Chemical Realkalization of Carbonated Concrete: Influence of Cement Composition on Alkalinity Restoration and Portlandite Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
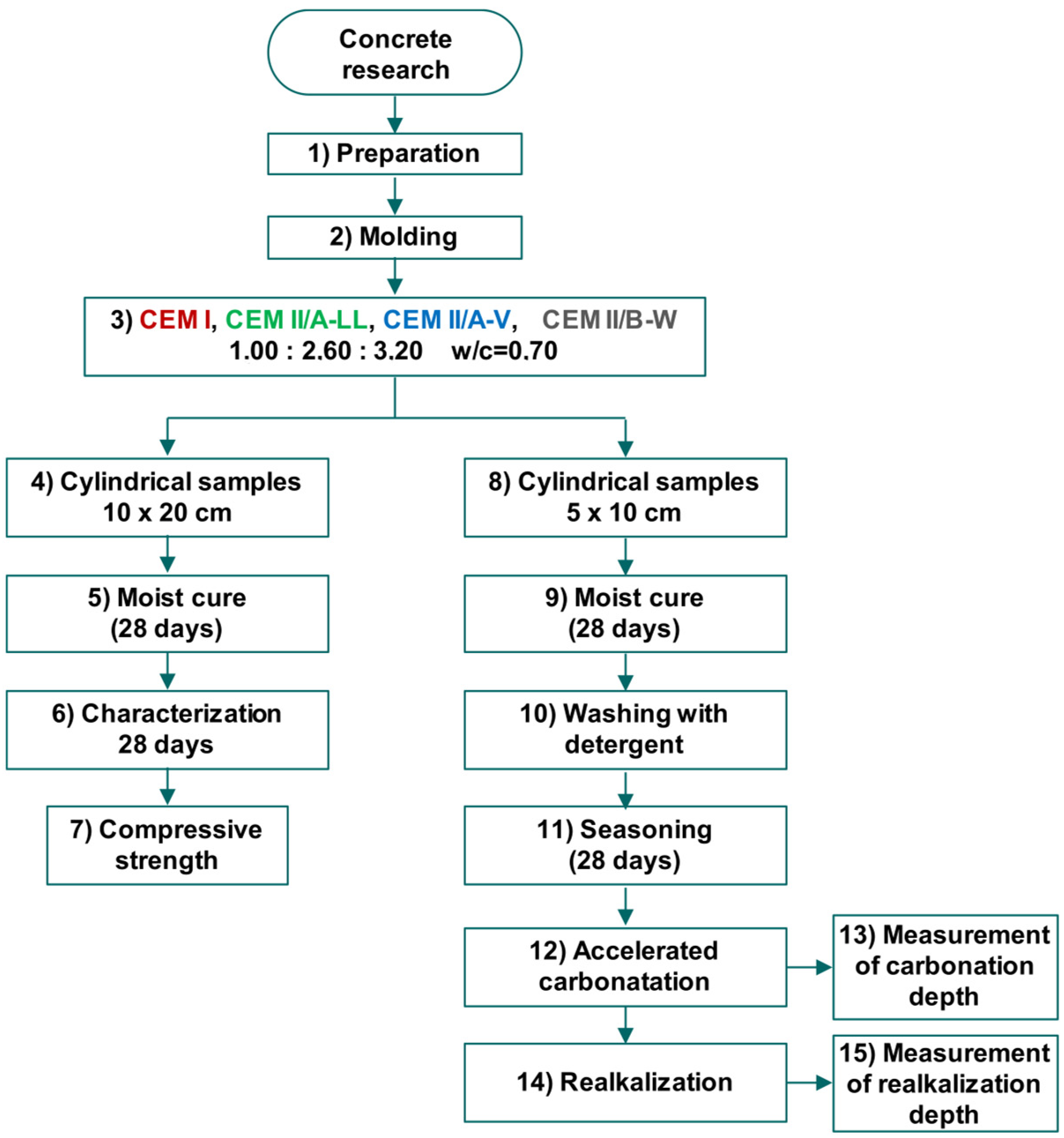
2.2. Study on Concrete Samples
2.2.1. Concrete Mixing and Specimen Preparation
2.2.2. Curing and Conditioning
2.2.3. Accelerated Carbonation
2.2.4. Realkalization Treatment
2.2.5. Measurement of Realkalization Depth
2.3. Study on Cement Paste Samples
2.3.1. Sample Preparation and Conditioning
2.3.2. X-Ray Diffraction (XRD) Analysis
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Apparent Porosity
2.3.5. pH Measurement
- Consistent and fine particle size distribution.
- Constant powder-to-water ratio (40–60% solid content).
- Standardized powder extraction and stirring methods.
- Stable temperature (±10 °C variation).
2.4. Thermodynamic Modelling
3. Results
3.1. Concrete Specimens Analysis
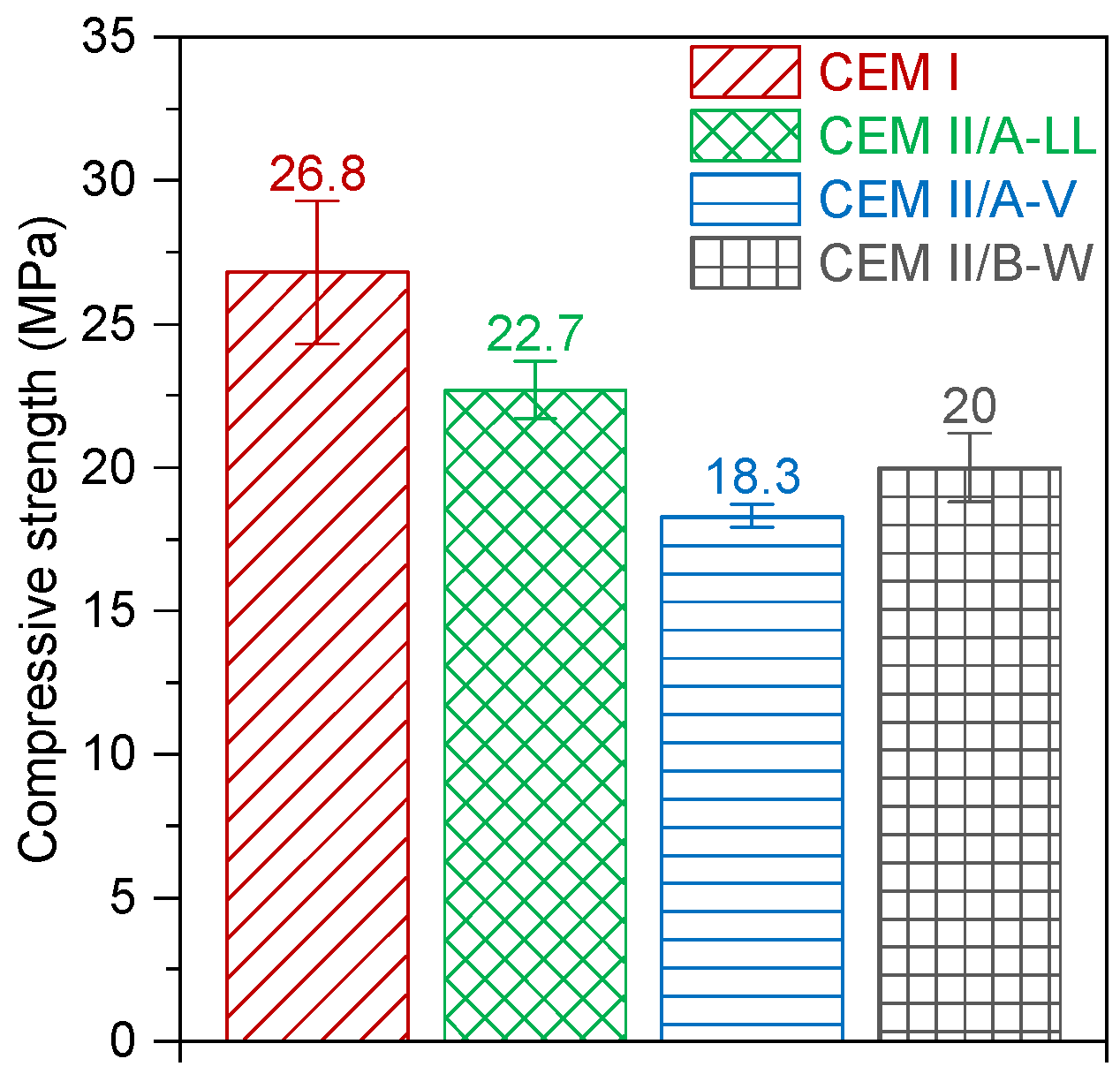
3.1.1. Compressive Strength
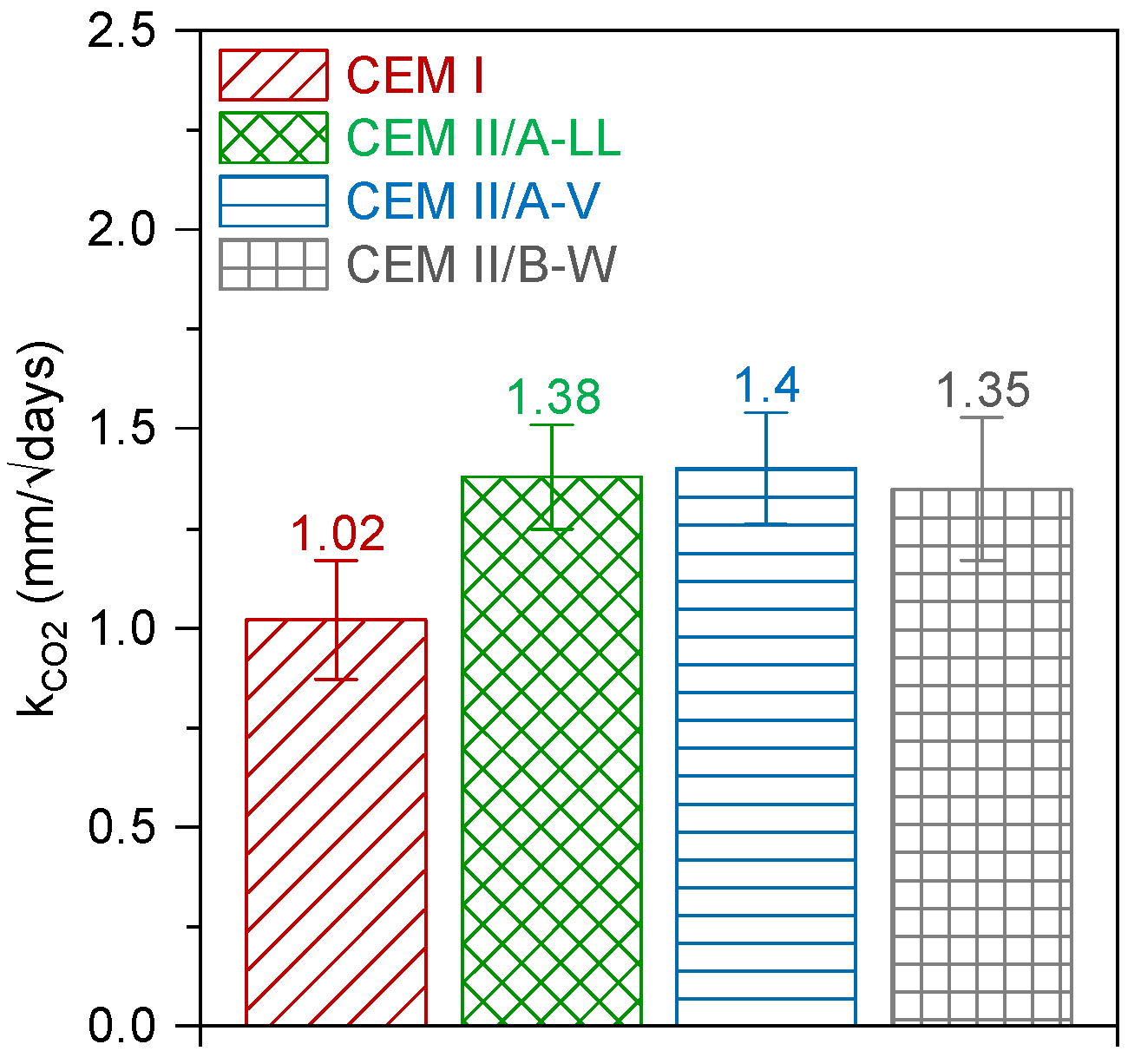
3.1.2. Carbonation Depth
3.1.3. Realkalization Depth
3.2. Cement Paste Analysis
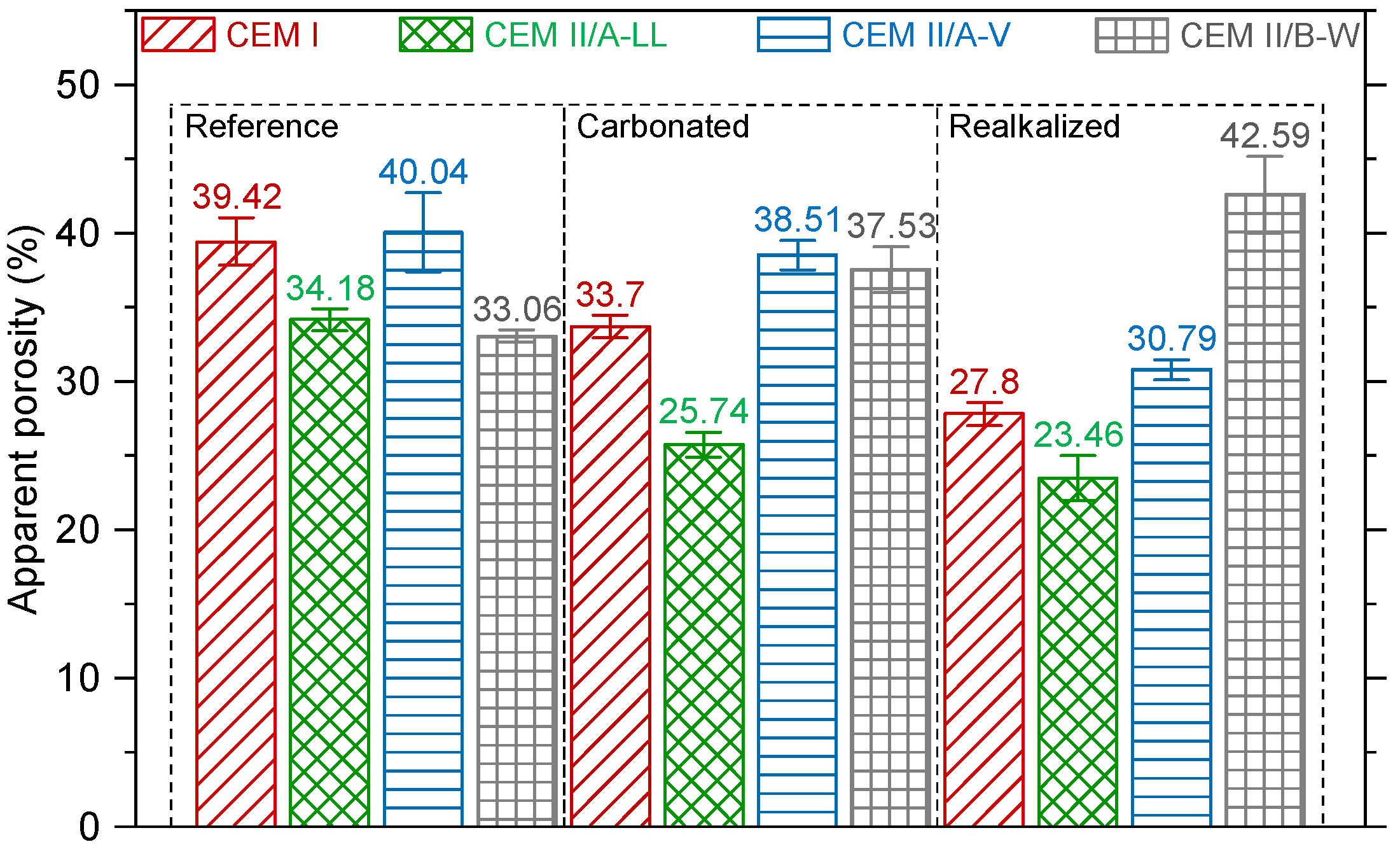
3.2.1. Apparent Porosity
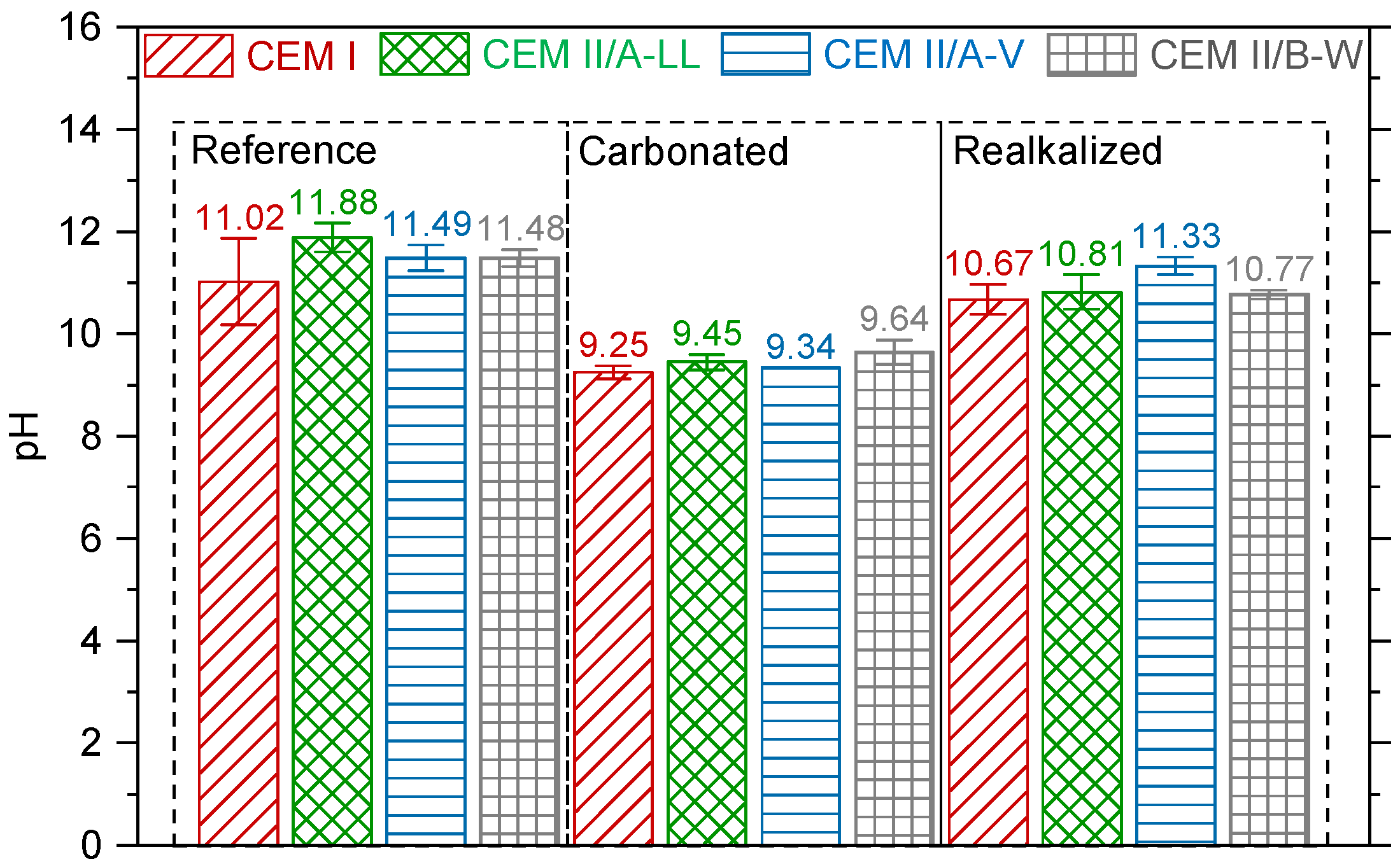
3.2.2. pH Measurements
3.2.3. X-Ray Diffraction (XRD)
3.2.4. Thermogravimetric Analysis (TGA)
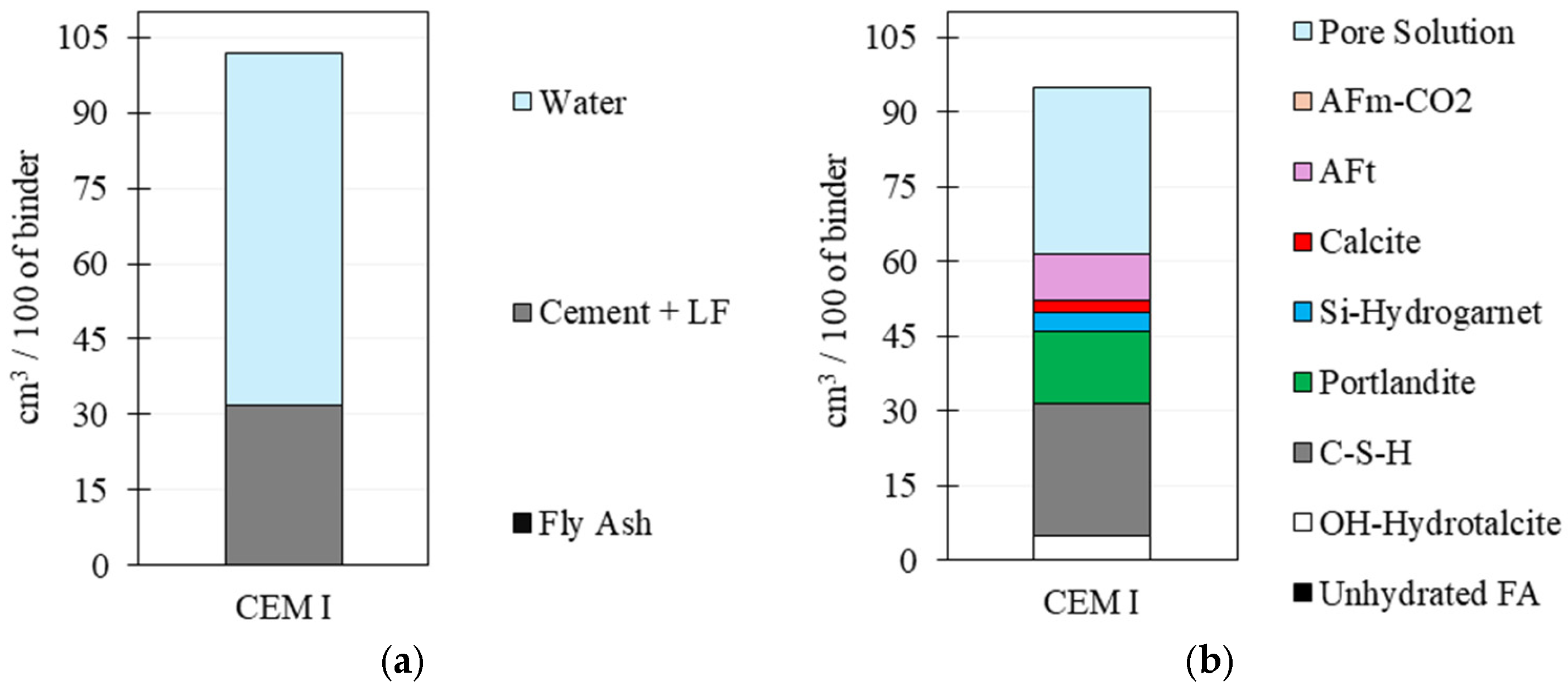
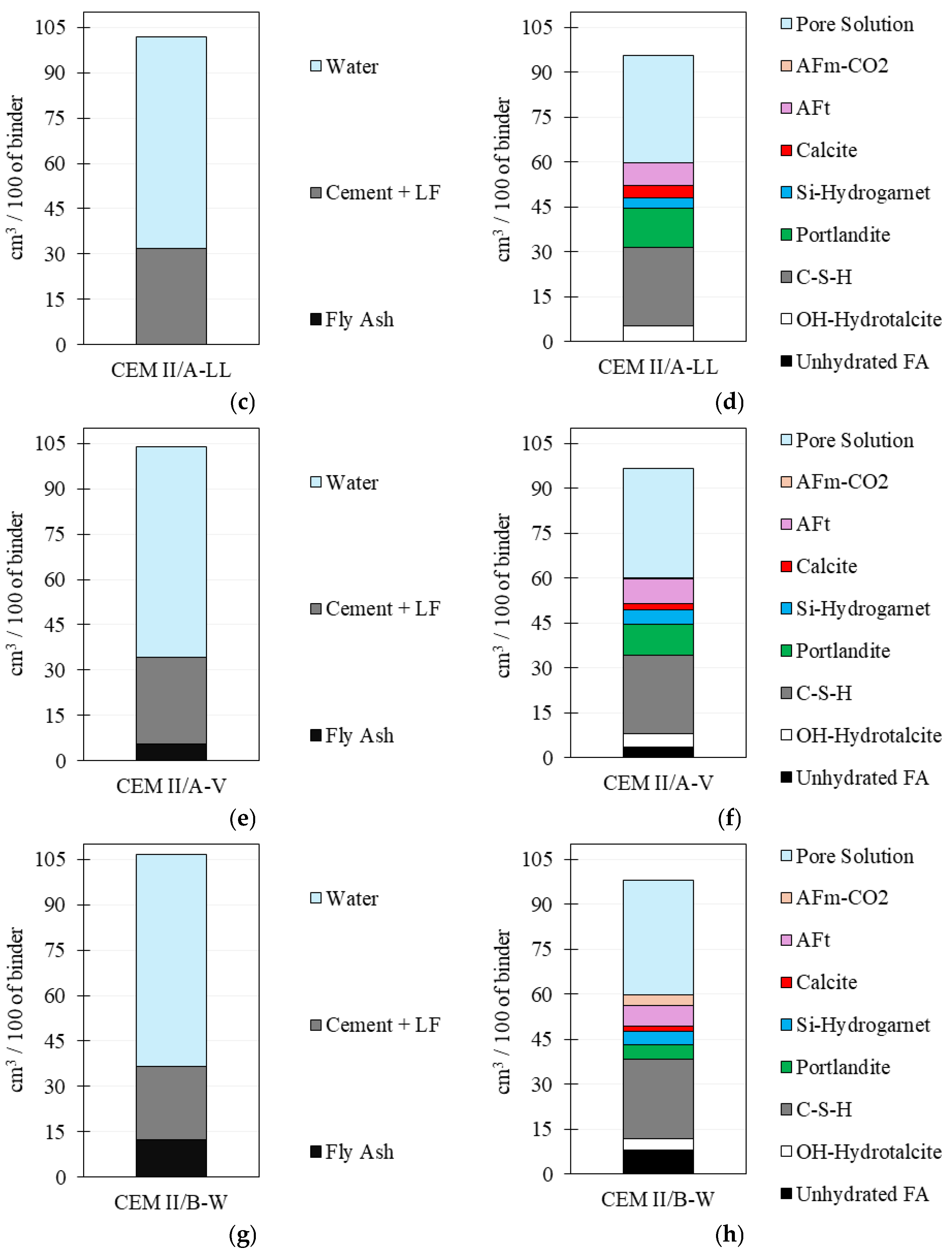
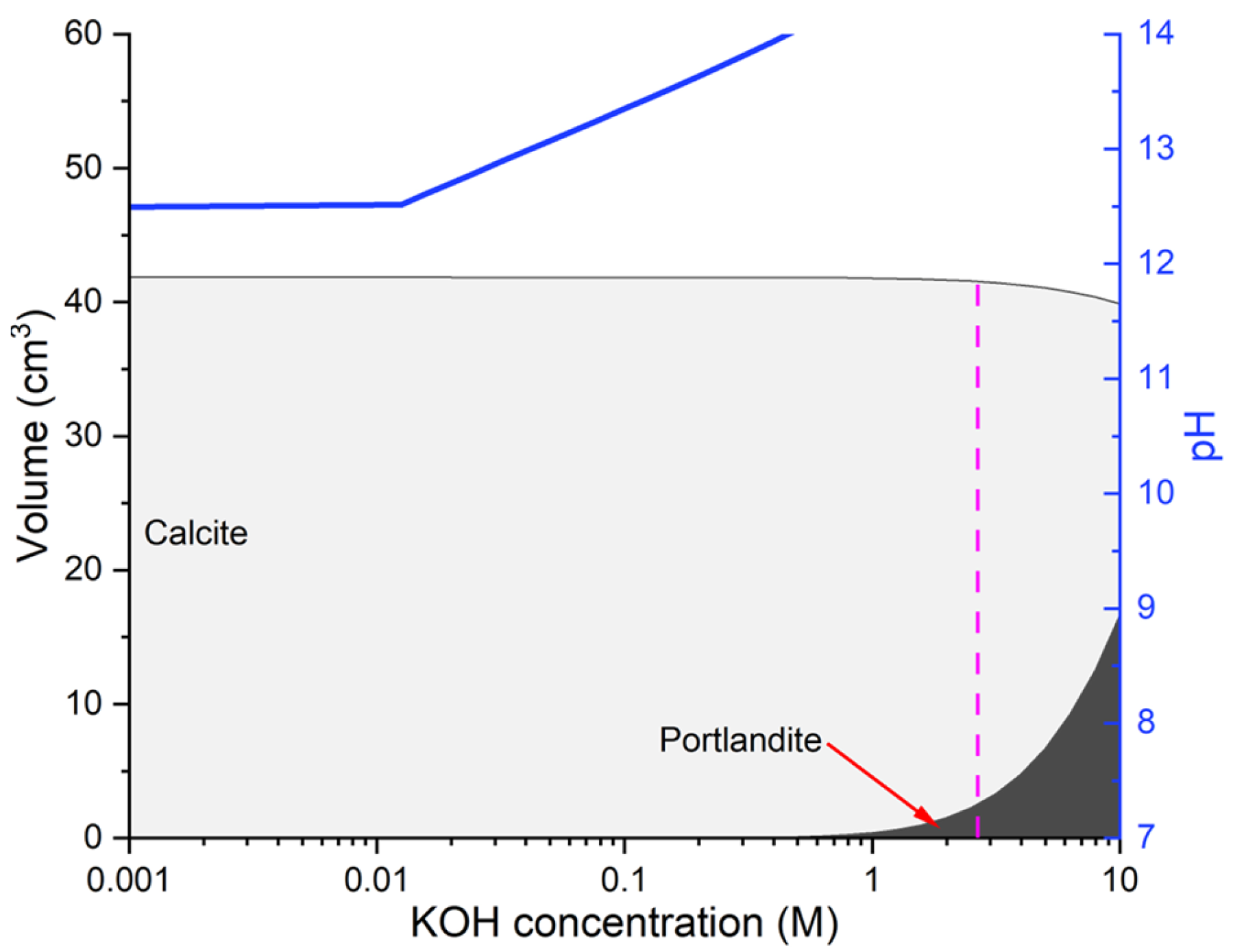
3.3. Thermodynamic Modelling
4. Discussions
4.1. Carbonation and Realkalization Dynamics
4.2. Microstructural Evolution: Porosity and pH
4.3. Phase Transformations: XRD and TGA Analyses
4.4. Thermodynamic Modelling: Phase Assemblage and Pore Solution Evolution
4.5. Implications for Realkalization Strategies
5. Conclusions
- In contrast, the realkalization process was more effective in blended cements, with CEM II/A-V showing the deepest realkalization depth, followed by CEM II/B-W. The higher porosity in these mixtures enhanced the penetration of alkaline solutions, contributing to deeper ionic transport and partial recovery of alkalinity. CEM I, with its lower porosity, exhibited the lowest realkalization depth and slower realkalization rate, further emphasizing the influence of cement microstructure on remediation efficiency.
- The phase transformation analysis, supported by thermogravimetric (TGA) and X-ray diffraction (XRD) results, revealed that carbonation led to the depletion of portlandite and the formation of calcium carbonate polymorphs. While realkalization resulted in some regeneration of portlandite, it did not fully reverse the carbonation-induced changes, which were further corroborated by thermodynamic simulations. These simulations also demonstrated the limited effect of high alkaline concentrations in dissolving carbonation products, reinforcing the partial nature of realkalization in restoring the original cement matrix.
- Based on the results, the 2.67 M KOH solution provided sufficient alkalinity to induce partial portlandite reformation and raise the pH. However, exposure durations should be tailored according to the cement composition. Blended cements, such as CEM II/A-V and CEM II/B-W, with higher permeability, responded well to shorter treatment periods (28–56 days), whereas denser systems, like CEM I, required prolonged immersion (up to 91 days) to achieve comparable pH recovery levels. These observations align with the thermodynamic modeling, which indicated that solution pH, rather than the dissolution of carbonation products, governed the potential for portlandite regeneration.
- Overall, this study underscores the importance of tailoring realkalization strategies to the specific characteristics of the cementitious materials used, considering both their carbonation resistance and realkalization potential. Future research should focus on optimizing realkalization methods, particularly the properties of alkaline solutions and exposure conditions, to improve the recovery of cementitious systems with varying compositions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vázquez, E.; Barra, M.; Aponte, D.; Jiménez, C.; Valls, S. Improvement of the Durability of Concrete with Recycled Aggregates in Chloride Exposed Environment. Constr. Build. Mater. 2014, 67, 61–67. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of Supplementary Cementing Materials on Concrete Resistance against Carbonation and Chloride Ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Machner, A.; Zajac, M.; Ben Haha, M.; Kjellsen, K.O.; Geiker, M.R.; De Weerdt, K. Stability of the Hydrate Phase Assemblage in Portland Composite Cements Containing Dolomite and Metakaolin after Leaching, Carbonation, and Chloride Exposure. Cem. Concr. Compos. 2018, 89, 89–106. [Google Scholar] [CrossRef]
- Al-Sodani, K.A.A.; Al-Amoudi, O.S.B.; Maslehuddin, M.; Shameem, M. Efficiency of Corrosion Inhibitors in Mitigating Corrosion of Steel under Elevated Temperature and Chloride Concentration. Constr. Build. Mater. 2018, 163, 97–112. [Google Scholar] [CrossRef]
- De Weerdt, K.; Wilson, W.; Machner, A.; Georget, F. Chloride Profiles—What Do They Tell Us and How Should They Be Used? Cem. Concr. Res. 2023, 173, 107287. [Google Scholar] [CrossRef]
- Kannan, V.; Ganesan, K. Chloride and Chemical Resistance of Self Compacting Concrete Containing Rice Husk Ash and Metakaolin. Constr. Build. Mater. 2014, 51, 225–234. [Google Scholar] [CrossRef]
- Houst, Y.F.; Wittmann, F.H. Influence of Porosity and Water Content on the Diffusivity of CO2 and O2 through Hydrated Cement Paste. Cem. Concr. Res. 1994, 24, 1165–1176. [Google Scholar] [CrossRef]
- Qiu, Q. A State-of-the-Art Review on the Carbonation Process in Cementitious Materials: Fundamentals and Characterization Techniques. Constr. Build. Mater. 2020, 247, 118503. [Google Scholar] [CrossRef]
- Bernal, S.A.; Dhandapani, Y.; Elakneswaran, Y.; Gluth, G.J.G.; Gruyaert, E.; Juenger, M.C.G.; Lothenbach, B.; Olonade, K.A.; Sakoparnig, M.; Shi, Z.; et al. Report of RILEM TC 281-CCC: A Critical Review of the Standardised Testing Methods to Determine Carbonation Resistance of Concrete; Springer: Dordrecht, The Netherlands, 2024; Volume 57, ISBN 0123456789. [Google Scholar]
- Medeiros, M.H.F.; Helene, P. Surface Treatment of Reinforced Concrete in Marine Environment: Influence on Chloride Diffusion Coefficient and Capillary Water Absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Macioski, G.; De Souza, D.J.; Capraro, A.P.B.; Medeiros, M.H.F. Analysis of Steel Bars Corrosion as a Function of the Environment PH. Alconpat J. 2016, 6, 141–151. [Google Scholar] [CrossRef][Green Version]
- Phung, Q.T.; Maes, N.; Jacques, D.; Bruneel, E.; Van Driessche, I.; Ye, G.; De Schutter, G. Effect of Limestone Fillers on Microstructure and Permeability Due to Carbonation of Cement Pastes under Controlled CO2 Pressure Conditions. Constr. Build. Mater. 2015, 82, 376–390. [Google Scholar] [CrossRef]
- Von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the Carbonation of Concrete with Supplementary Cementitious Materials: A Critical Review by RILEM TC 281-CCC. Mater. Struct. 2020, 53, 136. [Google Scholar] [CrossRef]
- Bier, T.A.; Kropp, J.; Hilsdorf, H.K. Carbonation and Realkalinization of Concrete and Hydrated Cement Paste. In Durability of Construction Materials; RILEM: Paris, France; Chapman & Hall Publishers: London, UK, 1987. [Google Scholar]
- Réus, G.C.; Medeiros, M.H.F. Chemical Realkalization for Carbonated Concrete Treatment: Alkaline Solutions and Application Methods. Constr. Build. Mater. 2020, 262, 120880. [Google Scholar] [CrossRef]
- Al-Jabari, M. Concrete Durability Problems: Physicochemical and Transport Mechanisms. In Integral Waterproofing of Concrete Structures; Woodhead Publishing: Sawston, UK, 2022; ISBN 978-0-12-824354-1. [Google Scholar]
- Chatterji, S. Simultaneous Chloride Removal and Realkalinization of Old Concrete Structures. Cem. Concr. Res. 1994, 24, 1051–1054. [Google Scholar] [CrossRef]
- Oliveira, R.L.N.; Bragança, M.O.G.P.; Medeiros-Junior, R.A. Effect of Coarse Aggregate Size on Corrosion of Reinforced Concrete Exposed to Carbonation and Chloride Ingress by Electrochemical Measurements. Constr. Build. Mater. 2022, 361, 129665. [Google Scholar] [CrossRef]
- Mazer, W.; Ferreira, E.O.; da Silva, M.T.Q.; Basso Tokarski, R. Rehabilitation Technologies. In Durability of Concrete Structures; Delgado, J.M.P.Q., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 63–83. ISBN 978-3-030-62825-3. [Google Scholar]
- de Oliveira, F.G.X.; de Oliveira, F.R.X. Evaluation and Repair of a Reinforced Concrete Structure: A Case Study; Rehabend; David Publishing: Wilmington, DE, USA, 2018; pp. 1913–1921. [Google Scholar] [CrossRef]
- Girčienė, O.; Ramanauskas, R.; Gudavičiūtė, L.; Martušienė, A. Inhibition Effect of Sodium Nitrite and Silicate on Carbon Steel Corrosion in Chloride-Contaminated Alkaline Solutions. Corrosion 2011, 67, 125001–125012. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Yang, X.; Yang, Z.; Zhang, Z. Assessing the Long-Term Chloride Resistance of Alkali-Activated Fly Ash/Slag Concrete by Natural Chloride Diffusion Test and Rapid Chloride Migration Test. Constr. Build. Mater. 2025, 472, 140823. [Google Scholar] [CrossRef]
- Wang, J.; Fu, M.; Zheng, K.; Zhang, K.; Fan, Y.; Niu, D. Ion Transport Mechanism and Diffusion Model Establishment in Recycled Aggregate Concrete Subjected to Magnesium, Sulfate, and Chloride Attack. Constr. Build. Mater. 2024, 428, 136273. [Google Scholar] [CrossRef]
- Saloni; Parveen; Lim, Y.Y.; Pham, T.M.; Negi, P. Performance Enhancement of Rubberised-Alkali-Activated-Concrete Utilising Ultra-Fine Slag and Fly Ash. Clean. Mater. 2022, 4, 100080. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X.; Liu, C.; Yu, W.; Bai, G. Study on the Chloride Ion Transport Mechanism of Recycled Mixed Aggregate Concrete Based on Evolution Characteristics of Pore Structure. Constr. Build. Mater. 2022, 353, 129101. [Google Scholar] [CrossRef]
- Xie, M.; Su, D.; Mayer, K.U.; MacQuarrie, K.T.B. Reactive Transport Investigations of the Long-Term Geochemical Evolution of a Multibarrier System Including Bentonite, Low-Alkali Concrete and Host Rock. Appl. Geochem. 2022, 143, 105385. [Google Scholar] [CrossRef]
- de Medeiros, M.H.F.; Raisdorfer, J.W.; Hoppe Filho, J.; Medeiros-Junior, R.A. Partial Replacement and Addition of Fly Ash in Portland Cement: Influences on Carbonation and Alkaline Reserve. J. Build. Pathol. Rehabil. 2017, 2, 4. [Google Scholar] [CrossRef]
- Filho, J.H.; Medeiros, M.D.; Pereira, E.; Helene, P.; Isaia, G.C. High-Volume Fly Ash Concrete with and without Hydrated Lime: Chloride Diffusion Coefficient from Accelerated Test. J. Mater. Civ. Eng. 2013, 25, 411–418. [Google Scholar] [CrossRef]
- Valcarce, M.B.; Vázquez, M. Carbon Steel Passivity Examined in Alkaline Solutions: The Effect of Chloride and Nitrite Ions. Electrochim. Acta 2008, 53, 5007–5015. [Google Scholar] [CrossRef]
- Chang, J.J. Bond Degradation Due to the Desalination Process. Constr. Build. Mater. 2003, 17, 281–287. [Google Scholar] [CrossRef]
- Anstice, D.J.; Page, C.L.; Page, M.M. The Pore Solution Phase of Carbonated Cement Pastes. Cem. Concr. Res. 2005, 35, 377–383. [Google Scholar] [CrossRef]
- Yohai, L.; Valcarce, M.B.; Vázquez, M. Testing Phosphate Ions as Corrosion Inhibitors for Construction Steel in Mortars. Electrochim. Acta 2016, 202, 316–324. [Google Scholar] [CrossRef]
- Tonoli, G.H.D.; Santos, S.F.; Savastano, H.; Delvasto, S.; Mejía De Gutiérrez, R.; Lopez De Murphy, M.D.M. Effects of Natural Weathering on Microstructure and Mineral Composition of Cementitious Roofing Tiles Reinforced with Fique Fibre. Cem. Concr. Compos. 2011, 33, 225–232. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary Cementitious Materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Sun, W.; Yan, H.D. Hydration of High-Volume Fly Ash Cement Pastes. Cem. Concr. Compos. 2000, 22, 445–452. [Google Scholar] [CrossRef]
- Medeiros, M.H.F.D.; Raisdorfer, J.W.; Hoppe Filho, J. Influence of silica fume and metakaolin on carbonation rate of concrete: Relationship with strength, absorption and w/c ratio. Ambient. Construído 2017, 17, 125–139. [Google Scholar] [CrossRef]
- Hoppe Filho, J.; Gobbi, A.; Pereira, E.; Tanaka, R.S.; De Medeiros, M.H.F. Atividade Pozolânica de Adições Minerais Para Cimento Portland (Parte Ii): Índice de Atividade Pozolânica Com Cimento Portland (IAP), Difração de Raios-x (DRX) e Termogravimetria (TG/DTG). Rev. Mater. 2017, 22, e11873. [Google Scholar] [CrossRef][Green Version]
- Kirchheim, A.P.; Rheinheimer, V.; Dal Molin, D.C.C. Comparative Study of White and Ordinary Concretes with Respect of Carbonation and Water Absorption. Constr. Build. Mater. 2015, 84, 320–330. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of Early Carbonation Curing on Chloride Penetration and Weathering Carbonation in Concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- Hoppe Filho, J.; Rodrigues, C.S.; Ribeiro, L.S.O.P.; Medeiros, M.H.F. Evaluation of Sample Drying Methods to Determine the Apparent Porosity and Estimation of Degree of Hydration of Portland Cement Pastes. J. Build. Pathol. Rehabil. 2021, 6, 1. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2016; ISBN 9781498738675. [Google Scholar]
- De Souza, D.J.; Antunes, L.R.; Sanchez, L.F.M. The Evaluation of Wood Ash as a Potential Preventive Measure against Alkali-Silica Reaction Induced Expansion and Deterioration. J. Clean. Prod. 2022, 358, 131984. [Google Scholar] [CrossRef]
- Pu, Q.; Jiang, L.; Xu, J.; Chu, H.; Xu, Y.; Zhang, Y. Evolution of PH and Chemical Composition of Pore Solution in Carbonated Concrete. Constr. Build. Mater. 2012, 28, 519–524. [Google Scholar] [CrossRef]
- Behnood, A.; Van Tittelboom, K.; De Belie, N. Methods for Measuring PH in Concrete: A Review. Constr. Build. Mater. 2016, 105, 176–188. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 3rd ed.; McGraw Hill: New York, NY, USA, 2013; ISBN 0-07-146289-9. [Google Scholar]
- Kulik, D.A.; Wagner, T.; Dmytrieva, S.V.; Kosakowski, G.; Hingerl, F.F.; Chudnenko, K.V.; Berner, U.R. GEM-Selektor Geochemical Modeling Package: Revised Algorithm and GEMS3K Numerical Kernel for Coupled Simulation Codes. Comput. Geosci. 2013, 17, 1–24. [Google Scholar] [CrossRef]
- Thoenen, T.; Hummel, W.; Berner, U.; Curti, E. The PSI/Nagra Chemical Thermodynamic Database 12/07, PSI Report 14-04; Paul Scherrer Institut: Villigen, Switzerland, 2014. [Google Scholar]
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.D.; Myers, R.J. Cemdata18: A Chemical Thermodynamic Database for Hydrated Portland Cements and Alkali-Activated Materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Kulik, D.A. Improving the Structural Consistency of C-S-H Solid Solution Thermodynamic Models. Cem. Concr. Res. 2011, 41, 477–495. [Google Scholar] [CrossRef]
- De Souza, D.J.; Sanchez, L.F.M.; Hoppe Filho, J.; Medeiros, M.H.F. Development of a Durability Indicator to Forecast the Efficiency of Preventive Measures against External Sulphate Attack. Cem. Concr. Compos. 2024, 145, 105349. [Google Scholar] [CrossRef]
- De Souza, D.J.; Medeiros, M.H.F.; Hoppe, J.; Sanchez, L.F.M. The Uses of Finely Ground Materials to Mitigate the External Sulphate Attack (ESA) on Cementitious Materials; Springer: Cham, Switzerland, 2020; Volume 21. [Google Scholar]
- De Souza, D.J. Capacity of Minerals Additions to Mitigate the Sodium and Magnesium Sulfate Attack in Portland Cement Mortars; Universidade Federal do Paraná: Curitiba, Brazil, 2016. [Google Scholar]
- Tuutti, K. Corrosion of Steel in Concrete; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 1982. [Google Scholar]







| CEM I | CEM II/A-LL | CEM II/A-V | CEM II/B-W | |
|---|---|---|---|---|
| Al2O3 | 4.34 | 4.19 | 6.44 | 9.51 |
| SiO2 | 18.56 | 18.75 | 23.31 | 29.06 |
| Fe2O3 | 2.86 | 2.74 | 3.16 | 4.16 |
| CaO | 61.56 | 61.96 | 53.62 | 45.18 |
| MgO | 3.67 | 3.94 | 3.12 | 2.67 |
| SO3 | 3.03 | 2.56 | 2.73 | 2.17 |
| Loss on ignition | 3.65 | 5.86 | 7.91 | 4.03 |
| Free CaO | 1.40 | 0.90 | 0.76 | 0.87 |
| Insoluble residue | 0.77 | 1.04 | 11.13 | 24.52 |
| Alkali equivalent | 0.67 | 0.68 | 0.87 | 1.17 |
| % of limestone filler | ≈5 | ≈11 | - | - |
| % of Fly Ash | - | - | ≈11 | ≈24 |
| Property | CEM I | CEM II/A-LL | CEM II/A-V | CEM II/B-W |
|---|---|---|---|---|
| Hot expansion (mm) | 0.00 | 1.00 | 0.50 | 0.00 |
| Initial set time (h) | 3:10 | 3:50 | 4:20 | 4:30 |
| Final set time (h) | 4:00 | 4:30 | 5:15 | 5:15 |
| Water for normal consistency (%) | 29.2 | 26.4 | 27.4 | 29.8 |
| Blaine Specific Surface Area (cm2/g) | 4410 | 3170 | 3620 | 4190 |
| % Sieve Retention #200 (%) | 0.10 | 1.70 | 2.60 | 0.40 |
| % Sieve Retention #350 (%) | 0.50 | 9.70 | 10.40 | 2.60 |
| Compressive strength 1 day (MPa) | 25.2 | 17.1 | 13.6 | 13.6 |
| Compressive strength 3 days (MPa) | 41.6 | 30.1 | 26.5 | 24.9 |
| Compressive strength 7 days (MPa) | 47.1 | 36.9 | 31.4 | 31.6 |
| Compressive strength 28 days (MPa) | 48.8 | 43.2 | 36.5 | 46.0 |
| Mixture | Chemically Bound Water (wt.%) | Portlandite (wt.%) | Carbonates, Calcite + Vaterite (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref | Carb | Realk | Ref | Carb | Realk | Ref | Carb | Realk | |
| CEM I | 15.1 | 8.9 | 7.9 | 21 | 0 | 1.2 | 15.5 | 60.5 | 61.3 |
| CEM II/A-LL | 12.7 | 8 | 6.9 | 18.5 | 0 | 1.2 | 17.9 | 61.12 | 60.6 |
| CEM II/A-V | 12.4 | 7.6 | 6.7 | 9.3 | 0 | 1.9 | 28.4 | 57.8 | 64.8 |
| CEM II/B-W | 11.9 | 7.3 | 6.3 | 8.6 | 0 | 1.3 | 22.3 | 48.4 | 47.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Réus, G.C.; Salvador, R.P.; Hoppe Filho, J.; De Souza, D.J.; de Medeiros, M.H.F. Chemical Realkalization of Carbonated Concrete: Influence of Cement Composition on Alkalinity Restoration and Portlandite Formation. Buildings 2025, 15, 1318. https://doi.org/10.3390/buildings15081318
Réus GC, Salvador RP, Hoppe Filho J, De Souza DJ, de Medeiros MHF. Chemical Realkalization of Carbonated Concrete: Influence of Cement Composition on Alkalinity Restoration and Portlandite Formation. Buildings. 2025; 15(8):1318. https://doi.org/10.3390/buildings15081318
Chicago/Turabian StyleRéus, Giovana Costa, Renan Pícolo Salvador, Juarez Hoppe Filho, Diego Jesus De Souza, and Marcelo H. F. de Medeiros. 2025. "Chemical Realkalization of Carbonated Concrete: Influence of Cement Composition on Alkalinity Restoration and Portlandite Formation" Buildings 15, no. 8: 1318. https://doi.org/10.3390/buildings15081318
APA StyleRéus, G. C., Salvador, R. P., Hoppe Filho, J., De Souza, D. J., & de Medeiros, M. H. F. (2025). Chemical Realkalization of Carbonated Concrete: Influence of Cement Composition on Alkalinity Restoration and Portlandite Formation. Buildings, 15(8), 1318. https://doi.org/10.3390/buildings15081318







