Finite Element Method Simulation Study on the Temperature Field of Mass Concrete with Phase Change Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phase Change Materials
2.2. Concrete Material
2.2.1. Materia
2.2.2. Equipment
- (1)
- Specimen mold: The thermodynamic thermostat performance test uses a 300 × 300 × 300 mm3 plastic mold, as shown in Figure 1a;
- (2)
- A-BF multi-channel temperature recorder: Dongguan Bufan Electronic Co., Ltd (Dongguan Guangdong China)., as shown in Figure 1b;
- (3)
- High- and low-temperature test chamber BC1300: Shanghai Yiheng Technology Co., Ltd (Shanghai China)., as shown in Figure 1c.
2.3. Finite-Element Modeling
- (1)
- Modeling: first of all, it is necessary to establish a three-dimensional model, including a structural model and material model. In the model, it is necessary to define the characteristics of the material itself, such as thermophysical parameters, thermal conductivity, specific heat capacity, etc.;
- (2)
- Setting boundary conditions: setting the boundary conditions of the model, including the boundary conditions of the structure and the boundary conditions of the analysis, such as the application of thermal loads. Different boundary conditions need to be set in different parts of the model to simulate the heat transfer process in practical engineering;
- (3)
- Define the construction management stage: define the time step and time course in the analysis process to ensure the accuracy and reliability of the analysis results;
- (4)
- Thermal analysis: the temperature field analysis, which is used to simulate the transient temperature field of the temperature change effect with time;
- (5)
- Results post-processing: view and post-process the analysis results of the calculated temperature values, including drawing temperature cloud maps, temperature change maps, etc.
3. Preparation and Properties of Phase Change Materials
3.1. Preparation of Phase Change Materials
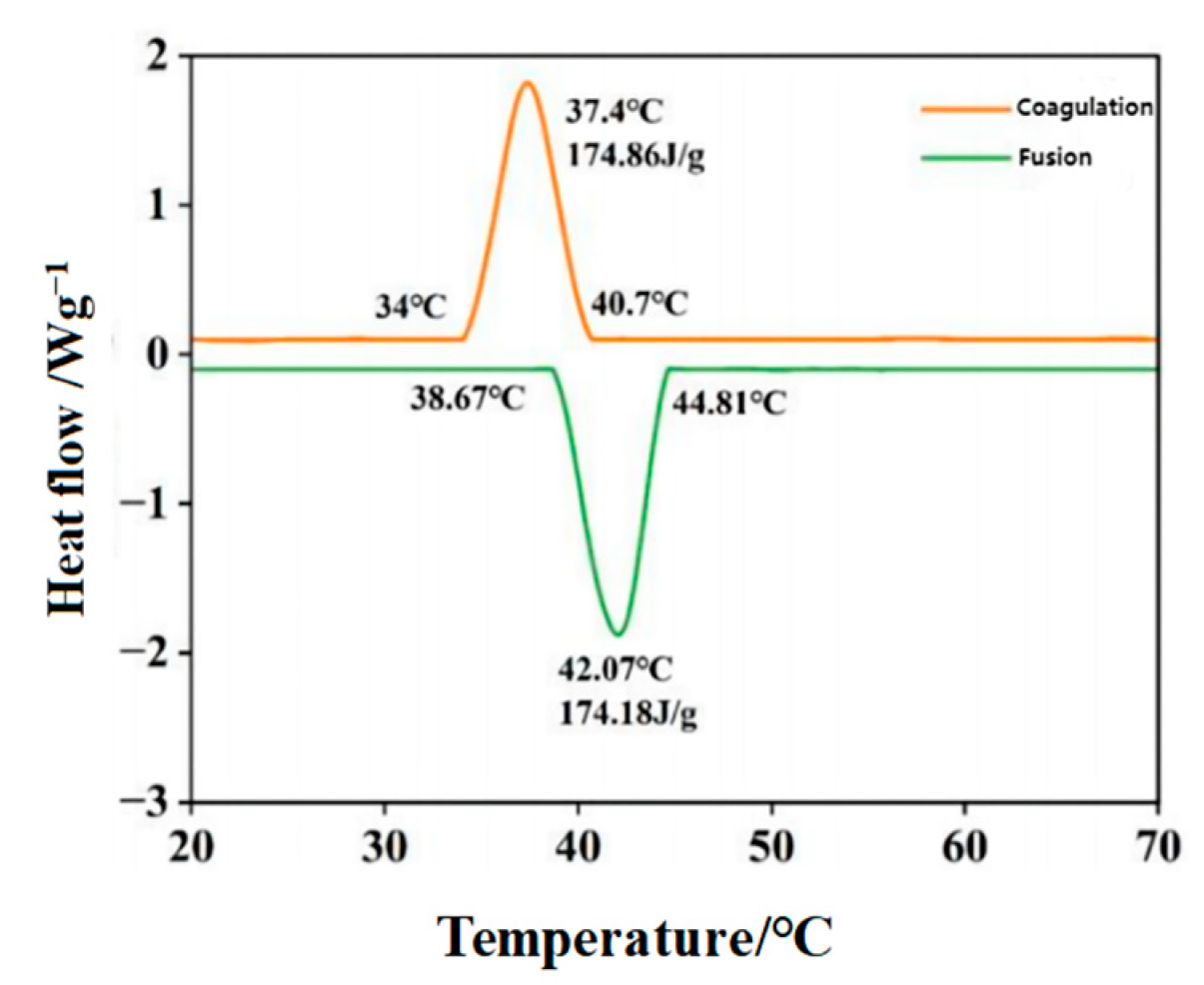
3.2. Phase Transition Properties
4. Study on Performance of Phase Change Concrete
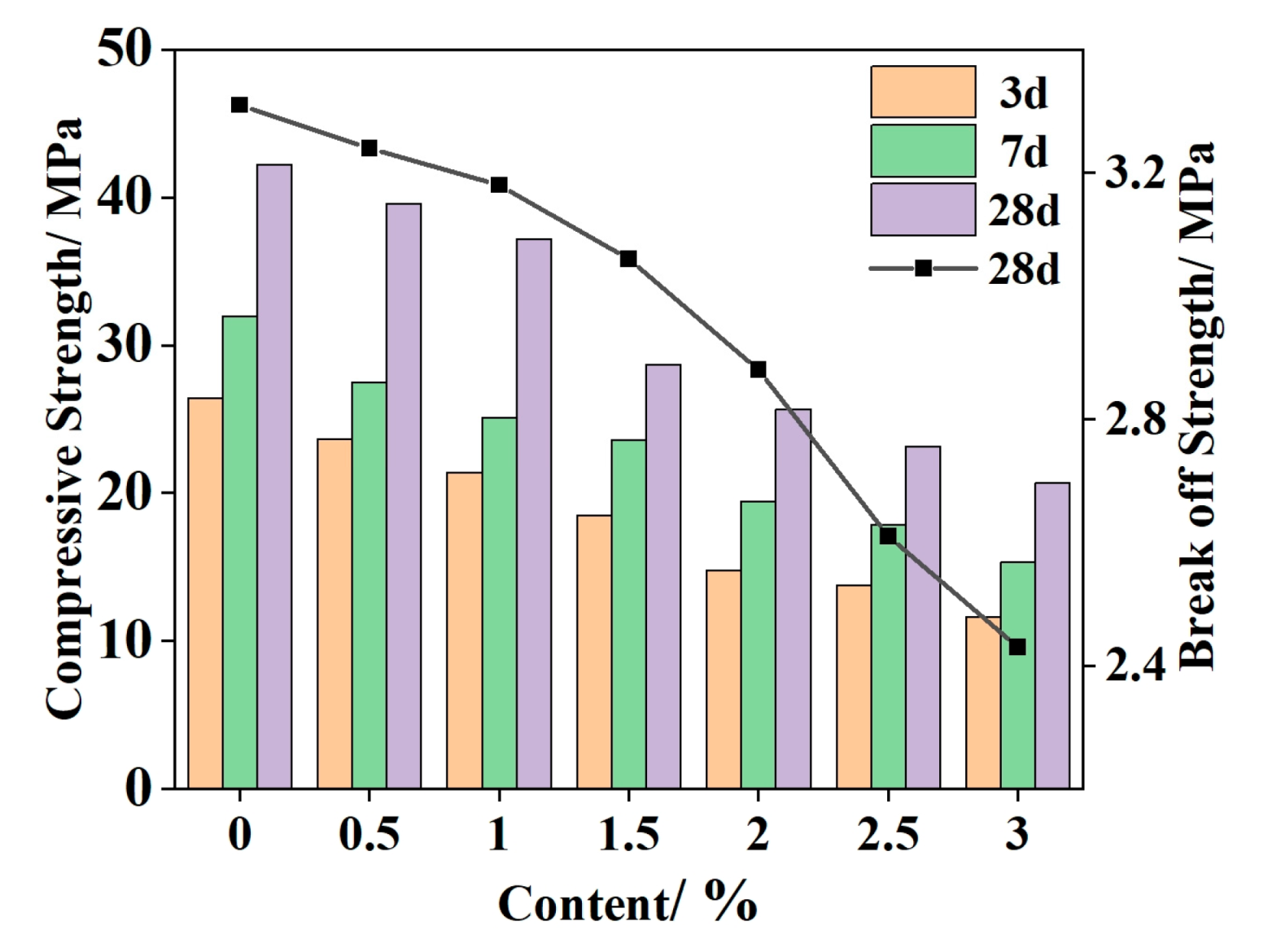
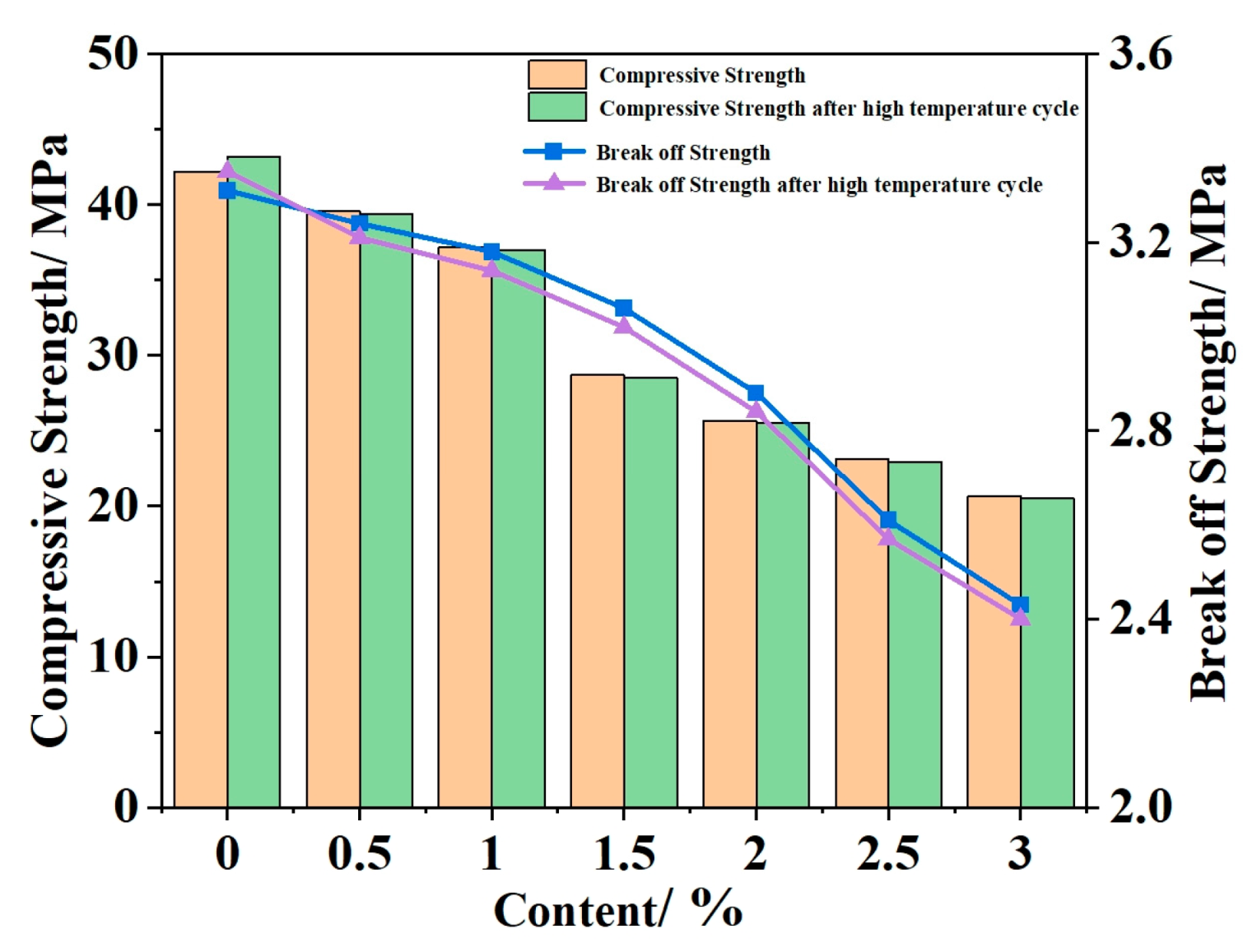
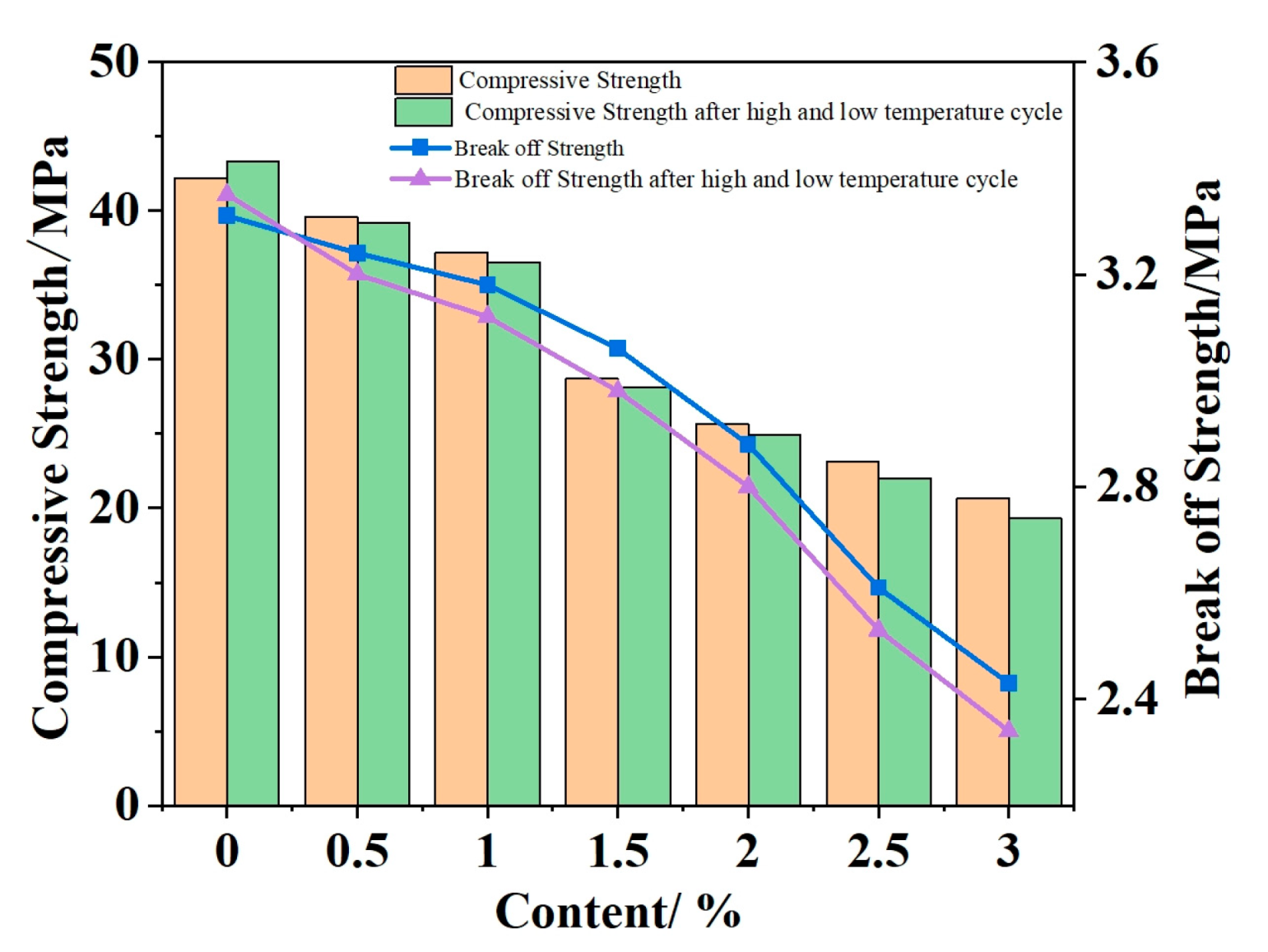
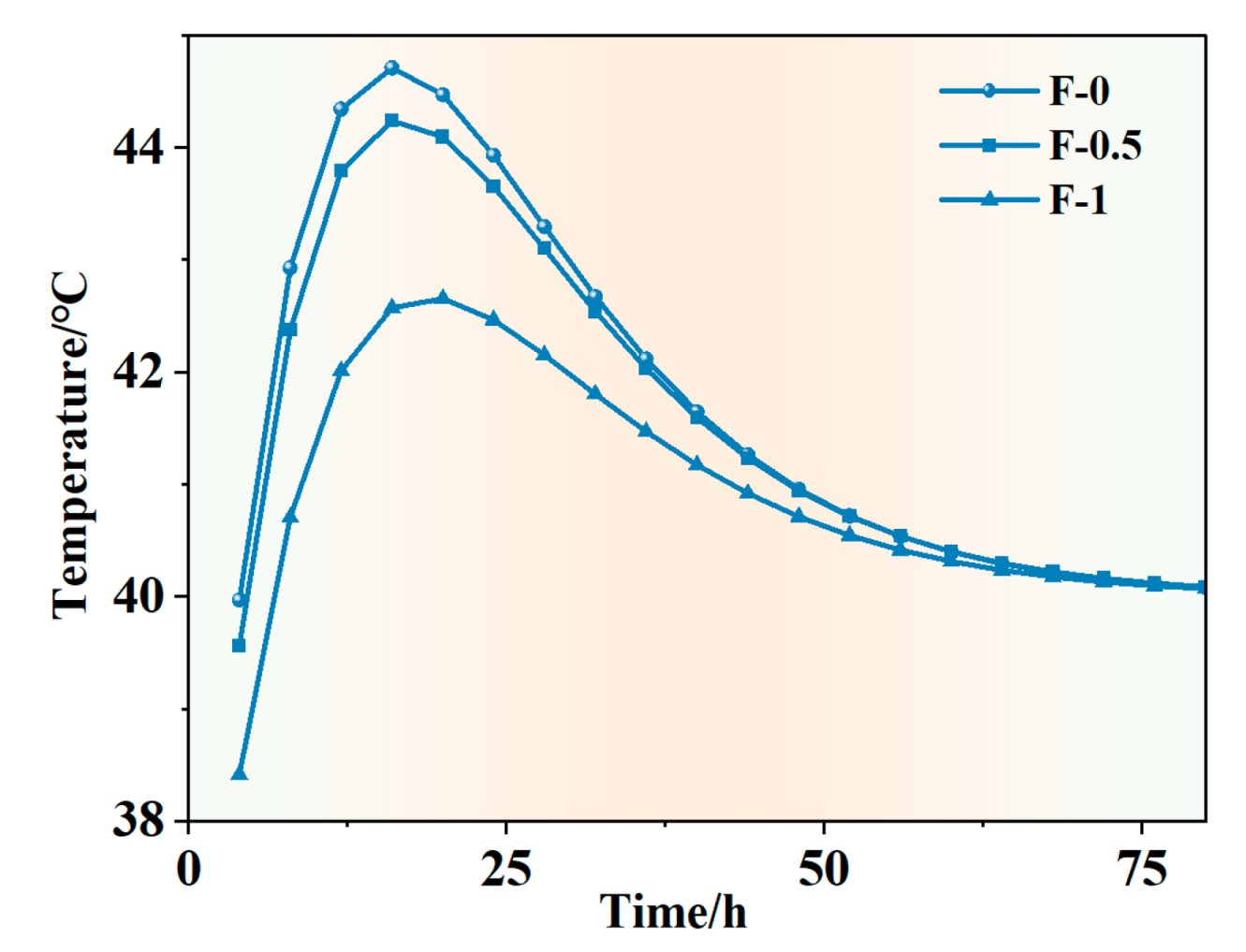
4.1. Mechanical and Temperature Tests of Ordinary Concrete Mixed with Phase Change Materials
4.2. Temperature Field Simulation of Ordinary Concrete Structure with Phase Change Materials
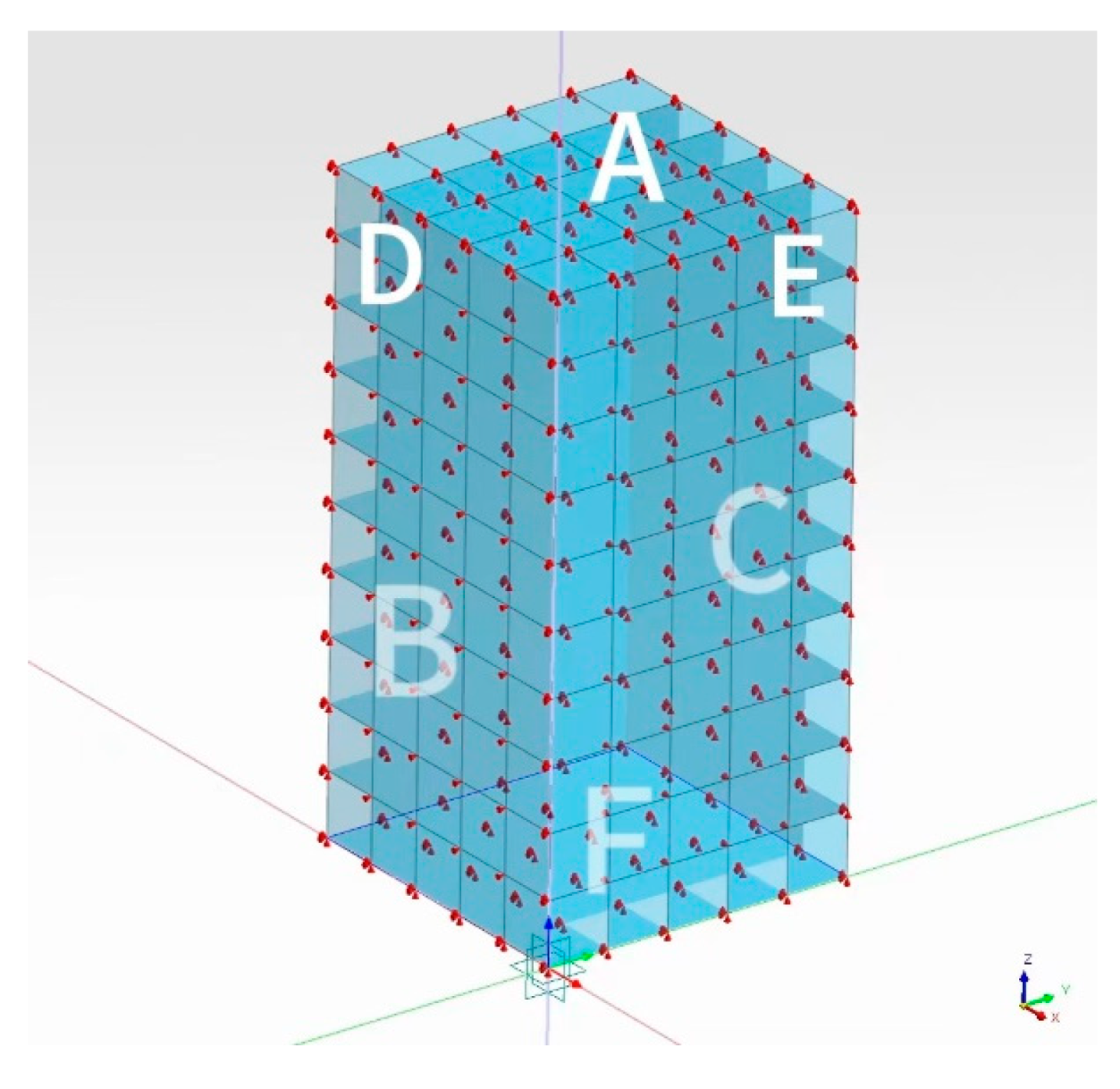
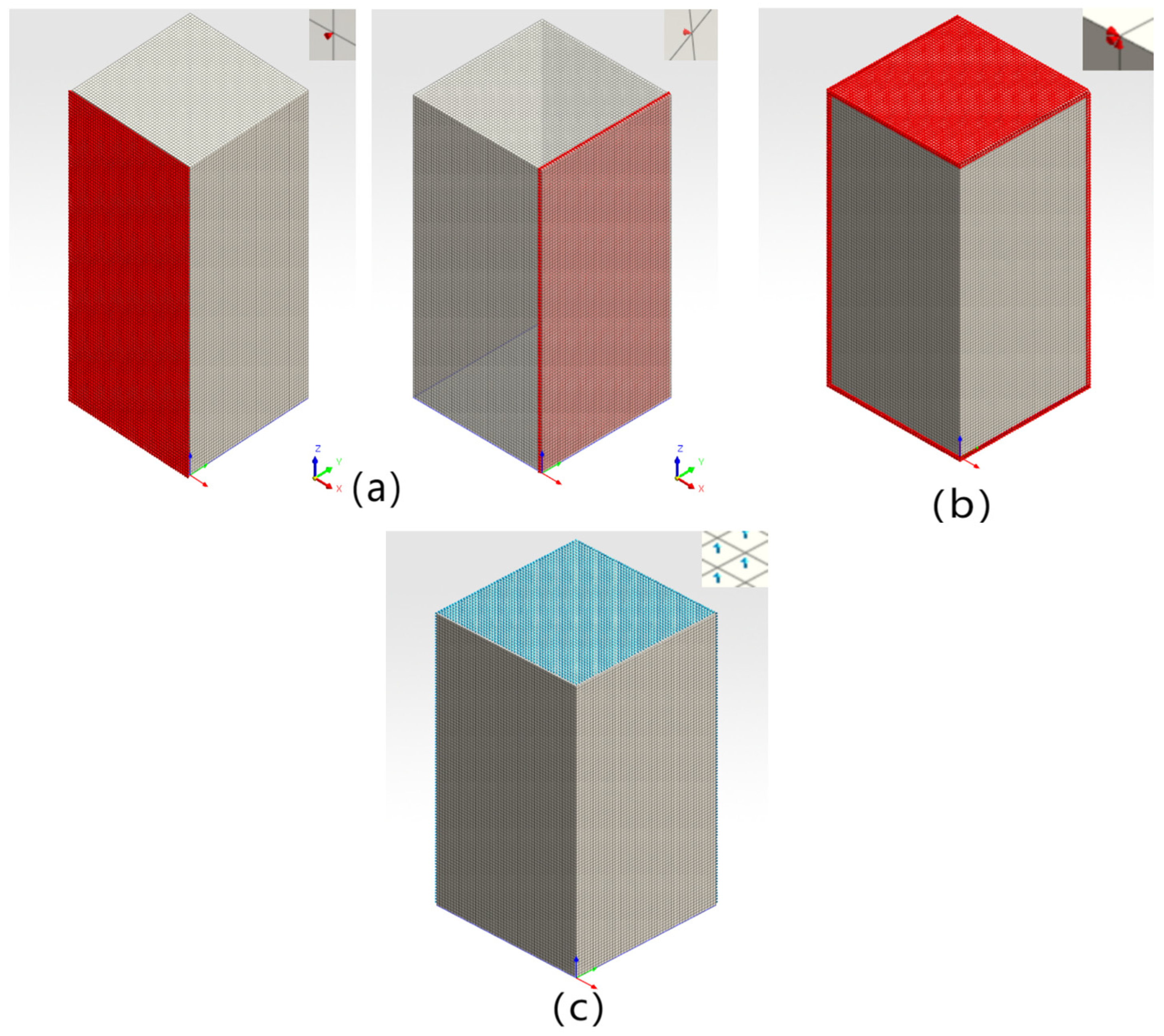
4.2.1. Model Construction and Parameter Setting
4.2.2. Boundary Condition Setting
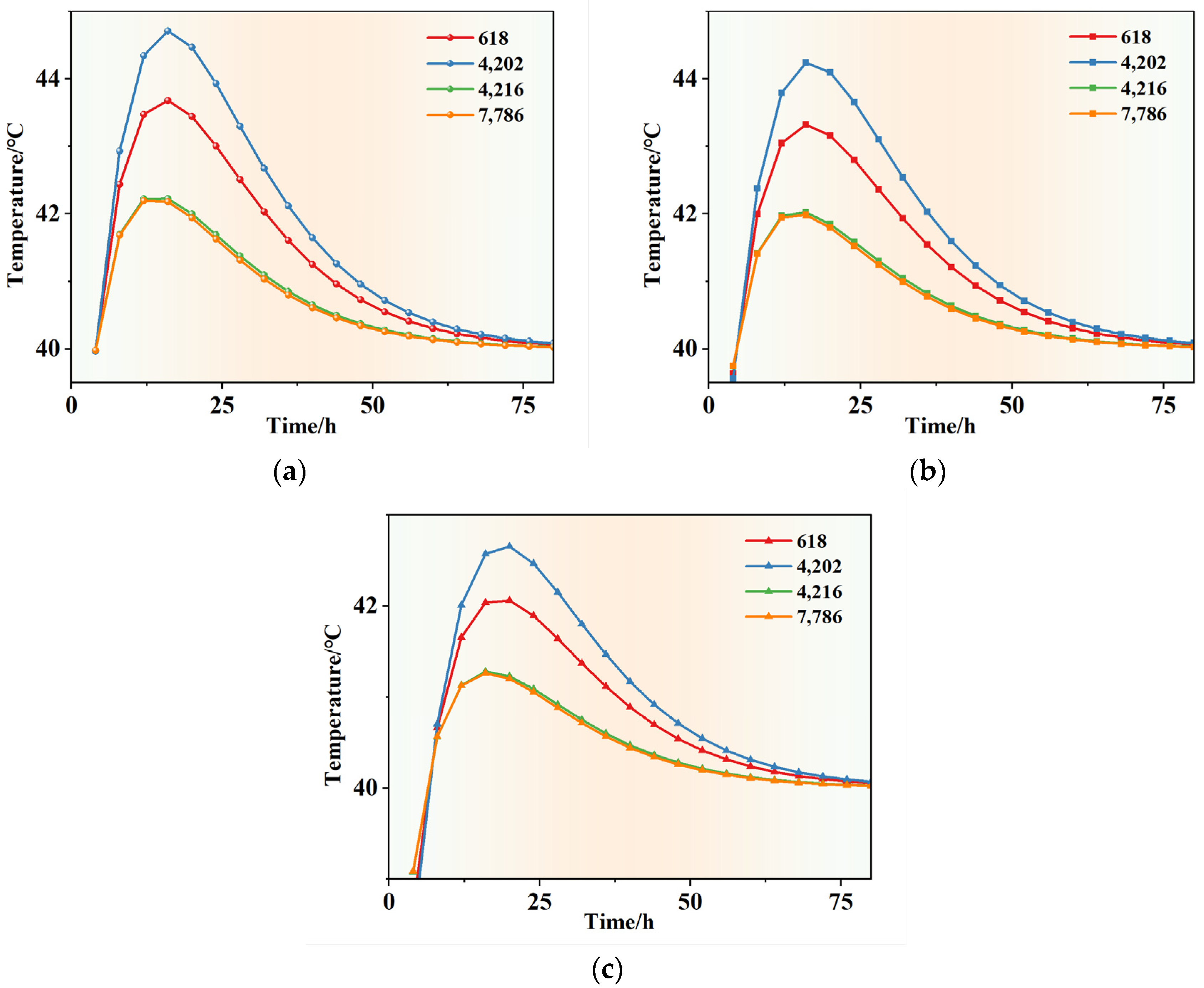
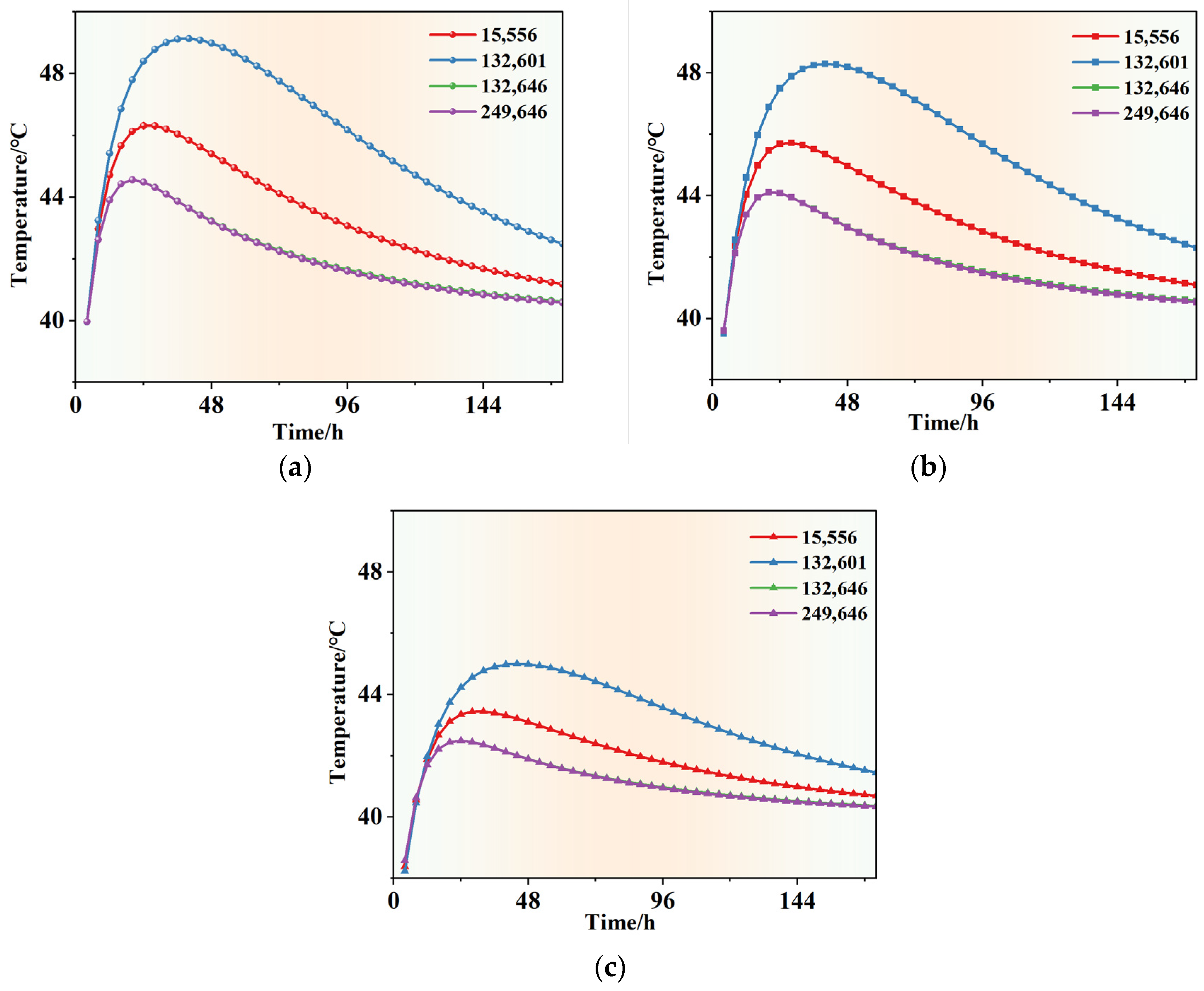
4.2.3. Simulation Analysis of Early Hydration Heat Temperature Field
5. Temperature Field Simulation of Mass Concrete Structure with Phase Change Materials
5.1. Model Construction and Parameter Setting
5.2. Boundary Condition Setting
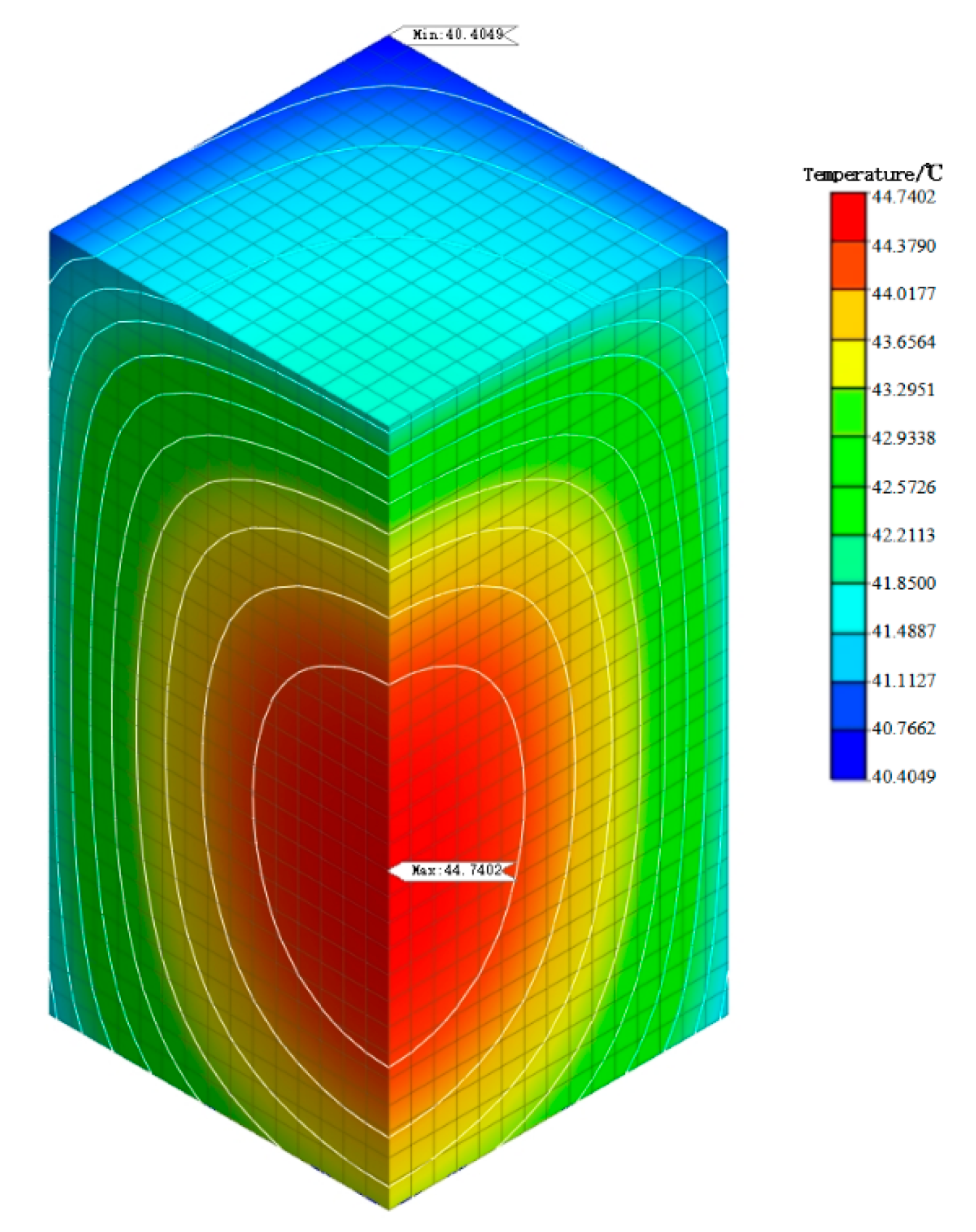
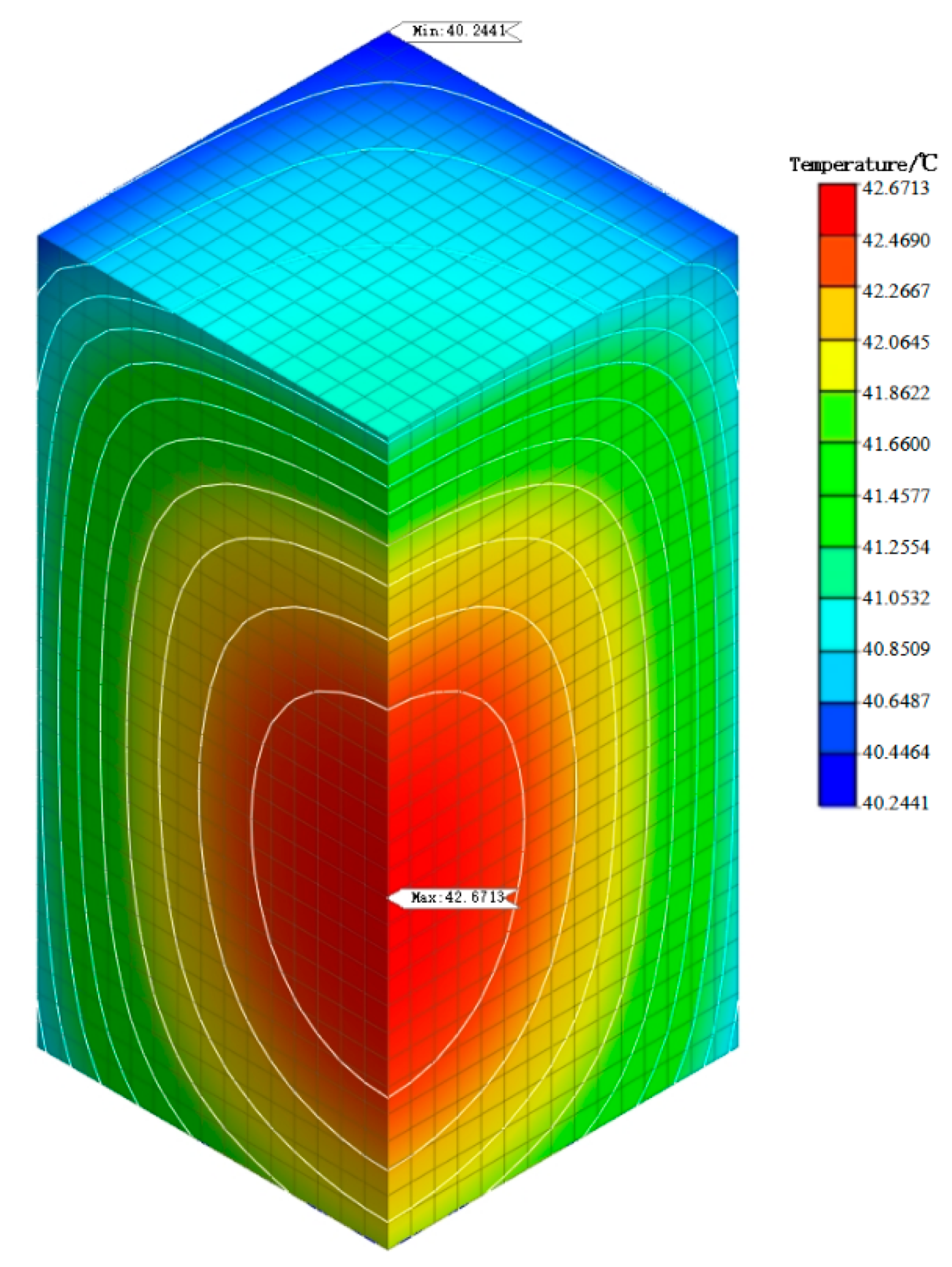
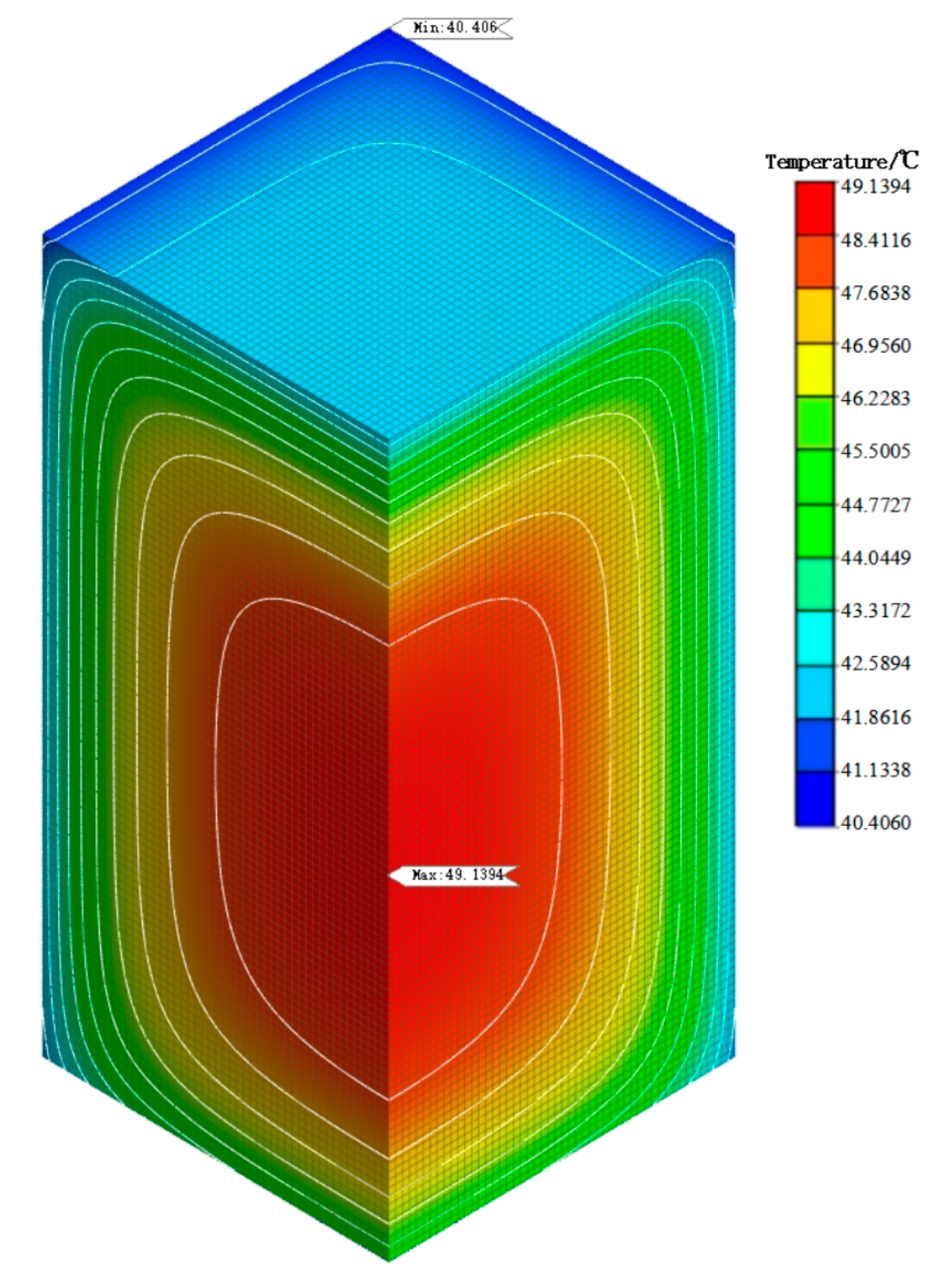
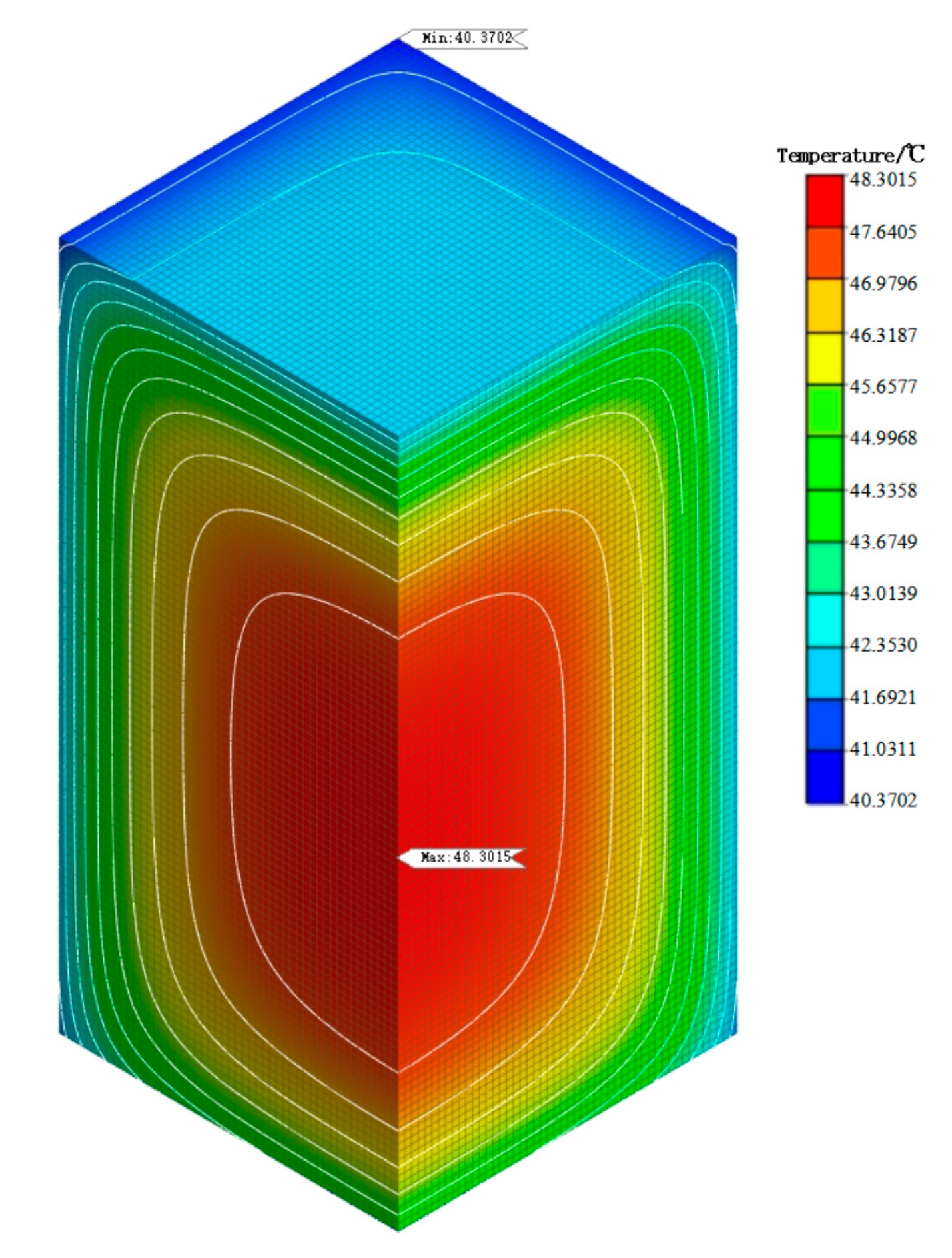
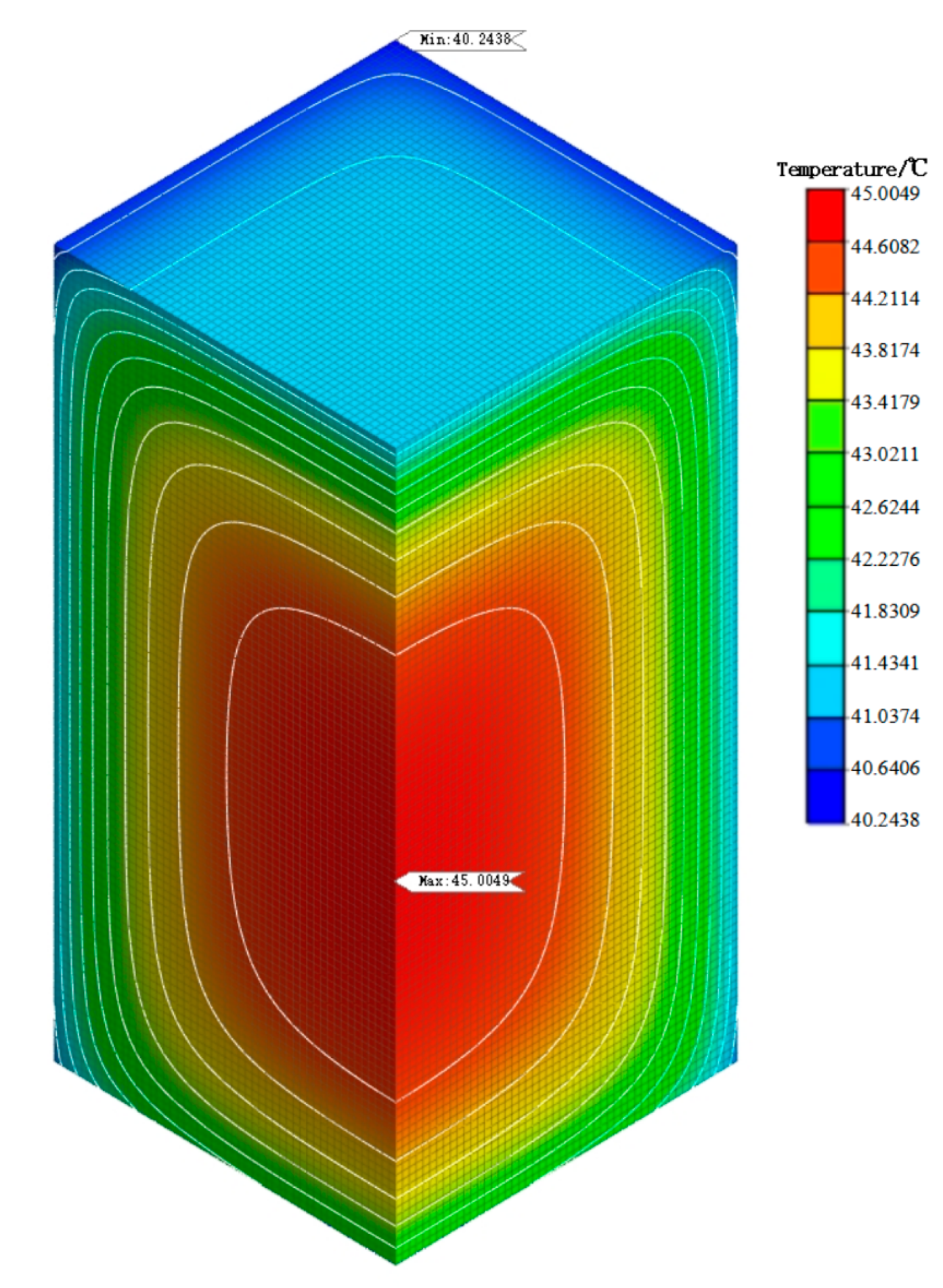
5.3. Simulation Analysis of Early Hydration Heat Temperature Field
6. Conclusions
- (1)
- According to the basic mechanical test results of phase change concrete, with an increase in the content of self-prepared composite phase change material, the compressive and flexural properties of concrete decrease but still meet the basic mechanical requirements; in the experiment of the thermal thermoregulation performance of phase change concrete, the self-prepared composite phase change material has a certain regulation and reduction effect on the peak temperature rise caused by the early hydration heat of concrete and, at the same time, delays the time taken to reach the peak temperature rise and realizes the effect of ‘high-temperature peak clipping’ and ‘delay effect’.
- (2)
- Using the adiabatic temperature rise calculation method of mass phase change concrete proposed in the existing literature, the temperature field simulation analysis of concrete mixed with self-prepared composite phase change materials is carried out. The simulation analysis results are similar to the experimental temperature regulation data of phase change concrete and ordinary concrete, indicating that the simulation analysis method in this paper is suitable for the simulation of the temperature field of a concrete structure mixed with composite phase change materials and the analysis of the temperature regulation effect.
- (3)
- From the analysis of the temperature field of mass concrete, it is found that the addition of self-prepared composite phase change material reduces the temperature peak of the mass concrete structure. Compared with the temperature field of the mass concrete structure without composite phase change material and with a content of 1%, it is found that the maximum temperature is reduced by 4.14 °C and the heating–cooling rate of concrete is slowed down. This shows that the composite phase change material reduces the internal and external temperature difference of the structure by reducing the temperature peak inside the mass concrete structure, thus alleviating the influence of the increase in temperature stress caused by the increase in internal and external temperature difference and reducing the possibility of cracks caused by temperature stress exceeding the structural design stress. Therefore, the self-prepared composite phase change has a certain control effect on the temperature field of the mass concrete structure.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dawei, J. Study on crack control of mass concrete. Build. Struct. 2023, 53, 2275–2279. [Google Scholar] [CrossRef]
- He, L.; Li, X.F.; Tang, B.F. Causes and Treatment Countermeasures of Mass Concrete Cracks in Bridge Construction. Highway 2019, 64, 98–101. [Google Scholar]
- Wang, P.H.; Xu, Q.G. Application of mass concrete crack control technology in construction engineering. Build. Struct. 2022, 52, 1015–1018. [Google Scholar] [CrossRef]
- Haidong, Z. Causes and control of non-load cracks in mass concrete in bridge engineering. Transp. World 2019, 94–95. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, S.H.; Cao, K.; Li, X.L.; Gu, W.W. Numerical simulation of energy release performance of solar phase change energy storage tank. Energy Storage Sci. Technol. 2013, 2, 377–382. [Google Scholar]
- Han, D. Preparation and Properties of Composite Phase Change Materials Suitable for Temperature Control of Mass Concrete. Master’s Thesis, Zhejiang Ocean University, Zhoushan, China, 2019. [Google Scholar]
- Wang, J. Theoretical Basis Research and Preparation of Phase Change Temperature Control Concrete. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2011. [Google Scholar]
- Song, W. Effect of initial temperature on hydration heat of mass concrete. J. Hunan City Univ. 2020, 29, 12–15. [Google Scholar]
- Zhao, M. Preparation and Properties of Palmitic Acid-Stearic Acid/Porous Graphite Form-Stable Phase Change. Master’s Thesis, Inner Mongolia University of Science and Technology, Baotou, China, 2023. [Google Scholar] [CrossRef]
- JGJ 55-2011; Specification for Mix Proportion Design of Ordinary Concrete. National Standards of the People’s Republic of China: Beijing, China, 2011.
- Geng, M.S.; Lin, E.J.; Lv, J.B.; Fu, B. Hydration heat analysis and temperature control of massive concrete pile caps. Concrete 2021, 50–55. [Google Scholar]
- Khan, R.M.A.; Shafighfard, T.; Ali, H.Q.; Mieloszyk, M.; Yildiz, M. Strength prediction and experimental damage investigations of plain woven CFRPs with interacting holes using multi-instrument measurements. Polym. Compos. 2023, 44, 3594–3609. [Google Scholar] [CrossRef]
- Zhang, X. Preparation and Properties of Stearic Acid/SiO2 Composite Phase Change Materials by Sol-Gel. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2012. [Google Scholar]
- Liu, D.; He, S.Y.; Dong, Y.X.; Sheng, J.F.; Li, X.F. Preparation and properties of calcium lactate composite phase change cold storage material. Cold Storage Technol. 2024, 47, 28–34. [Google Scholar] [CrossRef]
- Zhao, H.J.; Wan, X.; Lu, J.H.; Zhang, H.Y.; Guo, B.H. Development and application of phase change energy storage materials in the field of construction. China Plast. 2023, 37, 46–61. [Google Scholar] [CrossRef]
- Zhu, H.Z.; Chen, R.P.; Gou, S.; Wang, D.Q.; Hu, L.X. Preparation and properties of phase change materials for cement pavement temperature regulation at low temperature. Mater. Bull. 2021, 35, 14198–14203. [Google Scholar]
- Shi, W.; Hou, J. Study on temperature field distribution characteristics of paraffin phase change temperature control concrete. Silic. Bull. 2013, 32, 2183–2186. [Google Scholar] [CrossRef]
- Feng, X.; He, C. Principles of Chemical Engineering, 2nd ed.; Scientific Press: Christchurch, New Zealand, 2007. [Google Scholar]
- Zhu, B. Thermal Stress and Temperature Control of Mass Concrete; China Electric Power Press: Beijing, China, 2003. [Google Scholar]
- Wang, J.; Guo, X.; Qiu, W.H.; Kong, Y.M. Study on the influence of curing methods on the temperature field of mass concrete construction in summer. J. Xuchang Univ. 2023, 42, 90–95. [Google Scholar]
- Haonan, S. Research on Temperature Control of Mass Concrete Based on Phase Change Principle. Master’s Thesis, Shandong Jiaotong University, Jinan, China, 2024. [Google Scholar] [CrossRef]






| Test Reagent | Specification | Manufacturer |
|---|---|---|
| Palmitic acid (C16H32O2, PA) | Analytical pure (RG), 98%+ | Shanghai Titan Technology Co., Ltd. (Shanghai China). |
| Stearic acid (C18H36O2, SA) | Analytical pure (RG), 99% (Mixture) | Shanghai Titan Technology Co., Ltd. (Shanghai China). |
| Tetraethyl orthosilicate (C8H20O4Si, TEOS) | Analytical pure (AR) | Guangdong Wengjiang Chemical Reagent Co., Ltd. (Guangdong China). |
| Anhydrous ethanol (C2H5OH) | Analytically pure (AR), concentration ≥99.7% | Shanghai Titan Technology Co., Ltd. (Shanghai China). |
| Hydrochloric acid (HCL) | The concentration is 33~35% | Laiyang City Kant Chemical Co., Ltd. (Laiyang Yantai China). |
| Ammonia liquor (NH4OH) | Analysis pure (AR) concentration of 25~28% | Shanghai Titan Technology Co., Ltd. (Shanghai China). |
| Phase Transformation Form | Starting Temperature/°C | Peak Temperature/°C | Temperature Range/°C | Enthalpy Value/J·g−1 | |
|---|---|---|---|---|---|
| Palmitic acid | fusion | 53.83 | 58.23 | 53.83~59.20 | 202.17 |
| solidification | 50.26 | 52.07 | 50.26~53.90 | 198.05 | |
| Stearic acid | fusion | 62.43 | 64.73 | 62.43~66.10 | 234.20 |
| solidification | 58.16 | 60.13 | 58.16~60.73 | 235.16 |
| Materials | Remark |
|---|---|
| Cement | P.O42.5 grade ordinary Portland cement, Jinan Century Innovation Cement Co., Ltd (Jinan Shandong China). |
| Fly ash | Grade I |
| Mineral powder | Grade S95 |
| Sand | Ordinary test river sand |
| Cobble | 4.75–9.5 mm particle size and 9.5–19.0 mm particle size, accounting for 60% and 40%, respectively |
| Water reducing agent | Highly efficient polycarboxylic acid water reducer, water reduction rate of more than 25%, a content of 0.8% of cement mass |
| Water | Ordinary tap water |
| Cement | Fly Ash | Mineral Powder | Water | Sand | Cobble | Water Reducing Agent | |
|---|---|---|---|---|---|---|---|
| 4.75–9.5 | 9.5–19.0 | ||||||
| 213 | 98 | 16 | 151 | 601 | 488 | 733 | 1.7 |
| Concrete Material Parameters | Unit | Set Value |
|---|---|---|
| Elastic modulus | MPa | 31,991.10 |
| Poisson ratio | / | 0.2 |
| Weight density | kN·m−3 | 2549 |
| Thermal conductivity | W·m−1·K−1 | 2.78 |
| Thermal expansion coefficient | / | 1 × 10−5 |
| Convection coefficient [18] | W·m−2·K−1 | 20 |
| Specific heat | kJ·kg−1·K−1 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Shangguan, H.; Zhang, W.; Yang, K. Finite Element Method Simulation Study on the Temperature Field of Mass Concrete with Phase Change Material. Buildings 2024, 14, 2755. https://doi.org/10.3390/buildings14092755
Chen R, Shangguan H, Zhang W, Yang K. Finite Element Method Simulation Study on the Temperature Field of Mass Concrete with Phase Change Material. Buildings. 2024; 14(9):2755. https://doi.org/10.3390/buildings14092755
Chicago/Turabian StyleChen, Renshan, Haonan Shangguan, Wei Zhang, and Kaibo Yang. 2024. "Finite Element Method Simulation Study on the Temperature Field of Mass Concrete with Phase Change Material" Buildings 14, no. 9: 2755. https://doi.org/10.3390/buildings14092755
APA StyleChen, R., Shangguan, H., Zhang, W., & Yang, K. (2024). Finite Element Method Simulation Study on the Temperature Field of Mass Concrete with Phase Change Material. Buildings, 14(9), 2755. https://doi.org/10.3390/buildings14092755





