Current Susceptibility Surveillance and Distribution of Antimicrobial Resistance in N. gonorrheae within WHO Regions
Abstract
1. Introduction
2. Brief Description of the Most Important Mechanisms of Resistance in Gonococci
3. WHO Surveillance
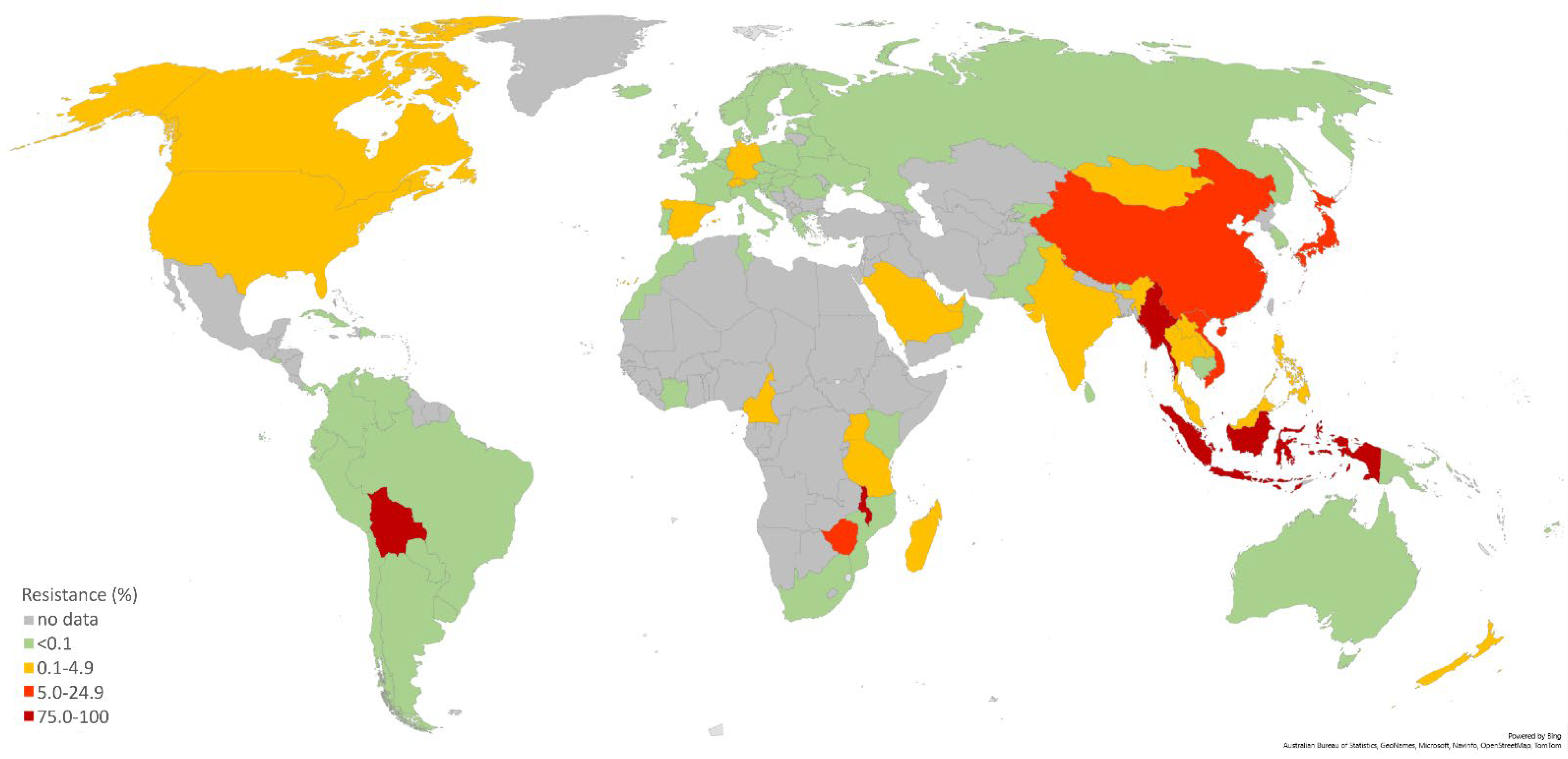
4. Current Surveillance Data in the WHO Regions
4.1. WHO European Region
4.2. WHO Western Pacific Region (WPR)
4.3. WHO African Region
4.4. WHO Eastern Mediterranean Region (EMR)
5. New Treatment Options for N. gonorrhoea Infections
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Lahra, M.M.; Cole, M.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.A.R.; Ramon-Pardo, P.; Bolan, G.; Wi, T. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): Review of new data and evidence to inform international collaborative actions and research efforts. Sex. Health 2019, 16, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Sexually Transmitted Disease Surveillance. 2020. Available online: https://www.cdc.gov/std/statistics/2020/default.htm (accessed on 17 October 2022).
- Chan, P.A.; Robinette, A.; Montgomery, M.; Almonte, A.; Cu-Uvin, S.; Lonks, J.R.; Chapin, K.C.; Kojic, E.M.; Hardy, E.J. Extragenital Infections Caused by Chlamydia trachomatis and Neisseria gonorrhoeae: A Review of the Literature. Infect. Dis. Obstet. Gynecol. 2016, 2016, 5758387. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yan, J. Antibiotic Resistance and Treatment Options for Multidrug-Resistant Gonorrhea. Infect. Microbes Dis. 2020, 2, 67–76. [Google Scholar] [CrossRef]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef]
- Golparian, D.; Ohlsson, A.K.; Janson, H.; Lidbrink, P.; Richtner, T.; Ekelund, O.; Fredlund, H.; Unemo, M. Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014. Euro Surveill. 2014, 19, 20862. [Google Scholar] [CrossRef]
- Unemo, M. Current and future antimicrobial treatment of gonorrhea—The rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect. Dis. 2015, 15, 364. [Google Scholar] [CrossRef]
- Barbee, L.A.; St Cyr, S.B. Management of Neisseria gonorrhoeae in the United States: Summary of Evidence from the Development of the 2020 Gonorrhea Treatment Recommendations and the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infection Treatment Guidelines. Clin. Infect. Dis. 2022, 74, S95–S111. [Google Scholar] [CrossRef]
- Unemo, M.; Ross, J.D.C.; Serwin, A.B.; Gomberg, M.; Cusini, M.; Jensen, J.S. Background review for the “2020 European guideline for the diagnosis and treatment of gonorrhoea in adults”. Int. J. STD AIDS 2021, 32, 108–126. [Google Scholar] [CrossRef]
- Golparian, D.; Rose, L.; Lynam, A.; Mohamed, A.; Bercot, B.; Ohnishi, M.; Crowley, B.; Unemo, M. Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, Ireland, August 2018. Euro Surveill. 2018, 23, 1800617. [Google Scholar] [CrossRef]
- Fifer, H.; Natarajan, U.; Jones, L.; Alexander, S.; Hughes, G.; Golparian, D.; Unemo, M. Failure of Dual Antimicrobial Therapy in Treatment of Gonorrhea. N. Engl. J. Med. 2016, 374, 2504–2506. [Google Scholar] [CrossRef]
- Whiley, D.M.; Jennison, A.; Pearson, J.; Lahra, M.M. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect. Dis. 2018, 18, 717–718. [Google Scholar] [CrossRef]
- Jennison, A.V.; Whiley, D.; Lahra, M.M.; Graham, R.M.; Cole, M.J.; Hughes, G.; Fifer, H.; Andersson, M.; Edwards, A.; Eyre, D. Genetic relatedness of ceftriaxone-resistant and highlevel azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Eurosurveillance 2019, 24, 1900118. [Google Scholar] [CrossRef]
- Sánchez-Busó, L.; Cole, M.J.; Spiteri, G.; Day, M.; Jacobsson, S.; Golparian, D.; Sajedi, N.; Yeats, C.A.; Abudahab, K.; Underwood, A.; et al. Europe-wide expansion and eradication of multidrug-resistant Neisseria gonorrhoeae lineages: A genomic surveillance study. Lancet Microbe 2022, 3, e452–e463. [Google Scholar] [CrossRef]
- Jacobsson, S.; Golparian, D.; Scangarella-Oman, N.; Unemo, M. In vitro activity of the novel triazaacenaphthylene gepotidacin (GSK2140944) against MDR Neisseria gonorrhoeae. J. Antimicrob. Chemother. 2018, 73, 2072–2077. [Google Scholar] [CrossRef]
- Scangarella-Oman, N.E.; Hossain, M.; Dixon, P.B.; Ingraham, K.; Min, S.; Tiffany, C.A.; Perry, C.R.; Raychaudhuri, A.; Dumont, E.F.; Huang, J.; et al. Microbiological analysis from a phase 2 randomized study in adults evaluating single oral doses of gepotidacin in the treatment of uncomplicated urogenital gonorrhea caused by neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2018, 62, e01221-18. [Google Scholar] [CrossRef]
- Basarab, G.S.; Kern, G.H.; McNulty, J.; Mueller, J.P.; Lawrence, K.; Vishwanathan, K.; Alm, R.A.; Barvian, K.; Doig, P.; Galullo, V.; et al. Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases. Sci. Rep. 2015, 5, 11827. [Google Scholar] [CrossRef]
- Unemo, M.; Nicholas, R.A. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 2012, 7, 1401–1422. [Google Scholar] [CrossRef]
- Aas, F.E.; Løvold, C.; Koomey, M. An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: Mechanism of action and links to Type IV pilus expression. Mol. Microbiol. 2002, 46, 1441–1450. [Google Scholar] [CrossRef]
- Hamilton, H.L.; Dillard, J.P. Natural transformation of Neisseria gonorrhoeae: From DNA donation to homologous recombination. Mol. Microbiol. 2006, 59, 376–385. [Google Scholar] [CrossRef]
- Bowler, L.D.; Zhang, Q.Y.; Riou, J.Y.; Spratt, B.G. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: Natural events and laboratory simulation. J. Bacteriol. 1994, 176, 333–337. [Google Scholar] [CrossRef]
- Igawa, G.; Yamagishi, Y.; Lee, K.I.; Dorin, M.; Shimuta, K.; Suematsu, H.; Nakayama, S.I.; Mikamo, H.; Unemo, M.; Ohnishia, M. Neisseria cinerea with high ceftriaxone MIC Is a source of ceftriaxone and cefixime resistance-mediating penA sequences in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2018, 62, e02069-17. [Google Scholar] [CrossRef] [PubMed]
- Demczuk, W.; Sidhu, S.; Unemo, M.; Whiley, D.M.; Allen, V.G.; Dillon, J.R.; Cole, M.; Seah, C.; Trembizki, E.; Trees, D.L.; et al. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. Gonorrhoeae strains. J. Clin. Microbiol. 2017, 55, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Turner, J.M.; Tomberg, J.; Fedarovich, A.; Unemo, M.; Nicholas, R.A.; Davies, C. Mutations in penicillin-binding protein 2 from cephalosporin-resistant Neisseria gonorrhoeae hinder ceftriaxone acylation by restricting protein dynamics. J. Biol. Chem. 2020, 295, 7529–7543. [Google Scholar] [CrossRef] [PubMed]
- Hagman, K.E.; Pan, W.; Spratt, B.G.; Balthazar, J.T.; Judd, R.C.; Shafer, W.M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 1995, 141 Pt 3, 611–622. [Google Scholar] [CrossRef]
- Zhao, S.; Tobiason, D.M.; Hu, M.; Seifert, H.S.; Nicholas, R.A. The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability. Mol. Microbiol. 2005, 57, 1238–1251. [Google Scholar] [CrossRef]
- Muhammad, I.; Golparian, D.; Dillon, J.A.R.; Johansson, Å.; Ohnishi, M.; Sethi, S.; Chen, S.-C.; Nakayama, S.-I.; Sundqvist, M.; Bala, M.; et al. Characterisation of bla TEM genes and types of β-lactamase plasmids in Neisseria gonorrhoeae—The prevalent and conserved bla TEM-135 has not recently evolved and existed in the Toronto plasmid from the origin. BMC Infect. Dis. 2014, 14, 454. [Google Scholar] [CrossRef]
- Kivata, M.W.; Mbuchi, M.; Eyase, F.; Bulimo, W.D.; Kyanya, C.K.; Oundo, V.; Mbinda, W.M.; Sang, W.; Andagalu, B.; Soge, O.O.; et al. Plasmid mediated penicillin and tetracycline resistance among Neisseria gonorrhoeae isolates from Kenya. BMC Infect. Dis. 2020, 20, 703. [Google Scholar] [CrossRef]
- Singh, R.; Perera, S.R.; Katselis, G.S.; Chumala, P.; Martin, I.; Kusalik, A.; Mitzel, K.M.; Dillon, J.A.R. A β-lactamase-producing plasmid from Neisseria gonorrhoeae carrying a unique 6 bp deletion in blaTEM-1 encoding a truncated 24 kDa TEM-1 penicillinase that hydrolyses ampicillin slowly. J. Antimicrob. Chemother. 2019, 74, 2904–2912. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, J.; van der Veen, S. High prevalence of TEM-135 expression from the Asian plasmid in penicillinase-producing Neisseria gonorrhoeae from Hangzhou, China. Int. J. Antimicrob. Agents 2019, 54, 361–366. [Google Scholar] [CrossRef]
- Shaskolskiy, B.; Kandinov, I.; Dementieva, E.; Gryadunov, D. Antibiotic Resistance in Neisseria gonorrhoeae: Challenges in Research and Treatment. Microorganisms 2022, 10, 1699. [Google Scholar] [CrossRef]
- Kirkcaldy, R.D.; Kidd, S.; Weinstock, H.S.; Papp, J.R.; Bolan, G.A. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: The Gonococcal Isolate Surveillance Project (GISP), January 2006-June 2012. Sex. Transm. Infect. 2013, 89 (Suppl. 4), iv5–iv10. [Google Scholar] [CrossRef]
- Kirkcaldy, R.D.; Harvey, A.; Papp, J.R.; del Rio, C.; Soge, O.O.; Holmes, K.K.; Hook, E.W.; Kubin, G.; Riedel, S.; Zenilman, J.; et al. Neisseria gonorrhoeae Antimicrobial Susceptibility Surveillance—The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014. MMWR. Surveill. Summ. 2016, 65, 1–24. [Google Scholar] [CrossRef]
- Martin, I.; Sawatzky, P.; Liu, G.; Allen, V.; Lefebvre, B.; Hoang, L.; Drews, S.; Horsman, G.; Wylie, J.; Haldane, D.; et al. Decline in Decreased Cephalosporin Susceptibility and Increase in Azithromycin Resistance in Neisseria gonorrhoeae, Canada. Emerg. Infect. Dis. 2016, 22, 65–67. [Google Scholar] [CrossRef]
- Gianecini, R.; De Las Mercedes Romero, M.; Oviedo, C.; Vacchino, M.; Galarza, P. Emergence and spread of neisseria gonorrhoeae isolates with decreased susceptibility to extended-spectrum cephalosporins in Argentina, 2009 to 2013. Sex. Transm. Dis. 2017, 44, 351–355. [Google Scholar] [CrossRef]
- Town, K.; Obi, C.; Quaye, N.; Chisholm, S.; Hughes, G. Drifting towards ceftriaxone treatment failure in gonorrhoea: Risk factor analysis of data from the Gonococcal Resistance to Antimicrobials Surveillance Programme in England and Wales. Sex. Transm. Infect. 2017, 93, 39–45. [Google Scholar] [CrossRef]
- EUCAST: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 29 September 2022).
- Unemo, M.; Ison, C.A.; Cole, M.; Spiteri, G.; Van De Laar, M.; Khotenashvili, L. Gonorrhoea and gonococcal antimicrobial resistance surveillance networks in the WHO European Region, including the independent countries of the former Soviet Union. Sex. Transm. Infect. 2013, 89 (Suppl. 4), iv42–iv46. [Google Scholar] [CrossRef]
- Boiko, I.; Golparian, D.; Krynytska, I.; Bezkorovaina, H.; Frankenberg, A.; Onuchyna, M.; Jacobsson, S.; Unemo, M. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates and treatment of gonorrhoea patients in Ternopil and Dnipropetrovsk regions of Ukraine, 2013–2018. APMIS 2019, 127, 503–509. [Google Scholar] [CrossRef]
- Day, M.J.; Jacobsson, S.; Spiteri, G.; Kulishev, C.; Sajedi, N.; Woodford, N.; Blumel, B.; van der Werf, M.J.; Amato-Gauci, A.J.; Unemo, M.; et al. Significant increase in azithromycin “resistance” and susceptibility to ceftriaxone and cefixime in Neisseria gonorrhoeae isolates in 26 European countries, 2019. BMC Infect. Dis. 2022, 22, 524. [Google Scholar] [CrossRef]
- Le, W.; Su, X.; Lou, X.; Li, X.; Gong, X.; Wang, B.; Genco, C.A.; Mueller, J.P.; Rice, P.A. Susceptibility trends of zoliflodacin against multidrug-resistant neisseria gonorrhoeae clinical isolates in nanjing, china, 2014 to 2018. Antimicrob. Agents Chemother. 2021, 65, e00863-20. [Google Scholar] [CrossRef]
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.A.R.; Ramon-Pardo, P.; Eremin, S.R.; Bolan, G.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 14, e1002344. [Google Scholar] [CrossRef] [PubMed]
- Workneh, M.; Hamill, M.M.; Kakooza, F.; Mande, E.; Wagner, J.; Mbabazi, O.; Mugasha, R.; Kajumbula, H.; Walwema, R.; Zenilman, J.; et al. Antimicrobial Resistance of Neisseria Gonorrhoeae in a Newly Implemented Surveillance Program in Uganda: Surveillance Report. JMIR Public Health Surveill. 2020, 6, e17009. [Google Scholar] [CrossRef] [PubMed]
- Maduna, L.D.; Kock, M.M.; van der Veer, B.M.J.W.; Radebe, O.; McIntyre, J.; van Alphen, L.B.; Peters, R.P.H. Antimicrobial resistance of Neisseria gonorrhoeae isolates from high-risk men in Johannesburg, South Africa. Antimicrob. Agents Chemother. 2020, 64, e00906-20. [Google Scholar] [CrossRef] [PubMed]
- Rambaran, S.; Naidoo, K.; Dookie, N.; Moodley, P.; Sturm, A.W. Resistance Profile of Neisseria gonorrhoeae in KwaZulu-Natal, South Africa Questioning the Effect of the Currently Advocated Dual Therapy. Sex. Transm. Dis. 2019, 46, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Crucitti, T.; Belinga, S.; Fonkoua, M.C.; Abanda, M.; Mbanzouen, W.; Sokeng, E.; Nzouankeu, A. Sharp increase in ciprofloxacin resistance of Neisseria gonorrhoeae in Yaounde, Cameroon: Analyses of a laboratory database period 2012–2018. Int. J. STD AIDS 2020, 31, 579–586. [Google Scholar] [CrossRef]
- Hançali, A.; Ndowa, F.; Bellaji, B.; Bennani, A.; Kettani, A.; Charof, R.; Aouad, E.R. Antimicrobial resistance monitoring in Neisseria gonorrhoeae and strategic use of funds from the Global Fund to set up a systematic Moroccan gonococcal antimicrobial surveillance programme. Sex. Transm. Infect. 2013, 89 (Suppl. 4), iv24–iv27. [Google Scholar] [CrossRef]
- Al-Maslamani, M.; Elmagboul, E.B.I.; Puthiyottil, A.; Chemaitelly, H.; Varghese, M.K.; Al Romaihi, H.E.; Al-Thani, M.H.; Al Khal, A.; Unemo, M.; Abu-Raddad, L.J. First characterisation of antimicrobial susceptibility and resistance of Neisseria gonorrhoeae isolates in Qatar, 2017–2020. PLoS ONE 2022, 17, e0264737. [Google Scholar] [CrossRef]
- Nguyen, P.T.T.; Pham, H.V.; Van, D.H.; Van Pham, L.; Nguyen, H.T.; Van Nguyen, H. Randomized controlled trial of the relative efficacy of high-dose intravenous ceftriaxone and oral cefixime combined with doxycycline for the treatment of Chlamydia trachomatis and Neisseria gonorrhoeae co-infection. BMC Infect. Dis. 2022, 22, 607. [Google Scholar] [CrossRef]
- Karim, S.; Bouchikhi, C.; Banani, A.; El Fatemi, H.; Souho, T.; Erraghay, S.; Bennani, B. Molecular Antimicrobial Resistance of Neisseria gonorrhoeae in a Moroccan Area. Infect. Dis. Obstet. Gynecol. 2018, 2018, 7263849. [Google Scholar] [CrossRef]
- Cole, M.J.; Quinten, C.; Jacobsson, S.; Day, M.; Amato-Gauci, A.J.; Woodford, N.; Spiteri, G.; Unemo, M.; Stary, A.; Haller, M.; et al. The European gonococcal antimicrobial surveillance programme (Euro-GASP) appropriately reflects the antimicrobial resistance situation for Neisseria gonorrhoeae in the European Union/European Economic Area. BMC Infect. Dis. 2019, 19, 1040. [Google Scholar] [CrossRef]
- Gonococcal Antimicrobial Susceptibility Surveillance in the European Union/European Economic Area. 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/gonococcal-antimicrobial-susceptibility-surveillance-2019 (accessed on 28 August 2022).
- Chisholm, S.A.; Unemo, M.; Quaye, N.; Johansson, E.; Cole, M.J.; Ison, C.A.; Van de Laar, M.J.W. Molecular epidemiological typing within the European Gonococcal Antimicrobial Resistance Surveillance Programme reveals predominance of a multidrugresistant clone. Eurosurveillance 2013, 18, 20358. [Google Scholar] [CrossRef]
- Harris, S.R.; Cole, M.J.; Spiteri, G.; Sánchez-Busó, L.; Golparian, D.; Jacobsson, S.; Goater, R.; Abudahab, K.; Yeats, C.A.; Bercot, B.; et al. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: A genomic survey. Lancet Infect. Dis. 2018, 18, 758–768. [Google Scholar] [CrossRef]
- Bignell, C.; Unemo, M. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int. J. STD AIDS 2013, 24, 85–92. [Google Scholar] [CrossRef]
- Cole, M.J.; Spiteri, G.; Jacobsson, S.; Woodford, N.; Tripodo, F.; Amato-Gauci, A.J.; Unemo, M.; Indra, A.; Maes, V.; Crucitti, T.; et al. Overall Low Extended-Spectrum Cephalosporin Resistance but high Azithromycin Resistance in Neisseria gonorrhoeae in 24 European Countries, 2015. BMC Infect. Dis. 2017, 17, 617. [Google Scholar] [CrossRef]
- Golparian, D.; Harris, S.R.; Sánchez-Busó, L.; Hoffmann, S.; Shafer, W.M.; Bentley, S.D.; Jensen, J.S.; Unemo, M. Genomic evolution of Neisseria gonorrhoeae since the preantibiotic era (1928-2013): Antimicrobial use/misuse selects for resistance and drives evolution. BMC Genomics 2020, 21, 116. [Google Scholar] [CrossRef]
- Unemo, M.; Ahlstrand, J.; Sánchez-Busó, L.; Day, M.; Aanensen, D.; Golparian, D.; Jacobsson, S.; Cole, M.J.; the European Collaborative Group; Torreblanca, R.A.; et al. High susceptibility to zoliflodacin and conserved target (GyrB) for zoliflodacin among 1209 consecutive clinical Neisseria gonorrhoeae isolates from 25 European countries, 2018. J. Antimicrob. Chemother. 2021, 76, 1221–1228. [Google Scholar] [CrossRef]
- Manoharan-Basil, S.S.; González, N.; Laumen, J.G.E.; Kenyon, C. Horizontal Gene Transfer of Fluoroquinolone Resistance-Conferring Genes From Commensal Neisseria to Neisseria gonorrhoeae: A Global Phylogenetic Analysis of 20,047 Isolates. Front. Microbiol. 2022, 13, 225. [Google Scholar] [CrossRef]
- Shaskolskiy, B.; Dementieva, E.; Kandinov, I.; Filippova, M.; Petrova, N.; Plakhova, X.; Chestkov, A.; Kubanov, A.; Deryabin, D.; Gryadunov, D. Resistance of Neisseria gonorrhoeae isolates to beta-lactam antibiotics (benzylpenicillin and ceftriaxone) in Russia, 2015–2017. PLoS ONE 2019, 14, e0220339. [Google Scholar] [CrossRef]
- Shaskolskiy, B.; Dementieva, E.; Leinsoo, A.; Petrova, N.; Chestkov, A.; Kubanov, A.; Deryabin, D.; Gryadunov, D. Tetracycline resistance of Neisseria gonorrhoeae in Russia, 2015–2017. Infect. Genet. Evol. 2018, 63, 236–242. [Google Scholar] [CrossRef]
- Kubanov, A.; Solomka, V.; Plakhova, X.; Chestkov, A.; Petrova, N.; Shaskolskiy, B.; Dementieva, E.; Leinsoo, A.; Gryadunov, D.; Deryabin, D. Summary and Trends of the Russian Gonococcal Antimicrobial Surveillance Programme, 2005 to 2016. J. Clin. Microbiol. 2019, 57, e02024-18. [Google Scholar] [CrossRef]
- Shaskolskiy, B.; Kandinov, I.; Kravtsov, D.; Vinokurova, A.; Gorshkova, S.; Filippova, M.; Kubanov, A.; Solomka, V.; Deryabin, D.; Dementieva, E.; et al. Hydrogel Droplet Microarray for Genotyping Antimicrobial Resistance Determinants in Neisseria gonorrhoeae Isolates. Polymers 2021, 13, 3889. [Google Scholar] [CrossRef] [PubMed]
- Pachulec, E.; van der Does, C. Conjugative Plasmids of Neisseria gonorrhoeae. PLoS ONE 2010, 5, e9962. [Google Scholar] [CrossRef] [PubMed]
- Rotman, E.; Seifert, H.S. The Genetics of Neisseria Species. Annu. Rev. Genet. 2014, 48, 405–431. [Google Scholar] [CrossRef] [PubMed]
- Aniskevich, A.; Shimanskaya, I.; Boiko, I.; Golubovskaya, T.; Golparian, D.; Stanislavova, I.; Jacobsson, S.; Adaskevich, A.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae isolates and gonorrhoea treatment in the Republic of Belarus, Eastern Europe, 2009–2019. BMC Infect. Dis. 2021, 21, 520. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Y.; Wang, Y.; Martin, I.; Demczuk, W.; Gu, W. Shanghai Neisseria gonorrhoeae Isolates Exhibit Resistance to Extended-Spectrum Cephalosporins and Clonal Distribution. Front. Microbiol. 2020, 11, 2392. [Google Scholar] [CrossRef]
- Sarafian, S.K.; Genco, C.A.; Roberts, M.C.; Knapp, J.S. Acquisition of beta-lactamase and TetM-containing conjugative plasmids by phenotypically different strains of Neisseria gonorrhoeae. Sex. Transm. Dis. 1990, 17, 67–71. [Google Scholar] [CrossRef]
- Li, X.; Le, W.; Lou, X.; Genco, C.A.; Rice, P.A.; Su, X. In Vitro Activity of Ertapenem against Neisseria gonorrhoeae Clinical Isolates with Decreased Susceptibility or Resistance to Extended-Spectrum Cephalosporins in Nanjing, China (2013 to 2019). Antimicrob. Agents Chemother. 2022, 66, e00109-22. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radovanovic, M.; Kekic, D.; Jovicevic, M.; Kabic, J.; Gajic, I.; Opavski, N.; Ranin, L. Current Susceptibility Surveillance and Distribution of Antimicrobial Resistance in N. gonorrheae within WHO Regions. Pathogens 2022, 11, 1230. https://doi.org/10.3390/pathogens11111230
Radovanovic M, Kekic D, Jovicevic M, Kabic J, Gajic I, Opavski N, Ranin L. Current Susceptibility Surveillance and Distribution of Antimicrobial Resistance in N. gonorrheae within WHO Regions. Pathogens. 2022; 11(11):1230. https://doi.org/10.3390/pathogens11111230
Chicago/Turabian StyleRadovanovic, Marina, Dusan Kekic, Milos Jovicevic, Jovana Kabic, Ina Gajic, Natasa Opavski, and Lazar Ranin. 2022. "Current Susceptibility Surveillance and Distribution of Antimicrobial Resistance in N. gonorrheae within WHO Regions" Pathogens 11, no. 11: 1230. https://doi.org/10.3390/pathogens11111230
APA StyleRadovanovic, M., Kekic, D., Jovicevic, M., Kabic, J., Gajic, I., Opavski, N., & Ranin, L. (2022). Current Susceptibility Surveillance and Distribution of Antimicrobial Resistance in N. gonorrheae within WHO Regions. Pathogens, 11(11), 1230. https://doi.org/10.3390/pathogens11111230






