Twenty Years after Bovine Vaccinia in Brazil: Where We Are and Where Are We Going?
Abstract
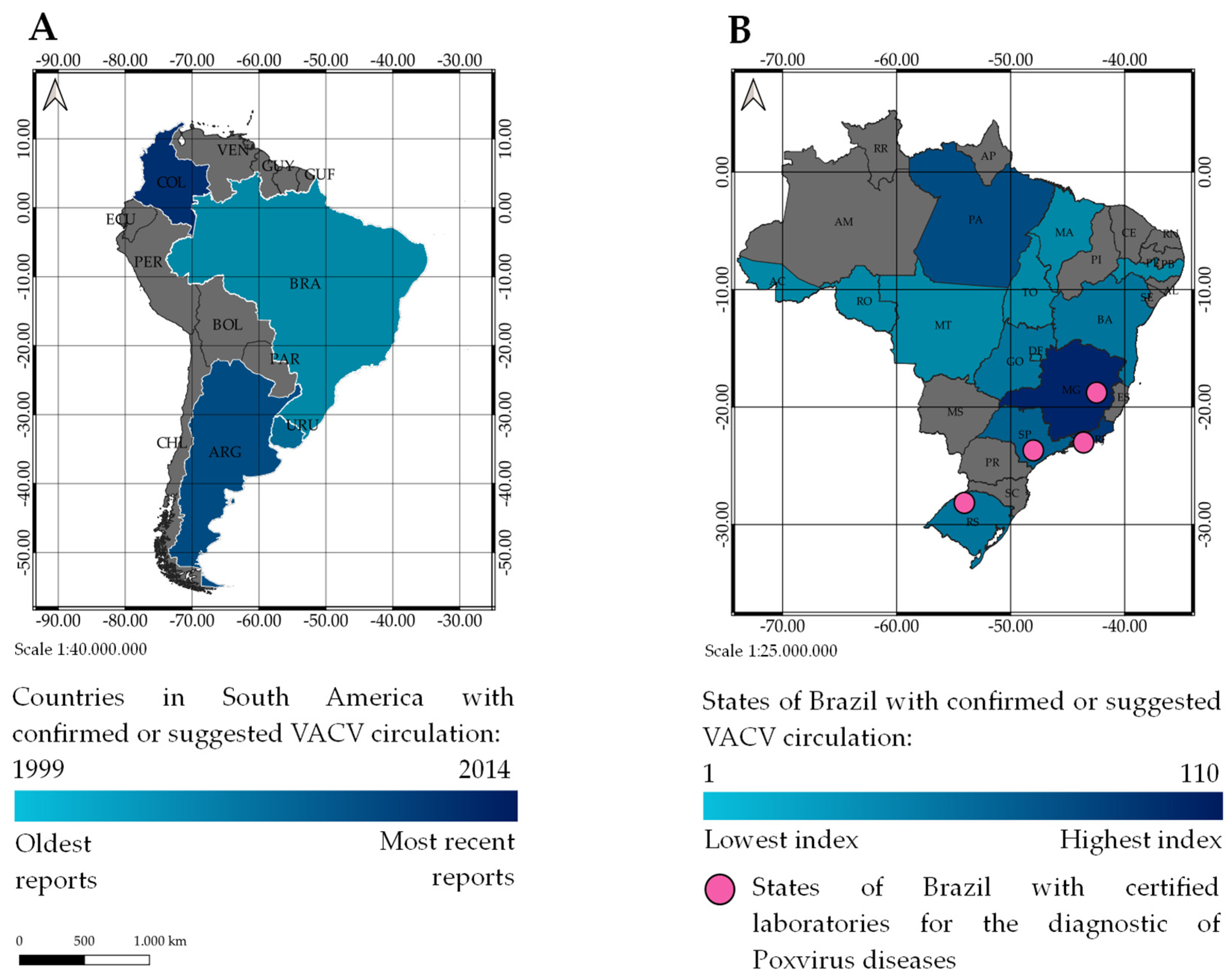
:1. Introduction
2. The Main Areas of BR-VACV Endemicity and the Epidemiological Profile
3. Genetic Characterization of Brazilian Vaccinia Viruses (BR-VACV)
4. Classic Transmission and Alternative Routes for Zoonotic BR-VACV Infections
5. The Transversal Impact of BR-VACV
6. Where Are We Going with the BV and/or VACV Threat?
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Kirby, T. WHO celebrates 40 years since eradication of smallpox. Lancet Infect. Dis. 2020, 20, 174. [Google Scholar] [CrossRef]
- Meyer, H.; Ehmann, R.; Smith, G.L. Smallpox in the Post-Eradication Era. Viruses 2020, 12, 138. [Google Scholar] [CrossRef] [Green Version]
- Baxby, D. Jenner’s Smallpox Vaccine: The Riddle of Vaccinia Virus and Its Origin. Am. Hist. Rev. 1981, 87, 1044–1045. [Google Scholar]
- Jenner, E. An Inquiry into the Causes and Effects of the Variolae Vaccinae; a Disease Discovered in some of the Western Counties of England, Particularly Gloucestershire, and Known by the Name of The Cow Pox, London. In Classics of Medicine and Surgery; Camac, C.N.B., Ed.; Dover: Mineola, NY, USA, 1959; pp. 213–240. [Google Scholar]
- Henderson, D.; Preston, R. Smallpox: The Death of a Disease—The Inside Story of Eradicating a Worldwide Killer. J. Hist. Med. Allied Sci. 2009, 66, 276–277. [Google Scholar]
- Pickup, D.J. Understanding Orthopoxviruses interference with host immune responses to inform novel vaccine design. Expert Rev. Vaccines 2007, 6, 87–95. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses—ICTV. 2020. Available online: https://talk.ictvonline.org/ (accessed on 23 August 2020).
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejías-Pérez, E.; García-Arriaza, J.; Di Pilato, M.; Esteban, M. The evolution of poxvirus vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.; Richter, M.; Hapke, C.; Stern, D.; Nitsche, A. Genomic expression libraries for the identification of cross-reactive Orthopoxvirus antigens. PLoS ONE 2011, 6, e21950. [Google Scholar] [CrossRef] [Green Version]
- Damaso, C.R.A.; Esposito, J.J.; Condit, R.C.; Moussatché, N. An emergent Poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology 2000, 277, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Trindade, G.S.; da Fonseca, F.G.; Marques, J.T.; Nogueira, M.L.; Mendes, L.C.N.; Borges, A.S.; Peiró, J.R.; Pituco, M.E.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Araçatuba virus: A vaccinia-like virus associated with infection in humans and cattle. Emerg. Infect. Dis. 2003, 9, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.C.D.; Rehfeld, I.S.; Guedes, M.I.M.C.; Lobato, Z.I.P. Bovine Vaccinia: Insights into the Disease in Cattle. Viruses 2018, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Leite, J.A.; Drumond, B.P.; Trindade, G.S.; Lobato, Z.I.P.; da Fonseca, F.G.; Santos, J.R.; Madureira, M.C.; Guedes, M.I.M.C.; Ferreira, J.M.S.; Bonjardim, C.A.; et al. Passatempo virus, a vaccinia virus strain, Brazil. Emerg. Infect. Dis. 2005, 11, 1935–1938. [Google Scholar] [CrossRef]
- Lobato, Z.; Trindade, G.S.; Frois, M.C.M.; Ribeiro, E.B.T.; Dias, G.R.C.; Teixeira, B.M.; Lima, F.A.; Almeida, G.M.F.; Kroon, E.G. Outbreak of exantemal disease caused by Vaccinia virus in human and cattle in Zona da Mata region, Minas Gerais. Arq. Bras. Med. Vet. Zootec. 2005, 57, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Kroon, E.G.; Mota, B.E.F.; Abrahão, J.S.; da Fonseca, F.G.; Trindade, G.S. Zoonotic Brazilian Vaccinia virus: From field to therapy. Antiviral. Res. 2011, 92, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.S.; Figueiredo, P.O.; Costa, G.B.; Assis, F.L.; Drumond, B.P.; da Fonseca, F.G.; Nogueira, M.L.; Kroon, E.G.; Trindade, G.S. Vaccinia virus Natural Infections in Brazil: The Good, The Bad, and The Ugly. Viruses 2017, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Silva-Fernandes, A.T.; Travassos, C.E.; Ferreira, J.M.; Abrahão, J.S.; Rocha, E.S.; Viana-Ferreira, F.; dos Santos, J.R.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Natural human infections with Vaccinia virus during bovine vaccinia outbreaks. J. Clin. Virol. 2009, 44, 308–313. [Google Scholar] [CrossRef]
- Silva, A.C.; Reis, B.B.; Ricci Junior, J.E.R.; Fernandes, F.S.; Correa, J.F.; Schatzmayr, H.G. Infecção em humanos por varíola bovina na microrregião de Itajubá, Estado de Minas Gerais: Relato de caso. Rev. Soc. Bras. Med. Trop. 2008, 41, 507–511. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Schatzmayr, H.G.; Costa, R.V.C.; Gonçalves, M.C.R.; Barreto, D.F.; Batista, V.H.; Silva, N.E.V.; Brust, L.A.C.; Barth, O.M. Human infections caused by vaccinia-like poxviruses in Brazil. Rev. Soc. Bras. Med. Trop. 2009, 42, 672–676. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.T.; Oliveira, G.P.; Assis, F.L.; Bretas de Oliveira, D.; Vaz, S.M.; Trindade, G.S.; Abrahão, J.S.; Kroon, E.G. Ocular Vaccinia Infection in Dairy Worker, Brazil. Emerg. Infect. Dis. 2018, 24, 161–162. [Google Scholar] [CrossRef] [Green Version]
- Borges, I.A.; McCollum, A.M.; Mehal, J.M.; Haberling, D.; Dutra, L.A.L.; Vieira, F.L.; Andrade, L.A.O.; Kroon, E.G.; Holman, R.C.; Reynolds, M.G.; et al. Dairy production practices and associated risks for bovine vaccinia exposure in cattle, Brazil. New Microb. New Infect. 2017, 20, 43–50. [Google Scholar] [CrossRef]
- Rehfeld, I.S.; Fraiha, A.L.S.; Matos, A.C.D.; Costa, A.G.; Gallinari, G.C.F.; Costa, E.A.; Guedes, M.I.M.C.; Lobato, Z.I.P. Short communication: Parapoxvirus and Orthopoxvirus coinfection in milk of naturally infected cows. J. Dairy Sci. 2018, 101, 7801–7803. [Google Scholar] [CrossRef]
- Franco-Luiz, A.P.; Fagundes-Pereira, A.; Costa, G.B.; Alves, P.A.; Oliveira, D.B.; Bonjardim, C.A.; Ferreira, P.C.; Trindade, G.S.; Panei, C.J.; Galosi, C.M.; et al. Spread of vaccinia virus to cattle herds, Argentina, 2011. Emerg. Infect. Dis. 2014, 20, 1576–1578. [Google Scholar] [CrossRef] [Green Version]
- Franco-Luiz, A.P.; Paim, L.M.; Oliveira, G.P.; Pereira, A.F.; de Almeida, G.M.; Figueiredo, L.B.; Tanus, A.; Trindade, G.S.; Ferreira, P.P.; Kroon, E.G.; et al. Horizontal study of vaccinia virus infections in an endemic area: Epidemiologic, phylogenetic and economic aspects. Arch. Virol. 2015, 160, 2703–2708. [Google Scholar]
- Franco-Luiz, A.P.; Oliveira, D.B.; Pereira, A.F.; Gasparini, M.C.; Bonjardim, C.A.; Ferreira, P.C.; Trindade, G.S.; Puentes, R.; Furtado, A.; Abrahão, J.S.; et al. Detection of Vaccinia Virus in Dairy Cattle Serum Samples from 2009, Uruguay. Emerg. Infect. Dis. 2016, 22, 2174–2177. [Google Scholar] [CrossRef] [Green Version]
- Styczynski, A.; Burgado, J.; Walteros, D.; Usme-Ciro, J.; Laiton, K.; Farias, A.P.; Nakazawa, Y.; Chapman, C.; Davidson, W.; Mauldin, M.; et al. Seroprevalence and Risk Factors Possibly Associated with Emerging Zoonotic Vaccinia Virus in a Farming Community, Colombia. Emerg. Infect. Dis. 2019, 25, 2169–2176. [Google Scholar] [CrossRef] [Green Version]
- Usme-Ciro, J.A.; Paredes, A.; Walteros, D.M.; Tolosa-Pérez, E.N.; Laiton-Donato, K.; Pinzón, M.D.; Petersen, B.W.; Gallardo-Romero, N.F.; Li, Y.; Wilkins, K.; et al. Detection and Molecular Characterization of Zoonotic Poxviruses Circulating in the Amazon Region of Colombia, 2014. Emerg. Infect. Dis. 2017, 23, 649–653. [Google Scholar] [CrossRef]
- Schatzmayr, H.G.; Costa, R.V.; Gonçalves, M.C.; D’Andrea, P.S.; Barth, O.M. Human and animal infections by vaccinia-like viruses in the state of Rio de Janeiro: A novel expanding zoonosis. Vaccine 2011, 29, 65–69. [Google Scholar] [CrossRef]
- Lima, M.T.; Oliveira, G.P.; Afonso, J.A.B.; Souto, R.J.C.; Mendonça, C.L.; Dantas, A.F.M.; Abrahão, J.S.; Kroon, E.G. An Update on the Known Host Range of the Brazilian Vaccinia Virus: An Outbreak in Buffalo Calves. Front. Microbiol. 2019, 9, 3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brum, M.C.S.; dos Anjos, B.L.; Nogueira, C.E.W.; Amaral, L.A.; Weiblen, R.; Flores, E.F. An outrbreak of orthopoxvirus-associated diseases in horses in southern Brazil. J. Vet. Diagn. Investig. 2010, 22, 143–147. [Google Scholar]
- Peres, M.G.; Bacchiegam, T.S.; Appolinário, C.M.; Vicente, A.F.; Mioni, M.S.R.; Ribeiro, B.L.D.; Fonseca, C.R.S.; Pelícia, V.C.; Ferreira, F.; Oliveira, G.P.; et al. Vaccinia Virus in Blood Samples of Humans, Domestic and Wild Mammals in Brazil. Viruses 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahão, J.S.; Guedes, M.I.; Trindade, G.S.; Fonseca, F.G.; Campos, R.K.; Mota, B.F.; Lobato, Z.I.; Silva-Fernandes, A.T.; Rodrigues, G.O.; Lima, L.S.; et al. One More Piece in the VACV Ecological Puzzle: Could Peridomestic Rodents Be the Link between Wildlife and Bovine Vaccinia Outbreaks in Brazil? PLoS ONE 2009, 4, e7428. [Google Scholar] [CrossRef] [Green Version]
- Campos, R.K.; Brum, M.C.; Nogueira, C.E.; Drumond, B.P.; Alves, P.A.; Siqueira-Lima, L.; Assis, F.L.; Trindade, G.S.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Assessing the variability of Brazilian Vaccinia virus isolates from a horse exanthematic lesion: Coinfection with distinct viruses. Arch. Virol. 2011, 156, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.G.; Bacchiega, T.S.; Appolinário, C.M.; Vicente, A.F.; Allendorf, S.D.; Antunes, J.M.; Moreira, S.A.; Legatti, E.; Fonseca, C.R.; Pituco, E.M.; et al. Serological study of vaccinia virus reservoirs in areas with and without official reports of outbreaks in cattle and humans in São Paulo, Brazil. Arch. Virol. 2013, 158, 2433–2441. [Google Scholar] [CrossRef] [Green Version]
- Abrahão, J.S.; de Souza Trindade, G.; Pereira-Oliveira, G.; de Oliveira Figueiredo, P.; Costa, G.; Moreira Franco-Luiz, A.P.; Lopes Assis, F.; Bretas de Oliveira, D.; Mattos Paim, L.R.; de Araújo Oliveira, C.E.; et al. Detection of Vaccinia virus during an outbreak of exanthemous oral lesions in Brazilian equids. Equine Vet. J. 2017, 49, 221–224. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Silva-Fernandes, A.T.; Lima, L.S.; Campos, R.K.; Guedes, M.I.M.C.; Cota, M.M.G.; Assis, F.L.; Borges, I.A.; Souza-Júnior, M.F.; Lobato, Z.I.P.; et al. Vaccinia virus infection in monkeys, Brazilian Amazon. Emerg. Infect. Dis. 2010, 16, 976–979. [Google Scholar] [CrossRef]
- Sant’ana, F.J.F.; Leal, A.A.; Rabelo, R.E.; Vulcani, V.A.S.; Ferreira Junior, J.A.; Cargnelutti, J.F.; Flores, E.F. Outbreaks of vesicular diseases caused by Vaccinia virus in dairy cattle from Goiás State, Brazil (2010–2012). Pesq. Vet. Bras. 2013, 33, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.B.; Assis, F.L.; Ferreira, P.C.; Bonjardim, C.A.; de Souza Trindade, G.; Kroon, E.G.; Abrahão, J.S. Group 1 Vaccinia virus Zoonotic Outbreak in Maranhão State, Brazil. Am. J. Trop. Med. Hyg. 2013, 89, 1142–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Assis, F.L.; Vinhote, W.M.; Barbosa, J.D.; de Oliveira, C.H.; de Oliveira, C.M.; Campos, K.F.; Silva, N.S.; de Souza Trindade, G.; Abrahão, J.S.; Kroon, E.G. Reemergence of Vaccinia Virus during Zoonotic Outbreak, Pará State, Brazil. Emerg. Infect. Dis. 2013, 19, 2017–2020. [Google Scholar] [CrossRef]
- Quixabeira-Santos, J.C.; Medaglia, M.L.; Pescador, C.A.; Damaso, C.R. Animal movement and establishment of vaccinia virus Cantagalo strain in Amazon biome, Brazil. Emerg. Infect. Dis. 2011, 4, 726–729. [Google Scholar] [CrossRef]
- De Souza Trindade, G.; Drumond, B.P.; Guedes, M.I.; Leite, J.A.; Mota, B.E. Zoonotic vaccinia virus infection in Brazil: Clinical description and implications for health professionals. J. Clin. Microbiol. 2007, 4, 1370–1372. [Google Scholar] [CrossRef] [Green Version]
- Costa, G.B.; Augusto, L.T.S.; Leite, J.A.; Ferreira, P.C.P.; Bonjardim, C.A.; Abrahão, J.S.; Kroon, E.G.; Moreno, E.C.; Trindade, G.S. Seroprevalence of Orthopoxvirus in rural Brazil: Insights into anti-OPV immunity status and its implications for emergent zoonotic OPV. Virol. J. 2016, 13, 121. [Google Scholar] [CrossRef] [Green Version]
- Resolução SES/MG Nº 6.532, de 05 de Dezembro de 2018. Available online: http://vigilancia.saude.mg.gov.br/index.php/download/resolucao-ses-mg-no-6-532-de-05-de-dezembro-de-2018/?wpdmdl=5990 (accessed on 12 November 2020). (In Portuguese)
- Resolução Estadual SES/GO nº 004-Doenças de Notificação Compulsória Estadual. Available online: https://www.saude.go.gov.br/images/imagens_migradas/upload/arquivos/2015-11/resolucao-estadual-no_-004-2013-doe2.pdf (accessed on 12 November 2020). (In Portuguese)
- Zanella, J.R.C. Zoonoses emergentes e reemergentes e sua importância para saúde e produção animal. Pesq. Agropec. Bras. 2016, 51, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Gibney, E. Brazilian science paralyzed by economic slump. Nature 2015, 526, 16–17. [Google Scholar] [CrossRef] [Green Version]
- Escobar, H. In Brazil, Researchers Struggle to Fend Off Deepening Budget Cuts. Available online: https://www.sciencemag.org/news/2017/10/brazil-researchers-struggle-fend-deepening-budget-cuts (accessed on 25 November 2020).
- Mota, B.E.F.; Trindade, G.S.; Diniz, T.C.; Silva-Nunes, M.; Braga, E.M.; Urbano-Ferreira, M.; Rodrigues, G.O.L.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Seroprevalence of orthopoxvirus in an Amazonian rural village, Acre, Brazil. Arch. Virol. 2010, 155, 1139–1144. [Google Scholar] [CrossRef]
- Figueiredo, P.O.; Silva-Fernandes, A.T.; Mota, B.E.F.; Costa, G.B.; Borges, I.A.; Ferreira, P.C.P.; Abrahão, J.S.; Braga, E.M.; Kroon, E.G.; Trindade, G.S. Evaluating anti-Orthopoxvirus antibodies in individuals from Brazilian rural areas prior to the bovine vaccinia era. Mem. Inst. Oswaldo Cruz. 2015, 110, 804–808. [Google Scholar] [CrossRef]
- Borges, I.A.; Reynolds, M.G.; McCollum, A.M.; Figueiredo, P.O.; Ambrosio, L.L.D.; Vieira, F.N.; Costa, G.B.; Matos, A.C.D.; de Andrade Almeida, V.M.; Ferreira, P.C.P.; et al. Serological Evidence of Orthopoxvirus Circulation Among Equids, Southeast Brazil. Front. Microbiolg. 2018, 9, 402. [Google Scholar] [CrossRef]
- Trindade, G.S.; Lobato, Z.I.P.; Drumond, B.P.; Leite, J.A.; Trigueiro, R.C.; Guedes, M.I.M.C.; da Fonseca, F.G.; dos Santos, J.R.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Short report: Isolation of two vaccinia virus strains from a single bovine vaccinia outbreak in rural area from Brazil: Implications on the emergence of zoonotic orthopoxviruses. Am. J. Trop. Med. Hyg. 2006, 75, 486–490. [Google Scholar] [CrossRef]
- Ferreira, J.M.S.; Drumond, B.P.; Guedes, M.I.M.C.; Pascoal-Xavier, M.A.; Almeida-Leite, C.M.; Arantes, R.M.E.; Mota, B.E.F.; Abrahão, J.S.; Alves, P.A.; Oliveira, F.M.; et al. Virulence in murine model shows the existence of two distinct populations of Brazilian Vaccinia virus strains. PLoS ONE 2008, 3, e3043. [Google Scholar] [CrossRef] [PubMed]
- Drumond, B.P.; Leite, J.A.; da Fonseca, F.G.; Bonjardim, C.A.; Ferreira, P.C.; Kroon, E.G. Brazilian Vaccinia virus strains are genetically divergent and differ from the Lister vaccine strain. Microbes Infect. 2008, 10, 185–197. [Google Scholar] [CrossRef]
- Oliveira, G.; Assis, F.; Almeida, G.; Albarnaz, J.; Lima, M.; Andrade, A.C.; Calixto, R.; Oliveira, C.; Diomedes Neto, J.; Trindade, G.S.; et al. From lesions to viral clones: Biological and molecular diversity amongst autochthonous Brazilian vaccinia virus. Viruses 2015, 7, 1218–1237. [Google Scholar] [CrossRef] [Green Version]
- Trindade, G.S.; Emerson, G.L.; Sammons, S.; Frace, M.; Govil, D.; Fernandes Mota, B.E.; Abrahão, J.S.; de Assis, F.L.; Olsen-Rasmussen, M.; Goldsmith, C.S.; et al. Serro 2 Virus Highlights the Fundamental Genomic and Biological Features of a Natural Vaccinia Virus Infecting Humans. Viruses 2016, 8, 328. [Google Scholar] [CrossRef] [Green Version]
- Assis, F.L.; Almeida, G.M.; Oliveira, D.B.; Franco-Luiz, A.P.; Campos, R.K.; Guedes, M.I.M.; Fonseca, F.G.; Trindade, G.S.; Drumond, B.P.; Kroon, E.G.; et al. Characterization of a New Vaccinia virus Isolate Reveals the C23L Gene as a Putative Genetic Marker for Autochthonous Group 1 Brazilian Vaccinia virus. PLoS ONE 2012, 7, e50413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calixto, R.; Oliveira, G.; Lima, M.; Andrade, A.C.; Trindade, G.S.; de Oliveira, D.B.; Kroon, E.G. A Model to Detect Autochthonous Group 1 and 2 Brazilian Vaccinia virus Coinfections: Development of a qPCR Tool for Diagnosis and Pathogenesis Studies. Viruses 2017, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Abrahão, J.S.; Silva-Fernandes, A.T.; Assis, F.L.; Guedes, M.I.; Drumond, B.P.; Leite, J.A.; Coelho, L.F.L.; Turrini, F.; Fonseca, F.G.; Lobato, Z.I.P. Human Vaccinia virus and Pseudocowpox virus co-infection: Clinical description and phylogenetic characterization. J. Clin. Virol. 2010, 48, 69–72. [Google Scholar] [CrossRef]
- Leite, J.A.; da Fonseca, F.G.; Trindade, G.S.; Abrahão, J.S.; Arantes, R.M.E.; Almeida-Leite, C.M.; Santos, J.R.; Guedes, M.I.M.C.; Ribeiro, B.M.; Bonjardim, C.A.; et al. A-type inclusion bodies: A factor influencing cowpox virus lesion pathogenesis. Arch. Virol. 2011, 156, 617–628. [Google Scholar] [CrossRef]
- Trindade, G.S.; Li, Y.; Olson, V.A.; Emerson, G.; Regnery, R.L.; Fonseca, F.G.; Kroon, E.G.; Damon, I. Real-time PCR assay to identify variants of Vaccinia virus: Implications for the diagnosis of bovine vaccinia in Brazil. J. Virol. Methods 2008, 152, 63–71. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Lima, L.S.; Assis, F.L.; Alves, P.A.; Silva-Fernandes, A.T.; Cota, M.M.G.; Ferreira, V.M.; Campos, R.K.; Mazur, C.; Lobato, Z.I.P.; et al. Nested-multiplex PCR detection of Orthopoxvirus and Parapoxvirus directly from exanthematic clinical samples. Virol. J. 2009, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Kroon, E.G.; Santos Abrahão, J.; de Souza Trindade, G.; Pereira Oliveira, G.; Moreira Franco Luiz, A.P.; Barbosa Costa, G.; Teixeira Lima, M.; Silva Calixto, R.; de Oliveira, D.B.; Drumond, B.P. Natural Vaccinia Virus Infection: Diagnosis, Isolation, and Characterization. Curr. Protoc. Microbiol. 2016, 42, 1–43. [Google Scholar]
- Medaglia, M.L.G.; Moussatché, N.; Nitsche, A.; Dabrowski, P.W.; Li, Y.; Damon, I.K.; Lucas, C.G.O.; Arruda, L.B.; Damaso, C.R. Genomic Analysis, Phenotype, and Virulence of the Historical Brazilian Smallpox Vaccine Strain IOC: Implications for the Origins and Evolutionary Relationships of Vaccinia Virus. J. Virol. 2015, 89, 11909–11925. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.R.V.; Luques, M.N.; Damaso, C.R. Genomic diversity of vaccinia virus strain Cantagalo isolated in southeastern Brazil during the early years of the outbreak, 1999–2006. Mem. Inst. Oswaldo Cruz. 2020, 115, e200521. [Google Scholar] [CrossRef]
- Miranda, J.B.; Borges, I.A.; Campos, S.P.S.; Vieira, F.N.; de Ázara, T.M.F.; Marques, F.A.; Costa, G.B.; Luiz, A.P.M.F.; Oliveira, J.S.; Ferreira, P.C.P.; et al. Serologic and Molecular Evidence of Vaccinia Virus Circulation among Small Mammals Serologic and Molecular Evidence of Vaccinia Virus Circulation among Small Mammals from Different Biomes, Brazil. Emerg. Infect. Dis. 2017, 23, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.B.; Miranda, J.B.; Almeida, G.G.; de Oliveira, J.S.; Pinheiro, M.S.; Gonçalves, S.A.; Pimenta dos Reis, J.K.; Gonçalves, R.; Ferreira, P.C.; Bonjardim, C.A.; et al. Detection of Vaccinia virus in urban domestic cats, Brazil. Emerg. Infect. Dis. 2017, 23, 360–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, G.B.; Ribeiro de Almeida, L.; Cerqueira, A.G.R.; Mesquita, W.U.; Silva de Oliveira, J.; Miranda, J.B.; Saraiva-Silva, A.T.; Abrahão, J.S.; Drumond, B.P.; Kroon, E.G.; et al. Vaccinia virus among Domestic Dogs and Wild Coatis, Brazil, 2013–2015. Emerg. Infect. Dis. 2018, 24, 2338–2342. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.M.S.; Abrahão, J.S.; Drumond, B.P.; Oliveira, F.M.; Alves, P.A.; Pascoal-Xavier, M.A.; Lobato, Z.I.P.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Vaccinia virus: Shedding and horizontal transmission in a murine model. J. Gen. Virol. 2008, 89, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M.I.; Rehfeld, I.S.; de Oliveira, T.M.; Assis, F.L.; Matos, A.C.; Abrahão, J.S.; Kroon, E.G.; Lobato, Z.I.P. Detection of Vaccinia Virus in Blood and Faeces of Experimentally Infected Cows. Transbound. Emerg. Dis. 2012, 8, 552–555. [Google Scholar]
- Abrahão, J.S.; de Souza Trindade, G.; Ferreira, J.M.S.; Campos, R.K.; Bonjardim, C.A.; Peregrino Ferreira, P.C.; Kroon, E.G. Long-lasting stability of Vaccinia virus strains in murine feces: Implications for virus circulation and environmental maintenance. Arch. Virol. 2009, 154, 1551–1553. [Google Scholar] [CrossRef]
- Peres, M.G.; Bacchiega, T.S.; Appolinário, C.M.; Vicente, A.F.; Mioni, M.S.R.; Ribeiro, B.L.D.; Fonseca, C.R.S.; Pelícia, V.C.; Ferreira, F.; Abrahão, J.S.; et al. Vaccinia virus in Feces and Urine of Wild Rodents from São Paulo State, Brazil. Viruses 2018, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutra, L.A.; de Freitas Almeida, G.M.; Oliveira, G.P.; Abrahão, J.S.; Kroon, E.G.; Trindade, G.S. Molecular evidence of Orthopoxvirus DNA in capybara (Hydrochoerus hydrochaeris) stool samples. Arch. Virol. 2017, 162, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.M.A.P.; Borges, I.A.; Trindade, G.S.; Kroon, E.G.; Cruvinel, T.M.A.; Peres, M.G.; Megid, J. Exposure of free-ranging capybaras (Hydrochoerus hydrochaeris) to the vaccinia virus. Transbound. Emerg. Dis. 2020, 67, 481–485. [Google Scholar] [CrossRef]
- Martins da Costa, P.S.P.; Oliveira, J.S.; Domingos, I.J.D.S.; Silva, P.H.B.; Dutra, A.G.S.; Amaral, C.D.; Abrahão, J.S.; Richini Pereira, V.B.; Kroon, E.G.; Barbosa Costa, G.; et al. Circulation of vaccinia virus in southern and south-eastern wildlife, Brazil. Transbound. Emerg. Dis. 2020, 67, 1781–1785. [Google Scholar]
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human monkeypox—After 40 years, an unintended consequence of smallpox eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef]
- Nguyen, P.Y.; Ajisegiri, W.S.; Costantino, V.; Chughtai, A.A.; MacIntyre, C.R. Reemergence of Human Monkeypox and Declining Population Immunity in the Context of Urbanization, Nigeria, 2017–2020. Emerg. Infect. Dis. 2021, 27, 1007–1014. [Google Scholar] [CrossRef]
- Costa, G.B.; Borges, I.A.; Alves, P.A.; Miranda, J.B.; Luiz, A.P.M.F.; Ferreira, P.C.P.; Abrahão, J.S.; Moreno, E.C.; Kroon, E.G.; Trindade, G.S. Alternative Routes of Zoonotic Vaccinia Virus Transmission, Brazil. Emerg. Infect. Dis. 2015, 21, 2244–2246. [Google Scholar] [CrossRef]
- Laiton-Donato, K.; Ávila-Robayo, P.; Páez-Martinez, A.; Benjumea-Nieto, P.; Usme-Ciro, J.A.; Pinzón-Nariño, N.; Giraldo, I.; Torres-Castellanos, D.; Nakazawa, Y.; Patel, N.; et al. Progressive Vaccinia Acquired through Zoonotic Transmission in a Patient with HIV/AIDS, Colombia. Emerg Infect Dis. 2020, 26, 601–605. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Oliveira, T.M.L.; Campos, R.K.; Madureira, M.C.; Kroon, E.G.; Lobato, Z.I.P. Bovine Vaccinia Outbreaks: Detection and Isolation of Vaccinia Virus in Milk Samples. Foodborne. Pathog. Dis. 2009, 6, 1141–1146. [Google Scholar] [CrossRef]
- Oliveira, T.M.L.; Rehfeld, I.S.; Siqueira, J.M.F.; Abrahão, J.S.; Campos, R.K.; Santos, A.K.R.; Cerqueira, M.M.O.P.; Kroon, E.G.; Lobato, Z.I.P. Vaccinia virus is not inactivated after thermal treatment and cheese production using experimentally contaminated milk. Foodborne Pathog. Dis. 2010, 7, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, I.S.; Fraiha, A.L.S.; Matos, A.C.D.; Guedes, M.I.M.C.; Costa, E.A.; de Souza, M.R.; Cavalcante, L.F.L.; Lobato, Z.I.P. Short communication: Survival of Vaccinia virus in inoculated cheeses during 60-day ripening. J. Dairy Sci. 2017, 100, 7051–7054. [Google Scholar] [CrossRef]
- Rehfeld, I.S.; Guedes, M.I.M.C.; Fraiha, A.L.S.; Costa, A.G.; Matos, A.C.D.; Fiúza, A.T.L.; Lobato, Z.I.P. Vaccinia virus Transmission through Experimentally Contaminated Milk Using a Murine Model. PLoS ONE 2015, 10, e0127350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, T.M.L.; Guedes, M.I.M.C.; Rehfeld, I.S.; Matos, A.C.D.; Rivetti Júnior, A.V.; da Cunha, A.F.; Cerqueira, M.M.O.P.; Abrahão, J.S.; Lobato, Z.I.P. Vaccinia virus detection in dairy products made with milk from experimentally infected cows. Transbound. Emerg. Dis. 2017, 65, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Empresa Brasileira de Pesquisa Agropecuária-EMBRAPA. Anuário Leite. 2020. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1124722/anuario-leite-2020-leite-de-vacas-felizes (accessed on 9 September 2020). (In Portuguese).
- Instituto Brasileiro de Geografia e Estatística—IBGE. 2018. Available online: https://www.ibge.gov.br/ (accessed on 14 September 2020). (In Portuguese)
- Instituto do Patrimônio Histórico Artístico e Artesanal—IPHAN; Departamento de Patrimônio Imaterial—DPI. Dossiê Do Queijo Artesanal. Minas Gerais. 2008. Available online: http://portal.iphan.gov.br/uploads/publicacao/Dossie_Queijo_de_Minas_web.pdf (accessed on 10 September 2020). (In Portuguese)
- Kamimura, B.A.; Magnani, M.; Luciano, W.A.; Campagnollo, F.B.; Pimentel, T.C.; Alvarenga, V.O.; Sant’Ana, A.S. Brazilian artisanal cheeses: An overview of their characteristics, main types and regulatory aspects. Comp. Rev. Food Sci. Food Saf. 2019, 18, 1636–1657. [Google Scholar] [CrossRef] [Green Version]
- Abrahão, J.S.; Campos, R.K.; Trindade, G.S.; Guimarães da Fonseca, F.; Ferreira, P.C.; Kroon, E.G. Outbreak of Severe Zoonotic Vaccinia Virus Infection, Southeastern Brazil. Emerg. Infect. Dis. 2015, 21, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.D.L.; Horodyski, G.S.; Passador, J.L. Food souvenirs in the perception of the tourist: The case of the artisanal minas Serro cheese. Rev. Bras. Pesq. Tur. 2017, 11, 347–364. [Google Scholar]
- Silva, T.G.; Lima, M.S.; Castro, A.M.M.G.; Martins, M.S.N.; Castiglioni, V.C.; Fava, C.D.; Pituco, E.M. Bovine Vaccinia in dairy cattle and suspicion of vesicular disease on milkers in Brazil. Cienc. Rural. 2018, 48, e20170723. [Google Scholar] [CrossRef]
- De Oliveira, J.S.; Costa, G.B.; Luiz, A.P.M.F.; Leite, J.A.; Bonjardim, C.A.; Abrahão, J.S.; Drumond, B.P.; Kroon, E.G.; Trindade, G.S. Cross-sectional study involving healthcare professionals in a Vaccinia virus endemic area. Vaccine 2017, 35, 3281–3285. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
José da Silva Domingos, I.; Silva de Oliveira, J.; Lorene Soares Rocha, K.; Bretas de Oliveira, D.; Geessien Kroon, E.; Barbosa Costa, G.; de Souza Trindade, G. Twenty Years after Bovine Vaccinia in Brazil: Where We Are and Where Are We Going? Pathogens 2021, 10, 406. https://doi.org/10.3390/pathogens10040406
José da Silva Domingos I, Silva de Oliveira J, Lorene Soares Rocha K, Bretas de Oliveira D, Geessien Kroon E, Barbosa Costa G, de Souza Trindade G. Twenty Years after Bovine Vaccinia in Brazil: Where We Are and Where Are We Going? Pathogens. 2021; 10(4):406. https://doi.org/10.3390/pathogens10040406
Chicago/Turabian StyleJosé da Silva Domingos, Iago, Jaqueline Silva de Oliveira, Kamila Lorene Soares Rocha, Danilo Bretas de Oliveira, Erna Geessien Kroon, Galileu Barbosa Costa, and Giliane de Souza Trindade. 2021. "Twenty Years after Bovine Vaccinia in Brazil: Where We Are and Where Are We Going?" Pathogens 10, no. 4: 406. https://doi.org/10.3390/pathogens10040406






