Animal Models of Trypanosoma cruzi Congenital Transmission
Abstract
:1. Introduction
2. Diagnosis and Burden of Congenital Transmission of T. cruzi in Humans
3. Characteristics and Classification of the Mammalian Placental Barrier
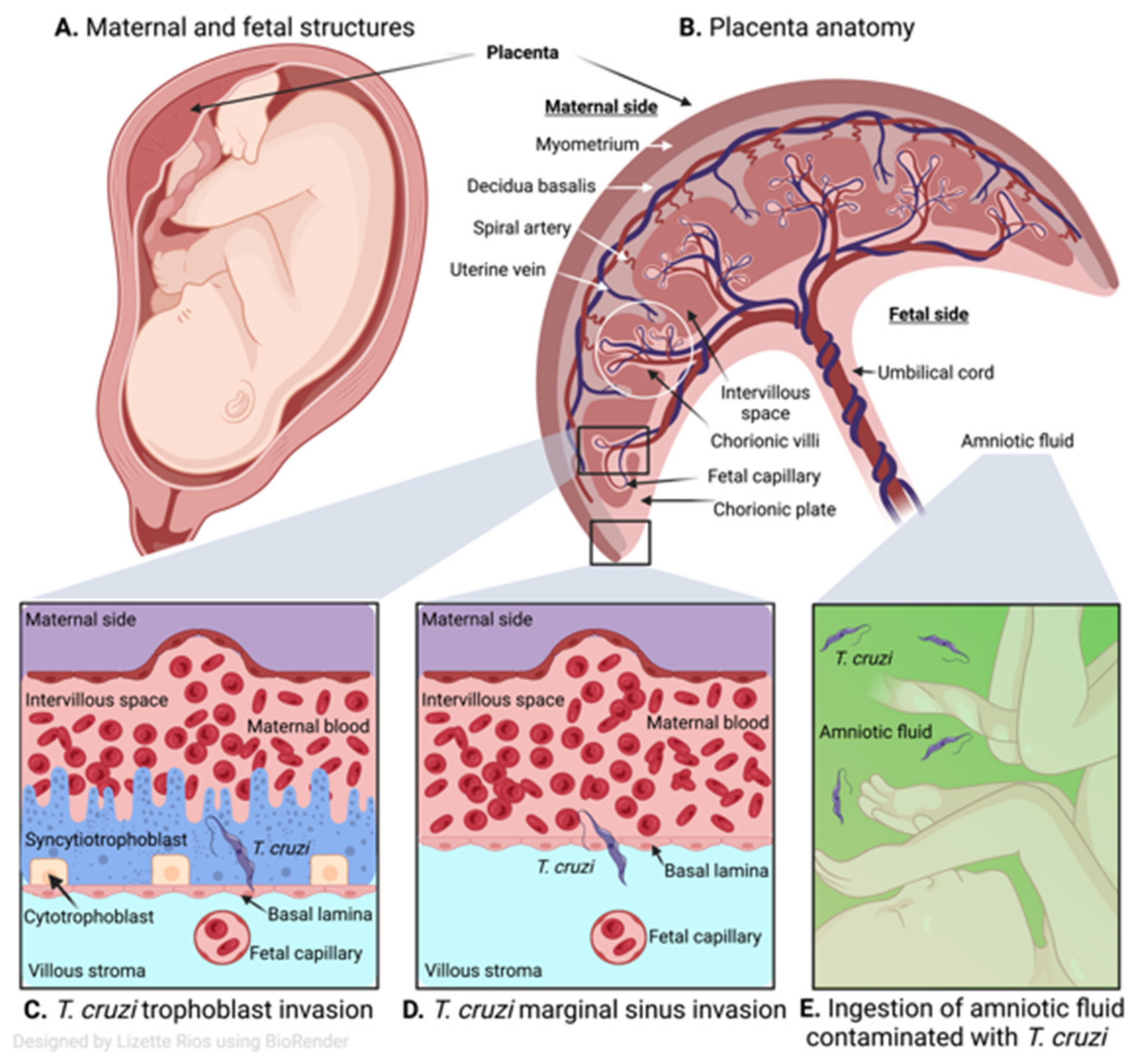
4. Human Placental Barrier and T. cruzi Invasion
5. Rodent Models of Congenital Infection by T. cruzi
6. Congenital Infection in Guinea Pigs and Chiropters
7. Congenital Transmission in Other Mammals
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coura, J.R.; Dias, J.C. Epidemiology, control, and surveillance of Chagas disease: 100 years after its discovery. Mem Inst. Oswaldo Cruz 2009, 104 (Suppl. 1), 31–40. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W. Basic cell biology of Trypanosoma cruzi. Curr. Pharm. Des. 2002, 8, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.S.; Ávila, A.R.; de Souza, W.; Motta, M.C.M.; Cavalcanti, D.P. Revisiting the Trypanosoma cruzi metacyclogenesis: Morphological and ultrastructural analyses during cell differentiation. Parasites Vectors 2018, 11, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard-Dias, W.; Alcantara, C.L.; Cunha-e-Silva, N.; de Souza, W.; Miranda, K. On the ultrastructural organization of Trypanosoma cruzi using cryopreparation methods and electron tomography. Histochem. Cell Biol. 2012, 138, 821–831. [Google Scholar] [CrossRef]
- D’Ávila, D.A.; Galvão, L.M.C.; Sousa, G.R.; Britto, C.; Moreira, O.C.; Chiari, E. Monitoring the parasite load in chronic Chagas disease patients: Comparison between blood culture and quantitative real time PCR. PLoS ONE 2018, 13, e0208133. [Google Scholar] [CrossRef]
- Meymandi, S.; Hernandez, S.; Park, S.; Sanchez, D.R.; Forsyth, C. Treatment of Chagas disease in the United States. Curr. Treat. Options Infect. Dis. 2018, 10, 373–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guhl, F.; Ramirez, J.D. Trypanosoma cruzi diversity i: Towards the need of genetic subdivision? Acta Trop. 2011, 119, 1–4. [Google Scholar] [CrossRef]
- Ramirez, J.D.; Hernandez, C. Trypanosoma cruzi ii: Towards the need of genetic subdivision? part ii. Acta Trop 2018, 184, 53–58. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci. Data 2022, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018, 184, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Cura, C.I.; Lucero, R.H.; Bisio, M.; Oshiro, E.; Formichelli, L.B.; Burgos, J.M.; Lejona, S.; Bruses, B.L.; Hernandez, D.O.; Severini, G.V.; et al. Trypanosoma cruzi discrete typing units in Chagas disease patients from endemic and non-endemic regions of Argentina. Parasitology 2012, 139, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Virreira, M.; Alonso-Vega, C.; Solano, M.; Jijena, J.; Brutus, L.; Bustamante, Z.; Truyens, C.; Schneider, D.; Torrico, F.; Carlier, Y.; et al. Congenital Chagas disease in Bolivia is not associated with DNA polymorphism of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2006, 75, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Del Puerto, R.; Nishizawa, J.E.; Kikuchi, M.; Iihoshi, N.; Roca, Y.; Avilas, C.; Gianella, A.; Lora, J.; Velarde, F.U.; Renjel, L.A.; et al. Lineage analysis of circulating Trypanosoma cruzi parasites and their association with clinical forms of Chagas disease in Bolivia. PLoS Negl. Trop. Dis. 2010, 4, e687. [Google Scholar] [CrossRef] [PubMed]
- Dorn, P.L.; McClure, A.G.; Gallaspy, M.D.; Waleckx, E.; Woods, A.S.; Monroy, M.C.; Stevens, L. The diversity of the Chagas parasite, Trypanosoma cruzi, infecting the main central American vector, Triatoma dimidiata, from Mexico to Colombia. PLoS Negl. Trop. Dis. 2017, 11, e0005878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva-Lizama, L.; Teh-Poot, C.; Majeau, A.; Herrera, C.; Dumonteil, E. Molecular genotyping of Trypanosoma cruzi by next-generation sequencing of the mini-exon gene reveals infections with multiple parasite discrete typing units in chagasic patients from Yucatan, Mexico. J. Infect. Dis. 2019, 219, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Abolis, N.G.; Araujo, S.M.; Toledo, M.J.; Fernandez, M.A.; Gomes, M.L. Trypanosoma cruzi I-III in southern Brazil causing individual and mixed infections in humans, sylvatic reservoirs and triatomines. Acta Trop. 2011, 120, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Corrales, R.M.; Mora, M.C.; Negrette, O.S.; Diosque, P.; Lacunza, D.; Virreira, M.; Breniere, S.F.; Basombrio, M.A. Congenital Chagas disease involves Trypanosoma cruzi sub-lineage IId in the northwestern province of Salta, Argentina. Infect. Genet. Evol. 2009, 9, 278–282. [Google Scholar] [CrossRef]
- Messenger, L.A.; Miles, M.A.; Bern, C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert. Rev. Anti-Infect. Ther. 2015, 13, 995–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izeta-Alberdi, A.; Ibarra-Cerdeña, C.N.; Moo-Llanes, D.A.; Ramsey, J.M. Geographical, landscape and host associations of Trypanosoma cruzi DTUs and lineages. Parasites Vectors 2016, 9, 631. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Chagas Disease: Control and Elimination; UNDP: New York, NY, USA; World Bank: Washington, DC, USA; WHO: Geneva, Switzerland, 2010; Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_17-en.pdf (accessed on 4 March 2022).
- World Health Organization. Chagas Disease (also Known as American Trypanosomiasis): Key Facts; UNDP: New York, NY, USA; World Bank: Washington, DC, USA; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 7 April 2022).
- Martinez-Ibarra, J.A.; Grant-Guillen, Y.; Morales-Corona, Z.Y.; Haro-Rodriguez, S.; Ventura-Rodriguez, L.V.; Nogueda-Torres, B.; Bustos-Saldana, R. Importance of species of triatominae (heteroptera: Reduviidae) in risk of transmission of Trypanosoma cruzi in western Mexico. J. Med. Entomol. 2008, 45, 476–482. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncayo, A. Chagas disease: Current epidemiological trends after the interruption of vectorial and transfusional transmission in the southern cone countries. Mem. Inst. Oswaldo Cruz 2003, 98, 577–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, L.; Campos, E.E.; Menon, R.; Zago, M.P.; Garg, N.J. Epidemiology and pathogenesis of maternal-fetal transmission of Trypanosoma cruzi and a case for vaccine development against congenital Chagas disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165591. [Google Scholar] [CrossRef]
- Garraud, O.; Andreu, G.; Elghouzzi, M.H.; Laperche, S.; Lefrere, J.J. Measures to prevent transfusion-associated protozoal infections in non-endemic countries. Travel Med. Infect. Dis. 2007, 5, 110–112. [Google Scholar] [CrossRef]
- Schmunis, G.A. Epidemiology of Chagas disease in non-endemic countries: The role of international migration. Mem. Inst. Oswaldo Cruz 2007, 102 (Suppl. 1), 75–85. [Google Scholar] [CrossRef] [Green Version]
- Gascon, J.; Bern, C.; Pinazo, M.J. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010, 115, 22–27. [Google Scholar] [CrossRef]
- Rodari, P.; Angheben, A.; Gennati, G.; Trezzi, L.; Bargiggia, G.; Maino, M.; Ruggeri, M.; Rampello, S.; Soavi, L.; Rizzi, M. Congenital Chagas disease in a non-endemic area: Results from a control programme in Bergamo province, northern Italy. Travel Med. Infect. Dis. 2018, 25, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Torrico, F.; Munoz, J.; Gascon, J. Congenital transmission of Chagas disease: A clinical approach. Expert. Rev. Anti-Infect. Ther. 2010, 8, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Carlier, Y.; Sosa-Estani, S.; Luquetti, A.O.; Buekens, P. Congenital Chagas disease: An update. Mem. Inst. Oswaldo Cruz 2015, 110, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Carlier, Y.; Truyens, C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta, and fetuses. Acta Trop. 2015, 151, 103–115. [Google Scholar] [CrossRef]
- Paricio-Talayero, J.M.; Benlloch-Muncharaz, M.J.; Ignacio Collar-del-Castillo, J.; Rubio-Soriano, A.; Serrat-Pérez, C.; Magraner-Egea, J.; Landa-Rivera, L.; Sánchez-Palomares, M.; Beseler-Soto, B.; Santos-Serrano, L.; et al. Vigilancia epidemiológica de la transmisión vertical de la enfermedad de chagas en tres maternidades de la comunidad valenciana. Enferm. Infecc. Microbiol. Clínica 2008, 26, 609–613. [Google Scholar] [CrossRef]
- Cevallos, A.M.; Hernández, R. Chagas disease: Pregnancy and congenital transmission. Biomed. Res. Int. 2014, 2014, 401864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buekens, P.; Cafferata, M.L.; Alger, J.; Althabe, F.; Belizan, J.M.; Bustamante, N.; Carlier, Y.; Ciganda, A.; Del Cid, J.H.; Dumonteil, E.; et al. Congenital transmission of Trypanosoma cruzi in Argentina, Honduras, and Mexico: An observational prospective study. Am. J. Trop. Med. Hyg. 2018, 98, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Moya, P.; Basso, B.; Moretti, E. Congenital Chagas disease in Cordoba, Argentina: Epidemiological, clinical, diagnostic, and therapeutic aspects. Experience of 30 years of follow up. Rev. Soc. Bras. Med. Trop. 2005, 38 (Suppl. 2), 33–40. [Google Scholar]
- Bustos, P.L.; Milduberger, N.; Volta, B.J.; Perrone, A.E.; Laucella, S.A.; Bua, J. Trypanosoma cruzi infection at the maternal-fetal interface: Implications of parasite load in the congenital transmission and challenges in the diagnosis of infected newborns. Front. Microbiol. 2019, 10, 1250. [Google Scholar] [CrossRef] [Green Version]
- Scapellato, P.G.; Bottaro, E.G.; Rodriguez-Brieschke, M.T. Mother-child transmission of Chagas disease: Could coinfection with human immunodeficiency virus increase the risk? Rev. Soc. Bras. Med. Trop. 2009, 42, 107–109. [Google Scholar] [CrossRef] [Green Version]
- Agosti, M.R.; Ercoli, P.; Dolcini, G.; Andreani, G.; Peralta, L.M.; Ayala, S.G. Two cases of mother-to-child transmission of HIV and Trypanosoma cruzi in Argentina-clinical key. Braz. J. Infect. Dis. 2012, 16, 398–399. [Google Scholar] [CrossRef] [Green Version]
- Carlier, Y.; Truyens, C.; Deloron, P.; Peyron, F. Congenital parasitic infections: A review. Acta Trop. 2012, 121, 55–70. [Google Scholar] [CrossRef]
- Arnal, A.; Waleckx, E.; Rico-Chavez, O.; Herrera, C.; Dumonteil, E. Estimating the current burden of Chagas disease in Mexico: A systematic review and meta-analysis of epidemiological surveys from 2006 to 2017. PLoS Negl. Trop. Dis. 2019, 13, e0006859. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, S.; Zulantay, I.; Solari, A.; Bisio, M.; Schijman, A.; Carlier, Y.; Apt, W. Presence of Trypanosoma cruzi in pregnant women and typing of lineages in congenital cases. Acta Trop. 2012, 124, 243–246. [Google Scholar] [CrossRef]
- Herrera, C.; Truyens, C.; Dumonteil, E.; Alger, J.; Sosa-Estani, S.; Cafferata, M.L.; Gibbons, L.; Ciganda, A.; Matute, M.L.; Zuniga, C.; et al. Phylogenetic analysis of Trypanosoma cruzi from pregnant women and newborns from Argentina, Honduras, and Mexico suggests an association of parasite haplotypes with congenital transmission of the parasite. J. Mol. Diagn. 2019, 21, 1095–1105. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; Messenger, L.A.; Luquetti, A.O.; Garcia, L.; Torrico, F.; Tavares, S.B.; Cheaib, B.; Derome, N.; Delepine, M.; Baulard, C.; et al. Deep sequencing of the Trypanosoma cruzi gp63 surface proteases reveals diversity and diversifying selection among chronic and congenital Chagas disease patients. PLoS Negl. Trop. Dis. 2015, 9, e0003458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgos, J.M.; Altcheh, J.; Petrucelli, N.; Bisio, M.; Levin, M.J.; Freilij, H.; Schijman, A.G. Molecular diagnosis and treatment monitoring of congenital transmission of Trypanosoma cruzi to twins of a triplet delivery. Diagn. Microbiol. Infect. Dis. 2009, 65, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Messenger, L.A.; Bern, C. Congenital Chagas disease: Current diagnostics, limitations, and future perspectives. Curr. Opin. Infect. Dis. 2018, 31, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Gomes, Y.M.; Lorena, V.M.; Luquetti, A.O. Diagnosis of Chagas disease: What has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem. Inst. Oswaldo Cruz 2009, 104 (Suppl. 1), 115–121. [Google Scholar] [CrossRef] [Green Version]
- Mora, M.C.; Sanchez Negrette, O.; Marco, D.; Barrio, A.; Ciaccio, M.; Segura, M.A.; Basombrio, M.A. Early diagnosis of congenital Trypanosoma cruzi infection using PCR, hemoculture, and capillary concentration, as compared with delayed serology. J. Parasitol. 2005, 91, 1468–1473. [Google Scholar] [CrossRef]
- Strout, R.G. A method for concentrating hemoflagellates. J. Parasitol. 1962, 48, 100. [Google Scholar] [CrossRef]
- Feilij, H.; Muller, L.; Gonzalez Cappa, S.M. Direct micromethod for diagnosis of acute and congenital Chagas’ disease. J. Clin. Microbiol. 1983, 18, 327–330. [Google Scholar] [CrossRef] [Green Version]
- De Rissio, A.M.; Riarte, A.R.; Garcia, M.M.; Esteva, M.I.; Quaglino, M.; Ruiz, A.M. Congenital Trypanosoma cruzi infection. Efficacy of its monitoring in an urban reference health center in a non-endemic area of Argentina. Am. J. Trop. Med. Hyg 2010, 82, 838–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balouz, V.; Aguero, F.; Buscaglia, C.A. Chagas disease diagnostic applications: Present knowledge and future steps. Adv. Parasitol. 2017, 97, 1–45. [Google Scholar]
- Eguez, K.E.; Alonso-Padilla, J.; Teran, C.; Chipana, Z.; Garcia, W.; Torrico, F.; Gascon, J.; Lozano-Beltran, D.F.; Pinazo, M.J. Rapid diagnostic tests duo as alternative to conventional serological assays for conclusive Chagas disease diagnosis. PLoS Negl. Trop. Dis. 2017, 11, e0005501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taibi, A.; Plumas-Marty, B.; Guevara-Espinoza, A.; Schöneck, R.; Pessoa, H.; Loyens, M.; Piras, R.; Aguirre, T.; Gras-Masse, H.; Bossus, M. Trypanosoma cruzi: Immunity-induced in mice and rats by trypomastigote excretory-secretory antigens and identification of a peptide sequence containing a T cell epitope with protective activity. J. Immunol. 1993, 151, 2676–2689. [Google Scholar] [PubMed]
- Pereiro, A.C. Guidelines for the diagnosis and treatment of Chagas disease. Lancet 2019, 393, 1486–1487. [Google Scholar] [CrossRef]
- Bua, J.; Volta, B.J.; Perrone, A.E.; Scollo, K.; Velazquez, E.B.; Ruiz, A.M.; De Rissio, A.M.; Cardoni, R.L. How to improve the early diagnosis of Trypanosoma cruzi infection: Relationship between validated conventional diagnosis and quantitative DNA amplification in congenitally infected children. PLoS Negl. Trop. Dis. 2013, 7, e2476. [Google Scholar] [CrossRef] [PubMed]
- Picado, A.; Cruz, I.; Redard-Jacot, M.; Schijman, A.G.; Torrico, F.; Sosa-Estani, S.; Katz, Z.; Ndung’u, J.M. The burden of congenital Chagas disease and implementation of molecular diagnostic tools in Latin America. BMJ Glob. Health 2018, 3, e001069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrico, F.; Alonso-Vega, C.; Suarez, E.; Rodriguez, P.; Torrico, M.C.; Dramaix, M.; Truyens, C.; Carlier, Y. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am. J. Trop. Med. Hyg. 2004, 70, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Schijman, A.G.; Bisio, M.; Orellana, L.; Sued, M.; Duffy, T.; Mejia Jaramillo, A.M.; Cura, C.; Auter, F.; Veron, V.; Qvarnstrom, Y.; et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl. Trop. Dis. 2011, 5, e931. [Google Scholar] [CrossRef]
- Saavedra, M.; Zulantay, I.; Apt, W.; Martinez, G.; Rojas, A.; Rodriguez, J. Chronic Chagas disease: PCR-xenodiagnosis without previous microscopic observation is a useful tool to detect viable Trypanosoma cruzi. Biol. Res. 2013, 46, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Murcia, L.; Simon, M.; Carrilero, B.; Roig, M.; Segovia, M. Treatment of infected women of childbearing age prevents congenital Trypanosoma cruzi infection by eliminating the parasitemia detected by PCR. J. Infect. Dis. 2017, 215, 1452–1458. [Google Scholar] [CrossRef] [Green Version]
- Torres-Vargas, J.; Jiménez-Coello, M.; Guzmán-Marín, E.; Acosta-Viana, K.Y.; Yadon, Z.E.; Gutiérrez-Blanco, E.; Guillermo-Cordero, J.L.; Garg, N.J.; Ortega-Pacheco, A. Quantitative and histological assessment of maternal-fetal transmission of Trypanosoma cruzi in guinea pigs: An experimental model of congenital Chagas disease. PLoS Negl. Trop. Dis. 2018, 12, e0006222. [Google Scholar] [CrossRef] [Green Version]
- Cura, C.I.; Ramirez, J.C.; Rodriguez, M.; Lopez-Albizu, C.; Irazu, L.; Scollo, K.; Sosa-Estani, S. Comparative study and analytical verification of PCR methods for the diagnosis of congenital Chagas disease. J. Mol. Diagn. 2017, 19, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Besuschio, S.A.; Llano Murcia, M.; Benatar, A.F.; Monnerat, S.; Cruz, I.; Picado, A.; Curto, M.L.A.; Kubota, Y.; Wehrendt, D.P.; Pavia, P.; et al. Analytical sensitivity and specificity of a loop-mediated isothermal amplification (lamp) kit prototype for detection of Trypanosoma cruzi DNA in human blood samples. PLoS Negl. Trop. Dis. 2017, 11, e0005779. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.; Bisio, M.; Velazquez, E.B.; Esteva, M.I.; Scollo, K.; Gonzalez, N.L.; Altcheh, J.; Ruiz, A.M. Rapid detection of Trypanosoma cruzi by colorimetric loop-mediated isothermal amplification (lamp): A potential novel tool for the detection of congenital Chagas infection. Diagn. Microbiol. Infect. Dis. 2017, 89, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Coello, M.; Shelite, T.; Castellanos-Gonzalez, A.; Saldarriaga, O.; Rivero, R.; Ortega-Pacheco, A.; Acevedo-Arcique, C.; Amaya-Guardia, K.; Garg, N.; Melby, P.; et al. Efficacy of recombinase polymerase amplification to diagnose Trypanosoma cruzi infection in dogs with cardiac alterations from an endemic area of Mexico. Vector Borne Zoonotic Dis. 2018, 18, 417–423. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Regnault, T.R.; Barker, P.L.; Botting, K.J.; McMillen, I.C.; McMillan, C.M.; Roberts, C.T.; Morrison, J.L. Placental adaptations in growth restriction. Nutrients 2015, 7, 360–389. [Google Scholar] [CrossRef] [Green Version]
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial vertical transmission during human pregnancy. Cell Host Microbe 2017, 21, 561–567. [Google Scholar] [CrossRef]
- Heerema-McKenney, A. Defense and infection of the human placenta. APMIS 2018, 126, 570–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Miglino, M.A.; Ambrosio, C.E.; dos Santos Martins, D.; Wenceslau, C.V.; Pfarrer, C.; Leiser, R. The carnivore pregnancy: The development of the embryo and fetal membranes. Theriogenology 2006, 66, 1699–1702. [Google Scholar] [CrossRef]
- Gundling, W.E., Jr.; Wildman, D.E. A review of inter- and intra-specific variation in the eutherian placenta. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140072. [Google Scholar] [CrossRef] [Green Version]
- Grosser, O. Vergleichende Anatomie und Entwicklungsgeschichte der Eihäute und der Placenta, Mit Besonderer Berücksichtigung des Menschen; Hardpress: Miami, FL, USA, 1909. [Google Scholar]
- Liempi, A.; Castillo, C.; Medina, L.; Galanti, N.; Maya, J.D.; Parraguez, V.H.; Kemmerling, U. Comparative ex vivo infection with Trypanosoma cruzi and Toxoplasma gondii of human, canine and ovine placenta: Analysis of tissue damage and infection efficiency. Parasitol. Int. 2020, 76, 102065. [Google Scholar] [CrossRef]
- Kemmerling, U.; Bosco, C.; Galanti, N. Infection and invasion mechanisms of Trypanosoma cruzi in the congenital transmission of Chagas disease: A proposal. Biol. Res. 2010, 43, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liempi, A.; Castillo, C.; Duaso, J.; Droguett, D.; Sandoval, A.; Barahona, K.; Hernandez, A.; Galanti, N.; Maya, J.D.; Kemmerling, U. Trypanosoma cruzi induces trophoblast differentiation: A potential local antiparasitic mechanism of the human placenta? Placenta 2014, 35, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Altemani, A.M.; Bittencourt, A.L.; Lana, A.M. Immunohistochemical characterization of the inflammatory infiltrate in placental Chagas disease: A qualitative and quantitative analysis. Am. J. Trop. Med. Hyg. 2000, 62, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaszkowska, J.; Goralska, K. Parasites and fungi as a threat for prenatal and postnatal human development. Ann. Parasitol. 2014, 60, 225–234. [Google Scholar]
- Fernandez-Aguilar, S.; Lambot, M.A.; Torrico, F.; Alonso-Vega, C.; Cordoba, M.; Suarez, E.; Noel, J.C.; Carlier, Y. Placental lesions in human Trypanosoma cruzi infection. Rev. Soc. Bras. Med. Trop. 2005, 38 (Suppl. 2), 84–86. [Google Scholar]
- Diaz-Luján, C.; Fernanda Triquell, M.; Mezzano, L.; Fretes, R.E. Placental infection by Trypanosoma cruzi, the causal agent of congenital Chagas disease. In Recent Advances in Research on the Human Placenta; Zheng, J., Ed.; Intechopen: London, UK, 2012; pp. 127–148. [Google Scholar] [CrossRef] [Green Version]
- Kemmerling, U.; Osuna, A.; Schijman, A.G.; Truyens, C. Congenital transmission of Trypanosoma cruzi: A review about the interactions between the parasite, the placenta, the maternal and the fetal/neonatal immune responses. Front. Microbiol. 2019, 10, 1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fretes, R.E.; Kemmerling, U. Mechanism of Trypanosoma cruzi placenta invasion and infection: The use of human chorionic villi explants. J. Trop. Med. 2012, 2012, 614820. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Lujan, C.; Triquell, M.F.; Castillo, C.; Hardisson, D.; Kemmerling, U.; Fretes, R.E. Role of placental barrier integrity in infection by Trypanosoma cruzi. Acta Trop. 2016, 164, 360–368. [Google Scholar] [CrossRef]
- Liempi, A.; Castillo, C.; Carrillo, I.; Munoz, L.; Droguett, D.; Galanti, N.; Maya, J.D.; Kemmerling, U. A local innate immune response against Trypanosoma cruzi in the human placenta: The epithelial turnover of the trophoblast. Microb. Pathog. 2016, 99, 123–129. [Google Scholar] [CrossRef]
- Abrahams, V.M. The role of the nod-like receptor family in trophoblast innate immune responses. J. Reprod. Immunol. 2011, 88, 112–117. [Google Scholar] [CrossRef]
- Ilekis, J.V.; Tsilou, E.; Fisher, S.; Abrahams, V.M.; Soares, M.J.; Cross, J.C.; Zamudio, S.; Illsley, N.P.; Myatt, L.; Colvis, C.; et al. Placental origins of adverse pregnancy outcomes: Potential molecular targets: An executive workshop summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obs. Gynecol. 2016, 215, S1–S46. [Google Scholar] [CrossRef]
- Castillo, C.; Carrillo, I.; Libisch, G.; Juiz, N.; Schijman, A.; Robello, C.; Kemmerling, U. Host-parasite interaction: Changes in human placental gene expression induced by Trypanosoma cruzi. Parasites Vectors 2018, 11, 479. [Google Scholar] [CrossRef]
- Triquell, M.F.; Díaz-Luján, C.; Romanini, M.C.; Ramirez, J.C.; Paglini-Oliva, P.; Schijman, A.G.; Fretes, R.E. Nitric oxide synthase and oxidative-nitrosative stress play a key role in placental infection by Trypanosoma cruzi. Am. J. Reprod. Immunol. 2018, 80, e12852. [Google Scholar] [CrossRef]
- Castillo, C.; Munoz, L.; Carrillo, I.; Liempi, A.; Medina, L.; Galanti, N.; Maya, J.D.; Kemmerling, U. Toll-like receptor-2 mediates local innate immune response against Trypanosoma cruzi in ex vivo infected human placental chorionic villi explants. Placenta 2017, 60, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Duaso, J.; Yanez, E.; Castillo, C.; Galanti, N.; Cabrera, G.; Corral, G.; Maya, J.D.; Zulantay, I.; Apt, W.; Kemmerling, U. Reorganization of extracellular matrix in placentas from women with asymptomatic Chagas disease: Mechanism of parasite invasion or local placental defense? J. Trop. Med. 2012, 2012, 758357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duaso, J.; Rojo, G.; Cabrera, G.; Galanti, N.; Bosco, C.; Maya, J.D.; Morello, A.; Kemmerling, U. Trypanosoma cruzi induces tissue disorganization and destruction of chorionic villi in an ex vivo infection model of human placenta. Placenta 2010, 31, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.A.; Rivera, I.M.; Moreno, S.C.; Alarcon, M.E.; Lugo-Yarbuh, A. Vertical transmission of Trypanosoma cruzi in wistar rats during the acute phase of infection. Investig. Clin. 2003, 44, 241–254. [Google Scholar]
- Alarcon, M.; Ruiz, G.; Yarbuh, A.L.; Guillen, C.B.; Moreno, E.; Aguilar, C.P.; Cruz, J. Congenital Trypanosoma cruzi transmission in pups of wistar rats with acute Chagas infection. Soc. Ven. Microbiol. 2010, 30, 114–120. [Google Scholar]
- Moreno, E.; Quintero, A.C.; Alarcon, M.; Yarbuh, A.L.; Moreno, S.C.; Araujo, S.A.; Borges, R. Investigaciones sobre la transmisión vertical de Trypanosoma cruzi en ratas wistar crónicamente infectadas. Boletín Malariol. Salud Ambient. 2006, 46, 149–160. [Google Scholar]
- Alarcon, M.; Moreno, E.; Colasante, C.; Yarbuh, A.L.; Caceres, K.; Araujo, S. Presence of Trypanosoma cruzi in tissues of experimentally infected wistar rats and their fetuses. Bol. Mal. Salud Amb. 2006, 46, 137–148. [Google Scholar]
- Perez, M.C.; Alarcon, M.; Goncalves, L.; Yarbuh, A.L.; Moreno, E.; Araujo, S.; Villarreal, J. Morphological and structural abnormalities in fetuses of mice with experimental acute Trypanosoma cruzi infection. Bol. Mal. Salud Amb. 2008, 48, 127–134. [Google Scholar]
- Alarcon, M.; Perez, M.C.; Villarreal, J.; Araujo, S.; Goncalves, L.; Gonzalez, A.; Moreno, E.; Lugo-Yarbuh, A. Detection of Trypanosoma cruzi DNA in the placenta and fetuses of mice with Chagasic acute infection. Investig. Clin. 2009, 50, 335–345. [Google Scholar]
- Cardoni, R.L.; Antunez, M.I. Outcome of Trypanosoma cruzi infection in pregnant BALB/c mice. Ann. Trop. Med. Parasitol. 2004, 98, 883–887. [Google Scholar] [CrossRef]
- Cencig, S.; Coltel, N.; Truyens, C.; Carlier, Y. Fertility, gestation outcome and parasite congenital transmissibility in mice infected with TcI, TcII and TcVI genotypes of Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2013, 7, e2271. [Google Scholar] [CrossRef] [Green Version]
- Mjihdi, A.; Lambot, M.A.; Stewart, I.J.; Detournay, O.; Noel, J.C.; Carlier, Y.; Truyens, C. Acute Trypanosoma cruzi infection in mouse induces infertility or placental parasite invasion and ischemic necrosis associated with massive fetal loss. Am. J. Pathol. 2002, 161, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Carlier, Y.; Rivera, M.T.; Truyens, C.; Puissant, F.; Milaire, J. Interactions between chronic murine Trypanosoma cruzi infection and pregnancy: Fetal growth retardation. Am. J. Trop. Med. Hyg. 1987, 37, 534–540. [Google Scholar] [CrossRef]
- De Araujo, S.M.; Chiari, E. Trypanosoma cruzi infection in offspring born to chagasic C3H/He mice. Mem. Inst. Oswaldo Cruz 1996, 91, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Badra, E.S.; Sala, M.A.; Lopes, R.A.; Prado, J.C., Jr.; Albuquerque, S.; Zucoloto, S.; Carraro-Abrahao, A.A. Histopathological changes in the placentas and fetuses of mice infected with Trypanosoma cruzi isolated from the Myotis nigricans nigricans bat. J. Comp. Pathol. 2008, 139, 108–112. [Google Scholar] [CrossRef]
- Andrade, S.G. The influence of the strain of Trypanosoma cruzi in placental infections in mice. Trans. R Soc. Trop. Med. Hyg. 1982, 76, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Cabeza Meckert, P.; Chambo, G.J.; Laguens, R.P. Congenital disease secondary to the chronic infection of mice with Trypanosoma cruzi. Experimental model of congenital Chagas disease. Medicina 1980, 40 (Suppl. 1), 40–44. [Google Scholar] [PubMed]
- Carter, A.M. Animal models of human placentation—A review. Placenta 2007, 28 (Suppl. A), S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J.; Cross, J.C. Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2001, 2, 538–548. [Google Scholar] [CrossRef]
- Mu, J.; Adamson, S.L. Developmental changes in hemodynamics of uterine artery, utero- and umbilicoplacental, and vitelline circulations in mouse throughout gestation. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1421–H1428. [Google Scholar] [CrossRef] [Green Version]
- Malassine, A.; Frendo, J.L.; Evain-Brion, D. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 2003, 9, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Adamson, S.L.; Lu, Y.; Whiteley, K.J.; Holmyard, D.; Hemberger, M.; Pfarrer, C.; Cross, J.C. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 2002, 250, 358–373. [Google Scholar] [CrossRef]
- Moreno, E.; Ramirez, M.; Alarcon, M.; Yarbuh, A.L.; Villarreal, J.; Araujo, S.; Mogollon, N.; Gonzalez, A.; Premoli, G. Congenital transmission of Trypanosoma cruzi in second generation wistar rats. Bol. Mal. Salud Amb. 2010, 50, 29–38. [Google Scholar]
- Carter, A.M. Animal models of human pregnancy and placentation: Alternatives to the mouse. Reproduction 2020, 160, R129–R143. [Google Scholar] [CrossRef]
- Sherlock, I.A.; Muniz, T.M. Transmission of Trypanosoma cruzi in three generations of Cavia porcellus without the participation of triatomines. Rev. Soc. Braz. Med. Trop. 1976, 10, 27–29. [Google Scholar] [CrossRef]
- Anez, N.; Crisante, G.; Soriano, P.J. Trypanosoma cruzi congenital transmission in wild bats. Acta Trop. 2009, 109, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Noya, B.A.; Diaz-Bello, Z.; Ruiz-Guevara, R.; Noya, O. Chagas disease expands its epidemiological frontiers from rural to urban areas. Front. Trop. Dis. 2022, 3, 799009. [Google Scholar] [CrossRef]
- Li, M.; Brokaw, A.; Furuta, A.M.; Coler, B.; Obregon-Perko, V.; Chahroudi, A.; Wang, H.Y.; Permar, S.R.; Hotchkiss, C.E.; Golos, T.G.; et al. Non-human primate models to investigate mechanisms of infection-associated fetal and pediatric injury, teratogenesis and stillbirth. Front. Genet. 2021, 12, 680342. [Google Scholar] [CrossRef]
- Siena, G.; Milani, C. Usefulness of maternal and fetal parameters for the prediction of parturition date in dogs. Animals 2021, 11, 878. [Google Scholar] [CrossRef] [PubMed]
- Kendricks, A.L.; Gray, S.B.; Wilkerson, G.K.; Sands, C.M.; Abee, C.R.; Bernacky, B.J.; Hotez, P.J.; Bottazzi, M.E.; Craig, S.L.; Jones, K.M. Reproductive outcomes in rhesus macaques (Macaca mulatta) with naturally acquired Trypanosoma cruzi infection. Comp. Med. 2020, 70, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.D.S. Transmissão intrauterina do Trypanosoma cruzi na infecção experimental do cão. An. Fac. Med. Univ. São Paulo 1928, 3, 35–39. [Google Scholar]
- Rodriguez-Morales, O.; Ballinas-Verdugo, M.A.; Alejandre-Aguilar, R.; Reyes, P.A.; Arce-Fonseca, M. Trypanosoma cruzi connatal transmission in dogs with Chagas disease: Experimental case report. Vector Borne Zoonotic Dis. 2011, 11, 1365–1370. [Google Scholar] [CrossRef]

| Species/Model | T. cruzi Strains | T. cruzi Dose | Route | Disease | Transmission Rate | Observations | References |
|---|---|---|---|---|---|---|---|
| Guinea pigs | H4 | 100 blood trypomastigotes | IP | Acute Chronic | 100% | Cell degeneration and necrosis in myocardial and skeletal tissues of fetuses, compromised fetal development | [62] |
| Human placenta explant | Tulahuen | 8 × 104 metacyclic trypomastigotes | NA | NA | NA | Chorionic villi denuded of syncytiotrophoblasts were more susceptible to infection. | [83,84,85] |
| Human placenta explant | NA | 1 × 104 metacyclic trypomastigotes | NA | NA | NA | Reprograming of human placental genes expression of the innate immune pathways by T. cruzi infection. | [88] |
| Human placenta explant | Tulahuen or Lucky | 1 × 105 or 1 × 106 metacyclic trypomastigotes | NA | NA | NA | Nitric oxide produced by syncytiotrophoblasts are relevant for the placental protection against T. cruzi. | [89] |
| Human placenta explant | NA | 1 × 105 metacyclic trypomastigotes | NA | NA | NA | T. cruzi induced IL-6 and IL-10 dysregulated the trophoblast turnover. | [90] |
| Human placenta | NA | NA | NA | Asymptomatic | 2/3 * | Placenta from T. cruzi-infected women showed reorganization of extracellular matrix tissue and trophoblastic cells and inflammatory immune response. | [91] |
| Wistar rats | Pr or YBM | 1 × 104 metacyclic trypomastigotes | ID | Acute | 9.1% 47.8% | Acute myocarditis, amastigote nests in fetal hearts. | [93] |
| Wistar rats | Planalto | 1.5 × 104 metacyclic trypomastigotes | IP | Acute | 24% | Parasite antigens detected in heart and skeletal muscle of 15% of pups. | [94] |
| Wistar rats | Y | 1 × 105 blood trypomastigotes | ID | Chronic | 44.6% | Virulence and inoculum size determines extent of vertical transmission of T. cruzi. | [95] |
| Wistar rats | Planalto | 1 × 105 blood trypomastigotes | IP | Chronic | 33% | Amastigote nests in fetal cardiac tissues, inflammatory infiltrate in placentas. | [96] |
| NMRI mice | Y | 22 × 103 metacyclic trypomastigotes | IP | Acute | 15% | Structural abnormalities in fetuses, loss of weight and growth retardation in pups, myocarditis in 10% of pups. | [97] |
| NMRI mice | Y | 22 × 103 metacyclic trypomastigotes | IP | Acute | 18% | Inflammatory infiltrate and amastigote nests in fetal hearts. | [98] |
| BALB/c mice | Tulahuen | 150 blood trypomastigotes | IP | Acute | 0% | Female resistance to T. cruzi infection prevented congenital transmission. | [99] |
| BALB/c mice | X10, Y or Tulahuen | 1 × 106 blood trypomastigotes | SC | Acute | 4% | Intra-uterine growth retardation of fetuses. | [100] |
| BALB/c mice | Tehuantepec | 100 blood trypomastigotes | IP SC | Acute | 0% | Placental invasion by parasites and injury, fetal growth retardation and death in the absence of congenital infection, 28% of embryos resorbed in early gestation. | [101] |
| BALB/c mice | Tehuantepec | 100 blood trypomastigotes | IP SC | Chronic | NA | Parasites were not detected in fetuses of chronically infected mice, yet fetal weight was significantly decreased. | [102] |
| C3H/He mice | Y | 500 blood trypomastigotes | IP | Acute | 1.18% | Maternal immune products transferred by placental and suckling routes modulate fetal immune response. | [103] |
| Swiss mice | Morc-1 | 1 × 105 blood trypomastigotes | IP | Acute | 30% | Amastigote nests in uterine muscle, striated muscle, and placental cells; intrauterine growth retardation and mortality in 10% of fetuses. | [104] |
| Swiss mice | Y, Peruvian (P), Honorina (H), Colombian (C) | Y: 144–157, P: 463, H: 72–500, C: 473 trypomastigotes | SC | Acute Chronic | 0% 0% | The incidence of placental parasitism-Y: 17%, H: 13.2%, C: 98%, Peruvian: 18.4%. Parasite strains play a role in congenital T. cruzi infection. | [105] |
| Swiss mice | Tulahuen | 25–30 blood trypomastigotes | NA | Chronic | 6.1% | Anti-T. cruzi maternal antibodies and parasite was rarely noted in newborns, myocytic lesions found in fetal tissues. | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avalos-Borges, E.E.; Rios, L.E.; Jiménez-Coello, M.; Ortega-Pacheco, A.; Garg, N.J. Animal Models of Trypanosoma cruzi Congenital Transmission. Pathogens 2022, 11, 1172. https://doi.org/10.3390/pathogens11101172
Avalos-Borges EE, Rios LE, Jiménez-Coello M, Ortega-Pacheco A, Garg NJ. Animal Models of Trypanosoma cruzi Congenital Transmission. Pathogens. 2022; 11(10):1172. https://doi.org/10.3390/pathogens11101172
Chicago/Turabian StyleAvalos-Borges, Eduardo E., Lizette E. Rios, Matilde Jiménez-Coello, Antonio Ortega-Pacheco, and Nisha J. Garg. 2022. "Animal Models of Trypanosoma cruzi Congenital Transmission" Pathogens 11, no. 10: 1172. https://doi.org/10.3390/pathogens11101172
APA StyleAvalos-Borges, E. E., Rios, L. E., Jiménez-Coello, M., Ortega-Pacheco, A., & Garg, N. J. (2022). Animal Models of Trypanosoma cruzi Congenital Transmission. Pathogens, 11(10), 1172. https://doi.org/10.3390/pathogens11101172






