Effects of Indoor Air Quality on Human Physiological Impact: A Review
Abstract
1. Introduction
- How do distinct categories of indoor air pollutants (e.g., gaseous pollutants, particulate matter, VOCs) differentially impact cardiovascular, respiratory, and neurological physiological indicators?
- What synergistic effects emerge from combined exposure to multiple pollutants, and how do these interactions modulate health risks?
- To what extent can current intervention strategies (e.g., ventilation optimization, air filtration) mitigate pollutant-induced physiological disturbances across diverse populations?
2. Materials and Methods
3. Effects of Gaseous Pollutants on Human Physiological Indicators
3.1. Effect of CO2 Content on Human Physiological Indicators
3.2. Effect of O3 Content on Human Physiological Indicators
3.3. Effects of Other Gaseous Pollutant Contents on Human Physiological Indicators
4. Effects of Particulate Matter on Human Physiological Indicators
4.1. Effects of PM2.5 on Human Physiological Indicators
4.2. Effects of Other Diameters of PM on Human Physiological Indicators
5. Effects of VOCs on Human Physiological Indicators
6. Effects of Other IAQ Factors on Human Physiological Indicators
7. Conclusions
- Gaseous pollutants for indoor air have a wide range of effects on human physiological indicators. The effects of CO2 on the human body cover indicators of the cardiovascular system, the respiratory system, and brain activity, while studies of O3 have focused on the effects on indicators of the human cardiovascular system. Since elevated aerosol concentrations for indoor air are often accompanied by the accumulation of other types of indoor pollutants, the simultaneous exposure of subjects to O3 and PM2.5 may cause synergistic effects in terms of changes in physiological indicators. Therefore, potential interactions between multiple indoor air pollutant exposures deserve further exploration in future studies.
- PM for indoor environments predominantly impacts the cardiovascular system, with adverse effects intensifying as particle size decreases and carbon content increases. Empirical evidence indicates that air filtration systems and ionization technologies can mitigate these effects by improving cardiovascular and respiratory biomarker profiles in settings with suboptimal IAQ. Future research should prioritize evaluating the efficacy of particulate purification devices across PM size fractions and compositional variations, while exploring integrated applications of IoT-based monitoring and AI-driven environmental control systems for enhanced indoor air management. Improvements in the performance of purification devices, IoT, and AI should be considered as a future challenge, which will improve the IAQ and promote the physiological health of people.
- VOCs for indoor air can also affect physiological indicators in a number of human systems. If the trend of VOCs for indoor air changes in the opposite direction, the effect on human physiological indicators may also have the opposite effect. The negative effects of VOCs on IAQ are more severe than those of other factors that affect IAQ. However, current research on indoor VOCs lacks standardized classification frameworks for specific chemical subgroups. Addressing these knowledge gaps requires large-scale controlled intervention trials employing longitudinal study designs to identify dominant VOC species and characterize their temporal concentration patterns. Such methodological refinements will enable comprehensive assessment of public health risks associated with targeted indoor air remediation strategies.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Author | Participants | Ages | Range | Task | Intervention | Physiological Index | Effect |
|---|---|---|---|---|---|---|---|
| Zhang et al. (2021) [25] | Adults N = 4 (4 males) | 24.20 ± 2.48 | 1626 ± 306 ppm, 3562 ± 259 ppm, 5087 ± 318 ppm | √ | — | HRV, EEG | Decreased SDNN standard deviation and increased PNN50 for HRV (1500 ppm: 8% vs. 3500 ppm: 9.6%); Increase in β relative power of EEG. |
| Kim et al. (2020) [29] | Adults N = 22 (16 males, 6 females) | 27.57 ± 0.09 | 1000–2000 ppm | — | — | Blood pressure | SBP increased (2000 ppm: 126.388 mmHg vs. 1000 ppm: 122.337 mmHg). |
| Zhang et al. (2017) [19] | University students N = 25 (10 males, 15 females) | 23 ± 2 | 500 ppm, 1000 ppm, 3000 ppm | √ | — | Respiration rate, HR, α-amylase, DBP | No change in respiration rate; HR reduced; significant increase in α-amylase concentration; DBP increased significantly. |
| Zhang et al. (2016) [30] | University students N = 10 (5 males, 5 females) | 25 ± 2 | 500 ppm, 5000 ppm | √ | — | Respiration rate, HR, α-amylase, cortisol | Respiratory rate, HR unchanged; α-amylase concentration increased; no difference in cortisol. |
| Snow et al. (2019) [14] | Employees or Students of the University N = 31 | 22.5 ± 4.8 | 830 ppm, 2700ppm | √ | — | EEG, HR, skin temperature, respiratory rate | EEG unchanged; HR increased. |
| Chen and Hsiao (2014) [16] | Youngster N = 10 (6 males, 4 females) | 26 ± 5 | More than 800 ppm, 800–1000 ppm, More than 1000 ppm | — | — | HR, SpO2, facial temperature | Increased HR; increased facial temperature; decreased SpO2. |
| Mishra et al. (2021) [22] | Adults N = 15 (8 males, 7 females) | 21~55 | 900 ppm, 1450ppm | √ | — | Respiration rate, ETCO2, FVC | Respiratory rate, ETCO2 unchanged; lung volume FVC decreased. |
| Shan et al. (2022) [35] | University students N = 25 (15 males, 15 females) | — | 1244 ± 70 ppm, 618 ± 45 ppm | √ | — | EEG | The relative power of both alpha and theta waves of EEG increased. |
| Jin et al. (2022) [24] | Adults N = 15 (8 males, 7 females) | 26.6 ± 3.4 | 4000 ppm, 40,000 ppm | — | — | EEG | EEG was affected. |
| Liu et al. (2017) [36] | University students N = 12 (6 males, 6 females) | 24.8 ± 2.6 | 380 ppm, 3000 ppm | — | — | Right ear tympanic membrane temperature, skin temperature, heart rate, blood pressure, SpO2, ETCO2 | All unchanged. |
| Kang et al. (2023) [37] | Adults N = 36 (16 males,16 females) | 23.7 ± 3.6 | 0–9999 ppm | √ | √ | Wrist skin temperature, blood pressure and pulse, oxygen saturation of blood, salivary biomarker | There was no significant difference. |
| Author | Participants | Ages | Range | Task | Intervention | Physiological Index | Effect |
|---|---|---|---|---|---|---|---|
| Urch et al. [40] | Healthy adults N = 23 (13 males, 10 females) | 32 ± 10 | 121 ± 3 ppb | — | — | DBP, HR | DBP increased from 1 mmHg to 6 mmHg when exposed in combination with PM2.5. |
| Brook et al. (2002) [41] | Healthy adults N = 25 (15 males, 10 females) | 34.9 ± 10 | 120 ppb | — | — | Blood pressure, FMD, NMD, BAD | BAD (−0.09 ± 0.15 mm vs. 0.01 ± 0.18 mm) was significantly lower, and there were no significant differences in FMD (0.29 ± 4.11% vs. −0.03 ± 6.63%), NMD (3.87 ± 5.43% vs. 3.46 ± 7.92%), and blood pressure. |
| Hoffmann et al. (2012) [42] | Diabetes mellitus type 2 (T2DM) N = 70 (53 males, 37 females) | Mean 64.4 | 13.3 ppb | — | — | Blood pressure | For each additional interquartile spacing, SBP, DBP, and central mean arterial blood pressure decreased by 4.0%, 2.0%, and 2.8%, respectively. |
| Urch et al. (2004) [43] | Healthy adults N = 24 (14 males, 10 females) | 35 ± 10 | 120 ppb | — | — | BAD | BAD reduced by 0.09 mm. Unable to assess. |
| Brook et al. (2009) [46] | Healthy adults N = 50 (19 males, 31 females) | 27 ± 8 | 120 ppb | — | — | DBP | There was little impact on DBP. |
| Fakhri et al. (2009) [44] | Adults N = 50 (24 males, 26 females) | 27.08 ± 7.13 | 113.9 ± 6.6 ppb | — | — | HRV, blood pressure, respiratory rate, HR | In synergistic exposure with O3 DBP increased by 2 mmHg and SDNN increased. |
| Power et al. (2008) [45] | Asthmatic N = 5 (1 males, 4 females) | Mean 37 | 200 ppb | — | Filtration | HRV | Combined particle and ozone exposure reduces SDNN in asthmatics. |
| Author | Participants | Ages | Range | Task | Intervention | Physiological Index | Effect |
|---|---|---|---|---|---|---|---|
| Dong et al. (2018) [50] | Older people N = 29 (29 females) | Mean 68.2 | 55.7 ± 55.4 μg/m3 | — | — | HRV | HRV’s HF, LF, and SDNN declined. |
| Lu et al. (2018) [21] | COPD patients N = 43 (40 males, 3 females) | 71.49 ± 6.40 | 58.01 ± 52.82 μg/m3 | — | — | HRV, HR | HF decreased by 34.85% in overweight patients compared to 2.01% in normal weight patients. |
| Power et al. (2008) [45] | Asthmatic N = 5 (1 males, 4 females) | Mean 37 | 313 ± 19.5 μg/m3 | — | Filtration | HRV | The SDNN standard deviation of HRV was significantly lower when exposed synergistically with O3; there was no significant change in HRV when exposed to particles only. |
| Jung et al. (2016) [17] | Office staff N = 115 (83 males, 32 females) | 34.2 ± 5.7 | 40.7 ± 29.1 μg/m3 | — | — | Blood pressure, HR | HR increased. |
| Rumchev et al. (2018) [8] | Adults N = 63 (28 males, 35 females) | Mean 61 | 18.74 μg/m3 | — | — | Blood pressure, HR | For each IQR increase in PM2.5, heart rate increases by 4–6 bpm. |
| Brook et al. (2015) [52] | Adults N = 65 (50 males, 15 females) | Mean 44.6 | 11.6 ± 8.5 μg/m3 | — | — | Blood pressure, HR, BAD and FMD | A 10 μg/m3 increase in PM2.5 was associated with a 1.41 mmHg increase in SBP after 1 day. |
| Fakhri et al. (2009) [44] | Adults N = 50 (24 males, 26 females) | 27.08 ± 7.13 | 121.6 ± 48.0 μg/m3 | — | — | HRV, blood pressure, respiratory rate, HR | DBP does not have a significant effect and SDNN increases when younger subjects are exposed only to PM2.5. |
| Jia et al. (2012) [56] | Healthy older people N = 30 (12 males, 18 females) | 57.9 ± 5.4 | 45.58 μg/m3 | — | — | HRV | HF and LF increased by 1.30% and 1.34%, respectively. |
| Lin et al. (2011) [57] | Healthy students N = 60 (30 males, 30 females) | Median age 25.0 | 23.65 ± 12.6 μg/m3 18.05 ± 8.45 μg/m3 | — | Filtration | Blood pressure, HR | Without filter, SBP, DBP, and HR increased by 4.11 mmHg, 2.78 mmHg, and 3.11 bpm, respectively; after filtering the air, BP, and HR did not change significantly with the rise of PM2.5. |
| Karottki et al. (2013) [58] | Older people N = 48 (22 males, 26 females) | 67 ± 6.5 | 4 μg/m3 8 μg/m3 | — | Filtration | Blood pressure, microvascular function, pulmonary function | There was no improvement in microvascular, functional lung function, and no significant reduction in systemic inflammation, monocyte activation, or lung cell injury. |
| Liu et al. (2018) [60] | Healthy older people, COPD patients N = 35 (20 males, 15 females) | 66.26 ± 7.71 | 58.24 μg/m3 37.99 μg/m3 | — | Activated carbon filtration | Blood pressure, HRV | For every 10 μg/m3 increase in PM2.5, there was a significant reduction of 1.34% for sham-filtered SDNN and a non-significant reduction of 0.81% for activated carbon filtration. |
| Liao et al. (1999) [70] | Older people N = 26 (7 males, 19 females) | Mean 81 | 9.8 ± 3.7 μg/m3 | — | — | HRV | HRV decreased. |
| Zhou et al. (2023) [59] | Adults N = 66 | 18–65 | 0–30 μg/m3; ±3 μg/m3. 30–1000 μg/m3; ± 10% | — | — | electrodermal activity, EDA and heart rate variability, HRV | Reducing significantly indoor elevated PM2.5 levels can improve some cognitive abilities in office workers. |
| Author | Participants | Ages | Range | Task | Intervention | Physiological Index | Effect |
|---|---|---|---|---|---|---|---|
| Kjærgaard et al. (1991) [64] | Adults N = 35 (15 males, 20 females) | Mean 41.25 | 25,000 µg/m3 | √ | — | PML, sebaceous sweat, mid-expiratory flow | PML increased; skin indicators regarding sebum and sweat, and mid-expiratory flow (FEF50) responded only in the SBS group. |
| Chuang et al. (2017) [53] | Healthy adults N = 200 (100 males, 100 females) | Mean 43.4 | 0.98 ± 0.56 ppm, 0.43 ± 0.21 ppm, 1.22 ± 0.81 ppm | — | Refrigeration | Blood pressure, Hs-CRP, 8-OHdG | BP, Hs-CRP, and 8-OHdG increased. |
| Chen et al. (2019) [15] | Older people N = 100 (50 males, 50 females) | Mean 67.3 | 347.5 ± 78.2 ppb, 748.4 ± 163.4 ppb | — | — | Blood pressure, HR | SBP and HR increased. |
| Mizukoshi et al. (2015) [20] | Chemically sensitized patients N = 8 (3 males, 5 females) | 44 ± 11 | 306 ± 148 µg/m3 | — | — | HRV | HF was significantly negatively correlated with it. |
| Jung et al. (2016) [17] | Office staff N = 115 (83 males, 32 females) | 34.2 ± 5.7 | 528.7 ± 315.4 μg/m3 | — | — | Blood pressure, HR | SBP levels were higher in overweight or obese subjects. |
| Mizukoshi et al. (2010) [65] | Adults N = 7 (4 males, 3 females) | 32 ± 13 | 63–1447 µg/m3 | — | — | HRV | ΔTVOC is negatively correlated with HF and positively correlated with LF/HF. |
| Bakó-Biró et al. (2005) [71] | Adults N = 60 (60 females) | Not mentioned | Not mentioned | √ | — | CO2 exhalation rate | The CO2 exhalation rate was significantly reduced by approximately 5%. |
| Wargocki et al. (1999) [72] | Healthy adults N = 30 (30 females) | Mean 24 | 165 ± 50 µg/m3, 195 ± 10 µg/m3, 220 ± 20 µg/m3 | √ | — | CO2 exhalation rate | The metabolic rate was 1.3, much higher than the relaxed sedentary normal of 1.2. |
| Nakayama et al. (2021) [67] | Healthy adults N = 168 | 20~50s | 3629 µg/m3, 55 µg/m3 | √ | — | EEG | The rate of increase/decrease in α/β values was significantly higher in the group with higher levels of VOCs than in the group with lower concentrations. |
| Lin et al. (2013) [48] | Healthy adults N = 300 (136 males, 164 females) | Mean 43.2 | 77.2 ± 27.3 ppb, 68.6 ± 23.1 ppb, 52.3 ± 20.4 ppb | — | Air conditioner | HRV, Hs-CRP, 8-OHdG, plasma fibrinogen | Hs-CRP, 8-OHdG and plasma fibrinogen were elevated. |
| Shim et al. (2023) [78] | Underground stores N = 454 | Over 20 | Not mentioned | — | — | eye irritation, respiratory, general symptoms | The concentrations of n-butanol, n-heptane, and xylene were associated with eye irritation symptoms, while those of n-heptane were associated with respiratory symptoms, and those of benzene, n-heptane, and decanal were associated with general symptoms. |
References
- Matson, P.A. Environmental Challenges for the Twenty-First Century: Interacting Challenges and Integrative Solutions. Ecol. Law Q. 2001, 27, 1179. [Google Scholar]
- Patial, S.; Nazim, M.; Khan, A.A.P.; Raizada, P.; Singh, P.; Hussain, C.M.; Asiri, A.M. Sustainable Solutions for Indoor Pollution Abatement During COVID Phase: A critical study on current technologies & challenges. J. Hazard. Mater. Adv. 2022, 7, 100097. [Google Scholar] [CrossRef]
- Madruga, D.G. A Comprehensive Review on the Indoor Air Pollution Problem, Challenges, and Critical Viewpoints. In Integrating IoT and AI for Indoor Air Quality Assessment; Saini, J., Dutta, M., Marques, G., Halgamuge, M.N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 9–26. [Google Scholar]
- Rudel, R.A.; Perovich, L.J. Endocrine Disrupting Chemicals in Indoor and Outdoor Air. Atmos. Environ. 2009, 43, 170–181. [Google Scholar] [CrossRef]
- Saini, J.; Dutta, M.; Marques, G. Chapter 6—Indoor Air Pollution: A Comprehensive Review of Public Health Challenges and Prevention Policies. In Current Trends and Advances in Computer-Aided Intelligent Environmental Data Engineering; Marques, G., Ighalo, J.O., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 105–126. [Google Scholar]
- Bako-Biro, Z.; Wargocki, P.; Weschler, C.J.; Fanger, P.O. Effects of Pollution from Personal Computers on Perceived Air Quality, SBS Symptoms and Productivity in Offices. Indoor Air 2004, 14, 178–187. [Google Scholar] [CrossRef]
- Wargocki, P.; Wyon, D.P. Providing Better Thermal and Air Quality Conditions in School Classrooms Would Be Cost-Effective. Build. Environ. 2013, 59, 581–589. [Google Scholar] [CrossRef]
- Rumchev, K.; Soares, M.; Zhao, Y.; Reid, C.; Huxley, R. The Association between Indoor Air Quality and Adult Blood Pressure Levels in a High-Income Setting. Int. J. Environ. Res. Public Health 2018, 15, 2026. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, T.; Pan, X.; Hu, M.; Lu, S.E.; Lin, Y.; Wang, T.; Zhang, Y.; Tang, X. Air Pollution and Autonomic and Vascular Dysfunction in Patients With Cardiovascular Disease: Interactions of Systemic Inflammation, Overweight, and Gender. Am. J. Epidemiol. 2012, 176, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Puett, R.C.; Hart, J.E.; Yanosky, J.D.; Paciorek, C.; Schwartz, J.; Suh, H.; Speizer, F.E.; Laden, F. Chronic Fine and Coarse Particulate Exposure, Mortality, and Coronary Heart Disease in the Nurses’ Health Study. Environ. Health Perspect. 2009, 117, 1697–1701. [Google Scholar] [CrossRef]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women. New Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef]
- Weichenthal, S.; Villeneuve, P.J.; Burnett, R.T.; van Donkelaar, A.; Martin, R.V.; Jones, R.R.; DellaValle, C.T.; Sandler, D.P.; Ward, M.H.; Hoppin, J.A. Long-Term Exposure to Fine Particulate Matter: Association with Nonaccidental and Cardiovascular Mortality in the Agricultural Health Study Cohort. Environ. Health Perspect. 2014, 122, 609–615. [Google Scholar] [CrossRef]
- Chen, J.-C.; Cavallari, J.M.; Stone, P.H.; Christiani, D.C. Obesity Is A Modifier of Autonomic Cardiac Responses to Fine Metal Particulates. Environ. Health Perspect. 2007, 115, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Snow, S.; Boyson, A.S.; Paas, K.H.W.; Gough, H.; King, M.-F.; Barlow, J.; Noakes, C.J.; Schraefel, M.C. Exploring the Physiological, Neurophysiological and Cognitive Performance Effects of Elevated Carbon Dioxide Concentrations Indoors. Build. Environ. 2019, 156, 243–252. [Google Scholar] [CrossRef]
- Chen, R.-Y.; Ho, K.-F.; Hong, G.-B.; Chuang, K.-J. Houseplant, Indoor Air Pollution, and Cardiovascular Effects Among Elderly Subjects in Taipei, Taiwan. Sci. Total Environ. 2020, 705, 135770. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Hsiao, T.-C. Physiological Responses to Different CO2 Levels in Poor Ventilation Room. In Proceedings of the 6th European Conference of the International Federation for Medical and Biological Engineering, Dubrovnik, Croatia, 7–11 September 2014; Springer: Cham, Switzerland, 2015; pp. 423–426. [Google Scholar]
- Jung, C.-C.; Su, H.-J.; Liang, H.-H. Association between Indoor Air Pollutant Exposure and Blood Pressure and Heart Rate in Subjects According to Body Mass Index. Sci. Total Environ. 2016, 539, 271–276. [Google Scholar] [CrossRef]
- Baumgartner, J.; Schauer, J.J.; Ezzati, M.; Lu, L.; Cheng, C.; Patz, J.A.; Bautista, L.E. Indoor Air Pollution and Blood Pressure in Adult Women Living in Rural China. Environ. Health Perspect. 2011, 119, 1390–1395. [Google Scholar] [CrossRef]
- Zhang, X.; Wargocki, P.; Lian, Z. Physiological Responses During Exposure to Carbon Dioxide and Bioeffluents at Levels Typically Occurring Indoors. Indoor Air 2017, 27, 65–77. [Google Scholar] [CrossRef]
- Mizukoshi, A.; Kumagai, K.; Yamamoto, N.; Noguchi, M.; Yoshiuchi, K.; Kumano, H.; Sakabe, K.; Yanagisawa, Y. In-situ Real-Time Monitoring of Volatile Organic Compound Exposure and Heart Rate Variability for Patients with Multiple Chemical Sensitivity. Int. J. Environ. Res. Public Health 2015, 12, 12446–12465. [Google Scholar] [CrossRef]
- Pan, L.; Wu, S.; Li, H.; Xu, J.; Dong, W.; Shan, J.; Yang, X.; Chen, Y.; Shima, M.; Deng, F.; et al. The Short-Term Effects of Indoor Size-Fractioned Particulate Matter and Black Carbon on Cardiac Autonomic Function in COPD Patients. Environ. Int. 2018, 112, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Schiavon, S.; Wargocki, P.; Tham, K.W. Respiratory Performance of Humans Exposed to Moderate Levels of Carbon Dioxide. Indoor Air 2021, 31, 1540–1552. [Google Scholar] [CrossRef]
- Kajtár, L.; Herczeg, L. Influence of Carbon-Dioxide Concentration on Human Well-Being and Intensity of Mental Work. Időjárás 2012, 116, 145–169. [Google Scholar]
- Jin, R.N.; Inada, H.; Négyesi, J.; Ito, D.; Nagatomi, R. Carbon Dioxide Effects on Daytime Sleepiness and EEG Signal: A Combinational Approach Using Classical Frequentist and Bayesian Analyses. Indoor Air 2022, 32, e13055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, X.; Wang, X.; Pang, L.; Liang, J.; Zhang, L. Physiological Responses to Elevated Carbon Dioxide Concentration and Mental Workload During Performing MATB Tasks. Build. Environ. 2021, 195, 107752. [Google Scholar] [CrossRef]
- Soltanpour, Z.; Mohammadian, Y.; Fakhri, Y. The Exposure to Formaldehyde in Industries and Health Care Centers: A Systematic Review and Probabilistic Health Risk Assessment. Environ. Res. 2022, 204, 112094. [Google Scholar] [CrossRef]
- Guais, A.; Brand, G.; Jacquot, L.; Karrer, M.; Dukan, S.; Grévillot, G.; Molina, T.J.; Bonte, J.; Regnier, M.; Schwartz, L. Toxicity of Carbon Dioxide: A Review. Chem. Res. Toxicol. 2011, 24, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kong, M.; Hong, T.; Jeong, K.; Lee, M. Physiological Response of Building Occupants Based on Their Activity and the Indoor Environmental Quality Condition Changes. Build. Environ. 2018, 145, 96–103. [Google Scholar] [CrossRef]
- Kim, J.; Hong, T.; Kong, M.; Jeong, K. Building Occupants’ Psycho-Physiological Response to Indoor Climate and CO2 Concentration Changes in Office Buildings. Build. Environ. 2020, 169, 106596. [Google Scholar] [CrossRef]
- Zhang, X.; Wargocki, P.; Lian, Z. Human Responses to Carbon Dioxide, a Follow-up Study at recommended exposure limits in non-industrial environments. Build. Environ. 2016, 100, 162–171. [Google Scholar] [CrossRef]
- Wargocki, P.; Wyon, D.P.; Clausen, G.; Fanger, P.O. The Effects of Outdoor Air Supply Rate in an Office on Perceived Air Quality, Sick Building Syndrome (SBS) Symptoms and Productivity. Indoor Air 2000, 10, 222–236. [Google Scholar] [CrossRef]
- Wojciech Zareba, E.p.; Maison-Blanche, P.; Locati, E.H.; Martínez Sande, J.L. Noninvasive Electrocardiology in Clinical Practice. Rev. Española Cardiol. 2001, 54, 1241. [Google Scholar] [CrossRef]
- Vehviläinen, T.; Lindholm, H.; Rintamäki, H.; Pääkkönen, R.; Hirvonen, A.; Niemi, O.; Vinha, J. High Indoor CO2 Concentrations in an Office Environment Increases the Transcutaneous CO2 Level and Sleepiness During Cognitive Work. J. Occup. Environ. Hyg. 2016, 13, 19–29. [Google Scholar] [CrossRef]
- Wilson, G.F. An Analysis of Mental Workload in Pilots During Flight Using Multiple Psychophysiological Measures. Int. J. Aviat. Psychol. 2009, 12, 3–18. [Google Scholar] [CrossRef]
- Shan, X.; Yang, E.-H.; Zhou, J.; Chang, V.W.C. Neural-Signal Electroencephalogram (EEG) Methods to Improve Human-Building Interaction under Different Indoor Air Quality. Energy Build. 2019, 197, 188–195. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, W.; Wargocki, P. Performance, Acute Health Symptoms and Physiological Responses During Exposure to High Air Temperature and Carbon Dioxide Concentration. Build. Environ. 2017, 114, 96–105. [Google Scholar] [CrossRef]
- Kang, M.; Yan, Y.; Guo, C.; Liu, Y.; Fan, X.; Wargocki, P.; Lan, L. Ventilation Causing an Average CO2 Concentration of 1,000 Ppm Negatively Affects Sleep: A Field-Lab Study on Healthy Young People. Build. Environ. 2024, 249, 111118. [Google Scholar] [CrossRef]
- Zhang, X.; Wargocki, P.; Lian, Z.; Thyregod, C. Effects of Exposure to Carbon Dioxide and Bioeffluents on Perceived Air Quality, Self-Assessed Acute Health Symptoms, and Cognitive Performance. Indoor Air 2017, 27, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Persily, A. Please Don’t Blame Standard 62.1 for 1000 ppm CO2. Ashrae J. 2021, 63, 1–2. [Google Scholar]
- Urch, B.; Silverman, F.; Corey, P.; Brook, J.R.; Lukic, K.Z.; Rajagopalan, S.; Brook, R.D. Acute Blood Pressure Responses in Healthy Adults During Controlled Air Pollution Exposures. Environ. Health Perspect. 2005, 113, 1052–1055. [Google Scholar] [CrossRef]
- Brook, R.D.; Brook, J.R.; Urch, B.; Vincent, R.; Rajagopalan, S.; Silverman, F. Inhalation of Fine Particulate Air Pollution and Ozone Causes Acute Arterial Vasoconstriction in Healthy Adults. Circulation 2002, 105, 1534–1536. [Google Scholar] [CrossRef]
- Hoffmann, B.; Luttmann-Gibson, H.; Cohen, A.; Zanobetti, A.; de Souza, C.; Foley, C.; Suh, H.H.; Coull, B.A.; Schwartz, J.; Mittleman, M.; et al. Opposing Effects of Particle Pollution, Ozone, and Ambient Temperature on Arterial Blood Pressure. Environ. Health Perspect. 2012, 120, 241–246. [Google Scholar] [CrossRef]
- Urch, B.; Brook, J.R.; Wasserstein, D.; Brook, R.D.; Rajagopalan, S.; Corey, P.; Silverman, F. Relative Contributions of PM2.5 Chemical Constituents to Acute Arterial Vasoconstriction in Humans. Inhal. Toxicol. 2008, 16, 345–352. [Google Scholar] [CrossRef]
- Fakhri, A.A.; Ilic, L.M.; Wellenius, G.A.; Urch, B.; Silverman, F.; Gold, D.R.; Mittleman, M.A. Autonomic Effects of Controlled Fine Particulate Exposure in Young Healthy Adults: Effect Modification by Ozone. Environ. Health Perspect. 2009, 117, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Power, K.L.; Balmes, J.; Solomon, C. Controlled Exposure to Combined Particles and Ozone Decreases Heart Rate Variability. J. Occup. Environ. Med. 2008, 50, 1253–1260. [Google Scholar] [CrossRef]
- Brook, R.D.; Urch, B.; Dvonch, J.T.; Bard, R.L.; Speck, M.; Keeler, G.; Morishita, M.; Marsik, F.J.; Kamal, A.S.; Kaciroti, N.; et al. Insights Into the Mechanisms and Mediators of the Effects of Air Pollution Exposure on Blood Pressure and Vascular Function in Healthy Humans. Hypertension 2009, 54, 659–667. [Google Scholar] [CrossRef]
- Tarkiainen, T.H.; Timonen, K.L.; Vanninen, E.J.; Alm, S.; Hartikainen, J.E.; Pekkanen, J. Effect of Acute Carbon Monoxide Exposure on Heart Rate Variability in Patients with Coronary Artery Disease. Clin. Physiol. Funct. Imaging 2003, 23, 98–102. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Chuang, H.-C.; Liu, I.J.; Chen, H.-W.; Chuang, K.-J. Reducing Indoor Air Pollution by Air Conditioning Is Associated with Improvements in Cardiovascular Health among the General Population. Sci. Total Environ. 2013, 463–464, 176–181. [Google Scholar] [CrossRef]
- Wang, D.; Yee, B.J.; Wong, K.K.; Kim, J.W.; Dijk, D.-J.; Duffin, J.; Grunstein, R.R. Comparing the Effect of Hypercapnia and Hypoxia on the Electroencephalogram During Wakefulness. Clin. Neurophysiol. 2015, 126, 103–109. [Google Scholar] [CrossRef]
- Dong, W.; Pan, L.; Li, H.; Miller, M.R.; Loh, M.; Wu, S.; Xu, J.; Yang, X.; Shan, J.; Chen, Y.; et al. Association of Size-Fractionated Indoor Particulate Matter and Black Carbon with Heart Rate Variability in Healthy Elderly Women in Beijing. Indoor Air 2018, 28, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Magari, S.R.; Hauser, R.; Schwartz, J.; Williams, P.L.; Smith, T.J.; Christiani, D.C. Association of Heart Rate Variability with Occupational and Environmental Exposure to Particulate Air Pollution. Circulation 2001, 104, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Bard, R.L.; Burnett, R.T.; Shin, H.H.; Vette, A.; Croghan, C.; Phillips, M.; Rodes, C.; Thornburg, J.; Williams, R. Differences in Blood Pressure and Vascular Responses Associated with Ambient Fine Particulate Matter Exposures Measured at the Personal Versus Community Level. Occup. Environ. Med. 2010, 68, 224–230. [Google Scholar] [CrossRef]
- Chuang, H.-C.; Ho, K.-F.; Lin, L.-Y.; Chang, T.-Y.; Hong, G.-B.; Ma, C.-M.; Liu, I.J.; Chuang, K.-J. Long-Term Indoor Air Conditioner Filtration and Cardiovascular Health: A Randomized Crossover Intervention Study. Environ. Int. 2017, 106, 91–96. [Google Scholar] [CrossRef]
- Sørensen, M.; Daneshvar, B.; Hansen, M.; Dragsted, L.O.; Hertel, O.; Knudsen, L.; Loft, S. Personal PM2.5 Exposure and Markers of Oxidative Stress in Blood. Environ. Health Perspect. 2002, 111, 161–165. [Google Scholar] [CrossRef]
- Devlin, R.B.; Ghio, A.J.; Kehrl, H.; Sanders, G.; Cascio, W. Elderly Humans Exposed to Concentrated Air Pollution Particles Have Decreased Heart Rate Variability. Eur. Respir. J. 2003, 21, 76s–80s. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Song, X.; Shima, M.; Tamura, K.; Deng, F.; Guo, X. Effects of Fine Particulate on Heart Rate Variability in Beijing: A Panel Study of Healthy Elderly Subjects. Int. Arch. Occup. Environ. Health 2011, 85, 97–107. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Chen, H.-W.; Su, T.-L.; Hong, G.-B.; Huang, L.-C.; Chuang, K.-J. The Effects of Indoor Particle Exposure on Blood Pressure and Heart Rate among Young Adults: An Air Filtration-Based Intervention Study. Atmos. Environ. 2011, 45, 5540–5544. [Google Scholar] [CrossRef]
- Karottki, D.G.; Spilak, M.; Frederiksen, M.; Gunnarsen, L.; Brauner, E.V.; Kolarik, B.; Andersen, Z.J.; Sigsgaard, T.; Barregard, L.; Strandberg, B.; et al. An Indoor Air Filtration Study in Homes of Elderly: Cardiovascular and Respiratory Effects of Exposure to Particulate Matter. Environ. Health 2013, 12, 116. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Huebner, G.; Zeng, Y.; Pei, Z.; Ucci, M. Short-Term Exposure to Indoor PM2.5 in Office Buildings and Cognitive Performance in Adults: An Intervention Study. Build. Environ. 2023, 233, 110078. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Zhao, Q.; Song, X.; Shao, D.; Meliefste, K.; Du, Y.; Wang, J.; Wang, M.; Wang, T.; et al. Cardiovascular Benefits of Short-Term Indoor Air Filtration Intervention in Elderly Living in Beijing: An Extended Analysis of BIAPSY Study. Environ. Res. 2018, 167, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, Q.; Wu, Y.; Song, Y.; Dong, W.; Chu, M.; Yang, D.; Zhang, X.; Zhang, J.; Chen, C.; et al. Metabolic Linkages between Indoor Negative Air Ions, Particulate Matter and Cardiorespiratory Function: A Randomized, Double-Blind Crossover Study among Children. Environ. Int. 2020, 138, 105663. [Google Scholar] [CrossRef]
- Chuang, K.-J.; Chan, C.-C.; Chen, N.-T.; Su, T.-C.; Lin, L.-Y. Effects of Particle Size Fractions on Reducing Heart Rate Variability in Cardiac and Hypertensive Patients. Environ. Health Perspect. 2005, 113, 1693–1697. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, B. Review on Portable EEG Technology in Educational Research. Comput. Hum. Behav. 2018, 81, 340–349. [Google Scholar] [CrossRef]
- Kjærgaard, S.K.; Mølhave, L.; Pedersen, O.F. Human Reactions to a Mixture of Indoor Air Volatile Organic Compounds. Atmos. Environ. Part A. Gen. Top. 1991, 25, 1417–1426. [Google Scholar] [CrossRef]
- Mizukoshi, A.; Kumagai, K.; Yamamoto, N.; Noguchi, M.; Yoshiuchi, K.; Kumano, H.; Yanagisawa, Y. A Novel Methodology to Evaluate Health Impacts Caused by VOC Exposures Using Real-Time VOC and Holter Monitors. Int. J. Environ. Res. Public Health 2010, 7, 4127–4138. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Liu, C.; Yang, J.; Zhang, J.; Peng, C. Monitoring, Human Health Risk Assessment and Optimized Management for Typical Pollutants in Indoor Air from Random Families of University Staff, Wuhan City, China. Sustainability 2017, 9, 1115. [Google Scholar] [CrossRef]
- Nakayama, Y.; Suzuki, N.; Nakaoka, H.; Tsumura, K.; Takaguchi, K.; Takaya, K.; Hanazato, M.; Todaka, E.; Mori, C. Assessment of Personal Relaxation in Indoor-Air Environments: Study in Real Full-Scale Laboratory Houses. Int. J. Environ. Res. Public Health 2021, 18, 10246. [Google Scholar] [CrossRef]
- Nishihara, N.; Wargocki, P.; Tanabe, S.-I. Cerebral Blood Flow, Fatigue, Mental Effort, and Task Performance in Offices with Two Different Pollution Loads. Build. Environ. 2014, 71, 153–164. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Eatough, D.J.; Gold, D.R.; Pang, Y.; Nielsen, K.R.; Nath, P.; Verrier, R.L.; Kanner, R.E. Acute Exposure to Environmental Tobacco Smoke and Heart Rate Variability. Environ. Health Perspect. 2001, 109, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Creason, J.; Shy, C.; Williams, R.; Watts, R.; Zweidinger, R. Daily Variation of Particulate Air Pollution and Poor Cardiac Autonomic Control in the Elderly. Environ. Health Perspect. 1999, 107, 521–525. [Google Scholar] [CrossRef]
- Bakó-Bíró, Z.; Wargocki, P.; Wyon, D.P.; Fanger, P.O. Poor Indoor Air Quality Slows Down Metabolic Rate of Office Workers. Proc. Indoor Air 2005, 1, 76–80. [Google Scholar]
- Wargocki, P.; Wyon, D.P.; Baik, Y.K.; Clausen, G.; Fanger, P.O. Perceived Air Quality, Sick Building Syndrome (SBS) Symptoms and Productivity in an Office with Two Different Pollution Loads. Indoor Air 1999, 9, 165–179. [Google Scholar] [CrossRef]
- Rovira, J.; Roig, N.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Human Health Risks of Formaldehyde Indoor Levels: An Issue of Concern. J. Environ. Sci. Health Part A 2016, 51, 357–363. [Google Scholar] [CrossRef]
- Duong, A.; Steinmaus, C.; McHale, C.M.; Vaughan, C.P.; Zhang, L. Reproductive and Developmental Toxicity of Formaldehyde: A Systematic Review. Mutat. Res./Rev. Mutat. Res. 2011, 728, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Jahan, S.A.; Lee, J.-T. Exposure to Formaldehyde and Its Potential Human Health Hazards. J. Environ. Sci. Health Part C 2011, 29, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Rhomberg, L.R.; Bailey, L.A.; Goodman, J.E.; Hamade, A.K.; Mayfield, D. Is Exposure to Formaldehyde in Air Causally Associated with Leukemia?—A Hypothesis-Based Weight-of-Evidence Analysis. Crit. Rev. Toxicol. 2011, 41, 555–621. [Google Scholar] [CrossRef]
- Goldstein, B.D. Hematological and Toxicological Evaluation of Formaldehyde as a Potential Cause of Human Leukemia. Hum. Exp. Toxicol. 2010, 30, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Shim, I.-K.; Kim, J.; Won, S.R.; Hwang, E.S.; Lee, Y.; Park, S.; Ryu, J.; Lee, J. Prevalence of Sick Building Syndrome Symptoms and Subjective–Objective Indoor Air Quality of Stores in Underground Shopping Districts of Korea. Build. Environ. 2023, 228, 109882. [Google Scholar] [CrossRef]
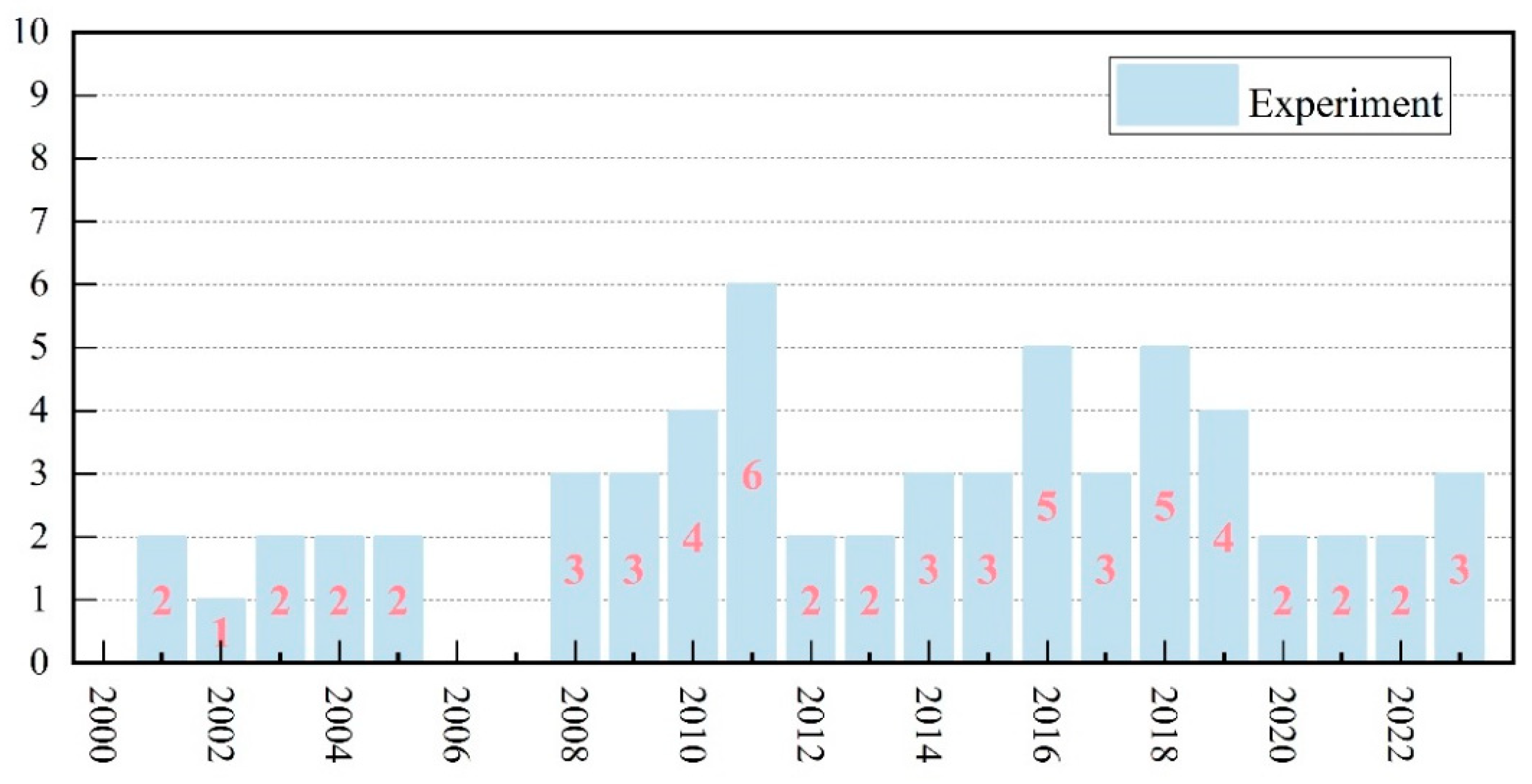
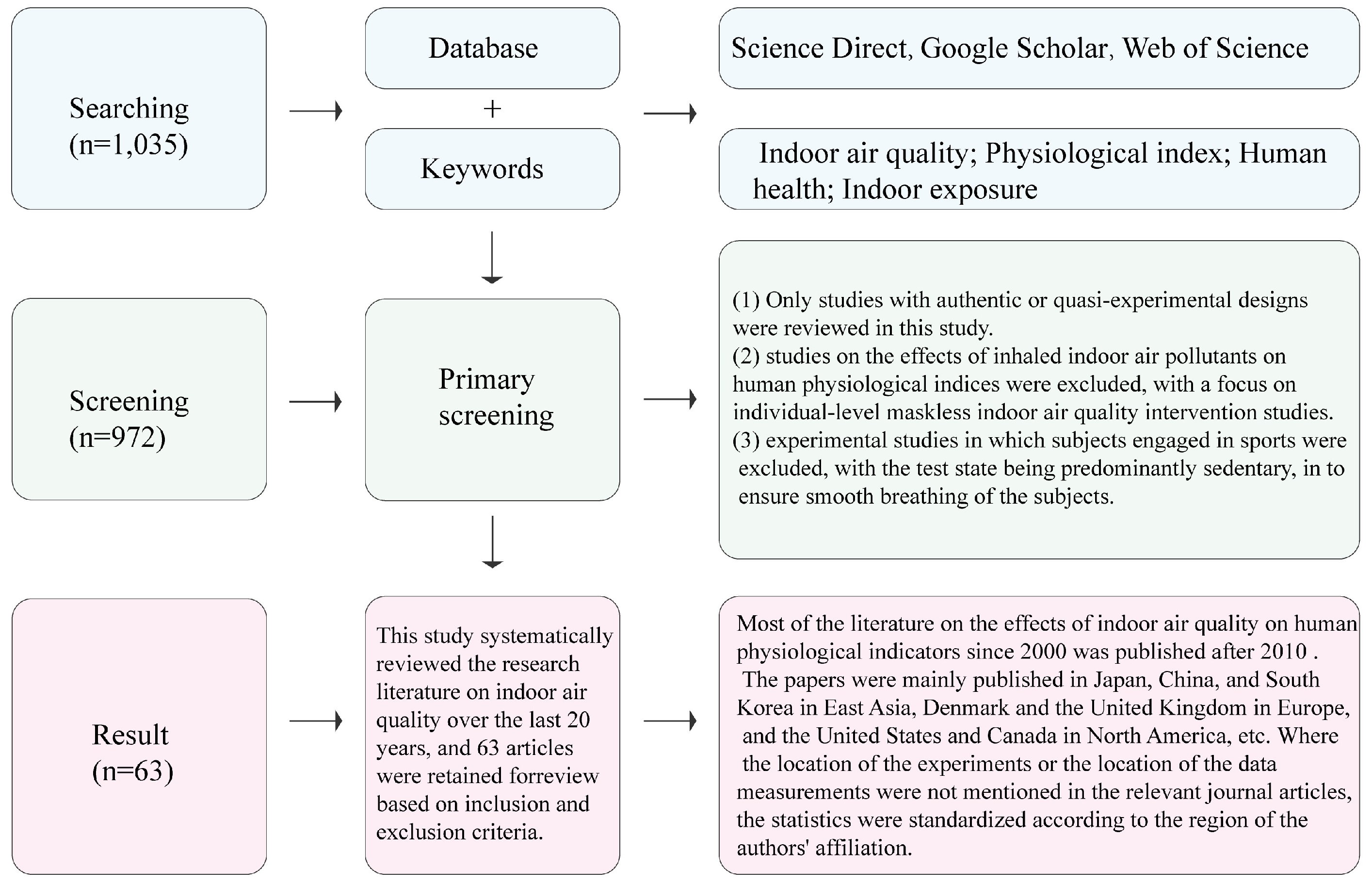
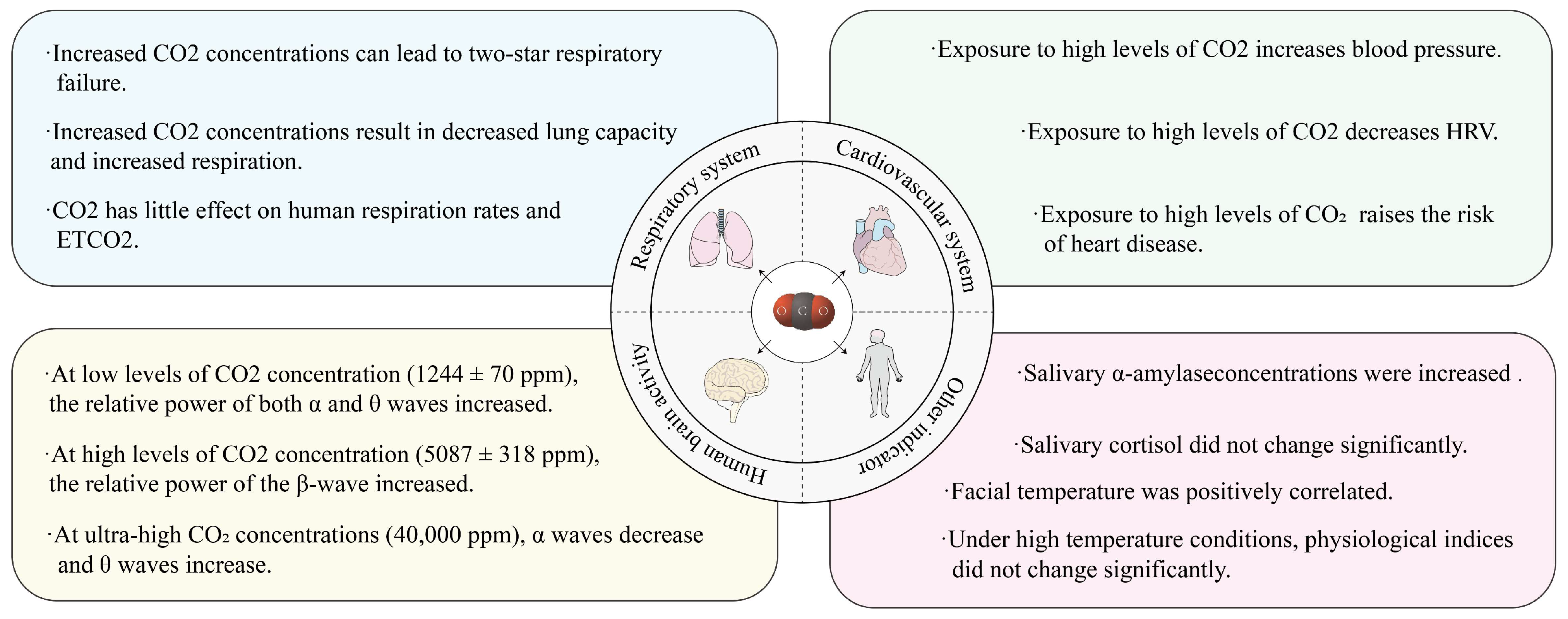

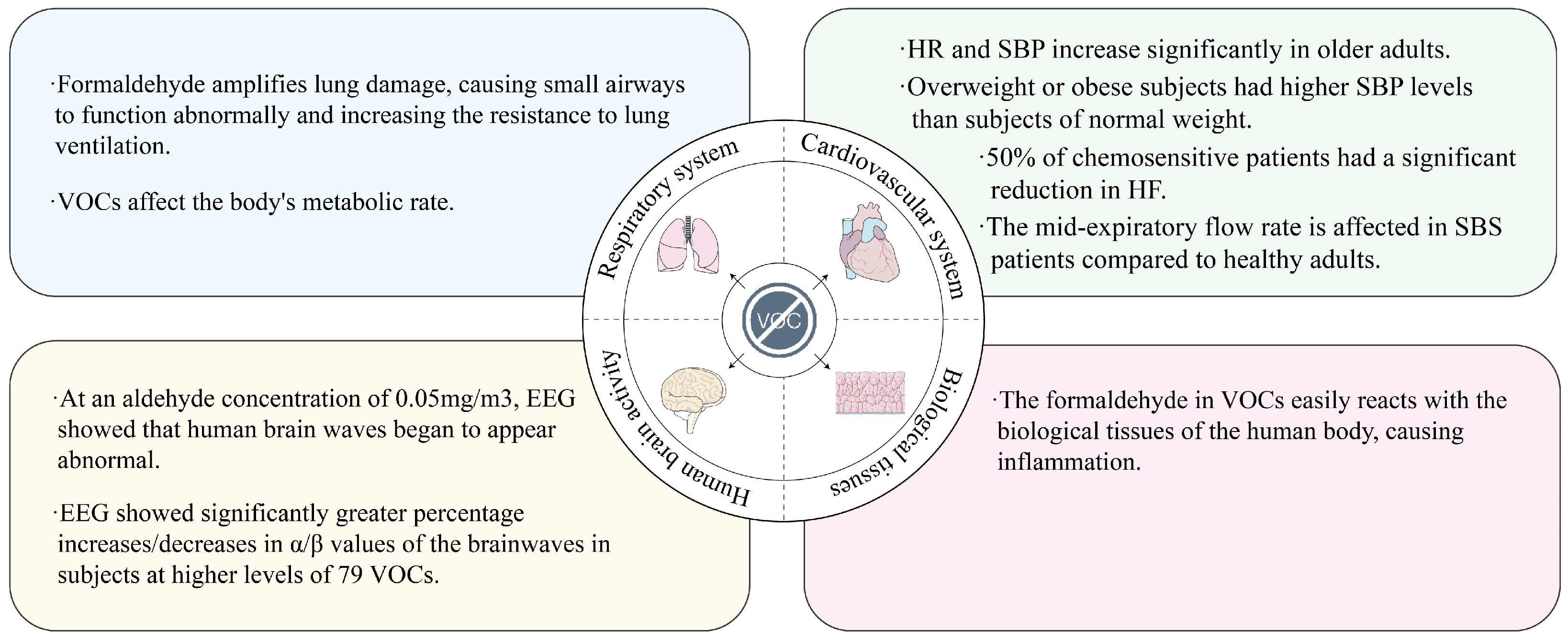
| Category | Keywords |
|---|---|
| Pollutants | “Indoor air quality”, “carbon dioxide”, “particulate matter”, “ozone”, or “volatile organic compounds” |
| Physiological Indicators | “Blood pressure”, “electroencephalogram”, “heart rate variability”, or “oximetry” |
| Exposure Scenarios | “Controlled experiment”, “human subjects”, “residential buildings”, or “office” |
| Journal Title | Number of Citations |
|---|---|
| Building and Environment | 12 |
| Environmental Health Perspectives | 11 |
| Indoor Air | 8 |
| International Journal of Environmental Research and Public Health | 4 |
| Science of The Total Environment | 3 |
| Atmospheric Environment | 3 |
| Environment International | 3 |
| Environmental Research | 2 |
| Other (1 each of 21 journals) | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, T.; Zhang, G.; Sun, Y.; Wang, W.; Wang, T.; Duan, H. Effects of Indoor Air Quality on Human Physiological Impact: A Review. Buildings 2025, 15, 1296. https://doi.org/10.3390/buildings15081296
Nie T, Zhang G, Sun Y, Wang W, Wang T, Duan H. Effects of Indoor Air Quality on Human Physiological Impact: A Review. Buildings. 2025; 15(8):1296. https://doi.org/10.3390/buildings15081296
Chicago/Turabian StyleNie, Tong, Guofu Zhang, Yinan Sun, Wenhao Wang, Tianai Wang, and Haoyan Duan. 2025. "Effects of Indoor Air Quality on Human Physiological Impact: A Review" Buildings 15, no. 8: 1296. https://doi.org/10.3390/buildings15081296
APA StyleNie, T., Zhang, G., Sun, Y., Wang, W., Wang, T., & Duan, H. (2025). Effects of Indoor Air Quality on Human Physiological Impact: A Review. Buildings, 15(8), 1296. https://doi.org/10.3390/buildings15081296




