Exploring the Potential of Meyerozyma guilliermondii on Physiological Performances and Defense Response against Fusarium Crown Rot on Durum Wheat
Abstract
:1. Introduction
2. Results
2.1. Effect of Seed Coating with M. guilliermondii and Infection with F. culmoum on Seed Germination and Seedling Growth of Wheat
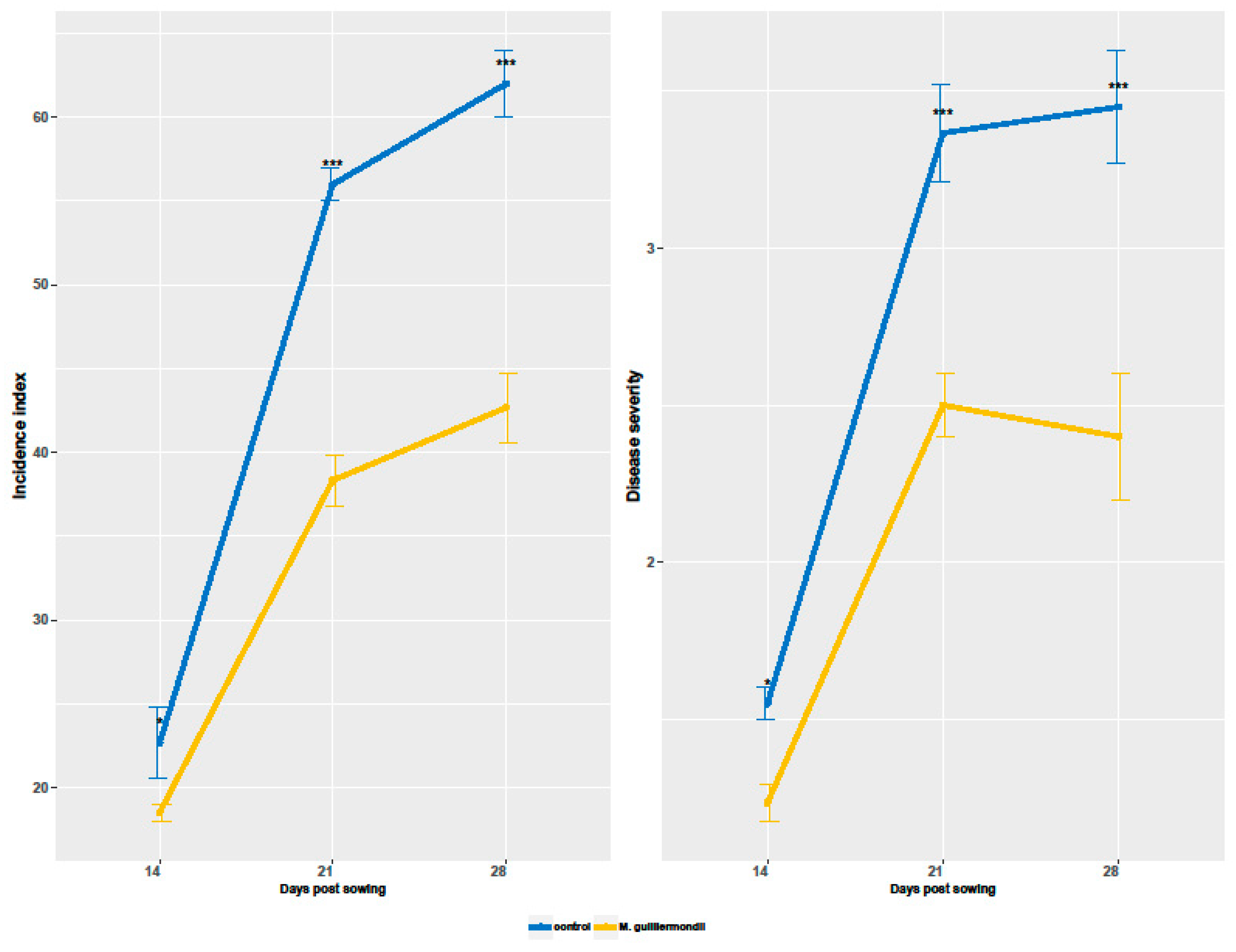
2.2. Effect of Seed Coating with M. guilliermondii on the Incidence and Severity of Fusarium Crown Rot
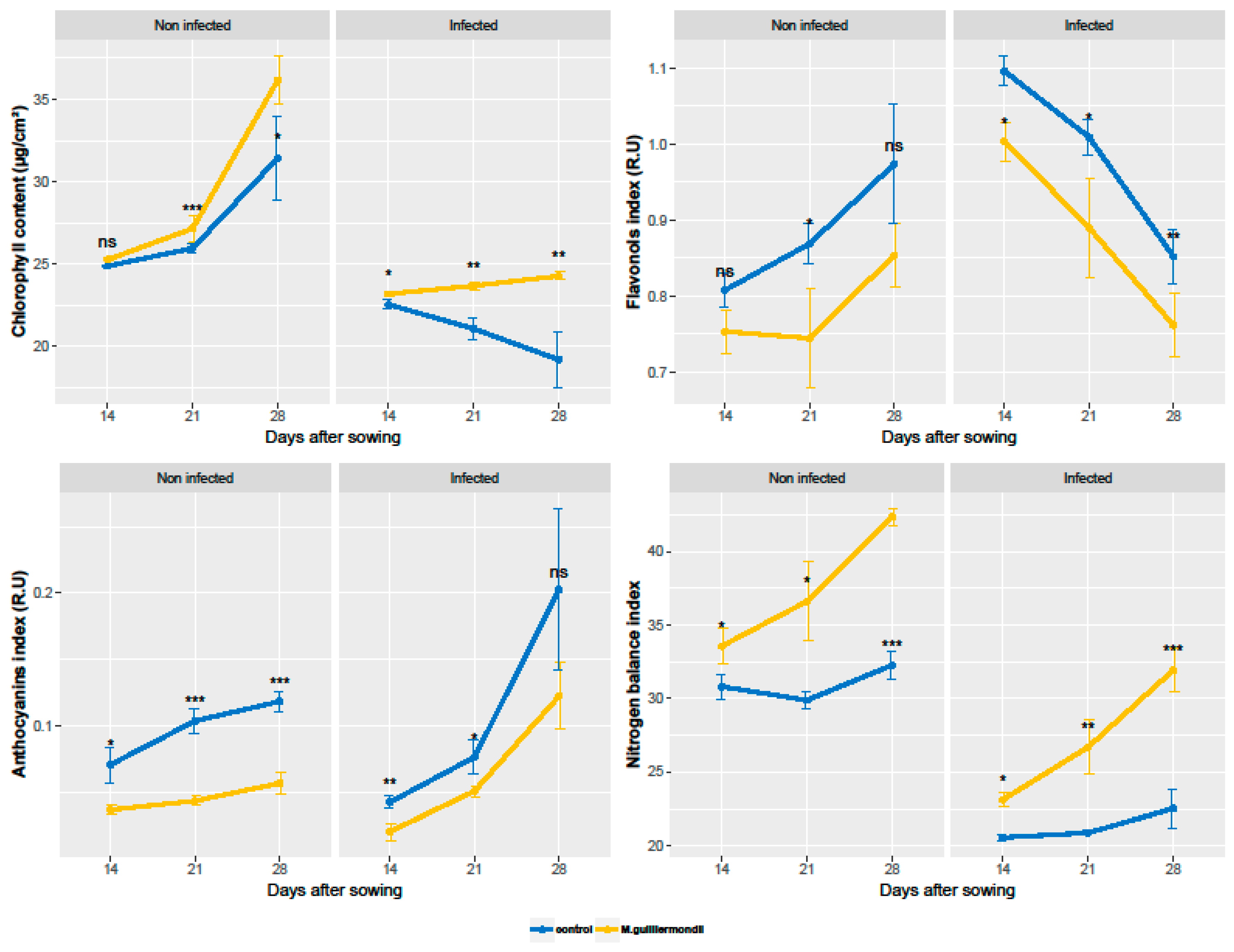
2.3. Effect of Seed Coating with M. guilliermondii and Infection with F. culmorum on the Change in Leaf Pigments
2.4. Effect of Seed Coating with M. guilliermondii and Infection with F. culmorum on the Leaf Gas Exchange, Photosynthesis, Stomatal Conductance (Sc), and ABA Content
2.5. The Interrelationship among the Physiological Traits and the Disease Severity and Incidence
3. Discussion
3.1. Effect of M. guilliermondii on Physiological Traits Under Non-Infected Conditions
3.2. Effect of the Infection with F. culmorum on the Physiological Traits in Control Plants
3.3. Effect of M. guilliermondii on the Physiological Traits Following to Fusarium-Infection
4. Materials and Methods
4.1. Yeast and Pathogen Material
4.2. Seed Coating and Infection with F. culmorum
4.3. Wheat Growth and Experimental Conditions
4.4. Measurements
4.5. Fusarium Crown Rot Disease Assessment
4.6. Dualex Sensor Measurements and Pigments Analysis
4.7. Leaf Gas Exchange
4.8. ABA Extraction and Quantification
4.9. Plant-Growth Traits
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fakhfakh, M.M.; Yahyaoui, A.; Rezgui, S.; Elias, E.M.; Daaloul, A. Identification and pathogenicity assessment of Fusarium spp. sampled from durum wheat fields in Tunisia. Afr. J. Biotechnol. 2011, 10, 6529–6539. [Google Scholar]
- Chekali, S.; Gargouri, S.; Berraies, S.; Gharbi, M.S.; Nicol, M.J.; Nasraoui, B. Impact of Fusarium foot and root rot on yield of cereals in Tunisia. Tunis. J. Plant Prot. 2013, 8, 75–86. [Google Scholar]
- Gargouri, S.; Hajlaoui, M.R.; Guermech, A.; Marrakchi, M. Identification des espèces fongiques associées à la pourriture du pied du blé et étude de leur répartition selon les étages bioclimatiques en Tunisie. EPPO Bull. 2001, 31, 499–503. [Google Scholar] [CrossRef]
- Kammoun, L.G.; Gargouri, S.; Hajlaoui, M.R.; Marrakchi, M. Occurrence and distribution of Microdochium and Fusarium species isolated from durum wheat in northern Tunisia and detection of mycotoxins in naturally infested grain. J. Phytopathol. 2009, 157, 546–551. [Google Scholar] [CrossRef]
- Balmas, V.; Scherm, B.; Marcello, A.; Beyer, M.; Hoffmann, L.; Migheli, Q.; Pasquali, M. Fusarium species and chemotypes associated with Fusarium head blight and Fusarium root rot on wheat in Sardinia. Plant Pathol. 2015, 64, 972–979. [Google Scholar] [CrossRef] [Green Version]
- Köycü, N.D. Effect on Fusarium culmorum of fungicides used in Wheat seed. In Proceedings of the International Congress on Engineering and Life Science, Kastamonu, Turkey, 26–29 April 2018; Kastamonu University: Kastamonu, Turkey, 2019; pp. 593–601. [Google Scholar]
- Wagacha, J.M.; Muthomi, J.W. Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Kthiri, Z.; Harbaoui, K.; Belguesmi, K.; Serret, M.D.; Araus, J.L.; Hamada, W. Seed coating with thyme essential oil or Paraburkholderia phytofirmans PsJN strain: Conferring Septoria leaf blotch resistance and promotion of yield and grain isotopic composition in wheat. Agronomy 2019, 9, 586. [Google Scholar] [CrossRef] [Green Version]
- Naguib, D.M. Control of Fusarium wilt in wheat seedlings by grain priming with defensin-like protein. Egypt. J. Biol. Pest Control 2018, 28, 68. [Google Scholar] [CrossRef]
- Djébali, N. Seed hydropriming effect on Triticum durum and Hordeum vulgare germination, seedling growth and resistance to Fusarium culmorum. Plant Pathol. J. 2012, 11, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Mnasri, N.; Chennaoui, C.; Gargouri, S.; Mhamdi, R.; Hessini, K.; Elkahoui, S.; Djébali, N. Efficacy of some rhizospheric and endophytic bacteria in vitro and as seed coating for the control of Fusarium culmorum infecting durum wheat in Tunisia. Eur. J. Plant Pathol. 2017, 147, 501–515. [Google Scholar] [CrossRef]
- Tyagi, B.; Dubey, A.; Verma, A.; Tiwari, S. Antibacterial activity of phenolics compounds against pathogenic bacteria. Int. J. Pharm. Sci. Rev. Res. 2015, 35, 16–18. [Google Scholar]
- Siranidou, E.; Kang, Z.; Buchenauer, H. Studies on symptom development, phenolic compounds and morphological defence responses in wheat cultivars differing in resistance to Fusarium head blight. J. Phytopathol. 2002, 150, 200–208. [Google Scholar] [CrossRef]
- Jan, F.G.; Hamayun, M.; Hussain, A.; Iqbal, A.; Jan, G.; Khan, S.A.; Khan, H.; Lee, I.J. A promising growth promoting Meyerozyma caribbica from Solanum xanthocarpum alleviated stress in maize plants. Biosci. Rep. 2019, 39, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.-P.; Luo, T.; Fu, H.W.; Wang, L.; Tan, Y.Y.; Huang, J.Z.; Wang, Q.; Ye, G.-Y.; Gatehouse, A.M.R.; Lou, Y.-G.; et al. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 2018, 4, 338–344. [Google Scholar] [CrossRef]
- Baghbani, F.; Lotfi, R.; Moharramnejad, S.; Bandehagh, A.; Roostaei, M.; Rastogi, A.; Kalaji, H.M. Impact of Fusarium verticillioides on chlorophyll fluorescence parameters of two maize lines. Eur. J. Plant Pathol. 2019, 2, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Li, X.; Chen, W.; Liu, T.; Zhong, S.; Ma, L.; Zhang, M.; Zhang, H.; Yu, D.; Luo, P. Wheat resistance to fusarium head blight is associated with changes in photosynthetic parameters. Plant Dis. 2016, 100, 847–852. [Google Scholar] [CrossRef]
- Lahlali, R.; Hamadi, Y.; Jijakli, M.H. Efficacy assessment of Pichia guilliermondii strain Z1, a new biocontrol agent, against citrus blue mould in Morocco under the influence of temperature and relative humidity. Biol. Control 2011, 56, 217–224. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, K.; Shao, X.; Jing, W.; Su, Z. Effects of the yeast Pichia guilliermondii against Rhizopus nigricans on tomato fruit. Postharvest Biol. Technol. 2008, 49, 113–120. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Nakatani, M.; Nishimura, M.; Arakawa, T.; Shimizu, M.; Hyakumachi, M. Suppression of rice blast, cabbage black leaf spot, and tomato bacterial wilt diseases by Meyerozyma guilliermondii TA-2 and the nature of protection. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 629–636. [Google Scholar]
- Nakayan, P.; Hameed, A.; Singh, S.; Young, L.S.; Hung, M.H.; Young, C.C. Phosphate-solubilizing soil yeast Meyerozyma guilliermondii CC1 improves maize (Zea mays L.) productivity and minimizes requisite chemical fertilization. Plant Soil 2013, 373, 301–315. [Google Scholar] [CrossRef]
- Sripontan, Y.; Tan, C.W.; Hung, M.H.; Young, C.C.; Hwang, S.Y. Effects of plant-growth-promoting microorganisms and fertilizers on growth of cabbage and tomato and Spodoptera litura performance. J. Asia Pac. Entomol. 2014, 17, 587–593. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Farran, I.; Larraya, L.; Ancin, M.; Arregui, L.M.; Veramendi, J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: Benefits for seedling development. Microbiol. Res. 2020, 237, 126480. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed biopriming with plant growth promoting rhizobacteria: A review. FEMS Microbiol. Ecol. 2016, 92, 8. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.A. The use of biologicals to enhance vegetable seed quality. Seed Technol. 1998, 20, 198–208. [Google Scholar]
- Shih-Yung, H. IAA Production by Streptomyces scabies and Its Role in Plant Microbe Interaction. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2010. [Google Scholar]
- Qi, P.F.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Wei, Y.M.; Zheng, Y.L.; Ouellet, T. Jasmonic acid and abscisic acid play important roles in host–pathogen interaction between Fusarium graminearum and wheat during the early stages of Fusarium head blight. Physiol. Mol. Plant Pathol. 2016, 93, 39–48. [Google Scholar] [CrossRef]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the basal role of ABA–roles outside of stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Zheng, X.; Apaliya, M.T.; Yang, Q.; Zhao, L.; Gu, X.; Zhang, H. Control of postharvest blue mold decay in pears by Meyerozyma guilliermondii and its effects on the protein expression profile of pears. Postharvest Biol. Technol. 2018, 136, 124–131. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Beccari, G.; Covarelli, L.; Nicholson, P. Infection processes and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant Pathol. 2011, 60, 671–684. [Google Scholar] [CrossRef]
- Kang, Z.; Buchenauer, H. Ultrastructural and immunocytochemical investigation of pathogen development and host responses in resistant and susceptible wheat spikes infected by Fusarium culmorum. Physiol. Mol. Plant Pathol. 2011, 57, 255–268. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, B.; Shaikh, F.I.; Friedt, W.; Gottwald, S. Wheat resistances to Fusarium root rot and head blight are both associated with deoxynivalenol-and jasmonate-related gene expression. Phytopathology 2018, 108, 602–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zobiole, L.H.S.; Kremer, R.J.; de Oliveira, R.S., Jr.; Constantin, J. Glyphosate effects on photosynthesis, nutrient accumulation, and nodulation in glyphosate-resistant soybean. J. Plant Nutr. Soil Sci. 2012, 175, 319–330. [Google Scholar] [CrossRef]
- Takahama, U.; Oniki, T. Flavonoids and some other phenolics as substrates of peroxidase: Physiological significance of the redox reactions. J. Plant Res. 2000, 113, 301–309. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L.; Barbottin, A.; Jeuffroy, M.H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop. Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Kangasjärvi, S.; Neukermans, J.; Li, S.; Aro, E.M.; Noctor, G. Photosynthesis, photorespiration, and light signalling in defence responses. J. Exp. Bot. 2012, 63, 1619–1636. [Google Scholar] [CrossRef] [Green Version]
- Bauriegel, E.; Giebel, A.; Herppich, W.B. Rapid Fusarium head blight detection on winter wheat ears using chlorophyll fluorescence imaging. J. Appl. Bot. Food Qual. 2010, 83, 196–203. [Google Scholar]
- Bauriegel, E.; Herppich, W.B. Hyperspectral and chlorophyll fluorescence imaging for early detection of plant diseases, with special reference to Fusarium spec. infections on wheat. Agriculture 2014, 4, 32–57. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Bakshi, P. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2019, 39, 509–531. [Google Scholar] [CrossRef]
- Nantawanit, N.; Chanchaichaovivat, A.; Panijpan, B.; Ruenwongsa, P. Induction of defense response against Colletotrichum capsici in chili fruit by the yeast Pichia guilliermondii strain R13. Biol. Control 2010, 52, 145–152. [Google Scholar] [CrossRef]
- Celis Zambrano, C.; Moreno Duran, G.; Sequeda-Castañeda, L.G.; García Caicedo, A.; Albarracín, D.M.; Barreto Charry, L.C. Determining the effectiveness of Candida guilliermondii in the biological control of Rhizopus stolonifer in postharvest tomatoes. Univ. Sci. 2014, 19, 51–62. [Google Scholar] [CrossRef]
- Pordel, R. The Role of Plant Hormones in Fusarium Head Blight of Wheat. Ph.D. Dissertation, Department of Biological Sciences, University of Lethbridge, Lethbridge, AB, Canada, 2017. [Google Scholar]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, M.; Usman, M.; Nadeem, F.; ur Rehman, H.; Wahid, A.; Basra, S.M.; Siddique, K.H. Seed priming in field crops: Potential benefits, adoption and challenges. Crop. Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]


| Traits | Germination % | Shoot Length (cm) | Root Length (cm) | Biomass (g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Infected | Infected | DR (%) | Non-Infected | Infected | DR (%) | Non-Infected | Infected | DR (%) | Non-Infected | Infected | DR (%) | ||
| Control | 46.7 ± 9.5 | 26.7 ± 9.2 | 42.8 | 52.0 ± 1.6 | 35.5 ± 0.4 | 31.73 | 24.0 ± 0.2 | 15.9 ± 0.8 | 33.75 | 9.3 ± 05 | 4.6 ± 0.6 | 50.53 | |
| M. guilliermondii | 93.3 ± 12.2 | 70.0 ± 9.4 | 24.97 | 73.6 ± 1.0 | 54.5 ± 0.4 | 25.95 | 40.6 ± 0.3 | 33.1 ± 0.5 | 18.47 | 10.8 ± 0.7 | 7.4 ± 0.7 | 31.48 | |
| ANOVA | Infection (I) | 408 ns | 181.7 *** | 63.02 *** | 27.90 *** | ||||||||
| Coating (C) | 3675 ** | 317.2 *** | 130.02 *** | 3.96 * | |||||||||
| C × I | 8 ns | 4.9 ns | 12.20 ** | 1.14 ns | |||||||||
| Chlorophyll(µg/cm²) | Flavonols (R.U) | Anthocyanins (R.U) | NBI (mg/g) | |
|---|---|---|---|---|
| Coating(C) | 53.5 *** | 0.090 *** | 0.0201 *** | 348.6 *** |
| Infection (I) | 339.6 *** | 0.093 *** | 0.0018 * | 894.1 *** |
| C × I | 102.0 *** | 0.018 * | 0.0427 *** | 175.7 *** |
| Traits | Photosynthesis Rate (μmol CO2 m−2 s−1) | Stomatal Conductance (mol H2O m−2 s−1) | Intercellular to Atmospheric CO2 Concentration Ci/Ca | Quantum Efficiency of Photosystem II (ɸPSII) | Electron Transport Rate (ETR) (µmol m−2 s−1) | Transpiration Rate (mmol H2O m−2 s−1) | Non-Photochemical Quenching (qN) | ABA (ng/g FW) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-infected | Infected | Non-infected | Infected | Non- Infected | Infected | Non- Infected | Infected | Non-Infected | Infected | Non-infected | Infected | Non-infected | Infected | Non-infected | Infected | ||
| Control | 18.30 b ± 3.20 | 9.40 c ± 0.25 | 0.399 b ± 0.01 | 0.12 c ± 0.01 | 287.66 ab ± 48.78 | 263.33 b ± 32.39 | 0.205 a ± 0.014 | 0.144 b ± 0.018 | 125722.5 b ± 733 | 94096.5 c ± 12567 | 4.94 b ± 0.99 | 2.32 c ± 0.53 | 1835.33 bc ± 78.38 | 2121.33 a ± 103.63 | 0.826 a ± 0.033 | 1.103 a ± 0.368 | |
| M. guilliermondii | 25.26 a ± 5.10 | 17.43 b ± 0.73 | 0.66 a ± 0.24 | 0.24 bc ± 0.08 | 334.33 a ± 6.65 | 162.66 c ± 50.95 | 0.206 a ± 0.019 | 0.170 b ± 0.005 | 145665.0a ± 3059 | 137664.5ab ± 5484 | 7.44 a ± 0.96 | 3.41 c ± 0.39 | 1729.33 c ± 37.58 | 1904.66 b ± 80.16 | 0.105 b ± 0.000 | 0.181 b ± 0.005 | |
| ANOVA | Coating (C) | 168.75 ** | 0.1114 * | 1519 ns | 0.000520 ns | 3.025e + 09 *** | 9.67 ** | 78085 ** | 2.0240 *** | ||||||||
| Infection (I) | 210.00 ** | 0.3626 ** | 31519 ** | 0.006960 *** | 1.178e + 09 ** | 33.17 *** | 159621*** | 0.0938 ns | |||||||||
| C × I | 0.85 ns | 0.0169 ns | 18330 ** | 0.000469 ns | 4.186e + 08 * | 1.52 ns | 9185 ns | 0.0301 ns | |||||||||
| Decrease Rate (%) | Increase Rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Traits | Photosynthesis rate (μmol CO2 m−2 s−1) | Stomatal conductance (mol H2O m−2 s−1) | Intercellular to atmospheric CO2 concentration Ci/Ca | Quantum efficiency of Photosystem II (ɸPSII) | Electron transport rate (ETR) (µmol m−2 s−1) | Transpiration rate (mmol H2O m−2 s−1) | Non-photochemical quenching (qN) | ABA (ng/g FW) |
| Control | 48.63 | 69.92 | 8.45 | 29.75 | 25.15 | 53.03 | 13.48 | 25.11 |
| M. guilliermondii | 30.99 | 42 | 51.34 | 17.47 | 5.49 | 54.16 | 9.20 | 41.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kthiri, Z.; Jabeur, M. B.; Chairi, F.; López-Cristoffanini, C.; López-Carbonell, M.; Serret, M. D.; Araus, J. L.; Karmous, C.; Hamada, W. Exploring the Potential of Meyerozyma guilliermondii on Physiological Performances and Defense Response against Fusarium Crown Rot on Durum Wheat. Pathogens 2021, 10, 52. https://doi.org/10.3390/pathogens10010052
Kthiri Z, Jabeur MB, Chairi F, López-Cristoffanini C, López-Carbonell M, Serret MD, Araus JL, Karmous C, Hamada W. Exploring the Potential of Meyerozyma guilliermondii on Physiological Performances and Defense Response against Fusarium Crown Rot on Durum Wheat. Pathogens. 2021; 10(1):52. https://doi.org/10.3390/pathogens10010052
Chicago/Turabian StyleKthiri, Zayneb, Maissa Ben Jabeur, Fadia Chairi, Camilo López-Cristoffanini, Marta López-Carbonell, Maria Dolores Serret, Jose Luis Araus, Chahine Karmous, and Walid Hamada. 2021. "Exploring the Potential of Meyerozyma guilliermondii on Physiological Performances and Defense Response against Fusarium Crown Rot on Durum Wheat" Pathogens 10, no. 1: 52. https://doi.org/10.3390/pathogens10010052





