Continuous Real-Time Motility Analysis of Acanthamoeba Reveals Sustained Movement in Absence of Nutrients
Abstract
:1. Introduction
2. Results
2.1. Total Distance
2.2. Max Distance
2.3. Displacement
2.4. Speed
3. Discussion
4. Materials and Methods
4.1. Acanthamoeba Trophozoite Culturing
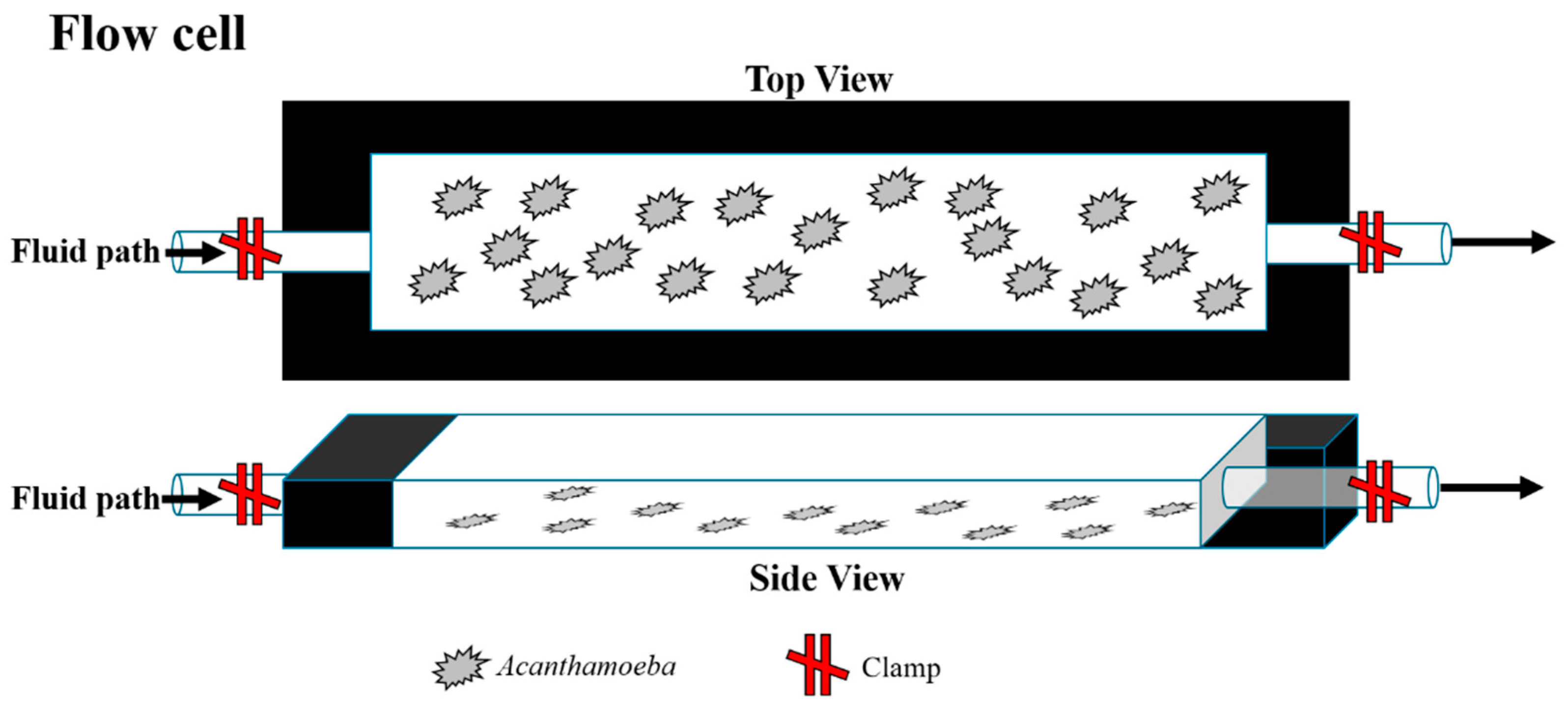
4.2. Acanthamoeba Flow Cell Suspension
4.3. Acanthamoeba Track Analysis
4.4. Tracking Algorithms
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szentmary, N.; Daas, L.; Shi, L.; Laurik, K.L.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba keratitis—Clinical signs, differential diagnosis and treatment. J. Curr. Ophthalmol. 2019, 31, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Carnt, N.; Hoffman, J.J.; Verma, S.; Hau, S.; Radford, C.F.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis: Confirmation of the uk outbreak and a prospective case-control study iden-tifying contributing risk factors. Br. J. Ophthalmol. 2018, 102, 1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verani, J.R.; Lorick, S.A.; Yoder, J.S.; Beach, M.J.; Braden, C.R.; Roberts, J.M.; Conover, C.S.; Chen, S.; McConnell, K.A.; Chang, D.C.; et al. National outbreak of acanthamoeba keratitis associated with use of a contact lens solution, united states. Emerg. Infect. Dis. 2009, 15, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Willcox, M.D.; Stapleton, F. In vivo efficacy of silver-impregnated barrel contact lens storage cases. Contact Lens Anterior Eye 2021, 44, 101357. [Google Scholar] [CrossRef] [PubMed]
- Tu, E.Y.; Joslin, C.E. Recent outbreaks of atypical contact lens-related keratitis: What have we learned? Am. J. Ophthalmol. 2010, 150, 602–608 e602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 14729:2001/A1. Ophthalmic Optics–Contact Lens Care Products–Microbiological Requirements and Test Methods for Products and Regimens for Hygienic Management of Contact Lenses; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- ASC Z80, Parent Committee Meeting, February 27. Available online: https://www.thevisioncouncil.org/sites/default/files/ASCZ80_ParentCommitteeMinutes_February_27_2018_FINALMar19-2018.pdf (accessed on 14 June 2021).
- Piltti, K.M.; Cummings, B.J.; Carta, K.; Manughian-Peter, A.; Worne, C.L.; Singh, K.; Ong, D.; Maksymyuk, Y.; Khine, M.; Anderson, A.J. Live-cell time-lapse imaging and single-cell tracking of in vitro cultured neural stem cells—Tools for analyzing dynamics of cell cycle, migration, and lineage selection. Methods 2018, 133, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.-M.; Medyukhina, A.; Belyaev, I.; Al-Zaben, N.; Figge, M.T. Untangling cell tracks: Quantifying cell migration by time lapse image data analysis. Cytom. Part A 2017, 93, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Paul, C.D.; Stahl, R.; Vanmeerbeeck, G.; Reumers, V.; Liu, C.; Konstantopoulos, K.; Lagae, L. Time-lapse lens-free imaging of cell migration in diverse physical microenvironments. Lab Chip 2016, 16, 3304–3316. [Google Scholar] [CrossRef]
- Reverey, J.F.; Jeon, J.-H.; Bao, H.; Leippe, M.; Metzler, R.; Selhuber-Unkel, C. Superdiffusion dominates intracellular particle motion in the supercrowded cytoplasm of pathogenic Acanthamoeba castellanii. Sci. Rep. 2015, 5, srep11690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukaya, S.; Aoki, K.; Kobayashi, M.; Takemura, M. Kinetic Analysis of the Motility of Giant Virus-Infected Amoebae Using Phase-Contrast Microscopic Images. Front. Microbiol. 2020, 10, 3014. [Google Scholar] [CrossRef]
- Rudell, J.; Gao, J.; Sun, Y.; Sun, Y.; Chodosh, J.; Schwab, I.; Zhao, M. Acanthamoeba Migration in an Electric Field. Investig. Opthalmol. Vis. Sci. 2013, 54, 4225–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campolo, A.; Shannon, P.; Crary, M. Evaluating Alternate Methods of Determining the Antimicrobial Efficacy of Contact Lens Care Products against Acanthamoeba Trophozoites. Pathogens 2021, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Crary, M.; Walters, R.; Shannon, P.; Gabriel, M. Variables Affecting the Recovery of Acanthamoeba Trophozoites. Pathogens 2021, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, B.A.; Quist, T.S.; Salinas, J.L.; Greiner, M.A. Notes from the field: Acanthamoeba keratitis cases—Iowa, 2002–2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 448–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radford, C.F.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis in England and Wales: Incidence, outcome, and risk factors. Br. J. Ophthalmol. 2002, 86, 536–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, D.F.; Kilvington, S.; Easty, D.L. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br. J. Ophthalmol. 1990, 74, 133–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuburich, N.A.; Adhikari, N.; Hadwiger, J.A. Acanthamoeba and Dictyostelium Use Different Foraging Strategies. Protist 2016, 167, 511–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnalich-Montiel, F.; Lumbreras-Fernández, B.; Martín-Navarro, C.M.; Valladares, B.; Lopez-Velez, R.; Morcillo-Laiz, R.; Lorenzo-Morales, J. Influence of Acanthamoeba Genotype on Clinical Course and Outcomes for Patients with Acanthamoeba Keratitis in Spain. J. Clin. Microbiol. 2014, 52, 1213–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledee, D.R.; Iovieno, A.; Miller, D.; Mandal, N.; Diaz, M.; Fell, J.; Fini, M.E.; Alfonso, E.C. Molecular Identification of T4 and T5 Genotypes in Isolates from Acanthamoeba Keratitis Patients. J. Clin. Microbiol. 2009, 47, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maghsood, A.H.; Sissons, J.; Rezaian, M.; Nolder, D.; Warhurst, D.; Khan, N.A. Acanthamoeba genotype T4 from the UK and Iran and isolation of the T2 genotype from clinical isolates. J. Med. Microbiol. 2005, 54, 755–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- TrackMate Algorithims. Available online: https://imagej.net/plugins/trackmate/algorithms (accessed on 18 June 2021).








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campolo, A.; Harris, V.; Walters, R.; Miller, E.; Patterson, B.; Crary, M. Continuous Real-Time Motility Analysis of Acanthamoeba Reveals Sustained Movement in Absence of Nutrients. Pathogens 2021, 10, 995. https://doi.org/10.3390/pathogens10080995
Campolo A, Harris V, Walters R, Miller E, Patterson B, Crary M. Continuous Real-Time Motility Analysis of Acanthamoeba Reveals Sustained Movement in Absence of Nutrients. Pathogens. 2021; 10(8):995. https://doi.org/10.3390/pathogens10080995
Chicago/Turabian StyleCampolo, Allison, Valerie Harris, Rhonda Walters, Elise Miller, Brian Patterson, and Monica Crary. 2021. "Continuous Real-Time Motility Analysis of Acanthamoeba Reveals Sustained Movement in Absence of Nutrients" Pathogens 10, no. 8: 995. https://doi.org/10.3390/pathogens10080995






