Solubility Characteristics and Microstructure of Bitumen: A Review
Abstract
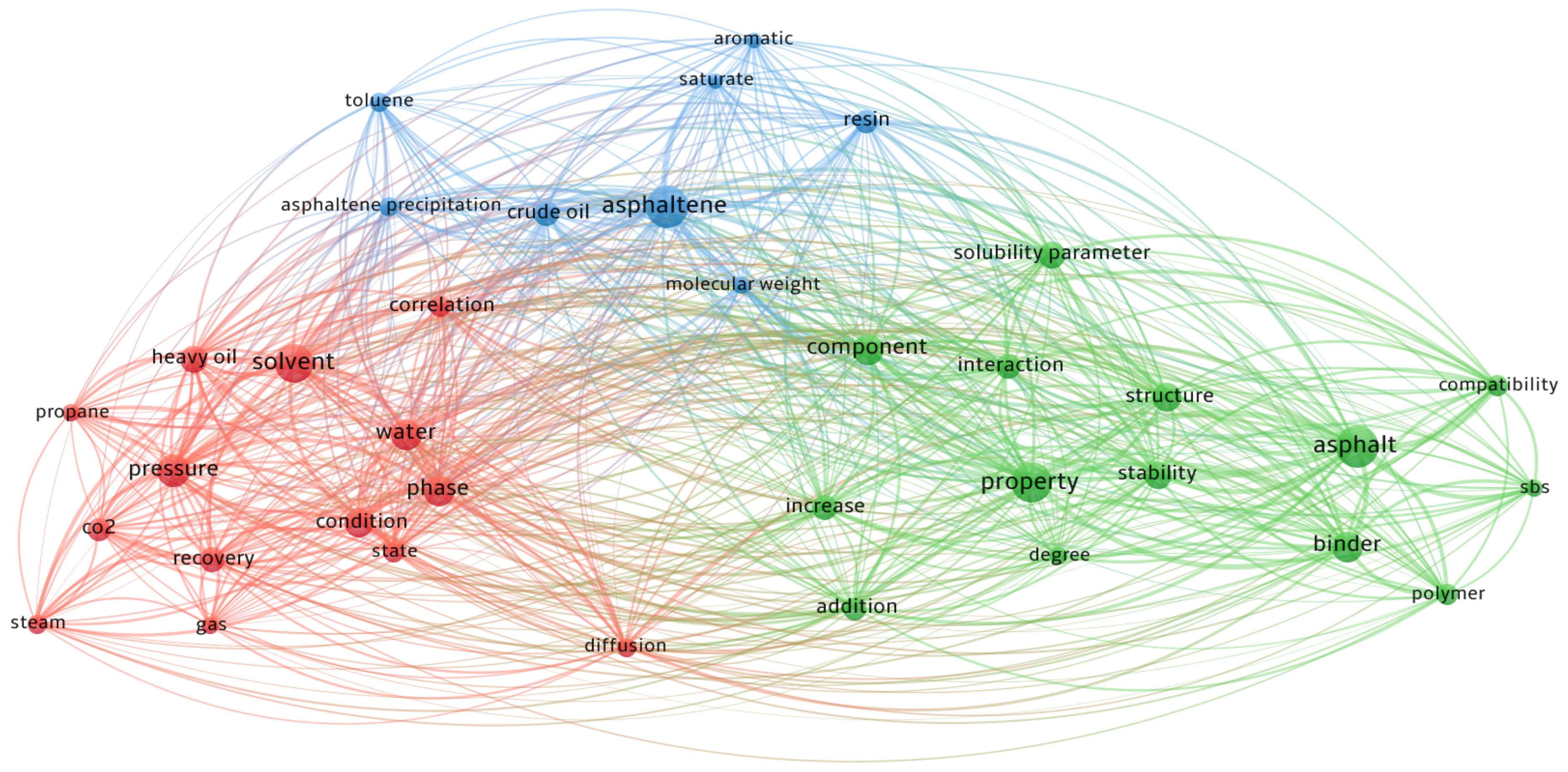
1. Introduction
2. Scope and Objective
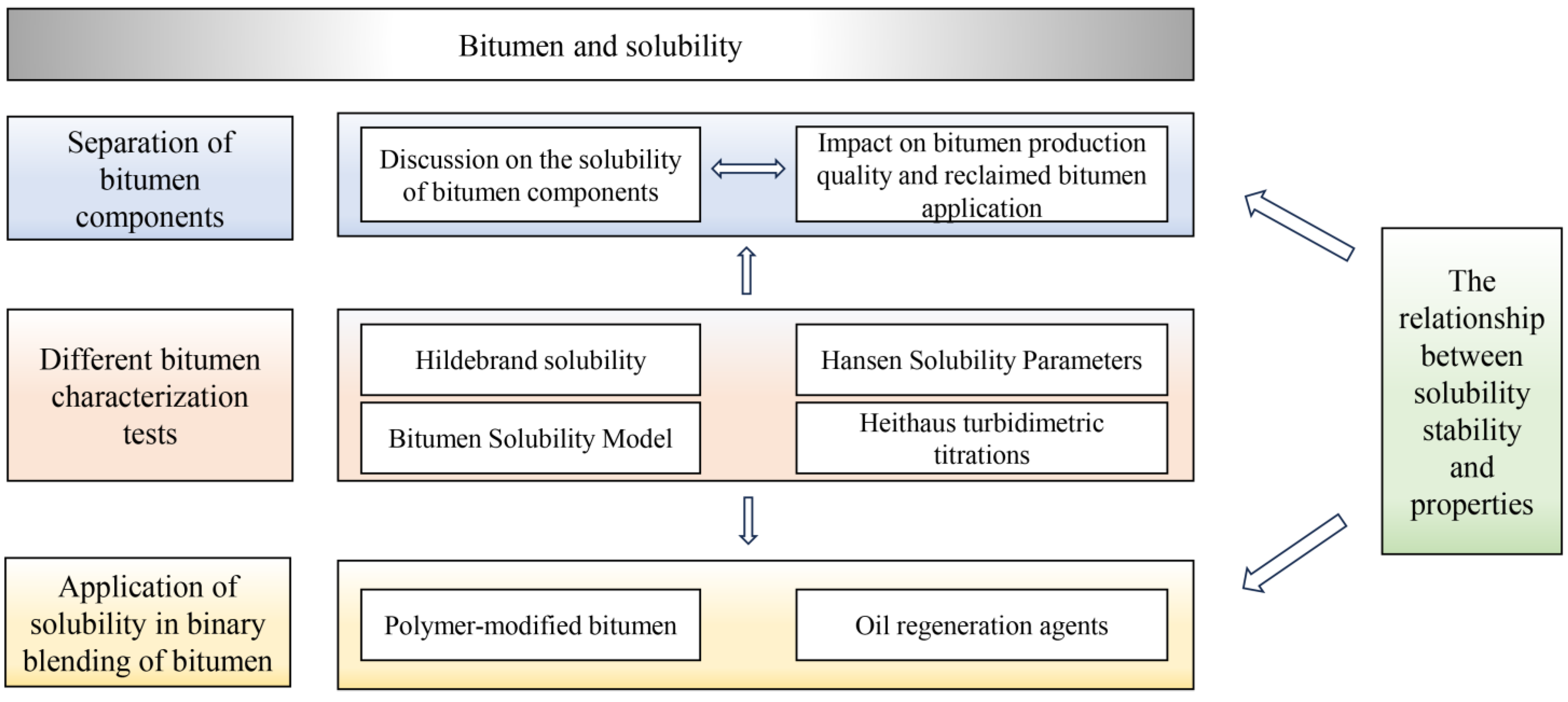
3. Separation of Bitumen Components Based on Solubility
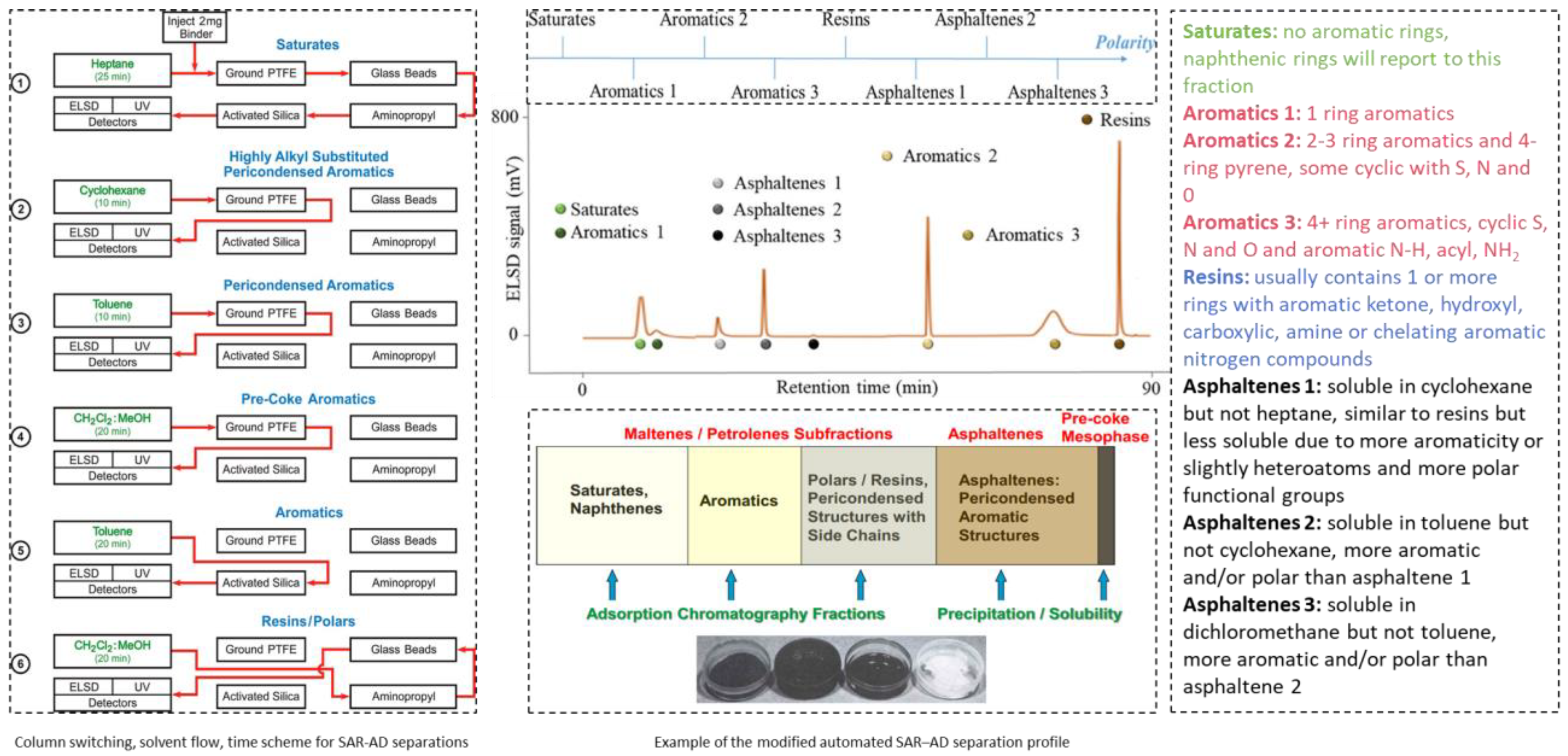
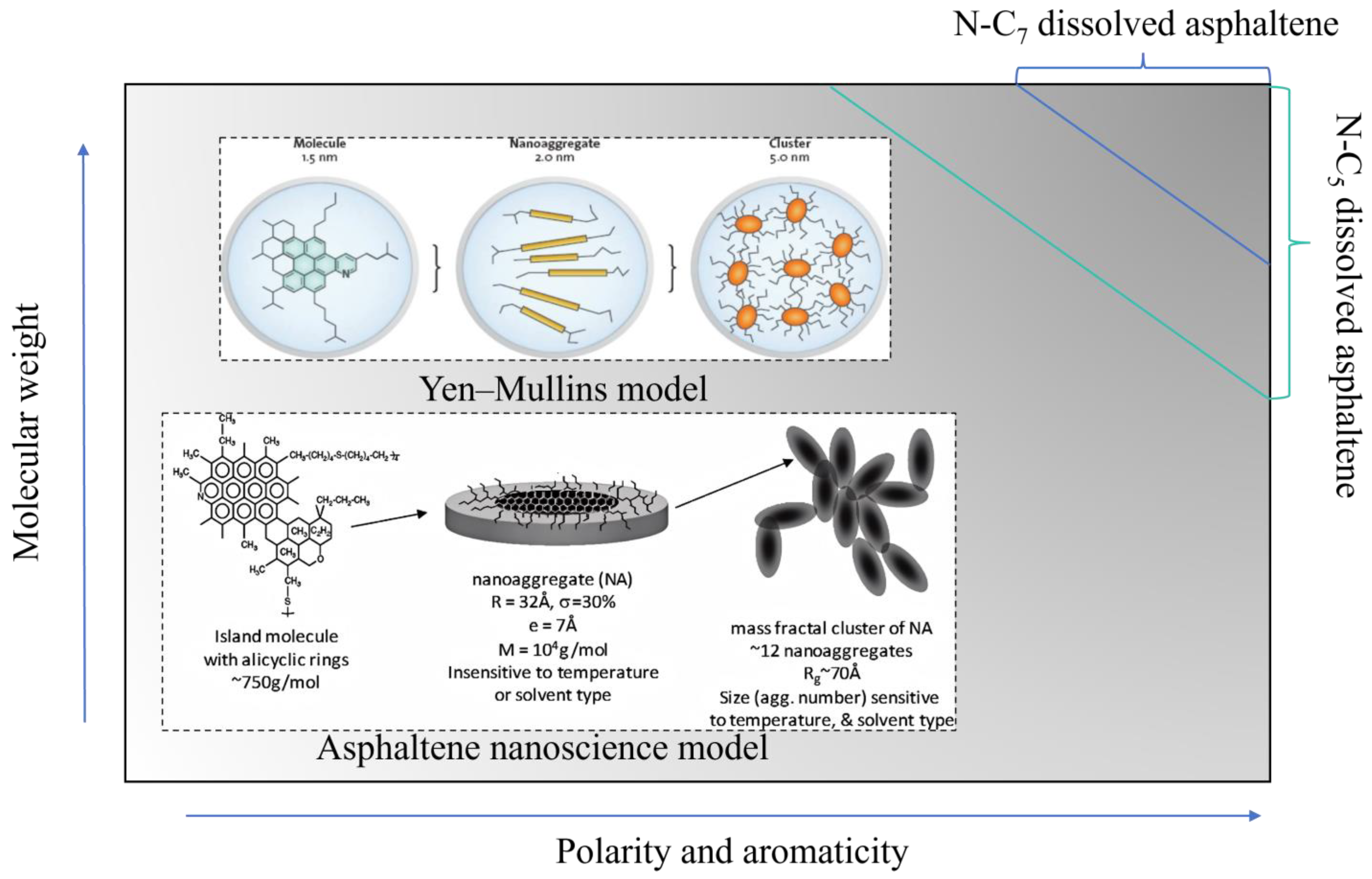
4. Solubility Theories and Different Bitumen Characterization Tests
5. The Relationship Between Solubility Stability and Properties
5.1. Internal Stability Changes Caused by Oxidation
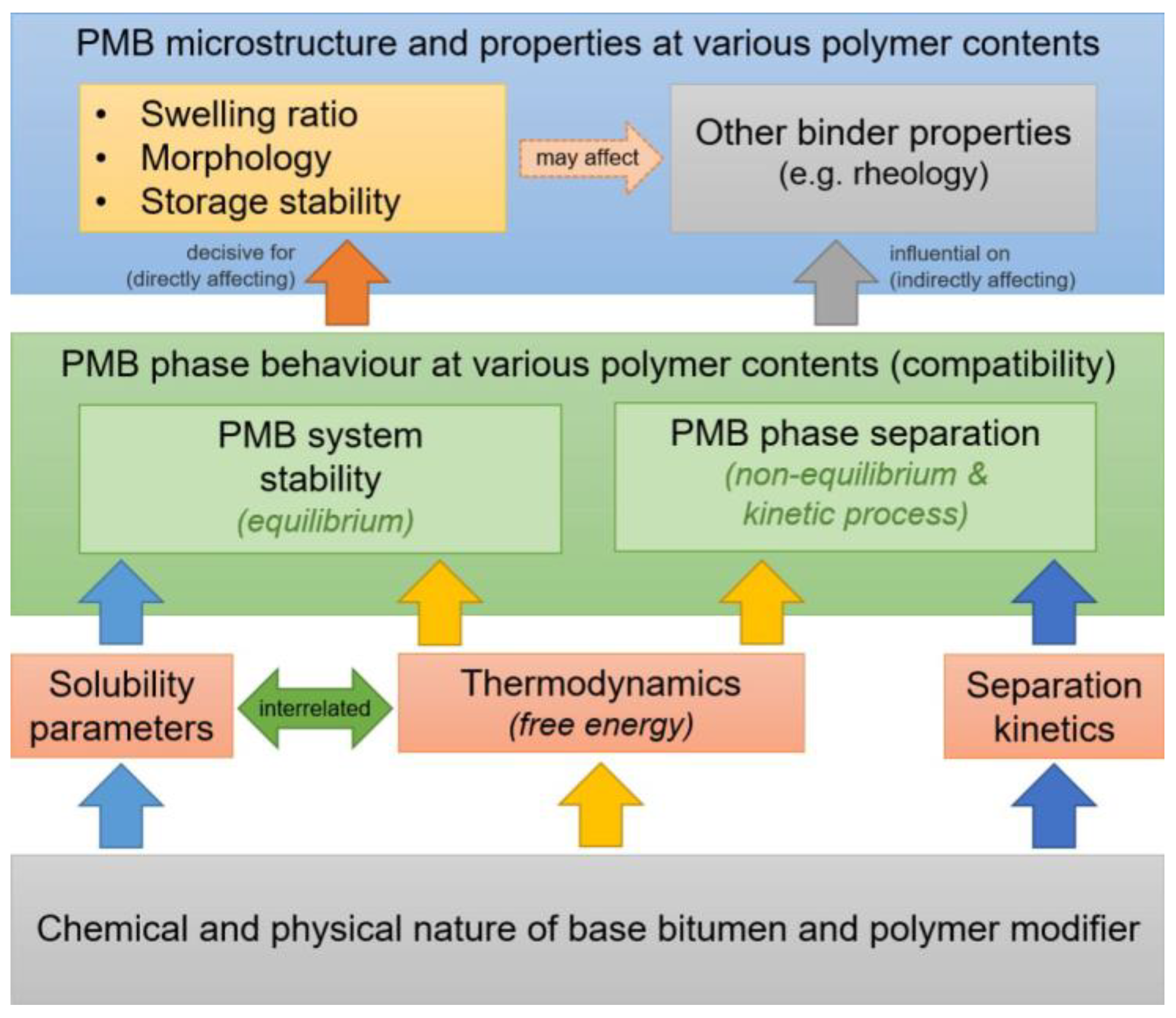
5.2. Application of Solubility in Binary Blending of Bitumen
6. Research Gaps and Future Development
7. Summary and Conclusions
- (1)
- Solvent fractionation and HSP are crucial for determining asphalt’s solubility behavior. These methods, combined with the Bitumen Solubility Model (BISOM) and turbidimetric titrations, provide insights into asphalt’s internal stability and mechanical behavior. While traditional methods like SARA fractionation have limitations in correlating chemical composition with properties, further separation of bitumen components based on various solubility methods can scientifically guide the production of high-quality asphalt.
- (2)
- The impact of aging on bitumen solubility is significant, affecting its rheological properties and stability. By analyzing the solubility profiles of different bitumen types and aging states, predictive models for bitumen behavior can be developed. These changes can be understood through various hypotheses, such as molecular condensation, increased polarity, and increased aromaticity. Moreover, the solubility domain concept and group-contribution methods offer valuable insights into the interactions between various bitumen components and solvents.
- (3)
- HSPs also aid in predicting the compatibility of bitumen with polymers, which is crucial for enhancing phase stability and performance in polymer-modified asphalt. Future advancements in analytical chemistry techniques will further refine our understanding of bitumen solubility.
Funding
Acknowledgments
Conflicts of Interest
References
- Mirwald, J.; Werkovits, S.; Camargo, I.; Maschauer, D.; Hofko, B.; Grothe, H. Investigating bitumen long-term-ageing in the laboratory by spectroscopic analysis of the SARA fractions. Constr. Build. Mater. 2020, 258, 119577. [Google Scholar] [CrossRef]
- Paliukaitė, M.; Vaitkus, A.; Zofka, A. Influence of bitumen chemical composition and ageing on pavement performance. Balt. J. Road Bridge Eng. 2015, 10, 97–104. [Google Scholar] [CrossRef]
- Tauste, R.; Moreno-Navarro, F.; Sol-Sánchez, M.; Rubio-Gámez, M.C. Understanding the bitumen ageing phenomenon: A review. Constr. Build. Mater. 2018, 192, 593–609. [Google Scholar] [CrossRef]
- Primerano, K.; Mirwald, J.; Hofko, B. Asphaltenes and maltenes in crude oil and bitumen: A comprehensive review of properties, separation methods, and insights into structure, reactivity and aging. Fuel 2024, 368, 131616. [Google Scholar] [CrossRef]
- Bruneau, L.; Tisse, S.; Michon, L.; Cardinael, P. Evaluation of Asphalt Aging Using Multivariate Analysis Applied to Saturates, Aromatics, Resins, and Asphaltene Determinator Data. ACS Omega 2023, 8, 24773–24785. [Google Scholar] [CrossRef]
- Bissada, K.K.; Tan, J.; Szymczyk, E.; Darnell, M.; Mei, M. Group-type characterization of crude oil and bitumen. Part I: Enhanced separation and quantification of saturates, aromatics, resins and asphaltenes (SARA). Org. Geochem. 2016, 95, 21–28. [Google Scholar] [CrossRef]
- Sultana, S.; Bhasin, A. Effect of chemical composition on rheology and mechanical properties of asphalt binder. Constr. Build. Mater. 2014, 72, 293–300. [Google Scholar] [CrossRef]
- Bowers, B.F.; Huang, B.; He, Q.; Shu, X.; Jia, X.; Miller, B.C. Investigation of sequential dissolution of asphalt binder in common solvents by FTIR and binder fractionation. J. Mater. Civ. Eng. 2015, 27, 04014233. [Google Scholar] [CrossRef]
- Koyun, A.N.; Zakel, J.; Kayser, S.; Stadler, H.; Keutsch, F.N.; Grothe, H. High resolution nanoscale chemical analysis of bitumen surface microstructures. Sci. Rep. 2021, 11, 13554. [Google Scholar] [CrossRef]
- Porto, M.; Angelico, R.; Caputo, P.; Abe, A.A.; Teltayev, B.; Rossi, C.O. The structure of bitumen: Conceptual models and experimental evidences. Materials 2022, 15, 905. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Z.-j.; Tan, Y.-q.; Liu, Z.-y. Investigating the interactions of the saturate, aromatic, resin, and asphaltene four fractions in asphalt binders by molecular simulations. Energy Fuels 2015, 29, 112–121. [Google Scholar] [CrossRef]
- Hu, D.; Gu, X.; Dong, Q.; Lyu, L.; Cui, B.; Pei, J. Investigating the bio-rejuvenator effects on aged asphalt through exploring molecular evolution and chemical transformation of asphalt components during oxidative aging and regeneration. J. Clean. Prod. 2021, 329, 129711. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, H.; Liu, H.; Qiu, Y. Thermoreversible aging in model asphalt binders. Constr. Build. Mater. 2021, 303, 124355. [Google Scholar] [CrossRef]
- Girdler, R. Constitution of asphaltenes and related studies. In Association of Asphalt Paving Technologists; The National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 1965; Volume 34, pp. 45–79. [Google Scholar]
- Wang, Y.; Wang, W.; Wang, L. Understanding the relationships between rheology and chemistry of asphalt binders: A review. Constr. Build. Mater. 2022, 329, 127161. [Google Scholar] [CrossRef]
- Pfeiffer, J.P.; Saal, R. Asphaltic bitumen as colloid system. J. Phys. Chem. 1940, 44, 139–149. [Google Scholar] [CrossRef]
- Saal, R.; Labout, J. Rheological Properties of Asphaltic Bitumen. J. Phys. Chem. 1940, 44, 149–165. [Google Scholar] [CrossRef]
- Dickie, J.P.; Yen, T.F. Macrostructures of the asphaltic fractions by various instrumental methods. Anal. Chem. 1967, 39, 1847–1852. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L.; et al. Advances in asphaltene science and the Yen–Mullins model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Moncayo-Riascos, I.; Taborda, E.; Hoyos, B.A.; Franco, C.A.; Cortés, F.B. Effect of resin/asphaltene ratio on the rheological behavior of asphaltene solutions in a de-asphalted oil and p-xylene: A theoretical–experimental approach. J. Mol. Liq. 2020, 315, 113754. [Google Scholar] [CrossRef]
- Sun, L. Structural Behavior of Asphalt Pavements: Intergrated Analysis and Design of Conventional and Heavy Duty Asphalt Pavement; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Burke, J. Solubility Parameters: Theory and Application; The American Institute for Conservation: Washington, DC, USA, 1984. [Google Scholar]
- Ren, S.; Liu, X.; Lin, P.; Gao, Y.; Erkens, S. Molecular dynamics simulation on bulk bitumen systems and its potential connections to macroscale performance: Review and discussion. Fuel 2022, 328, 125382. [Google Scholar] [CrossRef]
- Perianes-Rodriguez, A.; Waltman, L.; van Eck, N.J. Constructing bibliometric networks: A comparison between full and fractional counting. J. Informetr. 2016, 10, 1178–1195. [Google Scholar] [CrossRef]
- Rondón-Quintana, H.A.; Ruge-Cárdenas, J.C.; Zafra-Mejía, C.A. Natural Asphalts in Pavements. Sustainability 2023, 15, 2098. [Google Scholar] [CrossRef]
- Allen, R.G.; Little, D.N.; Bhasin, A.; Glover, C.J. The effects of chemical composition on asphalt microstructure and their association to pavement performance. Int. J. Pavement Eng. 2014, 15, 9–22. [Google Scholar] [CrossRef]
- Branthaver, J.; Huang, S.-C. Analytical separation methods in asphalt research. In Advances in Asphalt Materials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 31–57. [Google Scholar]
- Yuan, H.; Nili, A.; Chen, J.; Ding, H.; Liu, H.; Qiu, Y. Solubility and structural parameters of asphaltene subfractions separated from bitumen via an improved method. Fuel 2023, 344, 128113. [Google Scholar] [CrossRef]
- Petersen, J.C. Chemical composition of asphalt as related to asphalt durability. Dev. Pet. Sci. 2000, 40 Pt B, 363–399. [Google Scholar]
- Alvarez-Majmutov, A.; Gieleciak, R.; Chen, J. Enhanced Representation of Thermal Cracking Chemistry in the Context of Bitumen Partial Upgrading. Energy Fuels 2021, 35, 12005–12018. [Google Scholar] [CrossRef]
- Wen, C.S.; Chilingarian, G.V.; Yen, T.F. Properties and structure of bitumens. Dev. Pet. Sci. 1978, 7, 155–190. [Google Scholar]
- Petersen, J.; Robertson, R.; Branthaver, J.; Harnsberger, P.; Duvall, J.; Kim, S.; Anderson, D.A.; Christiansen, D.W.; Bahia, H.U. Binder Characterization and Evaluation; Strategic Highway Research Program, National Research Council: Rockville, MD, USA, 1994; Volume 1. [Google Scholar]
- O’Donnell, G.; Snider, L.; Rietz, E. Separating asphalt into its chemical constituents. Anal. Chem. 1951, 23, 894–898. [Google Scholar] [CrossRef]
- Qu, X.; Fan, Z.; Li, T.; Hong, B.; Zhang, Y.; Wei, J.; Wang, D.; Oeser, M. Understanding of asphalt chemistry based on the six-fraction method. Constr. Build. Mater. 2021, 311, 125241. [Google Scholar] [CrossRef]
- Adams, J.J.; Elwardany, M.D.; Planche, J.-P.; Boysen, R.B.; Rovani, J.F. Diagnostic Techniques for Various Asphalt Refining and Modification Methods. Energy Fuels 2019, 33, 2680–2698. [Google Scholar] [CrossRef]
- Boysen, R.B.; Schabron, J.F. The automated asphaltene determinator coupled with saturates, aromatics, and resins separation for petroleum residua characterization. Energy Fuels 2013, 27, 4654–4661. [Google Scholar] [CrossRef]
- Schabron, J.F.; Rovani, J.F. On-column precipitation and re-dissolution of asphaltenes in petroleum residua. Fuel 2008, 87, 165–176. [Google Scholar] [CrossRef]
- Adams, J.J.; Rovani, J.F.; Planche, J.-P.; Loveridge, J.; Literati, A.; Shishkova, I.; Palichev, G.; Kolev, I.; Atanassov, K.; Nenov, S.; et al. SAR-AD Method to Characterize Eight SARA Fractions in Various Vacuum Residues and Follow Their Transformations Occurring during Hydrocracking and Pyrolysis. Processes 2023, 11, 1220. [Google Scholar] [CrossRef]
- Salehzadeh, M.; Husein, M.M.; Ghotbi, C.; Dabir, B.; Taghikhani, V. In-depth characterization of light, medium and heavy oil asphaltenes as well as asphaltenes subfractions. Fuel 2022, 324, 124525. [Google Scholar] [CrossRef]
- Pagán Pagán, N.M.; Zhang, Z.; Nguyen, T.V.; Marciel, A.B.; Biswal, S.L. Physicochemical characterization of asphaltenes using microfluidic analysis. Chem. Rev. 2022, 122, 7205–7235. [Google Scholar] [CrossRef]
- Goual, L. Petroleum asphaltenes. In Crude Oil Emulsions—Composition Stability and Characterization; IntechOpen: London, UK, 2012; pp. 28–43. [Google Scholar]
- Lin, M.; Lunsford, K.; Glover, C.; Davison, R.; Bullin, J. The effects of asphaltenes on the chemical and physical characteristics of asphalt. In Asphaltenes: Fundamentals and Applications; Springer: Boston, MA, USA, 1995; pp. 155–176. [Google Scholar]
- Lobato, M.; Gamez, F.; Pedrosa, J. Evidence of the aggregation behaviour of asphaltenes according to the Yen-Mullins model by direct visualization of their Langmuir-Blodgett films. Fuel 2021, 299, 120872. [Google Scholar] [CrossRef]
- Schuler, B.; Zhang, Y.; Liu, F.; Pomerantz, A.E.; Andrews, A.B.; Gross, L.; Pauchard, V.; Banerjee, S.; Mullins, O.C. Overview of asphaltene nanostructures and thermodynamic applications. Energy Fuels 2020, 34, 15082–15105. [Google Scholar] [CrossRef]
- Musin, L.I.; Foss, L.E.; Shabalin, K.V.; Nagornova, O.A.; Borisova, Y.Y.; Borisov, D.N.; Yakubov, M.R. Simple methods for the separation of various subfractions from coal and petroleum asphaltenes. Energy Fuels 2020, 34, 6523–6543. [Google Scholar] [CrossRef]
- Wiehe, I.A. Asphaltene solubility and fluid compatibility. Energy Fuels 2012, 26, 4004–4016. [Google Scholar]
- Schabron, J.F.; Speight, J.G. The solubility and three-dimensional structure of asphaltenes. Pet. Sci. Technol. 1998, 16, 361–375. [Google Scholar] [CrossRef]
- Acevedo, S.; Ranaudo, M.A.; Pereira, J.C.; Castillo, J.; Fernández, A.; Pérez, P.; Caetano, M. Thermo-optical studies of asphaltene solutions: Evidence for solvent–solute aggregate formation. Fuel 1999, 78, 997–1003. [Google Scholar] [CrossRef]
- Luo, P.; Wang, X.; Gu, Y. Characterization of asphaltenes precipitated with three light alkanes under different experimental conditions. Fluid Phase Equilibria 2010, 291, 103–110. [Google Scholar] [CrossRef]
- Leontaritis, K.J.; Ali Mansoori, G. Asphaltene deposition: A survey of field experiences and research approaches. J. Pet. Sci. Eng. 1988, 1, 229–239. [Google Scholar] [CrossRef]
- Reynolds, J.G. Characterization of Heavy Residua by Application of a Modified D 2007 and Asphaltene Separation: Effect of Solvents on Physical and Chemical Properties of Fractions Derived from Hondo 850°F Residoom. Fuel Sci. Technol. Int. 1987, 5, 593–620. [Google Scholar] [CrossRef]
- Schabron, J.F.; Rovani, J.F., Jr.; Sanderson, M.M.; Loveridge, J.L.; Nyadong, L.; McKenna, A.M.; Marshall, A.G. Waxphaltene Determinator Method for Automated Precipitation and Redissolution of Wax and Asphaltene Components. Energy Fuels 2012, 26, 2256–2268. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, H.; Zhang, H.; Liu, D.; Qiu, Y.; Hussain, A. Separation of wax fraction in asphalt binder by an improved method and determination of its molecular structure. Fuel 2022, 322, 124081. [Google Scholar] [CrossRef]
- Ding, H.; Liu, H.; Qiu, Y.; Rahman, A. Effects of crystalline wax and asphaltene on thermoreversible aging of asphalt binder. Int. J. Pavement Eng. 2021, 23, 3997–4006. [Google Scholar] [CrossRef]
- Angle, C.W.; Long, Y.; Hamza, H.; Lue, L. Precipitation of asphaltenes from solvent-diluted heavy oil and thermodynamic properties of solvent-diluted heavy oil solutions. Fuel 2006, 85, 492–506. [Google Scholar] [CrossRef]
- Buckley, J.S.; Wang, J.; Creek, J.L. Solubility of the least-soluble asphaltenes. In Asphaltenes, Heavy Oils, and Petroleomics; Springer: New York, NY, USA, 2007; pp. 401–437. [Google Scholar]
- Glaser, R.; Planche, J.; Turner, F.; Boysen, R.; Schabron, J.; Delfosse, F.; Drouadaine, I.; Faucon-Dumont, S.; Largeaud, S.; Eckmann, B. Relationships between solubility and chromatographically defined bitumen fractions and physical properties. In Proceedings of the 6th Eurasphalt and Eurobitume Congress, Prague, Czech Republic, 1–3 June 2016. [Google Scholar]
- Speight, J.G. The Chemistry and Technology of Coal; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Mitchell, D.L.; Speight, J.G. Solubility of (athabasca bitumen) asphaltenes in (44) hydrocarbon solvents. Fuel 1973, 52, 149–152. [Google Scholar] [CrossRef]
- Hildebrand, J.; Scott, R. Solutions of nonelectrolytes. Annu. Rev. Phys. Chem. 1950, 1, 75–92. [Google Scholar] [CrossRef]
- AlQasas, N.; Eskhan, A.; Johnson, D. Hansen solubility parameters from surface measurements: A comparison of different methods. Surf. Interfaces 2023, 36, 102594. [Google Scholar] [CrossRef]
- Gallu, R.; Méchin, F.; Dalmas, F.; Gérard, J.-F.; Perrin, R.; Loup, F. On the use of solubility parameters to investigate phase separation-morphology-mechanical behavior relationships of TPU. Polymer 2020, 207, 122882. [Google Scholar] [CrossRef]
- Barton, A.F. CRC Handbook of Solubility Parameters and Other Cohesion Parameters; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Barton, A.F. Handbook of Poylmer-Liquid Interaction Parameters and Solubility Parameters; Routledge: Abingdon, UK, 2018. [Google Scholar]
- Gharagheizi, F.; Sattari, M.; Angaji, M.T. Effect of calculation method on values of Hansen solubility parameters of polymers. Polym. Bull. 2006, 57, 377–384. [Google Scholar] [CrossRef]
- Rahimian, I.; Zenke, G. Zum verhalten organischer loesemittel gegenueber bitumen. Bitumen 1986, 48, 2–8. [Google Scholar]
- Groenzin, H.; Mullins, O.C. Molecular size and structure of asphaltenes from various sources. Energy Fuels 2000, 14, 677–684. [Google Scholar] [CrossRef]
- Wiehe, I.A. Two-dimensional solubility parameter mapping of heavy oils. Fuel Sci. Technol. Int. 1996, 14, 289–312. [Google Scholar] [CrossRef]
- Hansen, C.M. The Three Dimensional Solubility Parameter-Key to Paint Component Affinities: III; Independent Calculation of the Parameter Components. J Paint Technol. 1967, 39, 511–514. [Google Scholar]
- Levin, M.; Redelius, P. Determination of three-dimensional solubility parameters and solubility spheres for naphthenic mineral oils. Energy Fuels 2008, 22, 3395–3401. [Google Scholar] [CrossRef]
- Bagley, E.; Nelson, T.; Scigliano, J. Three-dimensional solubility parameters and their relationship to internal pressure measurements in polar and hydrogen bonding solvents. J. Paint Technol. 1971, 43, 35–42. [Google Scholar]
- Bagley, E.; Scigliano, J. Polymer Solutions. Solutions and Solubilities; Wiley Interscience: New York, NY, USA, 1976; pp. 437–485. [Google Scholar]
- Ribeiro, W.C.; Martinez, P.F.M.; Lobosco, V. Solubility parameters analysis of Eucalyptus urograndis kraft lignin. BioResources 2020, 15, 8577. [Google Scholar] [CrossRef]
- Wilson, G.; Deal, C. Activity coefficients and molecular structure. Activity coefficients in changing environments—Solutions of groups. Ind. Eng. Chem. Fundam. 1962, 1, 20–23. [Google Scholar] [CrossRef]
- Howell, J.; Roesing, M.; Boucher, D. A functional approach to solubility parameter computations. J. Phys. Chem. B 2017, 121, 4191–4201. [Google Scholar] [CrossRef] [PubMed]
- Hagen, A.P.; Jones, R.; Hofener, R.M.; Randolph, B.B.; Johnson, M.P. Characterization of asphalt by solubility profiles. J. Assoc. Asph. Paving Technol. 1984, 53, 119–137. [Google Scholar]
- Lee, W.D. Part 1. Characterization of Roadway Asphalts by Solubility Studies. Part 2. Development of Concrete Applicant Organo-Functional Silanes. Ph.D. Thesis, The University of Oklahoma, Norman, OK, USA, 1993. [Google Scholar]
- Abbott, S.; Hansen, C.M. Hansen Solubility Parameters in Practice; Hansen-Solubility; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Levin, M.; Redelius, P. Determining the Hansen solubility parameter of three corrosion inhibitors and the correlation with mineral oil. Energy Fuels 2012, 26, 7243–7250. [Google Scholar] [CrossRef]
- Redelius, P. Bitumen solubility model using hansen solubility parameter. Energy Fuels 2004, 18, 1087–1092. [Google Scholar] [CrossRef]
- Redelius, P.G. Solubility parameters and bitumen. Fuel 2000, 79, 27–35. [Google Scholar] [CrossRef]
- ASTM D1370-20; Standard Test Method for Contact Compatibility Between Asphalt and Petroleum Base Fuels. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D4740-19; Standard Test Method for Cleanliness and Compatibility of Residual Fuels by Spot Test. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Sreeram, A.; Leng, Z.; Hajj, R.; Bhasin, A. Characterization of compatibility between aged and unaged binders in bituminous mixtures through an extended HSP model of solubility. Fuel 2019, 254, 115578. [Google Scholar] [CrossRef]
- Sreeram, A.; Leng, Z.; Hajj, R.; Ferreira, W.L.; Tan, Z.; Bhasin, A. Fundamental investigation of the interaction mechanism between new and aged binders in binder blends. Int. J. Pavement Eng. 2022, 23, 1317–1327. [Google Scholar] [CrossRef]
- Carrier, H.; Plantier, F.; Daridon, J.-L.; Lagourette, B.; Lu, Z. Acoustic method for measuring asphaltene flocculation in crude oils. J. Pet. Sci. Eng. 2000, 27, 111–117. [Google Scholar] [CrossRef]
- Escobedo, J.; Mansoori, G.A. Viscometric determination of the onset of asphaltene flocculation: A novel method. SPE Prod. Facil. 1995, 10, 115–118. [Google Scholar] [CrossRef]
- Fotland, P.; Anfindsen, H.; Fadnes, F.H. Detection of asphaltene precipitation and amounts precipitated by measurement of electrical conductivity. Fluid Phase Equilibria 1993, 82, 157–164. [Google Scholar] [CrossRef]
- Wiehe, I.A.; Kennedy, R.J. The oil compatibility model and crude oil incompatibility. Energy Fuels 2000, 14, 56–59. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Lai, C.H.; Kazarian, S.G. In situ chemical imaging of asphaltene precipitation from crude oil induced by n-heptane. Energy Fuels 2014, 28, 964–971. [Google Scholar] [CrossRef]
- Dufour, J.; Calles, J.A.; Marugán, J.; Giménez-Aguirre, R.; Peña, J.L.; Merino-García, D. Influence of hydrocarbon distribution in crude oil and residues on asphaltene stability. Energy Fuels 2010, 24, 2281–2286. [Google Scholar] [CrossRef]
- MacMillan, D.; Tackett, J., Jr.; Jessee, M.; Monger-McClure, T. A unified approach to asphaltene precipitation: Laboratory measurement and modeling. J. Pet. Technol. 1995, 47, 788–793. [Google Scholar] [CrossRef]
- Yudin, I.K.; Anisimov, M.A. Dynamic light scattering monitoring of asphaltene aggregation in crude oils and hydrocarbon solutions. In Asphaltenes, Heavy Oils, and Petroleomics; Springer: New York, NY, USA, 2007; pp. 439–468. [Google Scholar]
- Prunelet, A.; Fleury, M.; Cohen-Addad, J.-P. Detection of asphaltene flocculation using NMR relaxometry. Comptes Rendus Chim. 2004, 7, 283–289. [Google Scholar] [CrossRef]
- Pauli, A.T. Asphalt compatibility testing using the automated Heithaus titration test. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 1996, 41, 1276–1281. [Google Scholar]
- Liu, H.; Su, B.; Ding, H.; Qiu, Y. Critical analysis of using recycled wax oils in asphalt binder considering exudation effects. Fuel 2024, 373, 132380. [Google Scholar] [CrossRef]
- Hagen, A.P.; Ellis, D.A.; Lee, W.D. Chemical characterization of roadway asphalt. Fuel Sci. Technol. Int. 1992, 10, 595–606. [Google Scholar] [CrossRef]
- Soenen, H.; De Visscher, J.; Vanelstraete, A.; Redelius, P. Influence of thermal history on rheological properties of various bitumen. Rheol. Acta 2006, 45, 729–739. [Google Scholar] [CrossRef]
- Cui, Y.; Glover, C.J.; Braziunas, J.; Sivilevicius, H. Further exploration of the pavement oxidation model–diffusion-reaction balance in asphalt. Constr. Build. Mater. 2018, 161, 132–140. [Google Scholar] [CrossRef]
- Ma, L.; Varveri, A.; Jing, R.; Erkens, S. Comprehensive review on the transport and reaction of oxygen and moisture towards coupled oxidative ageing and moisture damage of bitumen. Constr. Build. Mater. 2021, 283, 122632. [Google Scholar] [CrossRef]
- Daly, W.H.; Negulescu, I.; Balamurugan, S.S. Implementation of GPC Characterization of Asphalt Binders at Louisiana Materials Laboratory. Louisiana Department of Transportation and Development: Baton Rouge, LA, USA, 2013.
- Lahjiri, F.; Mouillet, V.; Dony, A.; Ziyani, L.; Gazeau, S.; Delfosse, F.; Sztucki, M.; Dieudonne-George, P.; Henn, F. Relationships between Chemical Composition, Asphaltene Nanostructures, and Thermochemical Properties of Bitumen before and after Accelerated Oxidative Aging. Energy Fuels 2023, 37, 8444–8455. [Google Scholar] [CrossRef]
- Werkovits, S.; Bacher, M.; Mirwald, J.; Theiner, J.; Rosenau, T.; Hofko, B.; Grothe, H. The impact of field ageing on molecular structure and chemistry of bitumen. Fuel 2023, 343, 127904. [Google Scholar] [CrossRef]
- Petersen, J.C.; Glaser, R. Asphalt oxidation mechanisms and the role of oxidation products on age hardening revisited. Road Mater. Pavement Des. 2011, 12, 795–819. [Google Scholar] [CrossRef]
- Omairey, E.L.; Zhang, Y.; Al-Malaika, S.; Sheena, H.; Gu, F. Impact of anti-ageing compounds on oxidation ageing kinetics of bitumen by infrared spectroscopy analysis. Constr. Build. Mater. 2019, 223, 755–764. [Google Scholar] [CrossRef]
- Soenen, H.; Redelius, P. The effect of aromatic interactions on the elasticity of bituminous binders. Rheol. Acta 2014, 53, 741–754. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Lin, P.; Erkens, S.; Xiao, Y. Chemo-physical characterization and molecular dynamics simulation of long-term aging behaviors of bitumen. Constr. Build. Mater. 2021, 302, 124437. [Google Scholar] [CrossRef]
- van den Berg, F.G. History and Review of Dual Solvent Titration Methods. Energy Fuels 2022, 36, 8639–8648. [Google Scholar] [CrossRef]
- Redelius, P.; Soenen, H. Correlation between Bitumen Polarity and Rheology. Road Mater. Pavement Des. 2005, 6, 385–405. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Zhang, L.; Deng, W.; Que, G. Phase Separation and Colloidal Stability Change of Karamay Residue Oil during Thermal Reaction. Energy Fuels 2009, 23, 3002–3007. [Google Scholar] [CrossRef]
- Polacco, G.; Filippi, S.; Merusi, F.; Stastna, G. A review of the fundamentals of polymer-modified asphalts: Asphalt/polymer interactions and principles of compatibility. Adv. Colloid Interface Sci. 2015, 224, 72–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kringos, N. Towards the development of a viscoelastic model for phase separation in polymer-modified bitumen. Road Mater. Pavement Des. 2015, 16 (Suppl. S1), 39–49. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, X.; Kringos, N. Experimental investigation on storage stability and phase separation behaviour of polymer-modified bitumen. Int. J. Pavement Eng. 2018, 19, 832–841. [Google Scholar] [CrossRef]
- Zhu, J.; Balieu, R.; Wang, H. The use of solubility parameters and free energy theory for phase behaviour of polymer-modified bitumen: A review. Road Mater. Pavement Des. 2021, 22, 757–778. [Google Scholar] [CrossRef]
- Hu, Y.; Sreeram, A.; Xia, W.; Wang, H.; Zhou, L.; Si, W.; Airey, G.D. Use of Hansen Solubility Parameters (HSP) in the selection of highly effective rejuvenators for aged bitumen. Road Mater. Pavement Des. 2024. [Google Scholar] [CrossRef]
- Sun, Y.; Cannone Falchetto, A.; Zhang, F.; Wang, D.; Chen, W. Molecular Dynamics Simulation and the Regeneration and Diffusion Effects of Waste Engine Oil in Aged Asphalt Binder. Materials 2024, 17, 2212. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, Y.; Zhou, X.; Xu, X.; Chen, X. Quantifying the rejuvenation effects of soybean-oil on aged asphalt-binder using molecular dynamics simulations. J. Clean. Prod. 2021, 317, 128375. [Google Scholar] [CrossRef]
- Karlsson, R.; Isacsson, U. Bitumen structural stability characterization using turbidimetric titration. Energy Fuels 2003, 17, 1407–1415. [Google Scholar] [CrossRef]
- Karlsson, R.; Isacsson, U. Investigations on bitumen rejuvenator diffusion and structural stability (with discussion). J. Assoc. Asph. Paving Technol. 2003, 72, 463–501. [Google Scholar]
- Werkovits, S.; Bacher, M.; Theiner, J.; Rosenau, T.; Grothe, H. Multi-spectroscopic characterization of bitumen and its polarity-based fractions. Constr. Build. Mater. 2022, 352, 128992. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Z.; Liu, J.; Ma, F.; Peng, C.; Li, C.; Chang, X.; Zhao, P. Molecular dynamics simulation and experimental analysis on fluidity improvement of liquid rubber modified asphalt binder. Constr. Build. Mater. 2023, 402, 133027. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhang, X.; Yin, C.; Zhang, X. Properties of waste vegetable oil recycled asphalt-binder: A molecular simulation study. Mol. Simul. 2024, 50, 588–599. [Google Scholar] [CrossRef]
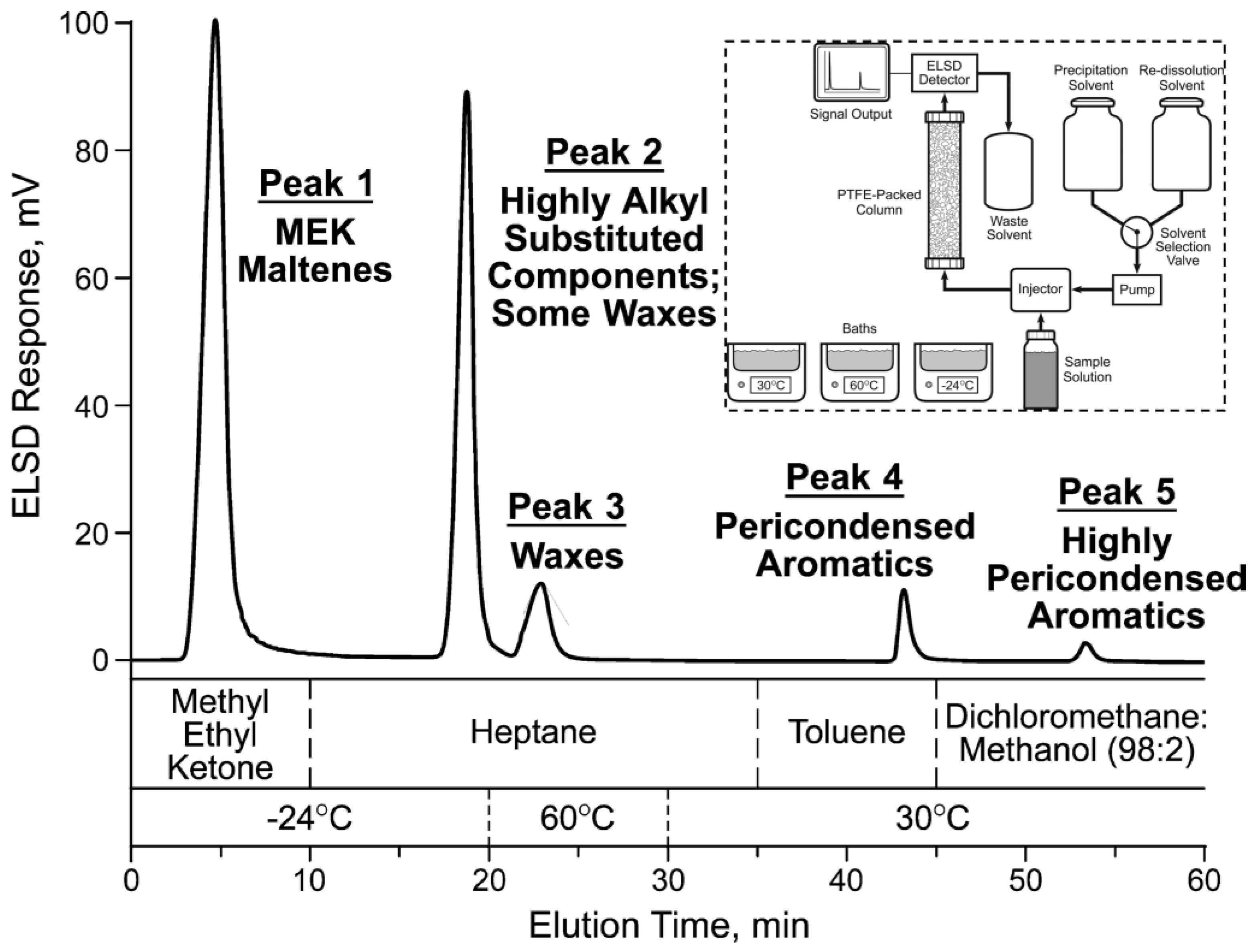
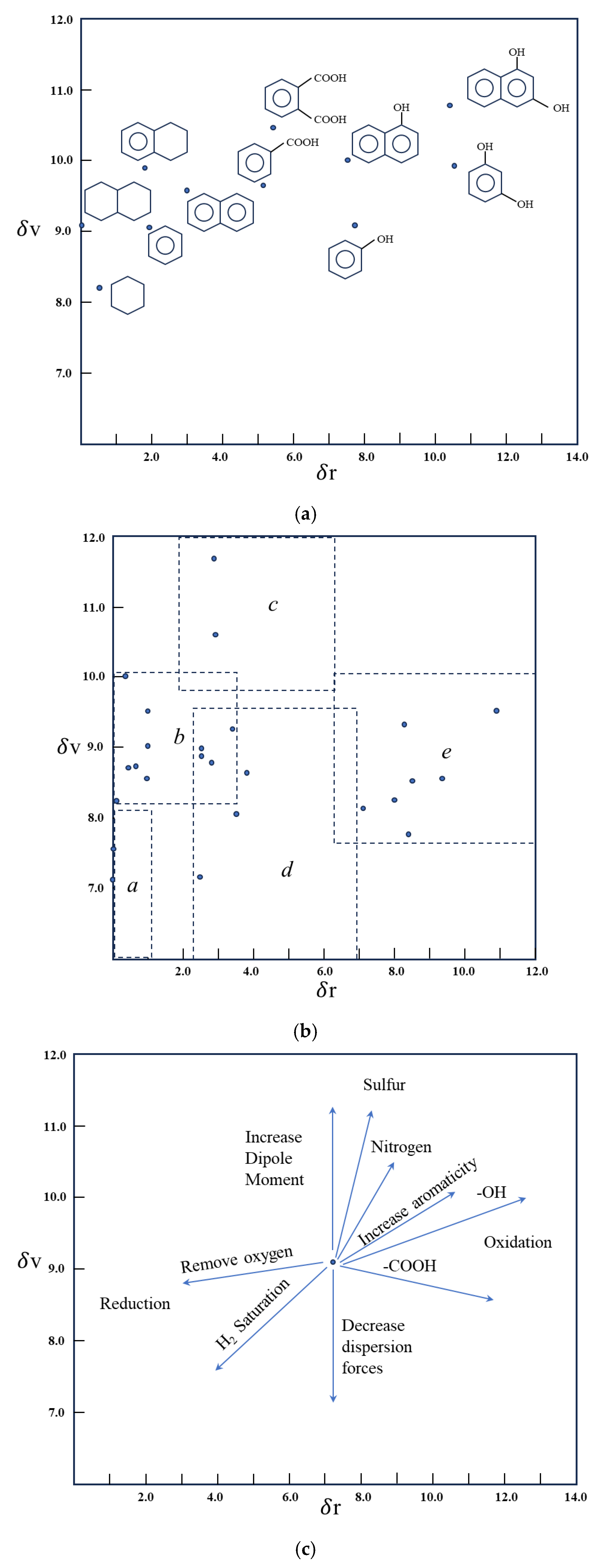
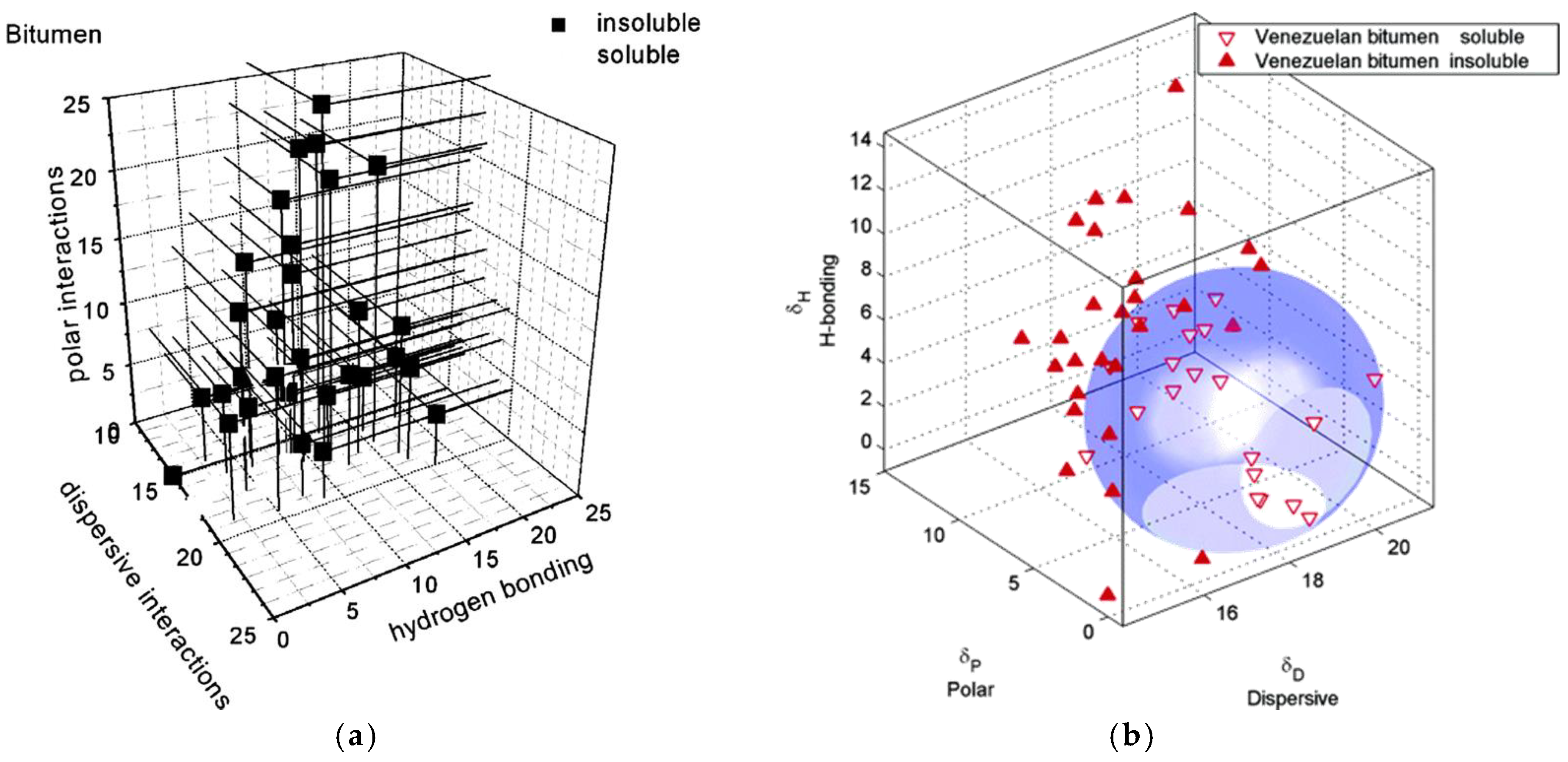
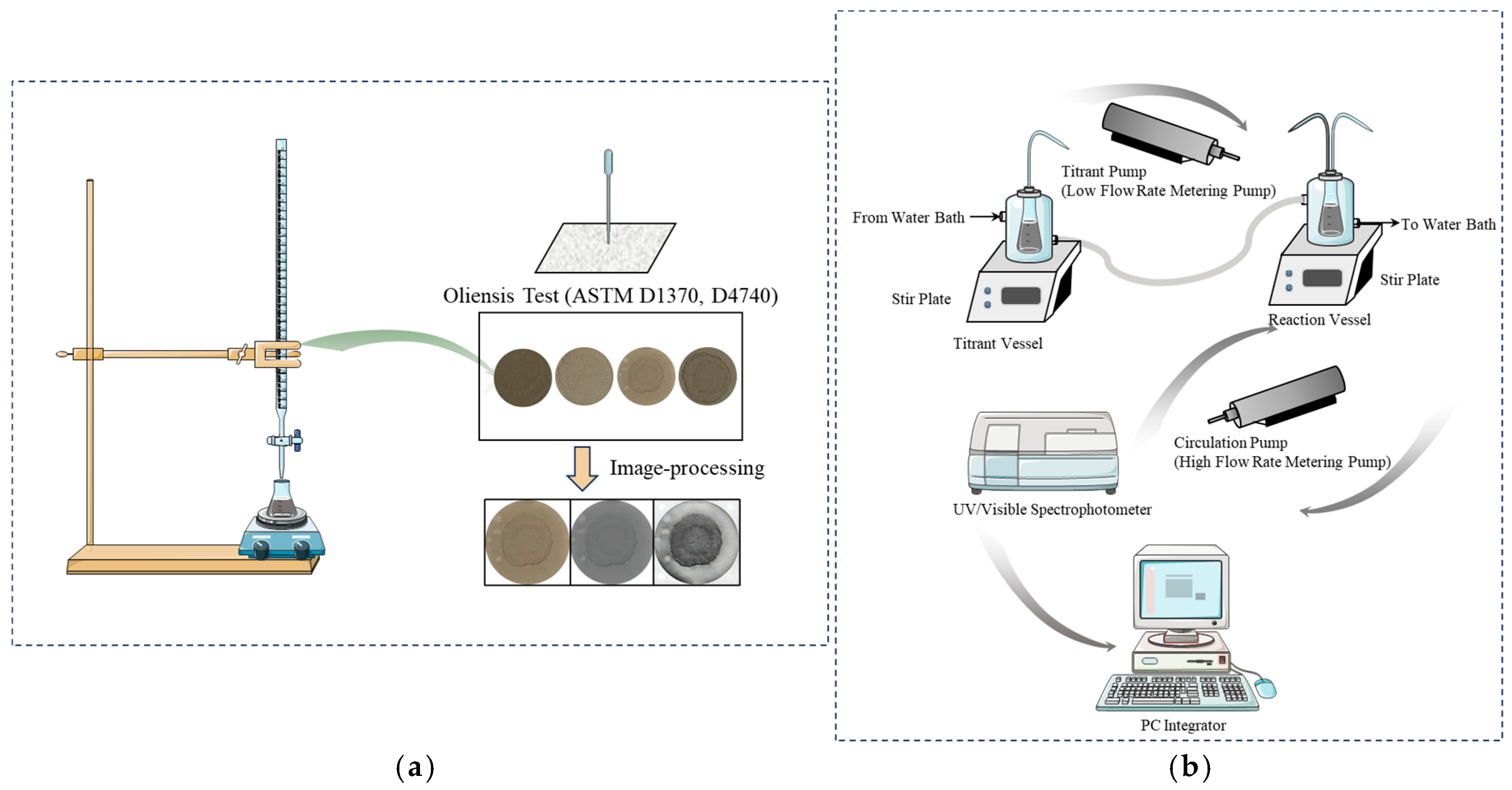


| Test Categories | Test Analysis |
|---|---|
| Fractionation by precipitation Fractionation by distillation | Solvent precipitation, Chemical precipitation Vacuum distillation, Thermogravimetric analysis |
| Chromatographic separation | Gas chromatography, Inverse gas-liquid chromatography, Liquid chromatography (adsorption, ion exchange, coordination, thin layer, size exclusion) |
| Chemical analysis | Spectrophotometric techniques (infrared, ultraviolet, nuclear magnetic resource, X-ray fluorescence, emission, neutron activation), Titrimetric and gravimetric techniques, Elemental analysis |
| Molecular weight analysis | Mass spectrometry, vapor pressure osmometry, and size exclusion chromatography |
| Indirect compositional analysis | Internal dispersion stability tests |
| Solvent | Hansen Parameters | Bagley and Scigliana Parameters | |||
|---|---|---|---|---|---|
| δD | δP | δH | δr | δv | |
| Cyclohexane | 8.2 | 0 | 0.1 | 0.1 | 8.3 |
| n-Heptane | 7.5 | 0 | 0 | 0 | 7.5 |
| n-Pentane | 7.1 | 0 | 0 | 0 | 7.1 |
| Diethyl ether | 7.1 | 1.4 | 2.5 | 2.5 | 7.2 |
| Ethyl acetate | 7.7 | 2.6 | 3.5 | 3.5 | 8.1 |
| 2-Butanol | 7.7 | 2.8 | 7.1 | 7.1 | 8.2 |
| 2-Propanol | 7.7 | 3.0 | 8.0 | 8.0 | 8.3 |
| 1-Propanol | 7.8 | 3.3 | 8.5 | 8.5 | 8.5 |
| Ethanol | 7.7 | 4.3 | 9.5 | 9.5 | |
| Methanol | 7.4 | 6.0 | 10.9 | 10.9 | 9 5 |
| Ethylene diamine * | 81 | 4.3 | 8.3 | 8.3 | 9.2 |
| Pyridine | 9.3 | 4.3 | 2.9 | 2.9 | 10.7 |
| Trichloroethylene | 8.8 | 1.5 | 2.6 | 2.6 | 8.9 |
| Carbon Disulfide | 10.0 | 0 | 0.3 | 0.3 | 10.0 |
| Chrorobenzene | 9.3 | 2.1 | 1.0 | 1.0 | 9.5 |
| Benzene | 9.0 | 0 | 1.0 | 1.0 | 9.0 |
| Carbon tetrachloride | 8.7 | 0 | 0.3 | 0.3 | 8.7 |
| Trichloroethane | 8.3 | 2.1 | 1.0 | 1.0 | 8.6 |
| 2-Butanone | 7.8 | 4.4 | 2.5 | 2.5 | 9.0 |
| Chloroform | 8.7 | 1.5 | 2.8 | 2.8 | 8.8 |
| Tetrahydrofuran | 8.2 | 2.8 | 3.9 | 3.9 | 8.7 |
| Acetone | 7.6 | 5.1 | 3.4 | 3.4 | 9 |
| Acetonitrile | 7.5 | 8.8 | 3.0 | 3.0 | 11.6 |
| Ethylbenzene | 8.7 | 0.3 | 0.7 | 0.7 | 8.7 |
| 1-Butanol | 7.8 | 2.8 | 7.7 | 8.3 | 7.7 |
| Petroleum | Pavement | |
|---|---|---|
| Solubility theory | Hildebrand and the Flory–Huggins theory | Hansen solubility theory |
| Solubility model | Asphaltenes model (size, shape, and specific intermolecular interaction) | Bitumen component compatibility model based on Hansen solubility |
| Characterization methods | Precipitation schemes | Miscibility studies |
| Research purposes | Precipitation of asphaltenes in crude oil | Durability of bitumen roads and stability of polymers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Ding, H.; Qiu, Y.; Grothe, H. Solubility Characteristics and Microstructure of Bitumen: A Review. Buildings 2025, 15, 135. https://doi.org/10.3390/buildings15010135
Liu H, Ding H, Qiu Y, Grothe H. Solubility Characteristics and Microstructure of Bitumen: A Review. Buildings. 2025; 15(1):135. https://doi.org/10.3390/buildings15010135
Chicago/Turabian StyleLiu, Han, Haibo Ding, Yanjun Qiu, and Hinrich Grothe. 2025. "Solubility Characteristics and Microstructure of Bitumen: A Review" Buildings 15, no. 1: 135. https://doi.org/10.3390/buildings15010135
APA StyleLiu, H., Ding, H., Qiu, Y., & Grothe, H. (2025). Solubility Characteristics and Microstructure of Bitumen: A Review. Buildings, 15(1), 135. https://doi.org/10.3390/buildings15010135








