Pathology of Urinary Bladder in Pearsonema spp. Infected Wildlife from Central Italy
Abstract
:1. Introduction
2. Results
2.1. Animals and Parasitology
2.2. Gross Pathology
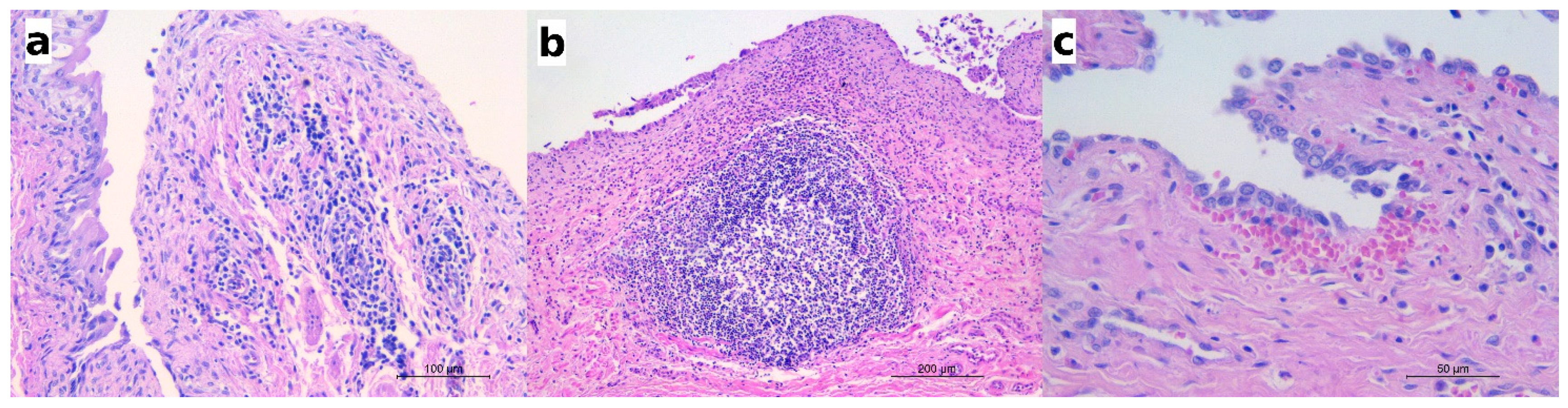
2.3. Histology
2.4. Association between Pearsonema sp. and Eosinophilic Cystitis
3. Discussion
4. Materials and Methods
4.1. Animals and Parasitology
4.2. Gross Pathology and Histology
4.3. Data Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basso, W.; Spänhauer, Z.; Arnold, S.; Deplazes, P. Capillaria plica (syn. Pearsonema plica) infection in a dog with chronic pollakiuria: Challenges in the diagnosis and treatment. Parasitol. Int. 2014, 63, 140–142. [Google Scholar] [CrossRef]
- Moravec, F. Proposal of a new systematic arrangement of nematodes of the family Capillariidae. Folia Parasitol. 1982, 29, 119–132. [Google Scholar]
- Beverley-Burton, M. The taxonomy of Capillaria spp. (Nematoda: Trichuroidea) in carnivorous mammals from Ontario, Canada. Syst. Parasitol. 1980, 1, 211–236. [Google Scholar] [CrossRef]
- Moravec, F.; Prokopic, J.; Shlikas, A.V. The biology of nematodes of the family Capillariidae Neveu-Lemaire, 1936. Folia Parasitol. 1987, 34, 39–56. [Google Scholar]
- Bowman, D.D.; Hendrix, C.M.; Lindsay, D.S.; Barr, S.C.; Bowman, D.D. (Eds.) Feline Clinical Parasitology; Iowa State University Press: Ames, IA, USA, 2002; ISBN 9780470376805. [Google Scholar]
- Krone, O.; Guminsky, O.; Meinig, H.; Herrmann, M.; Trinzen, M.; Wibbelt, G. Endoparasite spectrum of wild cats (Felis silvestris Schreber, 1777) and domestic cats (Felis catus L.) from the Eifel, Pfalz region and Saarland, Germany. Eur. J. Wildl. Res. 2008, 54, 95–100. [Google Scholar] [CrossRef]
- Bagrade, G.; Kirjuina, M.; Vismanis, K.; Ozoli, J. Helminth parasites of the wolf Canis lupus from Latvia. J. Helminthol. 2009, 83, 63–68. [Google Scholar] [CrossRef]
- Bružinskaite-Schmidhalter, R.; Sarkunas, M.; Malakauskas, A.; Mathis, A.; Torgerson, P.R.; Deplazes, P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology 2012, 139, 120–127. [Google Scholar] [CrossRef]
- Franssen, F.; Nijsse, R.; Mulder, J.; Cremers, H.; Dam, C.; Takumi, K.; Van Der Giessen, J. Increase in number of helminth species from Dutch red foxes over a 35-year period. Parasites Vectors 2014, 7, 166. [Google Scholar] [CrossRef] [Green Version]
- Takács, A.; Szabó, L.; Juhász, L.; Takács, A.; Lanszki, J.; Takács, P.; Heltai, M. Data on the parasitological status of golden jackal (Canis aureus L., 1758) in Hungary. Acta Vet. Hung. 2014, 62, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Varodi, E.I.; Malega, A.M.; Kuzmin, Y.I.; Kornyushin, V.V. Helminths of Wild Predatory Mammals of Ukraine. Nematodes. Vestn. Zool. 2017, 51, 187–202. [Google Scholar] [CrossRef] [Green Version]
- Davidson, R.K.; Gjerde, B.; Vikøren, T.; Lillehaug, A.; Handeland, K. Prevalence of Trichinella larvae and extra-intestinal nematodes in Norwegian red foxes (Vulpes vulpes). Vet. Parasitol. 2006, 136, 307–316. [Google Scholar] [CrossRef]
- Saeed, I.; Maddox-Hyttel, C.; Monrad, J.; Kapel, C.M.O. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006, 139, 168–179. [Google Scholar] [CrossRef]
- Bork-Mimm, S.; Rinder, H. High prevalence of Capillaria plica infections in red foxes (Vulpes vulpes) in Southern Germany. Parasitol. Res. 2011, 108, 1063–1067. [Google Scholar] [CrossRef]
- Alić, A.; Hodžić, A.; Kadrić, M.; Beširović, H.; Prašović, S. Pearsonema plica (Capillaria plica) infection and associated urinary bladder pathology in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasitol. Res. 2015, 114, 1933–1938. [Google Scholar] [CrossRef]
- Magi, M.; Guardone, L.; Prati, M.C.; Mignone, W.; Macchioni, F. Extraintestinal nematodes of the red fox Vulpes vulpes in north-west Italy. J. Helminthol. 2015, 89, 506–511. [Google Scholar] [CrossRef]
- Aleksić, J.; Stepanović, P.; Dimitrijević, S.; Gajić, B.; Bogunović, D.; Davidov, I.; Aleksić-Agelidis, A.; Ilić, T. Capillaria plica in Red Foxes (Vulpes vulpes) from Serbia: Epidemiology and Diagnostic Approaches to Urinary Capillariosis in Domestic Carnivores. Acta Parasitol. 2020, 65, 954–962. [Google Scholar] [CrossRef]
- Scott, D.M.; Berg, M.J.; Tolhurst, B.A.; Chauvenet, A.L.M.; Smith, G.C.; Neaves, K.; Lochhead, J.; Baker, P.J. Changes in the distribution of red foxes (Vulpes vulpes) in urban areas in Great Britain: Findings and limitations of a media-driven nationwide survey. PLoS ONE 2014, 9, e99059. [Google Scholar] [CrossRef] [Green Version]
- Mackenstedt, U.; Jenkins, D.; Romig, T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015, 4, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Pelligra, S.; Guardone, L.; Riggio, F.; Parisi, F.; Maestrini, M.; Mariacher, A.; Perrucci, S. Pearsonema spp. (Family Capillariidae, Order Enoplida) Infection in Domestic Carnivores in Central–Northern Italy and in a Red Fox Population from Central Italy. Animals 2020, 10, 1607. [Google Scholar] [CrossRef]
- Bédard, C.; Desnoyers, M.; Lavallée, M.C.; Poirier, D. Capillaria in the bladder of an adult cat. Can. Vet. J. 2002, 43, 973–974. [Google Scholar]
- Mariacher, A.; Millanta, F.; Guidi, G.; Perrucci, S. Urinary capillariosis in six dogs from Italy. Open Vet. J. 2016, 6, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Messina, N.; Ariti, G.; Riggio, F.; Perrucci, S. Symptomatic Capillaria plica infection in a young European cat. J. Feline Med. Surg. 2011, 13, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Waddell, A.H. Further observations on Capillaria feliscati Infections in the cat. Aust. Vet. J. 1968, 44, 33–34. [Google Scholar] [CrossRef]
- Senior, D.F.; Solomon, G.B.; Goldschmidt, M.H.; Joyce, T.; Bovee, K.C. Capillaria plica infection in dogs. J. Am. Vet. Med. Assoc. 1980, 176, 901–905. [Google Scholar]
- Wilson-Hanson, S.; Prescott, C.W. Capillaria in the bladder of the domestic cat. Aust. Vet. J. 1982, 59, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Hamir, A.N.; Rupprechtt, C.E. A Retrospective Histopathological Survey of Capillariasis in Raccoons from the Eastern United States. J. Parasitol. 1998, 84, 180. [Google Scholar] [CrossRef]
- Mariacher, A.; Eleni, C.; Fico, R.; Perrucci, S. Urinary capillariosis in a free-ranging Marsican brown bear (Ursus arctos marsicanus). Int. J. Parasitol. Parasites Wildl. 2018, 7, 429–431. [Google Scholar] [CrossRef]
- Fernández-Aguilar, X.; Mattsson, R.; Meijer, T.; Osterman-Lind, E.; Gavier-Widén, D. Pearsonema (syn Capillaria) plica associated cystitis in a Fennoscandian arctic fox (Vulpes lagopus): A case report. Acta Vet. Scand. 2010, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Mariacher, A.; Eleni, C.; Fico, R.; Ciarrocca, E.; Perrucci, S. Research Note. Pearsonema plica and Eucoleus böhmi infections and associated lesions in wolves (Canis lupus) from Italy. Helminthologia 2015, 52, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Shimalov, V.V.; Penkevich, V.A. Helminth Fauna of the wolf (Canis lupus linnaeus, 1758) in Belorussian Polesie. Parazitologiya 2012, 46, 118–126. [Google Scholar] [CrossRef]
- Segovia, J.M.; Torres, J.; Miquel, J.; Llaneza, L.; Feliu, C. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001, 75, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Milazzo, C.; Foronda, P.; Casanova, J.C. New data on helminths of stone marten, Martes foina (Carnivora, Mustelidae), in Italy. Helminthologia 2004, 41, 59–61. [Google Scholar]
- Visser, M.; Messner, C.; Rehbein, S. Massive infestation with fur mites (Lynxacarus mustelae) of a stone marten (Martes foina) from Tyrol. Wien. Klin. Wochenschr. 2011, 123, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.H.; Nielsen, S.T.; Larsen, G.; Holm, E.; Chriél, M. Prevalence of Capillaria plica in Danish wild carnivores. Int. J. Parasitol. Parasites Wildl. 2018, 7, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Segovia, J.M.; Torres, J.; Miquel, J.; Sospedra, E.; Guerrero, R.; Feliu, C. Analysis of helminth communities of the pine marten, Martes martes, in Spain: Mainland and insular data. Acta Parasitol. 2007, 52, 156–164. [Google Scholar] [CrossRef]
- Torres, J.; Miquel, J.; Fournier, P.; Fournier-Chambrillon, C.; Liberge, M.; Fons, R.; Feliu, C. Helminth communities of the autochthonous mustelids Mustela lutreola and M. putorius and the introduced Mustela vison in south-western France. J. Helminthol. 2008, 82, 349–355. [Google Scholar] [CrossRef]
- Torres, J.; Miquel, J.; Motjé, M. Helminth parasites of the eurasian badger (Meles meles L.) in Spain: A biogeographic approach. Parasitol. Res. 2001, 87, 259–263. [Google Scholar] [CrossRef]
- Takács, A.; Szemethy, L.; Takács, A.A.; Takács, T.P.; Heltai, M. Data on the parasitological state of the Eurasian badger (Meles meles) in Hungary. Magy. Allatorv. Lapja 2012, 134, 106–110. [Google Scholar]
- Seville, R.S.; Addison, E.M. Nongastrointestinal helminths in marten (Martes americana) from Ontario, Canada. J. Wildl. Dis. 1995, 31, 529–533. [Google Scholar] [CrossRef]
- Davidson, W.R.; Appel, M.J.; Doster, G.L.; Baker, O.E.; Brown, J.F. Diseases and parasites of red foxes, gray foxes, and coyotes from commercial sources selling to fox-chasing enclosures. J. Wildl. Dis. 1992, 28, 581–589. [Google Scholar] [CrossRef]
- Sréter, T.; Széll, Z.; Marucci, G.; Pozio, E.; Varga, I. Extraintestinal nematode infections of red foxes (Vulpes vulpes) in Hungary. Vet. Parasitol. 2003, 115, 329–334. [Google Scholar] [CrossRef]
- Del-Angel-Caraza, J.; Quijano-Hernández, I.A.; Soriano-Vargas, E.; Barbosa-Mireles, M.A.; Martínez-Castañeda, J.S. Urinary bladder worm (Pearsonema sp.) infection in domestic dogs and cats in Mexico at a high altitude. Parasitol. Res. 2018, 117, 1979–1983. [Google Scholar] [CrossRef] [PubMed]
- Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2017. Available online: https://www.r-project.org/ (accessed on 15 January 2021).




| Species | Pearsonema sp. Infection | ||
|---|---|---|---|
| No. Positive/Examined | % | 95% CI | |
| Red fox (Vulpes vulpes) | 21/28 | 75.00 | 58.96–91.04 |
| Wolf (Canis lupus) | 16/29 | 55.17 | 37.07–73.27 |
| Total Canidae = | 37/57 | 64.91 | 52.52–77.30 |
| Beech marten (Martes foina) | 1/3 | 33.33 | 0–86.68 |
| European badger (Meles meles) | 1/10 | 10.00 | 0–28.59 |
| Pine marten (Martes martes) | 0/2 | 0 | 0–0 |
| Total Mustelidae = | 2/15 | 13.33 | 0–30.54 |
| Total Carnivores = | 39/72 | 54.17 | 42.66–65.68 |
| Species | Pearsonema sp. Infection | Eosinophilic Cystitis | Other Histological Features | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Lymphocytes | Plasma Cells | Lymphoid Nodules | |||
| Canidae | Red fox (Vulpes vulpes) | Positive | 10 | 10 | 1 | 4 | 5 | ||
| Negative | 6 | 1 | |||||||
| Total | 16 | 11 | 1 | 4 | 5 | ||||
| Wolf (Canis lupus) | Positive | 1 | 8 | 5 | 2 | 9 | 8 | 5 | |
| Negative | 12 | 1 | |||||||
| Total | 13 | 9 | 5 | 2 | 9 | 8 | 5 | ||
| Total Canidae | Positive | 11 | 18 | 6 | 2 | 13 | 13 | 5 | |
| Negative | 18 | 2 | |||||||
| Total | 29 | 20 | 6 | 2 | 13 | 13 | 5 | ||
| Mustelidae | Beech marten (Martes foina) | Positive | 1 | ||||||
| Negative | 2 | ||||||||
| Total | 2 | 1 | |||||||
| European badger (Meles meles) | Positive | 1 | 1 | ||||||
| Negative | 6 | 3 | 3 | 2 | |||||
| Total | 6 | 4 | 4 | 2 | |||||
| Pine marten (Martes martes) | Positive | ||||||||
| Negative | 1 | 1 | 1 | 1 | |||||
| Total | 1 | 1 | 1 | 1 | |||||
| Total Mustelidae | Positive | 2 | 1 | ||||||
| Negative | 9 | 4 | 4 | 3 | |||||
| Total | 9 | 6 | 5 | 3 | |||||
| Total Carnivores | Positive | 11 | 20 | 6 | 2 | 14 | 13 | 5 | |
| Negative | 27 | 6 | 4 | 3 | |||||
| Total | 38 | 26 | 6 | 2 | 18 | 16 | 5 | ||
| Species | Number of C+/P+ Animals 1 | % | Cystitis Score (Mean ± SD) |
|---|---|---|---|
| Red fox (Vulpes vulpes) | 11/21 | 52.38 | 0.57 ± 0.60 |
| Wolf (Canis lupus) | 15/16 | 93.75 | 1.50 ± 0.82 |
| Total Canidae = | 26/37 | 70.27 | 0.97 ± 0.83 |
| Beech marten (Martes foina) | 1/1 | 100.00 | 1.0 ± 0.00 |
| European badger (Meles meles) | 1/1 | 100.00 | 1.0 ± 0.00 |
| Pine marten (Martes martes) | 0 | 0.00 | N.A. 2 |
| Total Mustelidae = | 2/2 | 100.00 | 1.0 ± 0.00 |
| Total Carnivores = | 28/39 | 71.74 | 0.93 ± 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleni, C.; Mariacher, A.; Grifoni, G.; Cardini, E.; Tonon, S.; Lombardo, A.; Barone, A.; Fichi, G. Pathology of Urinary Bladder in Pearsonema spp. Infected Wildlife from Central Italy. Pathogens 2021, 10, 474. https://doi.org/10.3390/pathogens10040474
Eleni C, Mariacher A, Grifoni G, Cardini E, Tonon S, Lombardo A, Barone A, Fichi G. Pathology of Urinary Bladder in Pearsonema spp. Infected Wildlife from Central Italy. Pathogens. 2021; 10(4):474. https://doi.org/10.3390/pathogens10040474
Chicago/Turabian StyleEleni, Claudia, Alessia Mariacher, Goffredo Grifoni, Elena Cardini, Sara Tonon, Andrea Lombardo, Antonino Barone, and Gianluca Fichi. 2021. "Pathology of Urinary Bladder in Pearsonema spp. Infected Wildlife from Central Italy" Pathogens 10, no. 4: 474. https://doi.org/10.3390/pathogens10040474
APA StyleEleni, C., Mariacher, A., Grifoni, G., Cardini, E., Tonon, S., Lombardo, A., Barone, A., & Fichi, G. (2021). Pathology of Urinary Bladder in Pearsonema spp. Infected Wildlife from Central Italy. Pathogens, 10(4), 474. https://doi.org/10.3390/pathogens10040474







