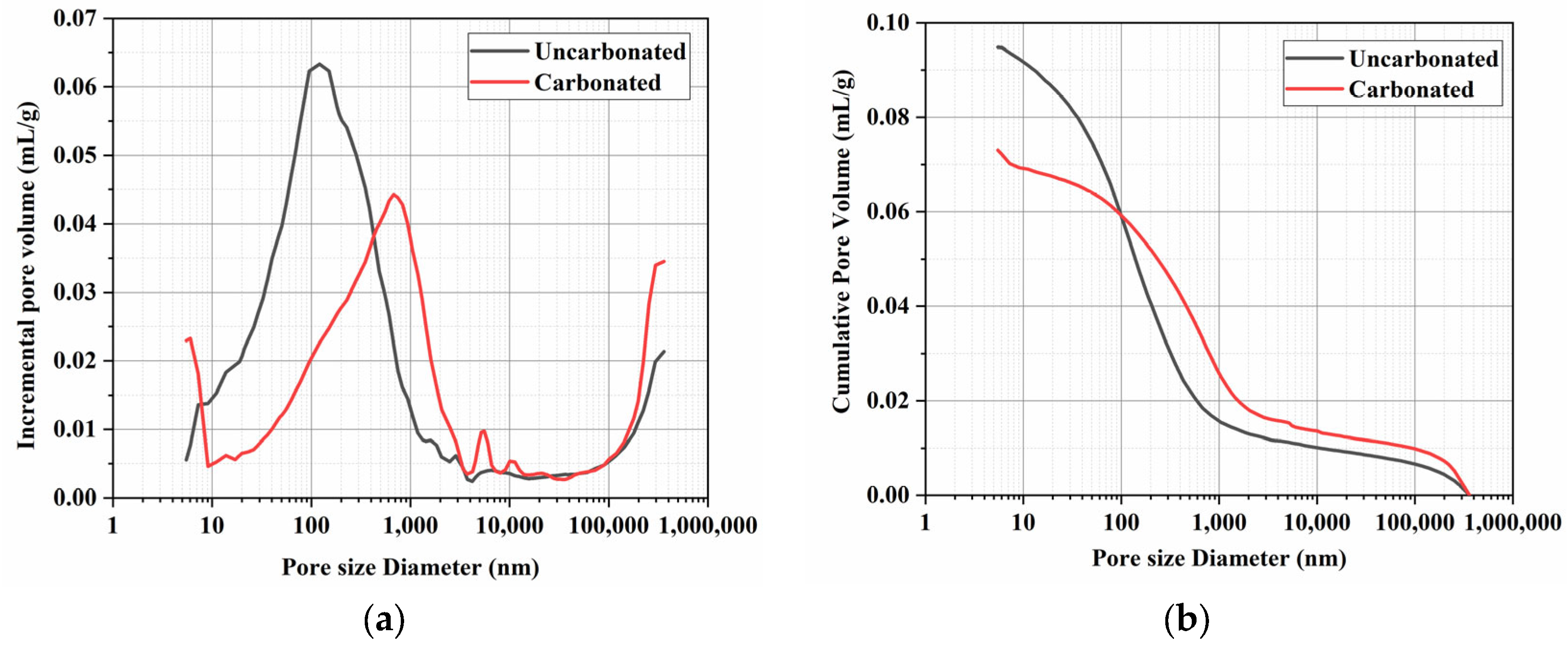
3.8. Pore Structure Analysis
4. Conclusions
- (1)
- The microwave pretreatment accelerates the hydration reaction rate, enhances the compressive strength of the specimens, and significantly reduces their moisture content, thereby facilitating faster penetration of CO2 into the specimens and enhancing carbonation efficiency. The initial increase in compressive strength and decrease in moisture content due to microwave pretreatment are rapid. After 1 h of microwave pretreatment, the compressive strength of the specimens increases from 12.12 MPa to 17.02 MPa, and the moisture content decreases from 8.64% to 5.29%. However, the effects start to diminish afterward. After 4 h of microwave pretreatment, the compressive strength reaches 21.77 MPa, and the moisture content eventually drops to 2.64%. After 36 h of carbonation curing, the specimen with 3 h of microwave pretreatment and a moisture content of 3.29% exhibits the highest compressive strength.
- (2)
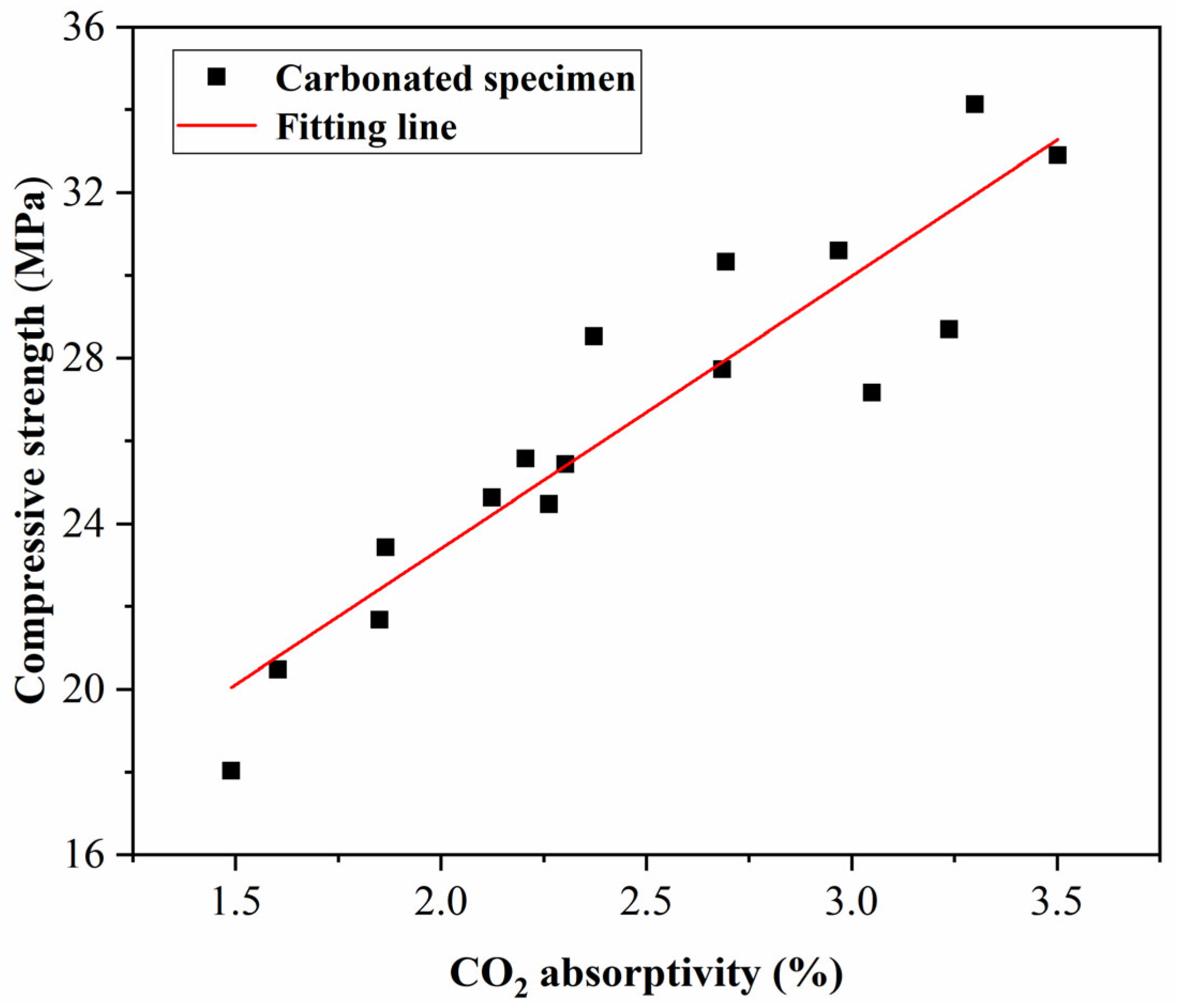
- The absorption of CO2 is relatively rapid in the early stage of carbonation curing, with over 50% of the CO2 absorption occurring within the 0–6 h period of carbonation curing, and during this stage, the variation in microwave pretreatment time has minimal impact on the absorption. In the later stage of carbonation curing, the carbonation rate significantly decreases, and specimens with longer microwave pretreatment times exhibit higher CO2 absorption. The carbonation rate and compressive strength of the specimens were linearly related to the CO2 absorption rate, and the fitting line was close to the origin of the coordinates.
- (3)
- The hydration products and microstructure of the uncarbonated part inside the specimens are generally consistent with the normal curing state. They are mainly influenced by microwave pretreatment, which promotes hydration reactions and is not affected by the external CO2 atmosphere. The fully carbonated part of the specimens lacks C-S-H, AFt, and Ca(OH)2, as they transform into CaCO3 during the carbonation process.
- (4)
- After microwave pretreatment and carbonation curing, some pores that were originally in the intermediate range differentiated, forming more extremely fine pores and large pores. The average pore size of the samples increased from 60 nm to 74 nm, but the porosity decreased from 19.65% to 15.77%. Although carbonation curing enlarges the average pore size of the samples, it also serves a filling function, making the samples more compact and reducing the porosity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Li, X.; Liu, H.; Boczkaj, G.; Cao, Y.; Wang, C. A review on carbon storage via mineral carbonation: Bibliometric analysis, research advances, challenges, and perspectives. Sep. Purif. Technol. 2024, 338, 126558. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, K.; Chen, L.; Yuan, Q. Review on accelerated carbonation of calcium-bearing minerals: Carbonation behaviors, reaction kinetics, and carbonation efficiency improvement. J. Build. Eng. 2024, 86, 108826. [Google Scholar] [CrossRef]
- Allen, M.; Mustafa, B.; Shukla, P. GLOBAL WARMING of 1.5 °C—An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts To Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Statistical Communiqué of the People’s Republic of China on the 2022 National Economic and Social Development; National Bureau of Statistics of China: Beijing, China, 2023; pp. 12–29.
- Monkman, S.; MacDonald, M. On carbon dioxide utilization as a means to improve the sustainability of ready-mixed concrete. J. Clean. Prod. 2017, 167, 365–375. [Google Scholar] [CrossRef]
- Zhang, R.; Scott, A.N.; Panesar, D.K. Carbonation and CO2 reabsorption of cement-based materials: Influence of limestone filler and ground-granulated blast-furnace slag. Constr. Build. Mater. 2024, 416, 135166. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, W.; Ye, J.; Li, J.; Lu, Z.; Ren, X. Mechanism of interaction between hydration–carbonation of C2S. Constr. Build. Mater. 2024, 412, 134891. [Google Scholar] [CrossRef]
- Ahmad, S.; Assaggaf, R.A.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Adekunle, S.K.; Ali, S.I. Effects of carbonation pressure and duration on strength evolution of concrete subjected to accelerated carbonation curing. Constr. Build. Mater. 2017, 136, 565–573. [Google Scholar] [CrossRef]
- Zajac, M.; Maruyama, I.; Iizuka, A.; Skibsted, J. Enforced carbonation of cementitious materials. Cem. Concr. Res. 2023, 174, 107285. [Google Scholar] [CrossRef]
- Fernández Bertos, M.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, J.; Tu, Z.; Wang, D. Progresses in CO2 Curing of Concrete. Cailiao Daobao/Mater. Rev. 2017, 31, 134–138. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Costa, J.O.; Milestone, N.B.; Lynsdale, C.J.; Streatfield, R.E. Carbonation of CH and C–S–H in composite cement pastes containing high amounts of BFS. Cem. Concr. Res. 2010, 40, 284–292. [Google Scholar] [CrossRef]
- Shi, C.; Liu, M.; He, P.; Ou, Z. Factors affecting kinetics of CO2 curing of concrete. J. Sustain. Cem. Based Mater. 2012, 1, 24–33. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y. Studies on some factors affecting CO2 curing of lightweight concrete products. Resour. Conserv. Recycl. 2008, 52, 1087–1092. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y. CO2 Curing of Concrete Blocks. Concr. Int. 2009, 31, 39–43. [Google Scholar]
- Junior, A.; Toledo Filho, R.; Fairbairn, E.; Dweck, J. CO2 sequestration by high initial strength Portland cement pastes. J. Therm. Anal. Calorim. 2013, 113, 1577–1584. [Google Scholar] [CrossRef]
- Jang, J.G.; Kim, G.M.; Kim, H.J.; Lee, H.K. Review on recent advances in CO2 utilization and sequestration technologies in cement-based materials. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Neves Junior, A.; Toledo Filho, R.D.; de Moraes Rego Fairbairn, E.; Dweck, J. The effects of the early carbonation curing on the mechanical and porosity properties of high initial strength Portland cement pastes. Constr. Build. Mater. 2015, 77, 448–454. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.; Shi, C. CO2 curing for improving the properties of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2013, 42, 1–8. [Google Scholar] [CrossRef]
- Shi, C.; He, P.; Tu, Z.; Cao, Z. Effect of pre-conditioning on process and microstructure of carbon dioxide cured concrete. Kuei Suan Jen Hsueh Pao/J. Chin. Ceram. Soc. 2014, 42, 996–1004. [Google Scholar] [CrossRef]
- Shi, C.; Wang, D.; He, F.; Liu, M. Weathering properties of CO2-cured concrete blocks. Resour. Conserv. Recycl. 2012, 65, 11–17. [Google Scholar] [CrossRef]
- Cho, J.M.; Oh, Y.-K.; Lee, J.; Chang, Y.K.; Park, W.-K. Development of dual strain microalgae cultivation system for the direct carbon dioxide utilization of power plant flue gas. Bioresour. Technol. 2024, 393, 130051. [Google Scholar] [CrossRef]
- Kikkinides, E.S.; Yang, R.T.; Cho, S.H. Concentration and recovery of carbon dioxide from flue gas by pressure swing adsorption. Ind. Eng. Chem. Res. 1993, 32, 2714–2720. [Google Scholar] [CrossRef]
- Elsener, B. Corrosion of Steel in Concrete. In Materials Science and Technology; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Galan, I.; Andrade, C.; Castellote, M. Natural and accelerated CO2 binding kinetics in cement paste at different relative humidities. Cem. Concr. Res. 2013, 49, 21–28. [Google Scholar] [CrossRef]
- Shi, C.; He, F.; Wu, Y. Effect of pre-conditioning on CO2 curing of lightweight concrete blocks mixtures. Constr. Build. Mater. 2012, 26, 257–267. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Yang, L.; Ding, Y. Microwave curing cement-fly ash blended paste. Constr. Build. Mater. 2021, 282, 122685. [Google Scholar] [CrossRef]
- Mishra, R.R.; Sharma, A.K. Microwave–material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos. Part A Appl. Sci. Manuf. 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Durability of concrete pipes subjected to combined steam and carbonation curing. Constr. Build. Mater. 2011, 25, 3345–3355. [Google Scholar] [CrossRef]
- Sharma, D.; Goyal, S. Accelerated carbonation curing of cement mortars containing cement kiln dust: An effective way of CO2 sequestration and carbon footprint reduction. J. Clean. Prod. 2018, 192, 844–854. [Google Scholar] [CrossRef]
- Liu, M. Microwave-Based Preconditioning Technique for Accelerated Carbonation Curing of Cementitious Materials. Ph.D. Thesis, University College London, London, UK, 2019. [Google Scholar]
- Al-Duri, B.; McIntyre, S. Comparison of drying kinetics of foods using a fan-assisted convection oven, a microwave oven and a combined microwave/convection oven. J. Food Eng. 1992, 15, 139–155. [Google Scholar] [CrossRef]
- Li, T.; Yue, Z.; Li, J.; Li, Q.; Li, Y.; Chen, G. Experimental study of microwave heating on mechanical properties of fly ash-based cementitious materials. J. Build. Eng. 2024, 83, 108454. [Google Scholar] [CrossRef]
- Zhou, F.; Pan, G.; Meng, H.; Mi, R. Effect of secondary curing on the performance of microwave cured concrete. Constr. Build. Mater. 2022, 330, 127256. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Zhang, L.; Shang, Y.; Wang, H. Experimental research on drying control condition with minimal effect on concrete strength. Constr. Build. Mater. 2017, 135, 194–202. [Google Scholar] [CrossRef]
- Salem, S.; Eissa, E.; Zarif, E.; Sherif, S.; Shazly, M. Influence of moisture content on the concrete response under dynamic loading. Ain Shams Eng. J. 2023, 14, 101976. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). State Administration for Maket Regulation, Standardization Administration of the P.R.C: Beijing, China, 2021.
- Mangat, P.S.; Grigoriadis, K.; Abubakri, S. Microwave curing parameters of in-situ concrete repairs. Constr. Build. Mater. 2016, 112, 856–866. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Yu, M.; Liu, T.; Zhu, J.; Yin, T.; Liu, K.; Yu, R. Exploring the effect of the micropore on the microstructure development of low water-cement ratio composite (LW/B-CC) subjected to microwave pre-curing. Constr. Build. Mater. 2024, 416, 135141. [Google Scholar] [CrossRef]
- Makul, N. Effect of low-pressure microwave-accelerated curing on the drying shrinkage and water permeability of Portland cement pastes. Case Stud. Constr. Mater. 2020, 13, e00358. [Google Scholar] [CrossRef]
- Monkman, S.; Kenward, P.A.; Dipple, G.; MacDonald, M.; Raudsepp, M. Activation of cement hydration with carbon dioxide. J. Sustain. Cem. Based Mater. 2018, 7, 160–181. [Google Scholar] [CrossRef]
- Wu, X.; Dong, J.; Tang, M. Microwave curing technique in concrete manufacture. Cem. Concr. Res. 1987, 17, 205–210. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Y.; Wang, Q.; Tian, H.; Liu, D. A study on strength characteristics of concrete under variable temperature curing conditions in ultra-high geothermal tunnels. Constr. Build. Mater. 2019, 229, 116989. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Ma, C.; Cai, C.; Wang, J. Effect of surface curing condition on the humidity field and moisture transfer in concrete. Constr. Build. Mater. 2024, 411, 134701. [Google Scholar] [CrossRef]
- Jerga, J. Physico-mechanical properties of carbonated concrete. Constr. Build. Mater. 2004, 18, 645–652. [Google Scholar] [CrossRef]
- Chen, K.-y.; Xia, J.; Wu, R.-j.; Shen, X.-y.; Chen, J.-j.; Zhao, Y.-x.; Jin, W.-l. An overview on the influence of various parameters on the fabrication and engineering properties of CO2-cured cement-based composites. J. Clean. Prod. 2022, 366, 132968. [Google Scholar] [CrossRef]
- Han, Y.; Park, K.-B.; Yang, B.; Wang, X.-Y. Optimizing and designing the effects of microwave pre-curing on the properties of ternary blended concrete with slag and fly ash using the simplex centroid method. Constr. Build. Mater. 2024, 419, 135443. [Google Scholar] [CrossRef]
- Makul, N.; Rattanadecho, P.; Agrawal, D.K. Applications of microwave energy in cement and concrete—A review. Renew. Sustain. Energy Rev. 2014, 37, 715–733. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, P.; Liu, S.; Gao, Z. Hydration and microstructure of cement-based materials under microwave curing. Constr. Build. Mater. 2016, 114, 831–838. [Google Scholar] [CrossRef]
- Asamoto, S.; Murano, K.; Kurashige, I.; Nanayakkara, A. Effect of carbonate ions on delayed ettringite formation. Constr. Build. Mater. 2017, 147, 221–226. [Google Scholar] [CrossRef]
- Bahafid, S.; Ghabezloo, S.; Duc, M.; Faure, P.; Sulem, J. Effect of the hydration temperature on the microstructure of Class G cement: C-S-H composition and density. Cem. Concr. Res. 2017, 95, 270–281. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Xian, X.; Mahoutian, M.; Zhang, D.; Shao, Y. Development of wet-cast Portland-cement-free concrete based on steel slag and ambient-pressure carbonation activation. Resour. Conserv. Recycl. 2024, 203, 107455. [Google Scholar] [CrossRef]
- Xian, X.; Shao, Y. Microstructure of cement paste subject to ambient pressure carbonation curing. Constr. Build. Mater. 2021, 296, 123652. [Google Scholar] [CrossRef]
- Xian, X.; Mahoutian, M.; Zhang, S.; Shao, Y.; Zhang, D.; Liu, J. Converting industrial waste into a value-added cement material through ambient pressure carbonation. J. Environ. Manag. 2023, 325, 116603. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Zhang, F.; Deng, M.; Jin, F.; Al-Tabbaa, A.; Wang, A. Accelerated carbonation and performance of concrete made with steel slag as binding materials and aggregates. Cem. Concr. Compos. 2017, 83, 138–145. [Google Scholar] [CrossRef]
- Kakali, G.; Tsivilis, S.; Aggeli, E.; Bati, M. Hydration products of C3A, C3S and Portland cement in the presence of CaCO3. Cem. Concr. Res. 2000, 30, 1073–1077. [Google Scholar] [CrossRef]
- Haga, K.; Shibata, M.; Hironaga, M.; Tanaka, S.; Nagasaki, S. Change in pore structure and composition of hardened cement paste during the process of dissolution. Cem. Concr. Res. 2005, 35, 943–950. [Google Scholar] [CrossRef]
- Guo, B.; Chu, G.; Yu, R.; Wang, Y.; Yu, Q.; Niu, D. Effects of sufficient carbonation on the strength and microstructure of CO2-cured concrete. J. Build. Eng. 2023, 76, 107311. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, Z.; Wang, F. Effect of Accelerated Carbonation Curing on Mechanical Property and Microstructure of High Volume Steel Slag Mortar. Kuei Suan Jen Hsueh Pao/J. Chin. Ceram. Soc. 2020, 48, 1801–1807. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, X.; Song, H. Morphological Investigation of Calcium Carbonate during Ammonification-Carbonization Process of Low Concentration Calcium Solution. J. Nanomater. 2014, 2014, 503696. [Google Scholar] [CrossRef]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Liu, Z.; Van den Heede, P.; Zhang, C.; Shi, X.; Wang, L.; Li, J.; Yao, Y.; Lothenbach, B.; De Belie, N. Carbonation of blast furnace slag concrete at different CO2 concentrations: Carbonation rate, phase assemblage, microstructure and thermodynamic modelling. Cem. Concr. Res. 2023, 169, 107161. [Google Scholar] [CrossRef]
- Han, X.; Wang, B.; Feng, J. Relationship between fractal feature and compressive strength of concrete based on MIP. Constr. Build. Mater. 2022, 322, 126504. [Google Scholar] [CrossRef]
- Fermani, S.; Njegić Džakula, B.; Reggi, M.; Falini, G.; Kralj, D. Effects of magnesium and temperature control on aragonite crystal aggregation and morphology. CrystEngComm 2017, 19, 2451–2455. [Google Scholar] [CrossRef]
- Shi, Z.; Lothenbach, B.; Geiker, M.R.; Kaufmann, J.; Leemann, A.; Ferreiro, S.; Skibsted, J. Experimental studies and thermodynamic modeling of the carbonation of Portland cement, metakaolin and limestone mortars. Cem. Concr. Res. 2016, 88, 60–72. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Xu, D.; Du, P.; Zhou, Z.; Yuan, L.; Cheng, X. Accelerated carbonation of hardened cement pastes: Influence of porosity. Constr. Build. Mater. 2019, 225, 159–169. [Google Scholar] [CrossRef]



| Composition | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | TiO2 | Na2O |
|---|---|---|---|---|---|---|---|---|---|
| Mass fraction/% | 61.16 | 20.89 | 5.29 | 4.32 | 2.59 | 2.88 | 1.21 | 0.36 | 0.30 |
| Carbonation Curing Time | Microwave Pretreatment for 0 h/mm | Microwave Pretreatment for 1 h/mm | Microwave Pretreatment for 2 h/mm | Microwave Pretreatment for 3 h/mm | Microwave Pretreatment for 4 h/mm |
|---|---|---|---|---|---|
| 6 h | 1.57 | 2.98 | 3.65 | 3.96 | 4.34 |
| 12 h | 2.54 | 3.68 | 4.03 | 4.39 | 4.48 |
| 24 h | 3.53 | 3.86 | 4.62 | 5.96 | 6.28 |
| 36 h | 4.09 | 4.28 | 5.07 | 6.63 | 8.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Li, M.; Wang, L.; Liu, S. Effect of Microwave Pretreatment on the Properties and Microstructure of Low-Concentration Carbon Dioxide Early Cured Cement-Based Materials. Buildings 2024, 14, 1074. https://doi.org/10.3390/buildings14041074
Liang X, Li M, Wang L, Liu S. Effect of Microwave Pretreatment on the Properties and Microstructure of Low-Concentration Carbon Dioxide Early Cured Cement-Based Materials. Buildings. 2024; 14(4):1074. https://doi.org/10.3390/buildings14041074
Chicago/Turabian StyleLiang, Xiao, Maosen Li, Lu Wang, and Shuhua Liu. 2024. "Effect of Microwave Pretreatment on the Properties and Microstructure of Low-Concentration Carbon Dioxide Early Cured Cement-Based Materials" Buildings 14, no. 4: 1074. https://doi.org/10.3390/buildings14041074
APA StyleLiang, X., Li, M., Wang, L., & Liu, S. (2024). Effect of Microwave Pretreatment on the Properties and Microstructure of Low-Concentration Carbon Dioxide Early Cured Cement-Based Materials. Buildings, 14(4), 1074. https://doi.org/10.3390/buildings14041074





