Rhodococcus equi—Occurrence in Goats and Clinical Case Report
Abstract
1. Introduction
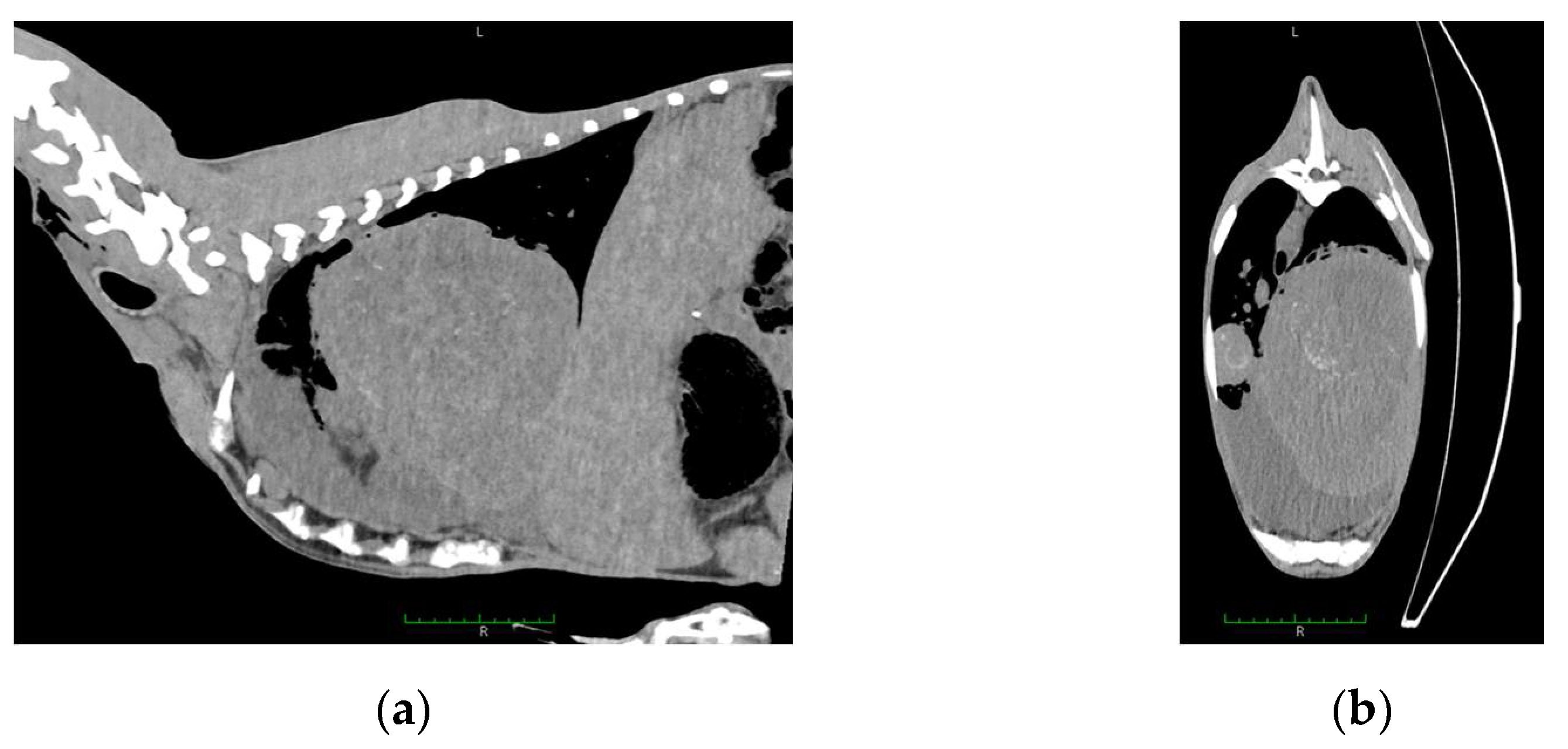
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giguere, S.; Cohen, N.D.; Chaffin, M.K.; Hines, S.A.; Hondalus, M.K.; Prescott, J.F.; Slovis, N.M. Rhodococcus equi: Clinical Manifestations, Virulence, and Immunity. J. Vet. Intern. Med. 2011, 25, 1221–1230. [Google Scholar] [CrossRef]
- Witkowski, L. Treatment and prevention of Rhodococcus equi in foals. Vet. Rec. 2019, 185, 16–18. [Google Scholar] [CrossRef]
- Rakowska, A.; Cywinska, A.; Witkowski, L. Current Trends in Understanding and Managing Equine Rhodococcosis. Animals 2020, 10, 1910. [Google Scholar] [CrossRef]
- Cohen, N.D. Rhodococcus equi foal pneumonia. Vet. Clin. N. Am. Equine Pract. 2014, 30, 609–622. [Google Scholar] [CrossRef]
- Muscatello, G. Rhodococcus equi pneumonia in the foal—Part 2: Diagnostics, treatment and disease management. Vet. J. 2012, 192, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, L.; Rzewuska, M.; Takai, S.; Chrobak-Chmiel, D.; Kizerwetter-Swida, M.; Feret, M.; Gawrys, M.; Witkowski, M.; Kita, J. Molecular characterization of Rhodococcus equi isolates from horses in Poland: pVapA characteristics and plasmid new variant, 85-kb type V. BMC Vet. Res. 2017, 13, 35. [Google Scholar] [CrossRef]
- Vazquez-Boland, J.A.; Giguere, S.; Hapeshi, A.; MacArthur, I.; Anastasi, E.; Valero-Rello, A. Rhodococcus equi: The many facets of a pathogenic actinomycete. Vet. Microbiol. 2013, 167, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Yamshchikov, A.V.; Schuetz, A.; Lyon, G.M. Rhodococcus equi infection. Lancet Infect. Dis. 2010, 10, 350–359. [Google Scholar] [CrossRef]
- Bryan, L.K.; Clark, S.D.; Diaz-Delgado, J.; Lawhon, S.D.; Edwards, J.F. Rhodococcus equi Infections in Dogs. Vet. Pathol. 2017, 54, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Kinne, J.; Madarame, H.; Takai, S.; Jose, S.; Wernery, U. Disseminated Rhodococcus equi infection in dromedary camels (Camelus dromedarius). Vet. Microbiol. 2011, 149, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Lechinski de Paula, C.; Silveira Silva, R.O.; Tavanelli Hernandes, R.; de Nardi Junior, G.; Babboni, S.D.; Trevizan Guerra, S.; Paganini Listoni, F.J.; Giuffrida, R.; Takai, S.; Sasaki, Y.; et al. First Microbiological and Molecular Identification of Rhodococcus equi in Feces of Nondiarrheic Cats. BioMed Res. Int. 2019, 2019, 4278598. [Google Scholar] [CrossRef]
- Lohr, C.V.; O’Neill, T.W.; Daw, D.N.; Pitel, M.O.; Schlipf, J.W. Pyogranulomatous enteritis and mesenteric lymphadenitis in an adult llama caused by Rhodococcus equi carrying virulence-associated protein A gene. J. Vet. Diagn. Investig. 2019, 31, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, R.; Moki, H.; Hayashi, K.; Ooniwa, K.; Tokuyama, K.; Kakuda, T.; Yoshioka, K.; Takai, S. A case report on disseminated Rhodococcus equi infection in a Japanese black heifer. J. Vet. Med. Sci. 2018, 80, 819–822. [Google Scholar] [CrossRef]
- Selim, S.A.; Mousa, W.M.; Mohamed, K.F.; Moussa, I.M. Synergistic Haemolytic Activity and Its Correlation to Phospholipase D Productivity by Corynebacteruim Pseudotuberculosis Egyptian Isolates from Sheep and Buffaloes. Braz. J. Microbiol. 2012, 43, 552–559. [Google Scholar] [CrossRef][Green Version]
- Stranahan, L.W.; Plumlee, Q.D.; Lawhon, S.D.; Cohen, N.D.; Bryan, L.K. Rhodococcus equi Infections in Goats: Characterization of Virulence Plasmids. Vet. Pathol. 2018, 55, 273–276. [Google Scholar] [CrossRef]
- Witkowski, L.; Rzewuska, M.; Takai, S.; Kizerwetter-Swida, M.; Kita, J. Molecular epidemiology of Rhodococcus equi in slaughtered swine, cattle and horses in Poland. BMC Microbiol. 2016, 16, 98. [Google Scholar] [CrossRef]
- Haanen, G.A.Y.; Lim, C.K.; Baird, A.N.; Sola, M.F.; Lenz, S.D. Disseminated Rhodococcus equi in an Anglo-Nubian goat. Vet. Radiol. Ultrasound 2020, 61, E22–E25. [Google Scholar] [CrossRef]
- Schlemmer, S.N.; Fratzke, A.P.; Gibbons, P.; Porter, B.F.; Mansell, J.; Ploeg, R.J.; Rodrigues Hoffmann, A.; Older, C.E.; Clark, S.D. Histoplasmosis and multicentric lymphoma in a Nubian goat. J. Vet. Diagn. Investig. 2019, 31, 770–773. [Google Scholar] [CrossRef]
- de Morais, A.B.C.; Bolanos, C.A.D.; Alves, A.C.; Ikuta, C.Y.; Lara, G.H.B.; Heinemann, M.B.; Giuffrida, R.; Listoni, F.P.; Mioni, M.D.R.; Motta, R.G.; et al. Identification of Mycobacterium species and Rhodococcus equi in peccary lymph nodes. Trop. Anim. Health Prod. 2018, 50, 1319–1326. [Google Scholar] [CrossRef]
- Saied, A.A.; Bryan, L.K.; Bolin, D.C. Ulcerative, granulomatous glossitis and enteritis caused by Rhodococcus equi in a heifer. J. Vet. Diagn. Investig. 2019, 31, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, M.J.; Links, I.J.; Morton, A.G. Rhodococcus equi Infection in Goats. Aust. Vet. J. 1988, 65, 331–332. [Google Scholar] [CrossRef]
- Rodriguez, J.L.; Acosta, B.; Navarro, R.; Gutierrez, C. Rhodococcus equi infection in goat: Apropos of two cases. J. Appl. Anim. Res. 2000, 18, 149–151. [Google Scholar] [CrossRef]
- Suzuki, Y.; Takahashi, K.; Takase, F.; Sawada, N.; Nakao, S.; Toda, A.; Sasaki, Y.; Kakuda, T.; Takai, S. Serological epidemiological surveillance for vapN-harboring Rhodococcus equi infection in goats. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101540. [Google Scholar] [CrossRef]
- Jeckel, S.; Holmes, P.; King, S.; Whatmore, A.M.; Kirkwood, I. Disseminated Rhodococcus equi infection in goats in the UK. Vet. Rec. 2011, 169, 56. [Google Scholar] [CrossRef]
- Kabongo, P.N.; Njiro, S.M.; Van Strijp, M.F.; Putterill, J.F. Caprine vertebral osteomyelitis caused by Rhodococcus equi. J. S. Afr. Vet. Assoc. 2005, 76, 163–164. [Google Scholar] [CrossRef][Green Version]
- Davis, W.P.; Steficek, B.A.; Watson, G.L.; Yamini, B.; Madarame, H.; Takai, S.; Render, J.A. Disseminated Rhodococcus equi infection in two goats. Vet. Pathol. 1999, 36, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Tkachuk-Saad, O.; Lusis, P.; Welsh, R.D.; Prescott, J.F. Rhodococcus equi infections in goats. Vet. Rec. 1998, 143, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.D.; Walker, R.D.; Parlor, K.W. Fatal Rhodococcus equi infection in an Angora goat. J. Vet. Diagn. Investig. 1994, 6, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Ojo, M.O.; Njoku, C.O.; Freitas, J.; Nurse, L.; Romain, H. Isolation of Rhodococcus equi from the liver abscess of a goat in Trinidad. Can. Vet. J. 1993, 34, 504. [Google Scholar]
- Aslam, M.W.; Lau, S.F.; Chin, C.S.L.; Ahmad, N.I.; Rahman, N.A.; Kuppusamy, K.; Omar, S.; Radzi, R. Clinicopathological and radiographic features in 40 cats diagnosed with pulmonary and cutaneous Rhodococcus equi infection (2012–2018). J. Feline Med. Surg. 2019, 22, 774–790. [Google Scholar] [CrossRef]
- Portilho, F.V.R.; Paes, A.C.; Megid, J.; Hataka, A.; Neto, R.T.; Headley, S.A.; Oliveira, T.E.S.; Colhado, B.S.; de Paula, C.L.; Guerra, S.T.; et al. Rhodococcus equi pVAPN type causing pneumonia in a dog coinfected with canine morbillivirus (Distemper virus) and Toxoplasma gondii. Microb. Pathog. 2019, 129, 112–117. [Google Scholar] [CrossRef]
- Alvarez-Narvaez, S.; Giguere, S.; Cohen, N.; Slovis, N.; Vazquez-Boland, J.A. Spread of Multidrug-Resistant Rhodococcus equi, United States. Emerg. Infect. Dis. 2021, 27, 529–537. [Google Scholar] [CrossRef]
- Alvarez-Narvaez, S.; Huber, L.; Giguere, S.; Hart, K.A.; Berghaus, R.D.; Sanchez, S.; Cohen, N.D. Epidemiology and Molecular Basis of Multidrug Resistance in Rhodococcus equi. Microbiol. Mol. Biol. Rev. 2021, 85, e00011-21. [Google Scholar] [CrossRef] [PubMed]
- Cisek, A.A.; Rzewuska, M.; Witkowski, L.; Binek, M. Antimicrobial resistance in Rhodococcus equi. Acta Biochim. Pol. 2014, 61, 633–638. [Google Scholar] [CrossRef]
- Erol, E.; Locke, S.; Saied, A.; Cruz Penn, M.J.; Smith, J.; Fortner, J.; Carter, C. Antimicrobial susceptibility patterns of Rhodococcus equi from necropsied foals with rhodococcosis. Vet. Microbiol. 2020, 242, 108568. [Google Scholar] [CrossRef]
- Witkowski, L.; Rzewuska, M.; Cisek, A.A.; Chrobak-Chmiel, D.; Kizerwetter-Swida, M.; Czopowicz, M.; Welz, M.; Kita, J. Prevalence and genetic diversity of Rhodococcus equi in wild boars (Sus scrofa), roe deer (Capreolus capreolus) and red deer (Cervus elaphus) in Poland. BMC Microbiol. 2015, 15, 110. [Google Scholar] [CrossRef]
- Golub, B.; Falk, G.; Spink, W.W. Lung Abscess Due to Corynebacterium equi—Report of First Human Infection. Ann. Intern. Med. 1967, 66, 1174. [Google Scholar] [CrossRef]
- Gundelly, P.; Suzuki, Y.; Ribes, J.A.; Thornton, A. Differences in Rhodococcus equi Infections Based on Immune Status and Antibiotic Susceptibility of Clinical Isolates in a Case Series of 12 Patients and Cases in the Literature. BioMed Res. Int. 2016, 2016, 2737295. [Google Scholar] [CrossRef]
- Marrie, T.J. Community acquired pneumonia. Praxis (Bern 1994) 2001, 90, 935–940. [Google Scholar]
- Topino, S.; Galati, V.; Grilli, E.; Petrosillo, N. Rhodococcus equi Infection in HIV-Infected Individuals: Case Reports and Review of the Literature. AIDS Patient Care STDs 2010, 24, 211–222. [Google Scholar] [CrossRef]
- Lin, W.V.; Kruse, R.L.; Yang, K.; Musher, D.M. Diagnosis and management of pulmonary infection due to Rhodococcus equi. Clin. Microbiol. Infect. 2019, 25, 310–315. [Google Scholar] [CrossRef]
- Gray, K.J.; French, N.; Lugada, E.; Watera, C.; Gilks, C.F. Rhodococcus equi and HIV-1 infection in Uganda. J. Infect. 2000, 41, 227–231. [Google Scholar] [CrossRef]
- Gundelly, P.; Thornton, A.; Greenberg, R.N.; McCormick, M.; Myint, T. Rhodococcus equi pericarditis in a patient living with HIV/AIDS. J. Int. Assoc. Provid. AIDS Care 2014, 13, 309–312. [Google Scholar] [CrossRef]
- Mikic, D.; Djordjevic, Z.; Sekulovic, L.; Kojic, M.; Tomanovic, B. Disseminated Rhodococcus equi infection in a patient with Hodgkin lymphoma. Vojnosanit. Pregl. 2014, 71, 317–324. [Google Scholar] [CrossRef]
- Nath, S.R.; Mathew, A.P.; Mohan, A.; Anila, K.R. Rhodococcus equi granulomatous mastitis in an immunocompetent patient. J. Med. Microbiol. 2013, 62, 1253–1255. [Google Scholar] [CrossRef]
- Takai, S.; Sawada, N.; Nakayama, Y.; Ishizuka, S.; Nakagawa, R.; Kawashima, G.; Sangkanjanavanich, N.; Sasaki, Y.; Kakuda, T.; Suzuki, Y. Reinvestigation of the virulence of Rhodococcus equi isolates from patients with and without AIDS. Lett. Appl. Microbiol. 2020, 71, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Lara, G.H.; Takai, S.; Sasaki, Y.; Kakuda, T.; Listoni, F.J.; Risseti, R.M.; de Morais, A.B.; Ribeiro, M.G. VapB type 8 plasmids in Rhodococcus equi isolated from the small intestine of pigs and comparison of selective culture media. Lett. Appl. Microbiol. 2015, 61, 306–310. [Google Scholar] [CrossRef]
- Ribeiro, M.G.; Lara, G.H.B.; da Silva, P.; Franco, M.M.J.; de Mattos-Guaraldi, A.L.; de Vargas, A.P.C.; Sakate, R.I.; Pavan, F.R.; Colhado, B.S.; Portilho, F.V.R.; et al. Novel bovine-associated pVAPN plasmid type in Rhodococcus equi identified from lymph nodes of slaughtered cattle and lungs of people living with HIV/AIDS. Transbound Emerg. Dis. 2018, 65, 321–326. [Google Scholar] [CrossRef]
- Rzewuska, M.; Witkowski, L.; Cisek, A.A.; Stefanska, I.; Chrobak, D.; Stefaniuk, E.; Kizerwetter-Swida, M.; Takai, S. Characterization of Rhodococcus equi isolates from submaxillary lymph nodes of wild boars (Sus scrofa), red deer (Cervus elaphus) and roe deer (Capreolus capreolus). Vet. Microbiol. 2014, 172, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Buergelt, C.D.; Layton, A.W.; Ginn, P.E.; Taylor, M.; King, J.M.; Habecker, P.L.; Mauldin, E.; Whitlock, R.; Rossiter, C.; Collins, M.T. The pathology of spontaneous paratuberculosis in the North American bison (Bison bison). Vet. Pathol. 2000, 37, 428–438. [Google Scholar] [CrossRef]
- Dvorska, L.; Parmova, I.; Lavickova, M.; Bartl, J.; Vrbas, V.; Pavlik, I. Isolation of Rhodococcus equi and atypical mycobacteria from lymph nodes of pigs and cattle in herds with the occurrence of tuberculoid gross changes in the Czech Republic over the period of 1996–1998. Vet. Med. 1999, 44, 321–330. [Google Scholar]
- Sahraoui, N.; Muller, B.; Guetarni, D.; Boulahbal, F.; Yala, D.; Ouzrout, R.; Berg, S.; Smith, N.H.; Zinsstag, J. Molecular characterization of Mycobacterium bovis strains isolated from cattle slaughtered at two abattoirs in Algeria. BMC Vet. Res. 2009, 5, 4. [Google Scholar] [CrossRef]
- Salazar-Rodriguez, D.; Aleaga-Santiesteban, Y.; Iglesias, E.; Plascencia-Hernandez, A.; Perez-Gomez, H.R.; Calderon, E.J.; Vazquez-Boland, J.A.; de Armas, Y. Virulence Plasmids of Rhodococcus equi Isolates from Cuban Patients with AIDS. Front. Vet. Sci. 2021, 8, 628239. [Google Scholar] [CrossRef]
- Didkowska, A.; Zmuda, P.; Kwiecien, E.; Rzewuska, M.; Klich, D.; Krajewska-Wedzina, M.; Witkowski, L.; Zychska, M.; Kaczmarkowska, A.; Orlowska, B.; et al. Microbiological assessment of sheep lymph nodes with lymphadenitis found during post-mortem examination of slaughtered sheep: Implications for veterinary-sanitary meat control. Acta Vet. Scand. 2020, 62, 48. [Google Scholar] [CrossRef]
- Flynn, O.; Quigley, F.; Costello, E.; O’Grady, D.; Gogarty, A.; Mc Guirk, J.; Takai, S. Virulence-associated protein characterisation of Rhodococcus equi isolated from bovine lymph nodes. Vet. Microbiol. 2001, 78, 221–228. [Google Scholar] [CrossRef]
- Szalus-Jordanow, O.; Kaba, J.; Czopowicz, M.; Witkowski, L.; Nowicki, M.; Nowicka, D.; Stefanska, I.; Rzewuska, M.; Sobczak-Filipiak, M.; Binek, M.; et al. Epidemiological features of Morel’s disease in goats. Pol. J. Vet. Sci. 2010, 13, 437–445. [Google Scholar] [PubMed]
- Rzewuska, M.; Kwiecien, E.; Chrobak-Chmiel, D.; Kizerwetter-Swida, M.; Stefanska, I.; Gierynska, M. Pathogenicity and Virulence of Trueperella pyogenes: A Review. Int. J. Mol. Sci. 2019, 20, 2737. [Google Scholar] [CrossRef]
- Kaba, J.; Nowicki, M.; Frymus, T.; Nowicka, D.; Witkowski, L.; Szalus-Jordanow, O.; Czopowicz, M.; Thrusfield, M. Evaluation of the risk factors influencing the spread of caseous lymphadenitis in goat herds. Pol. J. Vet. Sci. 2011, 14, 231–237. [Google Scholar] [CrossRef]
- Moroz, A.; Szalus-Jordanow, O.; Czopowicz, M.; Brodzik, K.; Petroniec, V.; Augustynowicz-Kopec, E.; Lutynska, A.; Roszczynko, M.; Golos-Wojcicka, A.; Korzeniowska-Kowal, A.; et al. Nasal carriage of various staphylococcal species in small ruminant lentivirus-infected asymptomatic goats. Pol. J. Vet. Sci. 2020, 23, 203–209. [Google Scholar] [CrossRef]
- Kaba, J.; Czopowicz, M.; Ganter, M.; Nowicki, M.; Witkowski, L.; Nowicka, D.; Szalus-Jordanow, O. Risk factors associated with seropositivity to small ruminant lentiviruses in goat herds. Res. Vet. Sci. 2013, 94, 225–227. [Google Scholar] [CrossRef]
- Czopowicz, M.; Szalus-Jordanow, O.; Mickiewicz, M.; Moroz, A.; Witkowski, L.; Markowska-Daniel, I.; Stefaniak, T.; Bagnicka, E.; Kaba, J. Haptoglobin and serum amyloid A in goats with clinical form of caprine arthritis-encephalitis. Small Rumin. Res. 2017, 156, 73–77. [Google Scholar] [CrossRef]
- Potarniche, A.V.; Cerbu, C.G.; Czopowicz, M.; Szalus-Jordanow, O.; Kaba, J.; Spinu, M. The epidemiological background of small ruminant lentivirus infection in goats from Romania. Vet. World 2020, 13, 1344–1350. [Google Scholar] [CrossRef]
- Szalus-Jordanow, O.; Bonecka, J.; Pankowski, F.; Barszcz, K.; Tarka, S.; Kwiatkowska, M.; Polguj, M.; Mickiewicz, M.; Moroz, A.; Czopowicz, M.; et al. Postmortem imaging in goats using computed tomography with air as a negative contrast agent. PLoS ONE 2019, 14, e0215758. [Google Scholar] [CrossRef]
- Szalus-Jordanow, O.; Czopowicz, M.; Witkowski, L.; Mickiewicz, M.; Moroz, A.; Kaba, J.; Sapierzynski, R.; Bonecka, J.; Jonska, I.; Garncarz, M.; et al. Malignant thymoma—The most common neoplasm in goats. Pol. J. Vet. Sci. 2019, 22, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Takai, S.; Ikeda, T.; Sasaki, Y.; Watanabe, Y.; Ozawa, T.; Tsubaki, S.; Sekizaki, T. Identification of virulent Rhodococcus equi by amplification of gene coding for 15- to 17-kilodalton antigens. J. Clin. Microbiol. 1995, 33, 1624–1627. [Google Scholar] [CrossRef]
- Ladrón, N.; Fernández, M.; Agüero, J.; González Zörn, B.; Vázquez-Boland, J.A.; Navas, J. Rapid identification of Rhodococcus equi by a PCR assay targeting the choE gene. J. Clin. Microbiol. 2003, 41, 3241–3245. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Sosa, A.A.; Lewis, D.A.; Navas, J.; Quigley, F.; Callejo, R.; Scortti, M.; Leadon, D.P.; Fogarty, U.; Vazquez-Boland, J.A. Molecular epidemiology of Rhodococcus equi based on traA, vapA, and vapB virulence plasmid markers. J. Infect. Dis. 2007, 196, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, C.; Bonella, H.; Renwick, L.; Dodson, H.I.; Alderson, G.; Goodfellow, M. Rapid determination of vapA/vapB genotype in Rhodococcus equi using a differential polymerase chain reaction method. Antonie Van Leeuwenhoek 2004, 85, 317–326. [Google Scholar] [CrossRef]




| Bacterium | No. of Affected Goats | No. (%) of Lymph Nodes from Which the Bacterium Was Isolated | ||
|---|---|---|---|---|
| Mesenteric (n = 89) | Submandibular (n = 122) | Mediastinal (n = 61) | ||
| Rhodococcus equi | 3 | 2 | 1 | 0 |
| (2.2) | (0.8) | (0.0) | ||
| Corynebacterium pseudotuberculosis | 2 | 0 | 1 | 2 |
| (0.0) | (0.8) | (3.3) | ||
| Staphylococcus spp. | 9 | 8 | 2 | 4 |
| (9.0) | (1.6) | (6.6) | ||
| Trueperella spp. | 1 | 1 | 0 | 1 |
| (1.1) | (0.0) | (1.6) | ||
| others | 2 | 1 | 2 | 0 |
| (1.1) | (1.6) | (0.0) | ||
| Target Gene | Gene Product | Primer Sequence (5′–3′) | Amplicon Size | Reference |
|---|---|---|---|---|
| choE | cholesterol oxidase | F-GTCAACAACATCGACCAGGCG | 959 bp | [66] |
| R-CGAGCCGTCCACGACGTACAG | ||||
| traA | protein of the conjugal transfer machinery | F-AGAGTTCATGCGTGACAACG | 959 bp | [67] |
| R-GTCCACAGGTCACCGTTCTT | ||||
| vapA | virulence-associated protein A | F-GACTCTTCACAAGACGGT | 564 bp | [65] |
| R-TAGGCGTTGTGCCAGCTA | ||||
| vapB | virulence-associated protein B | F-TGATGAAGGCTCTTCATAA | 589 bp | [68] |
| R-TTATGCAACCTCCCAGTTG | ||||
| vapN | virulence-associated protein N | F-GCACTCCAAAAATACCCCGGAAG | 625 bp | [9] |
| R-CTTTGCCAGGTCTTGCGAATGTTAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żychska, M.; Witkowski, L.; Klementowska, A.; Rzewuska, M.; Kwiecień, E.; Stefańska, I.; Czopowicz, M.; Szaluś-Jordanow, O.; Mickiewicz, M.; Moroz, A.; et al. Rhodococcus equi—Occurrence in Goats and Clinical Case Report. Pathogens 2021, 10, 1141. https://doi.org/10.3390/pathogens10091141
Żychska M, Witkowski L, Klementowska A, Rzewuska M, Kwiecień E, Stefańska I, Czopowicz M, Szaluś-Jordanow O, Mickiewicz M, Moroz A, et al. Rhodococcus equi—Occurrence in Goats and Clinical Case Report. Pathogens. 2021; 10(9):1141. https://doi.org/10.3390/pathogens10091141
Chicago/Turabian StyleŻychska, Monika, Lucjan Witkowski, Agnieszka Klementowska, Magdalena Rzewuska, Ewelina Kwiecień, Ilona Stefańska, Michał Czopowicz, Olga Szaluś-Jordanow, Marcin Mickiewicz, Agata Moroz, and et al. 2021. "Rhodococcus equi—Occurrence in Goats and Clinical Case Report" Pathogens 10, no. 9: 1141. https://doi.org/10.3390/pathogens10091141
APA StyleŻychska, M., Witkowski, L., Klementowska, A., Rzewuska, M., Kwiecień, E., Stefańska, I., Czopowicz, M., Szaluś-Jordanow, O., Mickiewicz, M., Moroz, A., Bonecka, J., & Kaba, J. (2021). Rhodococcus equi—Occurrence in Goats and Clinical Case Report. Pathogens, 10(9), 1141. https://doi.org/10.3390/pathogens10091141







