Exploring the Impact and Prevention of Epidemics Based on Inter-Animal Transmission from an Environmental Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Data
2.2. Analogue Basis and Parameter Measurements
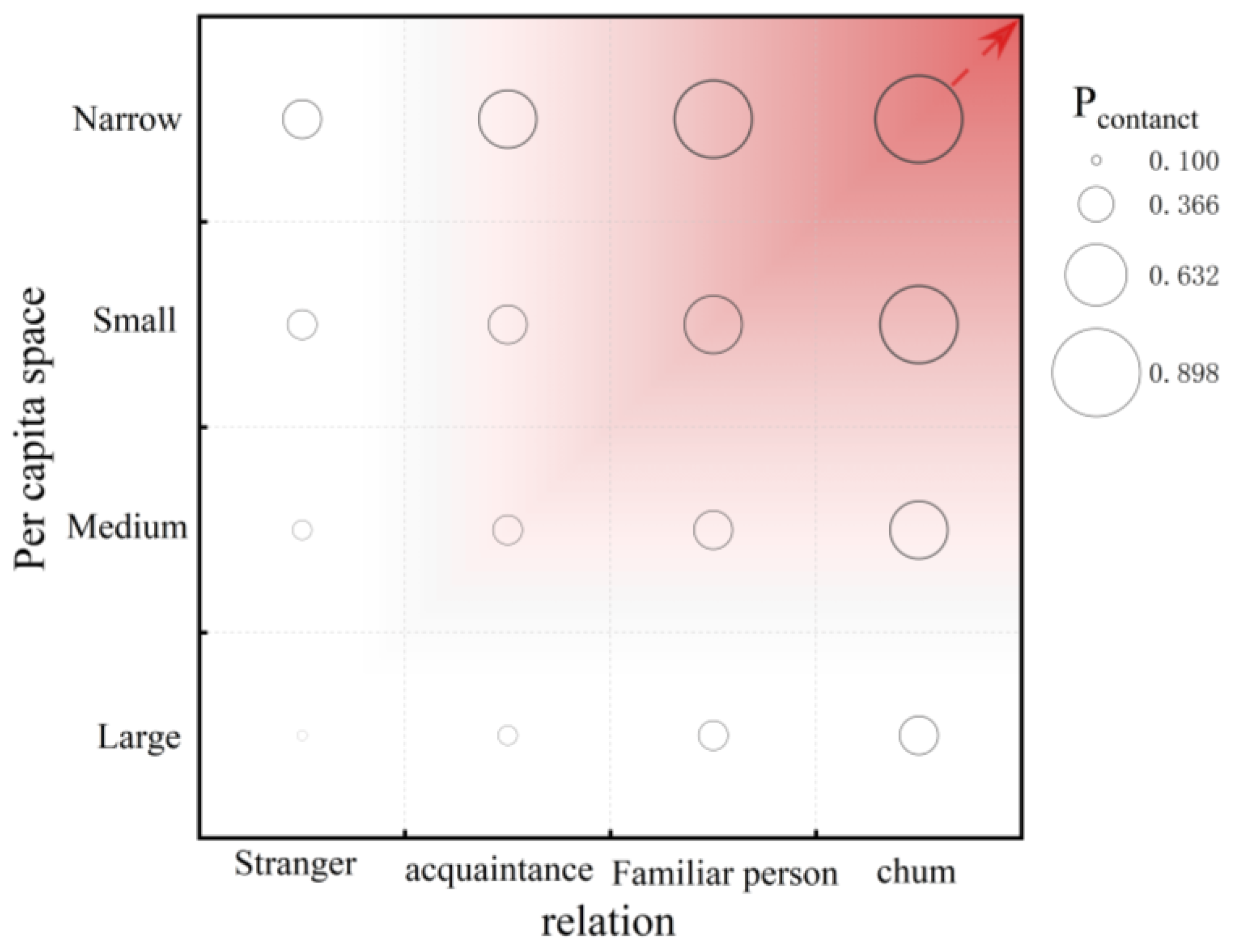
2.2.1. Probabilistic Contact Distance
2.2.2. Animal Distance Coefficient Calculation
2.2.3. Human Example Calculations
2.3. Models and Data Analysis Procedure
- Virus release intensity: More air changes will dilute the virus’s concentration, preventing airborne transmission. Nonetheless, it is crucial to take into account human comfort and prevent any negative effects brought on by unnecessarily high air change rates.
- Air change rate: A higher number of air changes will dilute the virus concentration and thus have the effect of suppressing airborne transmission. At the same time, it is necessary to consider the comfort of the human body and avoid the reaction caused by being too much.
3. Result and Discussion
3.1. Effect of Temperature Changes on Virus Transmission
3.2. Effect of Relative Humidity on Virus Transmission
3.3. Effect of the Number of Air Changes on Virus Transmission
3.4. Effect of the Number of Susceptible Persons on Virus Transmission
3.5. Statistical Analysis
3.6. Practical Applications and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Gu, Z.; Han, J.; Zhang, L.; Wang, H.; Luo, X.; Meng, X.; Zhang, Y.; Niu, X.; Lan, Y.; Wu, S.; et al. Unanswered questions on the airborne transmission of COVID-19. Environ. Chem. Lett. 2023, 21, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, Z. Dilution-based evaluation of airborne infection risk-Thorough expansion of Wells-Riley model. Build. Environ. 2021, 194, 107674. [Google Scholar] [CrossRef] [PubMed]
- Andrup, L.; Krogfelt, K.A.; Hansen, K.S.; Madsen, A.M. Transmission route of rhinovirus-the causative agent for common cold. A systematic review. Am. J. Infect. Control 2023, 51, 938–957. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, V.; Lowen, A.C.; Lakdawala, S.S. Block the Spread: Barriers to Transmission of Influenza Viruses. Annu. Rev. Virol. 2023, 10, 347–370. [Google Scholar] [CrossRef]
- Rawat, M.S.; Roberts, A.D.D.; Brown, D.M.M.; Ferro, A.R.R. Resuspension of Seeded Particles Containing Live Influenza A Virus in a Full-Scale Laboratory. Buildings 2023, 13, 1734. [Google Scholar] [CrossRef]
- Nicas, M. An Analytical Framework for Relating Dose, Risk, and Incidence: An Application to Occupational Tuberculosis Infection. Risk Anal. 1996, 16, 527–538. [Google Scholar] [CrossRef]
- Nazaroff, W.W.; Nicas, M.; Miller, S.L. Framework for Evaluating Measures to Control Nosocomial Tuberculosis Transmission. Indoor Air 1998, 8, 205–218. [Google Scholar] [CrossRef]
- Fisk, W.; Seppanen, O.; Faulkner, D.; Huang, J. Economic Benefits of an Economizer System: Energy Savings and Reduced Sick Leave. ASHRAE Trans. 2005, 111, 673–679. [Google Scholar]
- Franchimon, F.; Pernot, C.E.E.; Khoury, E.; Bronswijk, J.E.M.H. The feasibility of indoor humidity control against avian influenza. In Proceedings of the 11th International Conference on Indoor Air Quality and Climate (Indoor Air 2008), Copenhagen, Denmark, 17–22 August 2008. [Google Scholar]
- Gao, C.X.; Li, Y.; Wei, J.; Cotton, S.; Hamilton, M.; Wang, L.; Cowling, B.J. Multi-route respiratory infection: When a transmission route may dominate. medRxiv 2020, 752, 141856. [Google Scholar] [CrossRef]
- Aganovic, A.; Cao, G.; Kurnitski, J.; Melikov, A.; Wargocki, P. Zonal modeling of air distribution impact on the long-range airborne transmission risk of SARS-CoV-2. Appl. Math. Model. 2022, 112, 800–821. [Google Scholar] [CrossRef]
- Bougoffa, L.; Bougouffa, S.; Khanfer, A. Approximate and Parametric Solutions to SIR Epidemic Model. Axioms 2024, 13, 201. [Google Scholar] [CrossRef]
- Khyar, O.; Allali, K. Global dynamics of a multi-strain SEIR epidemic model with general incidence rates: Application to COVID-19 pandemic. Nonlinear Dyn. 2020, 102, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Kozyreff, G. Hospitalization dynamics during the first COVID-19 pandemic wave: SIR modelling compared to Belgium, France, Italy, Switzerland and New York City data. Infect. Dis. Model. 2021, 6, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Ram, V.; Schaposnik, L.P. A modified age-structured SIR model for COVID-19 type viruses. Sci. Rep. 2021, 11, 15194. [Google Scholar] [CrossRef]
- Aikebaier, S.; Song, Y.; Li, M.; Liu, J. Exploring the Impact and Prevention of Epidemics from a New Perspective: COVID-19 Transmission through Express Boxes. Int. J. Environ. Res. Public Health 2022, 19, 16884. [Google Scholar] [CrossRef]
- Onkhonova, G.; Gudymo, A.; Kosenko, M.; Marchenko, V.; Ryzhikov, A. Quantitative measurement of influenza virus transmission in animal model: An overview of current state. Biophys. Rev. 2023, 15, 1359–1366. [Google Scholar] [CrossRef]
- Sun, X.; Belser, J.A.; Pulit-Penaloza, J.A.; Brock, N.; Kieran, T.J.; Zeng, H.; Pappas, C.; Tumpey, T.M.; Maines, T.R.; Peiris, M. A naturally occurring HA-stabilizing amino acid (HA1-Y17) in an A(H9N2) low-pathogenic influenza virus contributes to airborne transmission. mBio 2024, 15, e0295723. [Google Scholar] [CrossRef]
- Port, J.R.; Morris, D.H.; Riopelle, J.C.; Yinda, C.K.; Avanzato, V.A.; Holbrook, M.G.; Bushmaker, T.; Schulz, J.E.; Saturday, T.A.; Barbian, K.; et al. Host and viral determinants of airborne transmission of SARS-CoV-2 in the Syrian hamster. eLife 2024, 12, RP87094. [Google Scholar] [CrossRef] [PubMed]
- Stauft, C.B.; Selvaraj, P.; D′Agnillo, F.; Meseda, C.A.; Liu, S.; Pedro, C.L.; Sangare, K.; Lien, C.Z.; Weir, J.P.; Starost, M.F.; et al. Intranasal or airborne transmission-mediated delivery of an attenuated SARS-CoV-2 protects Syrian hamsters against new variants. Nat. Commun. 2023, 14, 3393. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Brock, N.; Belser, J.A.; Sun, X.; Pappas, C.; Tumpey, T.M.; Maines, T.R. Kinetics and magnitude of viral RNA shedding as indicators for Influenza A virus transmissibility in ferrets. Commun. Biol. 2023, 6, 90. [Google Scholar] [CrossRef]
- Gudymo, A.; Onkhonova, G.; Danilenko, A.; Susloparov, I.; Danilchenko, N.; Kosenko, M.; Moiseeva, A.; Kolosova, N.; Svyatchenko, S.; Marchenko, V.; et al. Quantitative Assessment of Airborne Transmission of Human and Animal Influenza Viruses in the Ferret Model. Atmosphere 2023, 14, 471. [Google Scholar] [CrossRef]
- Kuehl, P.J.; Dearing, J.; Werts, A.; Cox, J.; Irshad, H.; Barrett, E.G.; Tucker, S.N.; Langel, S.N. Design and validation of an exposure system for efficient inter-animal SARS-CoV-2 airborne transmission in Syrian hamsters. Microbiol. Spectr. 2023, 11, e0471722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Q.; Kong, H.; Jiang, Y.; Gao, Y.; Deng, G.; Shi, J.; Tian, G.; Liu, L.; Liu, J.; et al. H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science 2013, 340, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Myung, H.; Joung, Y.S. Contribution of Particulates to Airborne Disease Transmission and Severity: A Review. Environ. Sci. Technol. 2024, 58, 6846–6867. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Su, B.; Dou, Z. Analysis of SARS-CoV-2 transmission in a university classroom based on real human close contact behaviors. Sci. Total Environ. 2024, 917, 170346. [Google Scholar] [CrossRef]
- Guo, Y.; Dou, Z.; Zhang, N.; Liu, X.; Su, B.; Li, Y.; Zhang, Y.; Bovell-Benjamin, A. Student close contact behavior and COVID-19 transmission in China’s classrooms. PNAS Nexus 2023, 2, 142. [Google Scholar] [CrossRef]
- Lowen, A.C.; Steel, J.; Mubareka, S.; Palese, P. High temperature (30 degrees C) blocks aerosol but not contact transmission of influenza virus. J. Virol. 2008, 82, 5650–5652. [Google Scholar] [CrossRef]
- Steel, J.; Lowen, A.C.; Mubareka, S.; Palese, P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 2009, 5, e1000252. [Google Scholar] [CrossRef]
- Lv, J.; Wei, B.; Yang, Y.; Yao, M.; Cai, Y.; Gao, Y.; Xia, X.; Zhao, X.; Liu, Z.; Li, X.; et al. Experimental transmission in guinea pigs of H9N2 avian influenza viruses from indoor air of chicken houses. Virus Res. 2012, 170, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Enqi, L.; Hailin, Y.; Weiwang, G. Medical Experimental zoology. Genetic 2008, 30, 918. [Google Scholar]
- Chen, S.C.; Chang, C.F.; Liao, C.M. Predictive models of control strategies involved in containing indoor airborne infections. Indoor Air 2006, 16, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Sze To, G.N.; Chao, C.Y. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air 2010, 20, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Reissner, J.; Siller, P.; Bartel, A.; Roesler, U.; Friese, A. Stability of Feline Coronavirus in aerosols and dried in organic matrices on surfaces at various environmental conditions. Sci. Rep. 2023, 13, 22012. [Google Scholar] [CrossRef] [PubMed]
- Doremalen, N.V.; Bushmaker, T.; Munster, V. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eurosurveillance 2013, 18, 20590. [Google Scholar] [CrossRef]
- Pyankov, O.V.; Bodnev, S.A.; Pyankova, O.G.; Agranovski, I.E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 2018, 115, 158–163. [Google Scholar] [CrossRef]
- Birgand, G.; Peiffer-Smadja, N.; Fournier, S.; Kerneis, S.; Lescure, F.X.; Lucet, J.C. Assessment of Air Contamination by SARS-CoV-2 in Hospital Settings. JAMA Netw. Open 2020, 3, e2033232. [Google Scholar] [CrossRef]
- Faridi, S.; Niazi, S.; Sadeghi, K.; Naddafi, K.; Yavarian, J.; Shamsipour, M.; Jandaghi, N.Z.S.; Sadeghniiat, K.; Nabizadeh, R.; Yunesian, M.; et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total. Environ. 2020, 725, 138401. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, H.; Strobl, E.; Li, H.; Li, R.; Jensen, S.S.; Zhang, Y. The role of weather conditions in COVID-19 transmission: A study of a global panel of 1236 regions. J. Clean. Prod. 2021, 292, 125987. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.-G.; Oh, J.S.; Nam, M.; Barrett, S.; Lee, S.; Hwang, W. The effects of indoor temperature and humidity on local transmission of COVID-19 and how it relates to global trends. PLoS ONE 2022, 17, e0271760. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Woiniakowski, G. Natural inactivation of African swine fever virus in tissues: Influence of temperature and environmental conditions on virus survival. Vet. Microbiol. 2020, 242, 108609. [Google Scholar] [CrossRef]
- Prussin, A.J., II; Schwake, D.O.; Lin, K.; Gallagher, D.L.; Buttling, L.; Marr, L.C. Survival of the Enveloped Virus Phi6 in Droplets as a Function of Relative Humidity, Absolute Humidity, and Temperature. Appl. Environ. Microbiol. 2018, 84, e00551-18. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Schulte, C.R.; Marr, L.C. Survival of MS2 and φ6 viruses in droplets as a function of relative humidity, pH, and salt, protein, and surfactant concentrations. PLoS ONE 2020, 15, e0243505. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of Air Temperature and Relative Humidity on Coronavirus Survival on Surfaces. Appl. Environ. Microbiol. 2010, 76, 2712–2717. [Google Scholar] [CrossRef] [PubMed]

| Contact Form | The Closest Distance (cm) | The Contact Probability Pcontact (%) | Literature Sources |
|---|---|---|---|
| collocation | 0 | 100 | Lowen A.C. [28] |
| Steel J. [29] | |||
| neighboring cage | 4 | 20 | Steel J. [29] |
| Long distance | 80 | 0 | Lv J. [30] |
| Physiological Parameters | Guinea Pig | Man |
|---|---|---|
| adult weight (g) | 250~350 | 45,000~70,000 |
| core temperature(°C) | 38.9~39.7 | 36.6–38 |
| lung ventilation rate (mL/min) | 82.8~197.6 | 4800~10,000 |
| Place | Temperature (°C) | Relative Humidity (%) | Air Exchange Rate (h−1) | o | Pcontact % | b | Exposure Time h | Calculation of Infection Rate | Actual Infection Rate | Literature Sources |
|---|---|---|---|---|---|---|---|---|---|---|
| School | 26 | 40 | 6 | 2.5 | 25 | 0.1314 | 6 | 0.0051 | 0.0052 | Chen, S.C. [32] |
| Airplane | 21 | 25 | 2 | 2.5 | 70 | 6.5873 | 3.33 | 0.276 | 0.27 | Sze To, G.N. [33] |
| Temperature (°C) | Presence of Infection (h) I > 2 | Outbreak (h) I > 10 | Total Infections (h) I = 75 |
|---|---|---|---|
| 10 | 2.08 | 2.54 | 2.74 |
| 15 | 2.38 | 2.9 | 3.13 |
| 20 | 2.6 | 3.2 | 3.44 |
| 25 | 2.82 | 3.44 | 3.7 |
| 30 | 3 | 3.67 | 3.95 |
| Relative Humidity (%) | Presence of Infection (h) I > 2 | Outbreak (h) I > 10 | Total Infections (h) I = 75 |
|---|---|---|---|
| 10 | 1.95 | 2.4 | 2.6 |
| 25 | 2.69 | 3.25 | 3.51 |
| 40 | 3.12 | 3.81 | 4.11 |
| 55 | 3.5 | 4.24 | 4.57 |
| 70 | 3.75 | 4.59 | 4.94 |
| Number of Air Changes (h−1) | Presence of Infection (h) I > 2 | Outbreak (h) I > 10 | Total Infections (h) I = 75 |
|---|---|---|---|
| 4 | 1.37 | 1.68 | 1.82 |
| 14 | 2.1 | 2.55 | 2.75 |
| 24 | 2.5 | 3.05 | 3.27 |
| 34 | 2.81 | 3.43 | 3.7 |
| 44 | 3.1 | 3.74 | 4.03 |
| Susceptible Persons | Presence of Infection (h) I > 2 | Outbreak (h) I > 10 | Total Infections (h) I = N |
|---|---|---|---|
| 115 | 1.69 | 2.06 | 2.19 |
| 95 | 2.08 | 2.52 | 2.7 |
| 75 | 2.43 | 3.01 | 3.25 |
| 55 | 2.92 | 3.62 | 3.93 |
| 35 | 3.66 | 4.5 | 4.99 |
| Independent Variable | Results | Presence of Infection I > 2 | Outbreak I > 10 | Total Infections I = N | Significance Ranking |
|---|---|---|---|---|---|
| Temperature (°C) | p-value | <0.001 | <0.001 | <0.001 | 1 |
| t | 18.658 | 17.592 | 18.009 | ||
| Beta | 0.996 | 0.995 | 0.995 | ||
| Relative humidity (%) | p-value | 0.003 | 0.002 | 0.003 | 3 |
| t | 8.504 | 9.503 | 9.421 | ||
| Beta | 0.98 | 0.984 | 0.984 | ||
| Number of air changes (h−1) | p-value | 0.004 | 0.004 | 0.004 | 4 |
| t | 8.219 | 7.926 | 8.157 | ||
| Beta | 0.979 | 0.977 | 0.978 | ||
| Susceptible people | p-value | 0.002 | 0.001 | 0.002 | 2 |
| t | −10.79 | −12.237 | −10.8 | ||
| Beta | −0.987 | −0.99 | −0.987 |
| Coefficient of Sensitivity | Interval | Presence of Infection I > 2 | Outbreak I > 10 | Total Infections I = N |
|---|---|---|---|---|
| Temperature (°C) | 10–15 | 0.38 | 0.37 | 0.37 |
| 15–20 | 0.28 | 0.31 | 0.3 | |
| 20–25 | 0.4 | 0.35 | 0.35 | |
| 25–30 | 0.32 | 0.34 | 0.34 | |
| Relative humidity (%) | 10–25 | 0.45 | 0.43 | 0.43 |
| 25–40 | 0.26 | 0.28 | 0.28 | |
| 40–55 | 0.39 | 0.37 | 0.36 | |
| 55–70 | 0.26 | 0.3 | 0.29 | |
| Number of air changes (h−1) | 4–14 | 0.48 | 0.47 | 0.47 |
| 14–24 | 0.26 | 0.27 | 0.26 | |
| 24–34 | 0.37 | 0.37 | 0.39 | |
| 34–44 | 0.35 | 0.3 | 0.3 | |
| Susceptible people | 115–95 | −0.88 | −0.86 | −0.89 |
| 95–75 | −0.79 | −0.92 | −0.96 | |
| 75–55 | −0.46 | −0.46 | −0.47 | |
| 55–35 | −0.69 | −0.66 | −0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Jia, Y.; Guo, L.; Cheng, Z.; Jiang, X.; Hu, W.; Long, E. Exploring the Impact and Prevention of Epidemics Based on Inter-Animal Transmission from an Environmental Perspective. Buildings 2024, 14, 2974. https://doi.org/10.3390/buildings14092974
Liao Y, Jia Y, Guo L, Cheng Z, Jiang X, Hu W, Long E. Exploring the Impact and Prevention of Epidemics Based on Inter-Animal Transmission from an Environmental Perspective. Buildings. 2024; 14(9):2974. https://doi.org/10.3390/buildings14092974
Chicago/Turabian StyleLiao, Yuxuan, Yonghong Jia, Luyao Guo, Zhu Cheng, Xingchi Jiang, Wenxin Hu, and Enshen Long. 2024. "Exploring the Impact and Prevention of Epidemics Based on Inter-Animal Transmission from an Environmental Perspective" Buildings 14, no. 9: 2974. https://doi.org/10.3390/buildings14092974
APA StyleLiao, Y., Jia, Y., Guo, L., Cheng, Z., Jiang, X., Hu, W., & Long, E. (2024). Exploring the Impact and Prevention of Epidemics Based on Inter-Animal Transmission from an Environmental Perspective. Buildings, 14(9), 2974. https://doi.org/10.3390/buildings14092974






