Recent Advances in the Rheumatic Fever and Rheumatic Heart Disease Continuum
Abstract
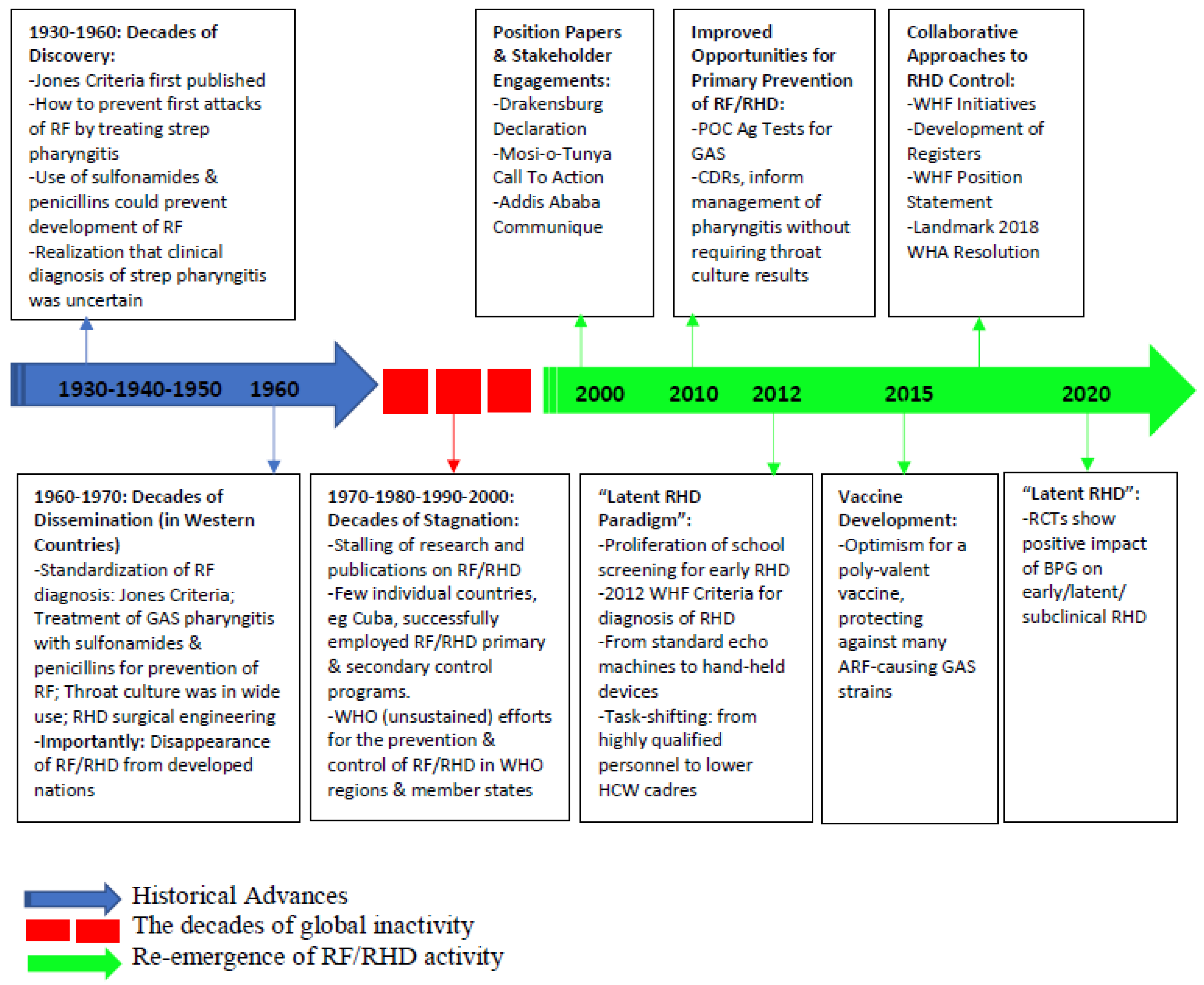
1. Rheumatic Fever and Rheumatic Heart Disease: A Historical Background
1.1. Decades of Discovery: 1930s–1950s
1.2. Decades of Dissemination: 1960s–1970s
1.3. Decades of Stagnation: 1970s–2000s
1.4. The Re-Awakening: From 2000 to Date
2. Advances in Understanding the Global Burden of RHD
2.1. Screening Echocardiography (Echo)
2.2. RHD Registries and Big Data
3. Advances in Understanding RF/RHD Pathogenesis
4. Advances in Understanding the Genetic Predisposition to RF/RHD
5. Advances in the RF/RHD Continuum
5.1. Advances in Primordial Prevention
Group A Streptococcus Vaccines
5.2. Advances in Primary Prevention
5.2.1. Diagnosis of Group A Strep Pharyngitis
5.2.2. Treatment of Group A Strep Pharyngitis
5.3. Advances in Secondary Prevention
5.4. Advances in Tertiary Prevention
5.4.1. Valve Surgery vs. Valve Repair
5.4.2. From Valvotomy to Balloon Mitral Valvuloplasty (BMV)
5.4.3. Transcatheter Aortic Valve Replacement (TAVR) for Rheumatic Aortic Stenosis
5.4.4. Medical Management of Clinical RHD
6. Rheumatic Heart Disease in Pregnancy
7. Other Recent Advances
7.1. Understanding of RHD-HIV Co-Infection
7.2. Understanding of RHD-Associated Costs in Endemic Regions
7.3. Global Efforts, Advocacy, and Stakeholder Engagement in the Fight against RHD
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferretti, J.; Köhler, W. History of Streptococcal Research Streptococcus pyogenes: Basic Biology to Clinical Manifestations; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Blevins, S.M.; Bronze, M.S. Robert Koch and the ‘golden age’ of bacteriology. Int. J. Infect. Dis. 2010, 14, e744–e751. [Google Scholar] [CrossRef]
- Armstrong, D.; Wheatley, G. Studies in Rheumatic Fever; Metropolitan Life Ins Co.: New York, NY, USA, 1944. [Google Scholar]
- Bland, E.F. Rheumatic fever: The way it was. Circulation 1987, 76, 1190–1195. [Google Scholar] [CrossRef]
- Swift, H.F. The Etiologyof Rheumatic Fever. Ann. Intern. Med. 1949, 31, 715–738. [Google Scholar] [CrossRef]
- Dajani, A.S.; Ayoub, E.; Bierman, F.Z.; Bisno, A.L.; Denny, F.W.; Durack, D.T.; Ferrieri, P.; Freed, M.; Gerber, M.; Kaplan, E.L. Guidelines for the diagnosis of rheumatic fever: Jones criteria, 1992 update. JAMA 1992, 268, 2069–2073. [Google Scholar] [CrossRef]
- Gewitz, M.H.; Baltimore, R.S.; Tani, L.Y.; Sable, C.A.; Shulman, S.T.; Carapetis, J.; Remenyi, B.; Taubert, K.A.; Bolger, A.F.; Beerman, L. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: A scientific statement from the American Heart Association. Circulation 2015, 131, 1806–1818. [Google Scholar] [CrossRef]
- Beaton, A.; Carapetis, J. The 2015 revision of the Jones criteria for the diagnosis of acute rheumatic fever: Implications for practice in low-income and middle-income countries. Heart Asia 2015, 7, 7–11. [Google Scholar] [CrossRef]
- Forbes, G.B.; Forbes, G.M. An historical note on chemotherapy of bacterial infections. Am. J. Dis. Child. 1970, 119, 6–8. [Google Scholar] [CrossRef]
- Hajar, R. Rheumatic fever and rheumatic heart disease a historical perspective. Heart Views 2016, 17, 120–126, Correction: Heart Views 2017, 18, 39. [Google Scholar] [CrossRef]
- Markowitz, M.; Hemphill, W. A comparison of oral benzathine penicillin g and sulfonamides for the prevention of streptococcal infections and recurrences of rheumatic fever. Pediatrics 1955, 15, 509–515. [Google Scholar] [CrossRef]
- Houser, H.B.; Clark, E.J.; Stolzer, B.L. Comparative effects of aspirin, ACTH and cortisone on the acute course of rheumatic fever in young adult males. Am. J. Med. 1954, 16, 168–180. [Google Scholar] [CrossRef]
- Denny, F.W.; Wannamaker, L.W.; Brink, W.R.; Rammelkamp, C.H.; Custer, E.A. Prevention of rheumatic fever: Treatment of the preceding streptococcic infection. J. Am. Med. Assoc. 1950, 143, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Wannamaker, L.W.; Denny, F.W.; Perry, W.D.; Rammelkamp, C.H., Jr.; Eckhardt, G.C.; Houser, H.B.; Hahn, E.O. The effect of penicillin prophylaxis on streptococcal disease rates and the carrier state. N. Engl. J. Med. 1953, 249, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stollerman, G.H. The use of antibiotics for the prevention of rheumatic fever. Am. J. Med. 1954, 17, 757–767. [Google Scholar] [CrossRef]
- Stollerman, G.H.; Rusoff, J.H.; Hirschfeld, I. Prophylaxis against group A streptococci in rheumatic fever: The use of single monthly injections of benzathine penicillin G. N. Engl. J. Med. 1955, 252, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Gale, A.; Gillespie, W.; Perry, C. Oral penicillin in the prophylaxis of streptococcal infection in rheumatic children. Lancet 1952, 260, 61–63. [Google Scholar] [CrossRef]
- Elias, W.; Price, A.H.; Merrion, H.J. N, N’-dibenzylethylenediamine penicillin: A new repository form of penicillin. Antibiot. Chemother. 1951, 1, 491–498. [Google Scholar]
- Stollerman, G.H.; Rusoff, J.H. Prophylaxis against group A streptococcal infections in rheumatic fever patients: Use of new repository penicillin preparation. J. Am. Med. Assoc. 1952, 150, 1571–1575. [Google Scholar] [CrossRef]
- Diehl, A.M.; Hamilton, T.R.; Keeling, I.C.; May, J.S. Long-acting repository penicillin in prophylaxis of recurrent rheumatic fever. J. Am. Med. Assoc. 1954, 155, 1466–1470. [Google Scholar] [CrossRef]
- Perry, C.B.; Gillespie, W. Intramuscular benzathene penicillin in the prophylaxis of streptococcal infection in rheumatic children. Br. Med. J. 1954, 2, 729. [Google Scholar] [CrossRef]
- Mccue, C.M.; Gibson, C.D., Jr.; Linde-mann, L.C. A comparison of intramuscular benzathine penicillin and oral sulfonamide in the control of rheumatic recurrences. J. Pediatrics 1955, 47, 450–460. [Google Scholar] [CrossRef]
- Markowitz, M.; Ferencz, C.; Bonet, A. A comparison of oral and intramuscular benzathine penicillin G for the prevention of streptococcal infections and recurrences of rheumatic fever. Pediatrics 1957, 19, 201–207. [Google Scholar] [CrossRef]
- Markowitz, M. The decline of rheumatic fever: Role of medical intervention: Lewis W. Wannamaker Memorial Lecture. J. Pediatr. 1985, 106, 545–550. [Google Scholar] [CrossRef]
- Gordis, L. Effectiveness of Comprehensive-Care Programs in Preventing Rheumatic Fever. N. Engl. J. Med. 1973, 289, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Gordis, L. The virtual disappearance of rheumatic fever in the United States: Lessons in the rise and fall of disease. T. Duckett Jones memorial lecture. Circulation 1985, 72, 1155–1162. [Google Scholar] [CrossRef]
- Beaton, A.; Taubert, K.A. Rheumatic Fever and the American Heart Association: The (Nearly) 100-Year War. Circulation 2021, 143, 2127–2128. [Google Scholar] [CrossRef]
- Gordis, L. Changing risk of rheumatic fever. In Management of Streptococcal pharyngitis in an Era of Declining Rheumatic Fever; Praeger Publishers: New York, NY, USA, 1984; p. 1322. [Google Scholar]
- Land, M.A.; Bisno, A.L. Acute rheumatic fever: A vanishing disease in suburbia. JAMA 1983, 249, 895–898. [Google Scholar] [CrossRef]
- Mohs, E. Infectious diseases and health in Costa Rica: The development of a new paradigm. Pediatric Infect. Dis. J. 1982, 1, 212. [Google Scholar] [CrossRef]
- Carapetis, J.R. Focus on research: Rheumatic heart disease in developing countries. N. Engl. J. Med. 2007, 357, 439–441. [Google Scholar] [CrossRef]
- Prevention WECoC and Diseases CoC. Community Prevention and Control of Cardiovascular Diseases: Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1986. [Google Scholar]
- World Health Organization. Community control of rheumatic heart disease in developing countries. 1. A major public health problem. WHO Chron. 1980, 34, 336–345. [Google Scholar]
- Strasser, T.; Dondog, N.; El Kholy, A.; Gharagozloo, R.; Kalbian, V.V.; Ogunbi, O.; Padmavati, S.; Stuart, K.; Dowd, E.; Bekessy, A. The community control of rheumatic fever and rheumatic heart disease: Report of a WHO international cooperative project. Bull. World Health Organ. 1981, 59, 285–294. [Google Scholar]
- World Health Organization. Rheumatic fever and rheumatic heart disease: Report of a WHO expert consultation. World Health Organ. Tech. Rep. Ser. 2004, 923, 1–122. [Google Scholar]
- Nordet, P. WHO programme for the prevention of rheumatic fever/rheumatic heart disease in 16 developing countries: Report from phase I (1986–90). Bull. World Health Organ. 1992, 70, 213–219. [Google Scholar]
- Marijon, E.; Ou, P.; Celermajer, D.S.; Ferreira, B.; Mocumbi, A.; Jani, D.; Paquet, C.; Jacob, S.; Sidi, D.; Jouven, X. Prevalence of Rheumatic Heart Disease Detected by Echocardiographic Screening. N. Engl. J. Med. 2007, 357, 470–476. [Google Scholar] [CrossRef]
- Sadiq, M.; Islam, K.; Abid, R.; Latif, F.; Rehman, A.U.; Waheed, A.; Azhar, M.; Khan, J.S. Prevalence of rheumatic heart disease in school children of urban Lahore. Heart 2009, 95, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Hardy, M.; Fakakovikaetau, T.; Taib, R.; Wilkinson, L.; Penny, D.J.; Steer, A.C. Evaluation of a screening protocol using auscultation and portable echocardiography to detect asymptomatic rheumatic heart disease in Tongan schoolchildren. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Paar, J.A.; Berrios, N.M.; Rose, J.D.; Cáceres, M.; Peña, R.; Pérez, W.; Chen-Mok, M.; Jolles, E.; Dale, J.B. Prevalence of Rheumatic Heart Disease in Children and Young Adults in Nicaragua. Am. J. Cardiol. 2010, 105, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Bhaya, M.; Panwar, S.; Beniwal, R.; Panwar, R.B. High prevalence of rheumatic heart disease detected by echocardiography in school children. Echocardiography 2010, 27, 448–453. [Google Scholar] [CrossRef]
- Mayosi, B.M. The Four Pillars of Rheumatic Heart Disease Control. S. Afr. Med. J. 2010, 100, 506. [Google Scholar] [CrossRef]
- Beaton, A.; Sable, C. Reducing rheumatic heart disease in Africa—Time for action. Nat. Rev. Cardiol. 2016, 13, 190–191. [Google Scholar] [CrossRef]
- Mayosi, B.; Robertson, K.; Volmink, J.; Adebo, W.; Akinyore, K.; Amoah, A.; Bannerman, C.; Biesman-Simons, S.; Carapetis, J.; Cilliers, A.; et al. The Drakensberg declaration on the control of rheumatic fever and rheumatic heart disease in Africa. S. Afr. Med. J. 2006, 96, 246. [Google Scholar]
- Mayosi, B.M.; Gamra, H.; Dangou, J.-M.; Kasonde, J. Rheumatic heart disease in Africa: The Mosi-o-Tunya call to action. Lancet Glob. Health 2014, 2, e438–e439. [Google Scholar] [CrossRef]
- Watkins, D.; Zuhlke, L.; Engel, M.; Daniels, R.; Francis, V.; Shaboodien, G.; Mayosi, B.M.; Kango, M.; Abul-Fadl, A.; Adeoye, A. Seven key actions to eradicate rheumatic heart disease in Africa: The Addis Ababa communiqué: Cardiovascular topics. Cardiovasc. J. Afr. 2016, 27, 184–187. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 71st World Health Assembly Adopts Resolution Calling for Greater Action on Rheumatic Heart Disease; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Reményi, B.; Wilson, N.; Steer, A.; Ferreira, B.; Kado, J.; Kumar, K.; Lawrenson, J.; Maguire, G.; Marijon, E.; Mirabel, M.; et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat. Rev. Cardiol. 2012, 9, 297–309. [Google Scholar] [CrossRef]
- Hunter, L.D.; Monaghan, M.; Lloyd, G.; Pecoraro, A.J.K.; Doubell, A.F.; Herbst, P.G. Screening for rheumatic heart disease: Is a paradigm shift required? Echo Res. Pract. 2017, 4, R43–R52. [Google Scholar] [CrossRef]
- Recommendation No. R (94) 11 on Screening as Tool of Preventive Medicine. 10 October 1994. Available online: http://hrlibrary.umn.edu/instree/coerecr94-11.html (accessed on 15 October 2021).
- Beaton, A.Z.; Okello, E.; Rwebembera, J.; Grobler, A.; Engelman, D.; Carapetis, J.; Canales, L.; Dewyer, A.; Lwabi, P.; Mirabel, M. A Randomized Controlled Trial of Secondary Antibiotic Prophylaxis for Latent Rheumatic Heart Disease. Circulation 2021, 144, A12503. [Google Scholar]
- Ali, F.; Hasan, B.; Ahmad, H.; Hoodbhoy, Z.; Bhuriwala, Z.; Hanif, M.; Ansari, S.U.; Chowdhury, D. Detection of subclinical rheumatic heart disease in children using a deep learning algorithm on digital stethoscope: A study protocol. BMJ Open 2021, 11, e044070. [Google Scholar] [CrossRef]
- Beaton, A.; Nascimento, B.R.; Diamantino, A.C.; Pereira, G.T.; Lopes, E.L.; Miri, C.O.; Bruno, K.K.; Chequer, G.; Ferreira, C.G.; Lafeta, L.C.; et al. Efficacy of a Standardized Computer-Based Training Curriculum to Teach Echocardiographic Identification of Rheumatic Heart Disease to Nonexpert Users. Am. J. Cardiol. 2016, 117, 1783–1789. [Google Scholar] [CrossRef]
- Nascimento, B.R.; Martins, J.F.B.; Nascimento, E.R.; Pappa, G.L.; Sable, C.A.; Beaton, A.Z.; Gomes, P.R.; Nunes, M.C.; Oliveira, K.K.; Franco, J.; et al. Deep learning for automatic identification of rheumatic heart disease in echocardiographic screening images: Data from the atmosphere-provar study. J. Am. Coll. Cardiol. 2020, 75, 3577. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Sable, C.; Nascimento, B.R.; de Lima, E.M.; da Silva, J.L.P.; Diamantino, A.C.; Oliveira, K.K.; Okello, E.; Aliku, T.; Lwabi, P.; et al. Simplified Echocardiography Screening Criteria for Diagnosing and Predicting Progression of Latent Rheumatic Heart Disease. Circ. Cardiovasc. Imaging 2019, 12, e007928. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.A.; Johnson, C.O.; Colquhoun, S.; Karthikeyan, G.; Beaton, A.; Bukhman, G.; Forouzanfar, M.H.; Longenecker, C.T.; Mayosi, B.M.; Mensah, G.A.; et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. New Engl. J. Med. 2017, 377, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, S.; Kalçık, M.; Gürsoy, M.O.; Güner, A.; Özkan, M. Diagnosis, treatment and management of prosthetic valve thrombosis: The key considerations. Expert Rev. Med Devices 2020, 17, 209–221. [Google Scholar] [CrossRef]
- Damasceno, A.; Mayosi, B.M.; Sani, M.; Ogah, O.S.; Mondo, C.; Ojji, D.; Dzudie, A.; Kouam, C.K.; Suliman, A.; Schrueder, N. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries: Results of the sub-Saharan Africa survey of heart failure. Arch. Intern. Med. 2012, 172, 1386–1394. [Google Scholar] [CrossRef]
- Kingué, S.; Ba, S.A.; Balde, D.; Diarra, M.B.; Anzouan-Kacou, J.-B.; Anisubia, B.; Damorou, J.-M.; Ndobo, P.; Menanga, A.; Kane, A.; et al. The VALVAFRIC study: A registry of rheumatic heart disease in Western and Central Africa. Arch. Cardiovasc. Dis. 2016, 109, 321–329. [Google Scholar] [CrossRef]
- Zuhlke, L.; Engel, M.; Karthikeyan, G.; Rangarajan, S.; Mackie, P.; Cupido, B.; Mauff, K.; Islam, S.; Joachim, A.; Daniels, R.; et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: The Global Rheumatic Heart Disease Registry (the REMEDY study). Eur. Hear. J. 2015, 36, 1115–1122. [Google Scholar] [CrossRef]
- Fabi, M.; Calicchia, M.; Palleri, D.; Ndikubwimana, I.; Conard, C.; Kamanzi, E.R.; Balducci, A.; Frabboni, I.; Dondi, A.; Gargiulo, G.; et al. Pediatric rheumatic carditis in Italy and Rwanda: The same disease, different socio-economic settings. Int. J. Cardiol. 2021, 338, 154–160. [Google Scholar] [CrossRef]
- Okello, E.; Wanzhu, Z.; Musoke, C.; Kakande, B.; Mondo, C.K.; Freers, J.; Twalib, A.; Lwabi, P.; Wilson, N.B.; Odoi-Adome, R. Cardiovascular complications in newly diagnosed rheumatic heart disease patients at Mulago Hospital, Uganda: Cardiovascular topics. Cardiovasc. J. Afr. 2013, 24, 82. [Google Scholar] [CrossRef]
- Essop, M.R.; Nkomo, V.T. Rheumatic and nonrheumatic valvular heart disease: Epidemiology, management, and prevention in Africa. Circulation 2005, 112, 3584–3591. [Google Scholar] [CrossRef]
- Scheel, A.; Ssinabulya, I.; Aliku, T.; Bradley-Hewitt, T.; Clauss, A.; Clauss, S.; Crawford, L.; Dewyer, A.; Donofrio, M.T.; Jacobs, M.; et al. Community study to uncover the full spectrum of rheumatic heart disease in Uganda. Heart 2018, 105, 60–66. [Google Scholar] [CrossRef]
- Baker, M.G.; Gurney, J.; Oliver, J.; Moreland, N.J.; A Williamson, D.; Pierse, N.; Wilson, N.; Merriman, T.R.; Percival, T.; Murray, C.; et al. Risk Factors for Acute Rheumatic Fever: Literature Review and Protocol for a Case-Control Study in New Zealand. Int. J. Environ. Res. Public Health 2019, 16, 4515. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.M.; Bond-Smith, D.; Seth, R.J.; Dempsey, K.; Cannon, J.; Stacey, I.; Wade, V.; de Klerk, N.; Greenland, M.; Sanfilippo, F.M.; et al. Contemporary Incidence and Prevalence of Rheumatic Fever and Rheumatic Heart Disease in Australia Using Linked Data: The Case for Policy Change. J. Am. Heart Assoc. 2020, 9, e016851. [Google Scholar] [CrossRef] [PubMed]
- Rwebembera, J.; Beaton, A.Z.; de Loizaga, S.R.; Rocha, R.T.; Doreen, N.; Ssinabulya, I.; Okello, E.; Fraga, C.L.; Galdino, B.F.; Nunes, M.C.P. The Global Impact of Rheumatic Heart Disease. Curr. Cardiol. Rep. 2021, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Beaton, A.; Cunningham, M.W.; Guilherme, L.; Karthikeyan, G.; Mayosi, B.M.; Sable, C.; Steer, A.; Wilson, N.; Wyber, R. Acute rheumatic fever and rheumatic heart disease. Nat. Rev. Dis. Primers 2016, 2, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Langshaw, E.; Hartas, J.; Lam, A.; Batzloff, M.R.; Good, M.F. A Synthetic M Protein Peptide Synergizes with a CXC Chemokine Protease To Induce Vaccine-Mediated Protection against Virulent Streptococcal Pyoderma and Bacteremia. J. Immunol. 2015, 194, 5915–5925. [Google Scholar] [CrossRef] [PubMed]
- Parks, T.; Smeesters, P.R.; Steer, A.C. Streptococcal skin infection and rheumatic heart disease. Curr. Opin. Infect. Dis. 2012, 25, 145–153. [Google Scholar] [CrossRef]
- Sikder, S.; Williams, N.L.; Sorenson, A.; Alim, A.; E Vidgen, M.; Moreland, N.; Rush, C.M.; Simpson, R.S.; Govan, B.L.; Norton, R.; et al. Group G Streptococcus Induces an Autoimmune Carditis Mediated by Interleukin 17A and Interferon γ in the Lewis Rat Model of Rheumatic Heart Disease. J. Infect. Dis. 2017, 218, 324–335. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Moreland, N.; Webb, R.H.; Upton, A.; Wilson, N.J. Acute Rheumatic Fever After Group A Streptococcus Pyoderma and Group G Streptococcus Pharyngitis. Pediatr. Infect. Dis. J. 2017, 36, 692–694. [Google Scholar] [CrossRef]
- Sikder, S. Determination of the Role of Group G Streptococcus in the Pathogenesis of Rheumatic Heart Disease Using a Rat Model. Ph.D. Thesis, James Cook University, Douglas, Australia, 2018. [Google Scholar]
- Essop, M.R.; Peters, F. Contemporary Issues in Rheumatic Fever and Chronic Rheumatic Heart Disease. Circulation 2014, 130, 2181–2188. [Google Scholar] [CrossRef]
- Dooley, L.M.; Ahmad, T.B.; Pandey, M.; Good, M.F.; Kotiw, M. Rheumatic heart disease: A review of the current status of global research activity. Autoimmun. Rev. 2020, 20, 102740. [Google Scholar] [CrossRef]
- Tashima, Y.; Stanley, P. Antibodies that detect O-linked β-DN-acetylglucosamine on the extracellular domain of cell surface glycoproteins. J. Biol. Chem. 2014, 289, 11132–11142. [Google Scholar] [CrossRef] [PubMed]
- Gorton, D.; Sikder, S.; Williams, N.L.; Chilton, L.; Rush, C.M.; Govan, B.L.; Cunningham, M.W.; Ketheesan, N. Repeat exposure to group A streptococcal M protein exacerbates cardiac damage in a rat model of rheumatic heart disease. Autoimmunity 2016, 49, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Dinkla, K.; Rohde, M.; Jansen, W.T.; Kaplan, E.L.; Chhatwal, G.S.; Talay, S.R. Rheumatic fever–associated Streptococcus pyogenes isolates aggregate collagen. J. Clin. Investig. 2003, 111, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Dinkla, K.; Talay, S.R.; Mörgelin, M.; Graham, R.M.; Rohde, M.; Nitsche-Schmitz, D.P.; Chhatwal, G.S. Crucial role of the CB3-region of collagen IV in PARF-induced acute rheumatic fever. PLoS ONE 2009, 4, e4666. [Google Scholar] [CrossRef]
- Guilherme, L.; Köhler, K.F.; Kalil, J. Rheumatic heart disease: Mediation by complex immune events. Adv. Clin. Chem. 2011, 53, 31–50. [Google Scholar]
- Ellis, N.M.J.; Li, Y.; Hildebrand, W.; Fischetti, V.A.; Cunningham, M.W. T Cell Mimicry and Epitope Specificity of Cross-Reactive T Cell Clones from Rheumatic Heart Disease. J. Immunol. 2005, 175, 5448–5456. [Google Scholar] [CrossRef]
- Guilherme, L.; Köhler, K.; Postol, E.; Kalil, J. Genes, autoimmunity and pathogenesis of rheumatic heart disease. Ann. Pediatr. Cardiol. 2011, 4, 13–21. [Google Scholar] [CrossRef]
- Guilherme, L.; Kalil, J. Rheumatic Fever and Rheumatic Heart Disease: Cellular Mechanisms Leading Autoimmune Reactivity and Disease. J. Clin. Immunol. 2010, 30, 17–23. [Google Scholar] [CrossRef]
- Toor, D.; Sharma, N. T cell subsets: An integral component in pathogenesis of rheumatic heart disease. Immunol. Res. 2017, 66, 18–30. [Google Scholar] [CrossRef]
- Excler, J.-L.; Kim, J.H. Accelerating the development of a group A Streptococcus vaccine: An urgent public health need. Clin. Exp. Vaccine Res. 2016, 5, 101–107. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Abu-Madi, M. The genetic control of the rheumatic heart: Closing the genotype-phenotype gap. Front. Med. 2021, 8, 611036. [Google Scholar] [CrossRef] [PubMed]
- Bryant, P.A.; Robins-Browne, R.; Carapetis, J.R.; Curtis, N. Some of the people, some of the time: Susceptibility to acute rheumatic fever. Circulation 2009, 119, 742–753. [Google Scholar] [CrossRef]
- Engel, M.E.; Stander, R.; Vogel, J.; Adeyemo, A.A.; Mayosi, B.M. Genetic Susceptibility to Acute Rheumatic Fever: A Systematic Review and Meta-Analysis of Twin Studies. PLoS ONE 2011, 6, e25326. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Steer, A.C.; Smeesters, P.; Keeble, J.; Inouye, M.; Carapetis, J.; Wicks, I.P. Post-infectious group A streptococcal autoimmune syndromes and the heart. Autoimmun. Rev. 2015, 14, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Muhamed, B.; Parks, T.; Sliwa, K. Genetics of rheumatic fever and rheumatic heart disease. Nat. Rev. Cardiol. 2020, 17, 145–154. [Google Scholar] [CrossRef]
- Auckland, K.; Mittal, B.; Cairns, B.J.; Garg, N.; Kumar, S.; Mentzer, A.J.; Kado, J.; Perman, M.L.; Steer, A.C.; Hill, A.V.S.; et al. The Human Leukocyte Antigen Locus and Rheumatic Heart Disease Susceptibility in South Asians and Europeans. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- El-Hagrassy, N.; El-Chennawi, F.; Zaki, M.E.-S.; Fawzy, H.; Zaki, A.; Joseph, N. HLA Class I and Class II HLA DRB Profiles in Egyptian Children with Rheumatic Valvular Disease. Pediatr. Cardiol. 2010, 31, 650–656. [Google Scholar] [CrossRef]
- Ayoub, E.M.; Barrett, D.J.; MacLaren, N.K.; Krischer, J.P. Association of class II human histocompatibility leukocyte antigens with rheumatic fever. J. Clin. Investig. 1986, 77, 2019–2026. [Google Scholar] [CrossRef]
- Guilherme, L.; Weidebach, W.; Kiss, M.H.; Snitcowsky, R.; Kalil, J. Association of human leukocyte class II antigens with rheumatic fever or rheumatic heart disease in a Brazilian population. . Circulation 1991, 83, 1995–1998. [Google Scholar] [CrossRef]
- Okello, E.; Beaton, A.; Mondo, C.K.; Kruszka, P.; Kiwanuka, N.; Odoi-Adome, R.; Freers, J. Rheumatic heart disease in Uganda: The association between MHC class II HLA DR alleles and disease: A case control study. BMC Cardiovasc. Disord. 2014, 14, 1–5. [Google Scholar] [CrossRef]
- Nana, M.L.A.; Anderson, J.L.; Carlquist, J.F.; Nanas, J.N. HLA-DR typing and lymphocyte subset evaluation in rheumatic heart disease: A search for immune response factors. Am. Hear. J. 1986, 112, 992–997. [Google Scholar] [CrossRef]
- Akkaya, E.; Berkowitsch, A.; Rieth, A.; Erkapic, D.; Hamm, C.W.; Neumann, T.; Kuniss, M. Clinical outcome and left atrial function after left atrial roof ablation using the cryoballoon technique in patients with symptomatic persistent atrial fibrillation. Int. J. Cardiol. 2019, 292, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tormin, J.P.; Nascimento, B.R.; Sable, C.A.; da Silva, J.L.P.; Brandao-De-Resende, C.; Rocha, L.P.C.; Pinto, C.H.; Neves, E.G.A.; Macedo, F.V.; Fraga, C.L.; et al. Cytokine gene functional polymorphisms and phenotypic expression as predictors of evolution from latent to clinical rheumatic heart disease. Cytokine 2021, 138, 155370. [Google Scholar] [CrossRef] [PubMed]
- Parks, T.; Network, P.I.R.H.D.G.; Mirabel, M.M.; Kado, J.; Auckland, K.; Nowak, J.; Rautanen, A.; Mentzer, A.J.; Marijon, E.; Jouven, X.; et al. Association between a common immunoglobulin heavy chain allele and rheumatic heart disease risk in Oceania. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Gray, L.-A.; D’Antoine, H.A.; Tong, S.Y.C.; McKinnon, M.; Bessarab, D.; Brown, N.; Reményi, B.; Steer, A.; Syn, G.; Blackwell, J.M.; et al. Genome-Wide Analysis of Genetic Risk Factors for Rheumatic Heart Disease in Aboriginal Australians Provides Support for Pathogenic Molecular Mimicry. J. Infect. Dis. 2017, 216, 1460–1470. [Google Scholar] [CrossRef]
- Machipisa, T.; Chong, M.; Muhamed, B.; Chishala, C.; Shaboodien, G.; Pandie, S.; de Vries, J.; Laing, N.; Joachim, A.; Daniels, R. Association of Novel Locus With Rheumatic Heart Disease in Black African Individuals: Findings From the RHDGen Study. JAMA Cardiol. 2021, 6, 1000–1011. [Google Scholar] [CrossRef]
- Visscher, P.M.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 years of GWAS discovery: Biology, function, and translation. Am. J. Hum. Genet. 2017, 101, 5–22. [Google Scholar] [CrossRef]
- Coffey, P.M.; Ralph, A.P.; Krause, V.L. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: A systematic review. PLoS Negl. Trop. Dis. 2018, 12, e0006577. [Google Scholar] [CrossRef]
- Franco, J.; Nascimento, B.R.; Oliveira, K.; Barbosa, M.; Arantes, N.F.; Beaton, A.Z.; Mello, L.A.; Nascif, M.C.L.; Oliveira, G.C.; Camargos, M.; et al. Evaluation of Family Risk of Rheumatic Heart Disease With Systematic Echocardiographic Screening: Data From the Provar+ Study. Circulation 2021, 144, A11802. [Google Scholar]
- Culliford-Semmens, N.; Tilton, E.; Wilson, N.; Stirling, J.; Doughty, R.; Gentles, T.; Peat, B.; Dimalapang, E.; Webb, R. Echocardiography for latent rheumatic heart disease in first degree relatives of children with acute rheumatic fever: Implications for active case finding in family members. EClinicalMedicine 2021, 37, 100935. [Google Scholar] [CrossRef]
- Wyber, R.; Noonan, K.; Halkon, C.; Enkel, S.; Cannon, J.; Haynes, E.; Mitchell, A.G.; Bessarab, D.C.; Katzenellenbogen, J.M.; Bond-Smith, D.; et al. Ending rheumatic heart disease in Australia: The evidence for a new approach. Med. J. Aust. 2020, 213, S3–S31. [Google Scholar] [CrossRef] [PubMed]
- Azuar, A.; Jin, W.; Mukaida, S.; Hussein, W.M.; Toth, I.; Skwarczynski, M. Recent Advances in the Development of Peptide Vaccines and Their Delivery Systems Against Group A Streptococcus. Vaccines 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B.; Walker, M.J. Update on group A streptococcal vaccine development. Curr. Opin. Infect. Dis. 2020, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Osowicki, J.; Azzopardi, K.I.; Baker, C.; Waddington, C.S.; Pandey, M.; Schuster, T.; Grobler, A.; Cheng, A.C.; Pollard, A.J.; McCarthy, J.; et al. Controlled human infection for vaccination against Streptococcus pyogenes (CHIVAS): Establishing a group A Streptococcus pharyngitis human infection study. Vaccine 2019, 37, 3485–3494. [Google Scholar] [CrossRef]
- Vekemans, J.; Gouvea-Reis, F.; Kim, J.H.; Excler, J.L.; Smeesters, P.R.; O’Brien, K.L.; Van Beneden, C.A.; Steer, A.C.; Carapetis, J.R.; Kaslow, D.C. The Path to Group A Streptococcus Vaccines: World Health Organization Research and Development Technology Roadmap and Preferred Product Characteristics. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, 877–883. [Google Scholar] [CrossRef]
- Wright, C.M.; Langworthy, K.; Manning, L. The Australian burden of invasive group A streptococcal disease: A narrative review. Intern. Med. J. 2021, 51, 835–844. [Google Scholar] [CrossRef]
- McNeil, S.A.; Halperin, S.A.; Langley, J.; Smith, B.; Baxendale, D.M.; Warren, A.; Sharratt, G.P.; Reddish, M.A.; Fries, L.F.; Vink, P.; et al. A double-blind, randomized phase II trial of the safety and immunogenicity of 26-valent group A streptococcus vaccine in healthy adults. Int. Congr. Ser. 2006, 1289, 303–306. [Google Scholar] [CrossRef]
- McNeil, S.A.; Halperin, S.A.; Langley, J.M.; Smith, B.; Warren, A.; Sharratt, G.P.; Baxendale, D.M.; Reddish, M.A.; Hu, M.C.; Stroop, S.D.; et al. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, 1114–1122. [Google Scholar] [CrossRef]
- Pastural, É.; McNeil, S.A.; MacKinnon-Cameron, D.; Ye, L.; Langley, J.M.; Stewart, R.; Martin, L.H.; Hurley, G.J.; Salehi, S.; Penfound, T.A.; et al. Safety and immunogenicity of a 30-valent M protein-based group a streptococcal vaccine in healthy adult volunteers: A randomized, controlled phase I study. Vaccine 2020, 38, 1384–1392. [Google Scholar] [CrossRef]
- Sekuloski, S.; Batzloff, M.R.; Griffin, P.; Parsonage, W.; Elliott, S.; Hartas, J.; O’Rourke, P.; Marquart, L.; Pandey, M.; Rubin, F.A.; et al. Evaluation of safety and immunogenicity of a group A streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLOS ONE 2018, 13, e0198658. [Google Scholar] [CrossRef]
- De Sá-Rocha, L.C.; Demarchi, L.; Postol, E.; Sampaio, R.O.; de Alencar, R.E.; Kalil, J.; Guilherme, L. StreptInCor, a Group A Streptococcal Adsorbed Vaccine: Evaluation of Repeated Intramuscular Dose Toxicity Testing in Rats. Front. Cardiovasc. Med. 2021, 8, 643317. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.E.; Cohen, K.; Gounden, R.; Kengne, A.P.; Barth, D.; Whitelaw, A.C.; Francis, V.; Badri, M.; Stewart, A.; Dale, J.; et al. The Cape Town Clinical Decision Rule for Streptococcal Pharyngitis in Children. Pediatr. Infect. Dis. J. 2017, 36, 250–255. [Google Scholar] [CrossRef]
- Joachim, L.; Smeesters, P.R.; Campos, D., Jr. Pragmatic Scoring System for Pharyngitis in Low-Resource Settings. Pediatrics 2010, 126, e608–e614. [Google Scholar] [CrossRef] [PubMed]
- Irlam, J.; Mayosi, B.M.; Engel, M.; Gaziano, T.A. Primary prevention of acute rheumatic fever and rheumatic heart disease with penicillin in South African children with pharyngitis: A cost-effectiveness analysis. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 343–351. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, W.J.; White, D.; Tannenbaum, D.; E Low, D. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. Can. Med Assoc. J. 1998, 158, 75–83. [Google Scholar]
- Janovsky, K. The Management of Acute Respiratory Infections in Children: Practical Guidelines for Outpatient Care; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Dougherty, S.; Carapetis, J.; Zuhlke, L.; Wilson, N. Primary Prevention of Acute Rheumatic Fever and Rheumatic Heart Disease. In Acute Rheumatic Fever and Rheumatic Heart Disease; Elsevier: Amsterdam, The Netherlands, 2020; p. 195. [Google Scholar]
- Le Marechal, F.; Martinot, A.; Duhamel, A.; Pruvost, I.; Dubos, F. Streptococcal pharyngitis in children: A meta-analysis of clinical decision rules and their clinical variables. BMJ Open 2013, 3, e001482. [Google Scholar] [CrossRef]
- Shaikh, N.; Swaminathan, N.; Hooper, E.G. Accuracy and Precision of the Signs and Symptoms of Streptococcal Pharyngitis in Children: A Systematic Review. J. Pediatr. 2012, 160, 487–493.e3. [Google Scholar] [CrossRef]
- Cohen, J.F.; Cohen, R.; Levy, C.; Thollot, F.; Benani, M.; Bidet, P.; Chalumeau, M. Selective testing strategies for diagnosing group A streptococcal infection in children with pharyngitis: A systematic review and prospective multicentre external validation study. Can. Med. Assoc. J. 2015, 187, 23–32. [Google Scholar] [CrossRef]
- Walker, C.L.F.; Rimoin, A.W.; Hamza, H.; Steinhoff, M.C. Comparison of clinical prediction rules for management of pharyngitis in settings with limited resources. J. Pediatr. 2006, 149, 64–71. [Google Scholar] [CrossRef]
- Toepfner, N.; Henneke, P.; Berner, R.; Hufnagel, M. Impact of technical training on rapid antigen detection tests (RADT) in group A streptococcal tonsillopharyngitis. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 609–611. [Google Scholar] [CrossRef]
- Stewart, E.H.; Davis, B.; Clemans-Taylor, B.L.; Littenberg, B.; Estrada, C.A.; Centor, R.M. Rapid Antigen Group A Streptococcus Test to Diagnose Pharyngitis: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e111727. [Google Scholar] [CrossRef]
- Adler, L.; Parizade, M.; Koren, G.; Yehoshua, I. Oral cavity swabbing for diagnosis of group a Streptococcus: A prospective study. BMC Fam. Pr. 2020, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.A.; Shulman, S.T. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin. Microbiol. Rev. 2004, 17, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Bertille, N.; Cohen, R.; Chalumeau, M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst. Rev. 2016, 2016, CD010502. [Google Scholar] [CrossRef]
- Plainvert, C.; Duquesne, I.; Touak, G.; Dmytruk, N.; Poyart, C. In vitro evaluation and comparison of 5 rapid antigen detection tests for the diagnosis of beta-hemolytic group A streptococcal pharyngitis. Diagn. Microbiol. Infect. Dis. 2015, 83, 105–111. [Google Scholar] [CrossRef]
- Thompson, T.Z.; McMullen, A.R. Group A streptococcus testing in pediatrics: The move to point-of-care molecular testing. J. Clin. Microbiol. 2020, 58, e01494-19. [Google Scholar] [CrossRef]
- Anderson, N.W.; Buchan, B.W.; Mayne, D.; Mortensen, J.E.; Mackey, T.-L.A.; Ledeboer, N.A. Multicenter clinical evaluation of the illumi gene group A Streptococcus DNA amplification assay for detection of group A Streptococcus from pharyngeal swabs. J. Clin. Microbiol. 2013, 51, 1474–1477. [Google Scholar] [CrossRef]
- Henson, A.M.; Carter, D.; Todd, K.; Shulman, S.T.; Zheng, X. Detection of Streptococcus pyogenes by Use of Illumigene Group A Streptococcus Assay. J. Clin. Microbiol. 2013, 51, 4207–4209. [Google Scholar] [CrossRef]
- Luo, R.; Sickler, J.; Vahidnia, F.; Lee, Y.-C.; Frogner, B.; Thompson, M. Diagnosis and Management of Group a Streptococcal Pharyngitis in the United States, 2011–2015. BMC Infect. Dis. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Cantera, J.L.; White, H.; Diaz, M.H.; Beall, S.G.; Winchell, J.M.; Lillis, L.; Kalnoky, M.; Gallarda, J.; Boyle, D.S. Assessment of eight nucleic acid amplification technologies for potential use to detect infectious agents in low-resource settings. PLoS ONE 2019, 14, e0215756. [Google Scholar] [CrossRef]
- Lu, H.-W.; Roskos, K.; Hickerson, A.I.; Carey, T.; Niemz, A. System for portable nucleic acid testing in low resource settings. In Microfluidics, BioMEMS, and Medical Microsystems XI; International Society for Optics and Photonics: Bellingham, WA, USA, 2013; Volume 8615, p. 0I–86150I-12. [Google Scholar] [CrossRef]
- Singh, S.; Kaushal, A.; Khare, S.; Kumar, P.; Kumar, A. Gold–mercaptopropionic acid–polyethylenimine composite based DNA sensor for early detection of rheumatic heart disease. Anal. 2014, 139, 3600–3606. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, N.; Białobrzeska, W.; Łęga, T.; Pałka, K.; Dziąbowska, K.; Żołędowska, S.; Czaczyk, E.; Pala, K.; Nidzworski, D. Antibody Modified Gold Electrode as an Impedimetric Biosensor for the Detection of Streptococcus pyogenes. Sensors 2020, 20, 5324. [Google Scholar] [CrossRef] [PubMed]
- Askarian, B.; Yoo, S.-C.; Chong, J.W. Novel Image Processing Method for Detecting Strep Throat (Streptococcal Pharyngitis) Using Smartphone. Sensors 2019, 19, 3307. [Google Scholar] [CrossRef]
- Van, T.T.; Mata, K.; Bard, J.D. Automated Detection of Streptococcus pyogenes Pharyngitis by Use of Colorex Strep A CHROMagar and WASPLab Artificial Intelligence Chromogenic Detection Module Software. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef] [PubMed]
- Farhan, S.; Alshraideh, M.; Mahafza, T. A medical decision support system for ent disease diagnosis using artificial neural networks. Int. J. Artif. Intell. Mechatron. 2015, 4, 45–54. [Google Scholar]
- Spinks, A.; Glasziou, P.P.; Del Mar, C.B. Antibiotics for sore throat. Cochrane Database Syst. Rev. 2013, 11. [Google Scholar] [CrossRef]
- Driel, M.L.; De Sutter, A.I.; Thorning, S.; Christiaens, T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst. Rev. 2021, 3. [Google Scholar]
- De Dassel, J.L.; de Klerk, N.; Carapetis, J.R.; Ralph, A.P. How Many Doses Make a Difference? An Analysis of Secondary Prevention of Rheumatic Fever and Rheumatic Heart Disease. J. Am. Heart Assoc. 2018, 7, e010223. [Google Scholar]
- Manyemba, J.; Mayosi, B.M. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst Rev. 2002, 2002, Cd002227. [Google Scholar] [CrossRef]
- Musoke, C.; Mondo, C.K.; Okello, E.; Zhang, W.Z.; Kakande, B.; Nyakoojo, W.; Freers, J. Benzathine penicillin adherence for secondary prophylaxis among patients affected with rheumatic heart disease attending Mulago Hospital: Cardiovascular topics. Cardiovasc. J. Afr. 2013, 24, 124–129. [Google Scholar] [CrossRef]
- Bennett, J.; Rentta, N.; Leung, W.; Anderson, A.; Oliver, J.; Wyber, R.; Harwod, M.; Webb, R.; Malcom, J.; Baker, M.G. Structured review of primary interventions to reduce group A streptococcal infections, acute rheumatic fever and rheumatic heart disease. J. Paediatr. Child Health 2021, 57, 797–802. [Google Scholar] [CrossRef]
- Russell, K.; Nicholson, R.; Naidu, R. Reducing the pain of intramuscular benzathine penicillin injections in the rheumatic fever population of C ounties M anukau D istrict H ealth B oard. J. Paediatr. Child Health 2014, 50, 112–117. [Google Scholar] [CrossRef]
- Huck, D.M.; Nalubwama, H.; Longenecker, C.T.; Frank, S.H.; Okello, E.; Webel, A.R. A Qualitative Examination of Secondary Prophylaxis in Rheumatic Heart Disease: Factors Influencing Adherence to Secondary Prophylaxis in Uganda. Glob. Heart 2015, 10, 63–69.e1. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.-M.F.; Mohamed, M.A.; Abosedira, M.M.; Al-Harbi, K.M.; Abdelaziz, N.A.; Khosh Hal, S.Q.; Boraey, N.F.; Aly, S.H.; Hassan, I.A.; Sharaf, A.E. Lidocaine as a Diluent for Benzathine Penicillin G Reduces Injection Pain in Patients with Rheumatic Fever: A Prospective, Randomized Double-Blinded Crossover Study. Aust. J. Basic Appl. Sci. 2012, 6, 236–240. [Google Scholar]
- Ndagire, E.; Kawakatsu, Y.; Nalubwama, H.; Atala, J.; Sarnacki, R.; Pulle, J.; Kyarimpa, R.; Mwima, R.; Kansiime, R.; Okello, E.; et al. Examining the Ugandan health system’s readiness to deliver rheumatic heart disease-related services. PLoS Negl. Trop. Dis. 2021, 15, e0009164. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Zühlke, L.J. Global research priorities in rheumatic fever and rheumatic heart disease. Ann. Pediatr Cardiol. 2011, 4, 4–12. [Google Scholar] [CrossRef]
- Maguire, G.P.; Carapetis, J.; Walsh, W.F.; Brown, A.D.H. The future of acute rheumatic fever and rheumatic heart disease in Australia. Med J. Aust. 2012, 197, 133–134. [Google Scholar] [CrossRef]
- Holanda e Silva, K.; Xavier-Junior, F.; Farias, I.; Caldas Neto, J.; Silva, A.; Nakashima-Junior, T.; Araujo, I.; Oliveira, A.; Medeiros, A.; Tabosa do Egito, E. A new insight about pharmaceutical dosage forms for benzathine penicillin G. J. Basic Appl. Pharm. Sci. 2006, 27, 21–26. [Google Scholar]
- Wyber, R.; Taubert, K.; Marko, S.; Kaplan, E.L. Benzathine penicillin G for the management of RHD: Concerns about quality and access, and opportunities for intervention and improvement. Glob. Heart 2013, 8, 227–234. [Google Scholar] [CrossRef]
- Taubert, K.; Marko, S.B. Access to essential medicines: Illuminating disparities in the global supply of benzathine penicillin G in the context of rheumatic fever/rheumatic heart disease prevention. J. Am. College Cardiol. 2013, 61, E2004. [Google Scholar] [CrossRef]
- Musser, J.M.; Beres, S.B.; Zhu, L.; Olsen, R.J.; Vuopio, J.; Hyyryläinen, H.-L.; Gröndahl-Yli-Hannuksela, K.; Kristinsson, K.G.; Darenberg, J.; Henriques-Normark, B.; et al. Reduced In Vitro Susceptibility of Streptococcus pyogenes to β-Lactam Antibiotics Associated with Mutations in the pbp2x Gene Is Geographically Widespread. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Nordet, P.; Lopez, R.; Duenas, A.; Sarmiento, L. Prevention and control of rheumatic fever and rheumatic heart disease: The Cuban experience (1986–1996–2002): Cardiovascular topic. Cardiovasc. J. Afr. 2008, 19, 135–140. [Google Scholar] [PubMed]
- Arguedas, A.; Mohs, E. Prevention of rheumatic fever in Costa Rica. J. Pediatr. 1992, 121, 569–572. [Google Scholar] [CrossRef]
- Matheka, D.M.; Murgor, M.; Kibochi, E.; Nigel, S.; Nderitu, J.; Selnow, G. O028 Role of Technology In Creating Rheumatic Heart Disease Awareness Among School-Going Children In Kenya. Glob. Heart 2014, 9, e7–e8. [Google Scholar] [CrossRef]
- Engel, M.; Muhamed, B.; Whitelaw, A.C.; Musvosvi, M.; Mayosi, B.; Dale, J.B. Group A Streptococcal emm Type Prevalence Among Symptomatic Children in Cape Town and Potential Vaccine Coverage. Pediatr. Infect. Dis. J. 2014, 33, 208–210. [Google Scholar] [CrossRef]
- DeWyer, A.; Scheel, A.; Webel, A.R.; Longenecker, C.T.; Kamarembo, J.; Aliku, T.; Engel, M.E.; Bowen, A.; Bwanga, F.; Hovis, I.; et al. Prevalence of group A β-hemolytic streptococcal throat carriage and prospective pilot surveillance of streptococcal sore throat in Ugandan school children. Int. J. Infect. Dis. 2020, 93, 245–251. [Google Scholar] [CrossRef]
- Hill, H.R. Group A Streptococcal Carrier versus Acute Infection: The Continuing Dilemma. Clin. Infect. Dis. 2010, 50, 491–492. [Google Scholar] [CrossRef]
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012, 55, e86–e102. [Google Scholar] [CrossRef]
- Jones, T.D. The Diagnosis of Rheumatic Fever. J. Am. Med. Assoc. 1944, 126, 481–484. [Google Scholar] [CrossRef]
- Ralph, A.P.; Webb, R.; Moreland, N.J.; McGregor, R.; Bosco, A.; Broadhurst, D.; Lassmann, T.; Barnett, T.C.; Benothman, R.; Yan, J.; et al. Searching for a technology-driven acute rheumatic fever test: The START study protocol. BMJ Open 2021, 11, e053720. [Google Scholar] [CrossRef]
- Wannamaker, L.W.; Ayoub, E.M. Antibody titers in acute rheumatic fever. Circulation 1960, 21, 598–614. [Google Scholar] [CrossRef] [PubMed]
- Shet, A.; Kaplan, E.L. Clinical use and interpretation of group A streptococcal antibody tests: A practical approach for the pediatrician or primary care physician. Pediatric Infect. Dis. J. 2002, 21, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kotby, A.A.; Habeeb, N.M.; Ezz El Arab, S. Antistreptolysin O titer in health and disease: Levels and significance. Pediatric Rep. 2012, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Okello, E.; Murali, M.; Rwebembera, J.; Atala, J.; Bowen, A.; Harik, N.; Kaudha, G.; Kitooleko, S.; Longenecker, C.; Ndagire, E.; et al. Cross-sectional study of population-specific streptococcal antibody titres in Uganda. Arch. Dis. Child. 2020, 105, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Chen, C.-Y.; Wu, V.C.-C.; Chou, A.-H.; Cheng, Y.-T.; Chang, S.-H.; Chu, P.-H. Mitral valve repair versus replacement in patients with rheumatic heart disease. J. Thorac. Cardiovasc. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- David, T.E. Commentary: Repair or replace rheumatic mitral valves? J. Thorac. Cardiovasc. Surg. 2021, 162, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, Y.; Zhang, H.; Han, J.; Jiao, Y.; Du, J.; Meng, X. Outcomes of mitral valve repair compared with replacement for patients with rheumatic heart disease. J. Thorac. Cardiovasc. Surg. 2021, 162, 72–82.e7. [Google Scholar] [CrossRef]
- Kumar, R.K.; Antunes, M.J.; Beaton, A.; Mirabel, M.; Nkomo, V.T.; Okello, E.; Regmi, P.R.; Reményi, B.; Sliwa-Hähnle, K.; Zühlke, L.J.; et al. Contemporary Diagnosis and Management of Rheumatic Heart Disease: Implications for Closing the Gap: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e337–e357. [Google Scholar] [CrossRef]
- Russell, E.A.; Walsh, W.F.; Reid, C.M.; Tran, L.; Brown, A.; Bennetts, J.S.; A Baker, R.; Tam, R.; Maguire, G.P. Outcomes after mitral valve surgery for rheumatic heart disease. Heart Asia 2017, 9, e010916. [Google Scholar] [CrossRef]
- Nunes, M.C.; Tan, T.C.; Elmariah, S.; do Lago, R.; Margey, R.; Cruz-Gonzalez, I.; Zheng, H.; Handschumacher, M.D.; Inglessis, I.; Palacios, I.F.; et al. The echo score revisited: Impact of incorporating commissural morphology and leaflet displacement to the prediction of outcome for patients undergoing percutaneous mitral valvuloplasty. Circulation 2014, 129, 886–895. [Google Scholar] [CrossRef]
- Howard, C.; Jullian, L.; Joshi, M.; Noshirwani, A.; Bashir, M.; Harky, A. TAVI and the future of aortic valve replacement. J. Card. Surg. 2019, 34, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.E.; Fadel, B.; Al-Sergani, H.; Al Amri, M.; Hassan, W.; Abdulbaki, K.; Shoukri, M.; Canver, C. Long-Term Results (Up to 16.5 Years) of Mitral Balloon Valvuloplasty in a Series of 518 Patients and Predictors of Long-Term Outcome. J. Interv. Cardiol. 2007, 20, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.A.; Tran, L.; A Baker, R.; Bennetts, J.S.; Brown, A.; Reid, C.M.; Tam, R.; Walsh, W.F.; Maguire, G.P. A review of valve surgery for rheumatic heart disease in Australia. BMC Cardiovasc. Disord. 2014, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Rotman, O.M.; Bianchi, M.; Ghosh, R.P.; Kovarovic, B.; Bluestein, D. Principles of TAVR valve design, modelling, and testing. Expert Rev. Med Devices 2018, 15, 771–791. [Google Scholar] [CrossRef]
- Zilla, P.; Williams, D.F.; Bezuidenhout, D. TAVR for Patients With Rheumatic Heart Disease: Opening the Door for the Many? J. Am. Coll Cardiol. 2021, 77, 1714–1716. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Ntsekhe, M.; Scherman, J. TAVI for rheumatic aortic stenosis–The next frontier? Int J. Cardiol. 2019, 280, 51–52. [Google Scholar] [CrossRef]
- Mentias, A.; Saad, M.; Desai, M.Y.; Krishnaswamy, A.; Menon, V.; Horwitz, P.A.; Kapadia, S.; Sarrazin, M.V. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Rheumatic Aortic Stenosis. J. Am. Coll. Cardiol. 2021, 77, 1703–1713. [Google Scholar] [CrossRef]
- Brennan, P.; Santos, A.; Johnston, N.; Owens, C.; Jeganathan, R.; Manoharan, G.; Spence, M. Extending the role of TAVR to rheumatic aortic stenosis: Our 10 year experience in Belfast. J. Am. Coll. Cardiol. 2019, 73, 1242. [Google Scholar] [CrossRef]
- Russell, E.A.; Walsh, W.F.; Costello, B.; McLellan, A.J.A.; Brown, A.; Reid, C.M.; Tran, L.; Maguire, G.P. Medical Management of Rheumatic Heart Disease: A Systematic Review of the Evidence. Cardiol. Rev. 2018, 26, 187–195. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, H.P.; Lopes, R.D.; Silva, P.G.D.B.E.; Liporace, I.L.; Sampaio, R.O.; Tarasoutchi, F.; Hoffmann-Filho, C.R.; Patriota, R.D.L.S.; Leiria, T.L.; Lamprea, D.; et al. Rivaroxaban in Patients with Atrial Fibrillation and a Bioprosthetic Mitral Valve. N. Engl. J. Med. 2020, 383, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, G.; Connolly, S.J.; Ntsekhe, M.; Benz, A.; Rangarajan, S.; Lewis, G.; Yun, Y.; Sharma, S.K.; Maklady, F.; Elghamrawy, A.E.; et al. The INVICTUS rheumatic heart disease research program: Rationale, design and baseline characteristics of a randomized trial of rivaroxaban compared to vitamin K antagonists in rheumatic valvular disease and atrial fibrillation. Am. Heart J. 2020, 225, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ene, G.; Garcia-Raso, A.; Gonzalez-Dominguez Weber, A.; Hidalgo-Vega, A.; Llamas, P. Cost of vitamin K antagonist anticoagulant treatment in patients with metallic prosthetic valve in mitral position. SAGE Open Med. 2016, 4. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- French, K.A.; Poppas, A. Rheumatic Heart Disease in Pregnancy: Global Challenges and Clear Opportunities. Circulation 2018, 137, 817–819. [Google Scholar] [CrossRef]
- Beaton, A.; Okello, E.; Scheel, A.; DeWyer, A.; Ssembatya, R.; Baaka, O.; Namisanvu, H.; Njeri, A.; Matovu, A.; Namagembe, I.; et al. Impact of heart disease on maternal, fetal and neonatal outcomes in a low-resource setting. Heart 2019, 105, 755–760. [Google Scholar] [CrossRef]
- Frank, T.D.; Carter, A.; Jahagirdar, D.; Biehl, M.H.; Douwes-Schultz, D.; Larson, S.L.; Arora, M.; Dwyer-Lindgren, L.; Steuben, K.M.; Abbastabar, H.; et al. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: A systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019, 6, e831–e859. [Google Scholar] [CrossRef]
- Gleason, B.; Mirembe, G.; Namuyonga, J.; Longenecker, C.T.; Okello, E.; Salata, R.; Mugyenyi, P.; Musiime, V.; Costa, M.; Kityo, C. O086 Does HIV infection modify the risk of RHD? Initial echocardiographic screening experience at the Joint Clinical Re-search Centre in Kampala, Uganda. Glob. Heart 2014, 9, e55. [Google Scholar] [CrossRef]
- Hovis, I.W.; Namuyonga, J.; Kisitu, G.P.; Ndagire, E.; Okello, E.; Longenecker, C.T.; Sanyahumbi, A.; Sable, C.A.; Penny, D.J.; Lwabi, P.; et al. Decreased Prevalence of Rheumatic Heart Disease Confirmed Among HIV-positive Youth. Pediatr. Infect. Dis. J. 2019, 38, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Gleason, B.; Mirembe, G.; Namuyonga, J.; Okello, E.; Lwabi, P.; Lubega, I.; Lubega, S.; Musiime, V.; Kityo, C.; Salata, R.A. Prevalence of latent rheumatic heart disease among HIV-infected children in Kampala, Uganda. J. Acquir. Immune Defic. Syndr. 2016, 71, 196. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Rwebembera, J.; Bendavid, E.; Okello, E.; Barry, M.; Beaton, A.Z.; Haeffele, C.; Webel, A.R.; Kityo, C.; Longenecker, C.T. Clinical Outcomes, Echocardiographic Findings, and Care Quality Metrics for People Living with HIV and Rheumatic Heart Disease in Uganda. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Health UMo. Uganda Population-Based HIV Impact Assessment (UPHIA) 2016–2017; Ministry of Health: Kampala, Uganda, 2019.
- Benjamin, L.A.; Bryer, A.; Emsley, H.; Khoo, S.; Solomon, T.; Connor, M.D. HIV infection and stroke: Current perspectives and future directions. Lancet Neurol. 2012, 11, 878–890. [Google Scholar] [CrossRef]
- Vorkoper, S.; Kupfer, L.E.; Anand, N.; Patel, P.; Beecroft, B.; Tierney, W.M.; Ferris, R.; El-Sadr, W.M. Building on the HIV chronic care platform to address noncommunicable diseases in sub-Saharan Africa: A research agenda. AIDS 2018, 32, S107. [Google Scholar] [CrossRef]
- Longenecker, C.T.; Morris, S.R.; Aliku, T.O.; Beaton, A.; Costa, M.A.; Kamya, M.R.; Kityo, C.; Lwabi, P.; Mirembe, G.; Nampijja, D. Rheumatic heart disease treatment cascade in Uganda. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004037. [Google Scholar] [CrossRef]
- Sanyahumbi, A.; Beaton, A.; Ms, D.G.; Hosseinipour, M.C.; Karlsten, M.; Minard, C.G.; Penny, D.J.; Sable, C.A.; Kazembe, P.N. Two-year evolution of latent rheumatic heart disease in Malawi. Congenit. Heart Dis. 2019, 14, 614–618. [Google Scholar] [CrossRef]
- Watkins, D.; Lubinga, S.J.; Mayosi, B.; Babigumira, J.B. A Cost-Effectiveness Tool to Guide the Prioritization of Interventions for Rheumatic Fever and Rheumatic Heart Disease Control in African Nations. PLoS Negl. Trop. Dis. 2016, 10, e0004860. [Google Scholar] [CrossRef]
- Irlam, J.H.; Mayosi, B.M.; Engel, M.E.; Gaziano, T.A. A cost-effective strategy for primary prevention of acute rheumatic fever and rheumatic heart disease in children with pharyngitis. South Afr. Med J. 2013, 103, 894. [Google Scholar] [CrossRef][Green Version]
- Watkins, D.A.; Mvundura, M.; Nordet, P.; Mayosi, B.M. A Cost-Effectiveness Analysis of a Program to Control Rheumatic Fever and Rheumatic Heart Disease in Pinar del Rio, Cuba. PLoS ONE 2015, 10, e0121363. [Google Scholar] [CrossRef]
- Manji, R.A.; Witt, J.; Tappia, P.S.; Jung, Y.; Menkis, A.H.; Ramjiawan, B. Cost–effectiveness analysis of rheumatic heart disease prevention strategies. Expert Rev. Pharm. Outcomes Res. 2013, 13, 715–724. [Google Scholar] [CrossRef]
- Roberts, K.; Cannon, J.; Atkinson, D.; Brown, A.; Maguire, G.; Remenyi, B.; Wheaton, G.; Geelhoed, E.; Carapetis, J.R. Echocardiographic Screening for Rheumatic Heart Disease in Indigenous Australian Children: A Cost–Utility Analysis. J. Am. Heart Assoc. 2017, 6, e004515. [Google Scholar] [CrossRef] [PubMed]
- Zachariah JP and Samnaliev, M. Echo-based screening of rheumatic heart disease in children: A cost-effectiveness Markov model. J. Med. Econ. 2015, 18, 410–419. [Google Scholar] [CrossRef]
- Ubels, J.; Sable, C.; Beaton, A.Z.; Nunes, M.C.P.; Oliveira, K.K.B.; Castro, L.C.; Teixeira, I.M.; Ruiz, G.Z.L.; Rabelo, L.M.M.; Tompsett, A.R.; et al. Cost-Effectiveness of Rheumatic Heart Disease Echocardiographic Screening in Brazil: Data from the PROVAR+ Study: Cost-effectiveness of RHD screening in Brazil. Glob. Heart 2020, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.V.; Maguire, G.P.; Brown, A.; Atkinson, D.N.; Remenyi, B.; Wheaton, G.; Ilton, M.; Carapetis, J. Rheumatic heart disease in Indigenous children in northern Australia: Differences in prevalence and the challenges of screening. Med J. Aust. 2015, 203, 221. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.M.; Sliwa, K.; A Watkins, D.; Zühlke, L.; Perel, P.; Berteletti, F.; Eiselé, J.-L.; Klassen, S.L.; Kwan, G.F.; O Mocumbi, A.; et al. An investment case for the prevention and management of rheumatic heart disease in the African Union 2021–30: A modelling study. Lancet Glob. Heath 2021, 9, e957–e966. [Google Scholar] [CrossRef]
- Opara, C.C.; Du, Y.; Kawakatsu, Y.; Atala, J.; Beaton, A.Z.; Kansiime, R.; Nakitto, M.; Ndagire, E.; Nalubwama, H.; Okello, E. Household Economic Consequences of Rheumatic Heart Disease in Uganda. Front. Cardiovasc. Med. 2021, 680. [Google Scholar]
- Oyebamiji, O. The Household Economic Impact of Rheumatic Heart Disease (RHD) in South Africa. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2018. [Google Scholar]
- Hellebo, A.G.; Zuhlke, L.J.; Watkins, D.A.; Alaba, O. Health system costs of rheumatic heart disease care in South Africa. BMC Public Health 2021, 21, 1303. [Google Scholar] [CrossRef]
- Mocumbi, A.O. Out-of-pocket costs in rheumatic heart disease care: A major barrier to equity in cardiovascular health. Indian Heart J. 2021, 73, 141–142. [Google Scholar] [CrossRef]
- Arvind, B.; Saxena, A.; Kazi, D.S.; Bolger, A.F. Out-of-pocket expenditure for administration of benzathine penicillin G injections for secondary prophylaxis in patients with rheumatic heart disease: A registry-based data from a tertiary care center in Northern India. Indian Heart J. 2021, 73, 169–173. [Google Scholar] [CrossRef]
- Watkins, D.; Daskalakis, A. The economic impact of rheumatic heart disease in developing countries. Lancet Glob. Health 2015, 3, S37. [Google Scholar] [CrossRef]
- White, A. WHO Resolution on rheumatic heart disease. Eur. Heart J. 2018, 39, 4233. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Rheumatic fever and rheumatic heart disease. In Report by the Director General. Seventy-First World Health Assembly; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA71/A71_25-en.pdf (accessed on 15 October 2021).
- Ogola, E.; Omokhodion, S.; Palweni, C.; Pearce, A.; Salo, A.; Thomas, B.; Walker, K.; Wiysonge, C.; Zaher, S. The Drakensberg declaration on the control of rheumatic fever and rheumatic heart disease in Africa. South Afr. Med. J. 2006, 96, 246. [Google Scholar]
- Remenyi, B.; Carapetis, J.; Wyber, R.; Taubert, K.; Mayosi, B.M. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat. Rev. Cardiol. 2013, 10, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Beaton, A.; Kamalembo, F.B.; Dale, J.; Kado, J.H.; Karthikeyan, G.; Kazi, D.S.; Longenecker, C.T.; Mwangi, J.; Okello, E.; Ribeiro, A.L.P.; et al. The American Heart Association’s Call to Action for Reducing the Global Burden of Rheumatic Heart Disease: A Policy Statement From the American Heart Association. Circulation 2020, 142, e358–e368. [Google Scholar] [CrossRef] [PubMed]
| Author | Countries Involved | Sample | Platform | Key Findings |
|---|---|---|---|---|
| Auckland et al., 2020 [93] | Fiji, India, UK | 822 cases; 1800 controls | HumanCore- 24 BeadChip (Illumina Inc., San Diego, CA, USA) UK Biobank Axiom Array (Affymetrix, USA) | HLA class III PBX2 region rs201026476, chromosome 14 (OR 1.99, 95% CI 1.58–2.51, p = 7.45 × 10−9) reached GWS Independent association was maintained in HLA class III region after conditioning and results replicated by validating through UK dataset. |
| Parks et al., 2017 [101] | Eight Oceanian countries | 1006 cases; 1846 controls | Low-density 300 k Illumina HumanCore platform | IGHV4-61 02 rs11846409 on chromosome 14 (1.4× risk) (OR 1.43, 95% CI 1.27–1.61, p = 4.1 × 10−9) that reached GWS |
| Gray et al., 2017 [102] | Northern territory of Australia | 398 cases; 865 controls | HumanCore-24 Bead Chip (Illumina Inc., San Diego, CA, USA) | HLA-DQA1 rs9272622 on chromosome 6 (protective) (OR = 0.90, p = 1.86 × 10−7) did not reach GWS |
| Machipisa et al., 2021 [103] | 8 African countries | 2548 cases; 2261 controls Family group -348 participants (118 trio families) | Infinium Human Omni 2.5–8 (Illumina Inc., San Diego, CA, USA) | GWS association at 11q24.1 (rs1219406); (OR 1.65; 95%CI, 1.48– 1.82; p = 4.36 × 10−8) for black African individuals but not admixed African individuals or other external datasets Replicated rs11846409 IGH locus GWAS [101] in admixed African individuals |
| Type of Vaccine | Stage of Development |
|---|---|
| StreptAvax: 26-valent vaccine | Phase I and II trials demonstrated good safety, tolerance and immunogenicity [114,115]; however further studies stopped for commercial reasons |
| StreptAnova *: 4 recombinant proteins | The 4 recombinant proteins represent 30 different M-types prevalent in North America and Europe Phase I trial: demonstrated good tolerance and immunogenicity in adults [116]. |
| MJ8VAX **: based on C-terminus of the M protein | Phase I trial: demonstrated that a single intramuscular dose of the vaccine was safe, well tolerated and immunogenic, but anti-J8 IgG concentration decreased after 180 days post immunization [117]. |
| StreptInCor: peptide vaccine containing T and B cell epitopes of the M protein CRR | Good results in models [118]. |
| Multi-component vaccines [110] | 3-Combo: SpyCEP, SpyAD, SLO; provides protection in models 5-Combo: ADI, TF, C5a peptidase, SpyCEP and SLO 5-CP: demonstrated protection against intranasal, skin and systemic challenges of GAS Spy-7: showed significant reduction in the dissemination of types M1 and M3 GAS |
| (Ambitious) Aims of Global Efforts in the Fight against RHD |
|---|
| • Developing a collaborative agenda to address the key health impacts of RHD worldwide (premature morbidity and mortality, maternal deaths) • Improving access to basic healthcare, from prophylaxis to more advanced therapies • Sensitizing stakeholders and the political sector on the importance of RHD and the need for reducing its burden, especially in the hardest hit regions • Developing position statements, guidelines, and calls-to-action focused on RHD • Including RHD as a priority in the world agenda, establishing action plans and goals led by international organizations such as the WHO, WHF, and key cardiovascular societies |
| Key Charges from the 2018 WHA Resolution to Member States [47,224,225] |
| • Accelerate multisectoral efforts to improve socioeconomic determinants of RHD • Estimate the burden of disease and implement multisectoral RHD programs • Improve access to primary health care, including RHD prevention and control • Ensure access to cost-effective essential laboratory technologies and medicines for RHD • Strengthen national and international cooperation to address RHD. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rwebembera, J.; Nascimento, B.R.; Minja, N.W.; de Loizaga, S.; Aliku, T.; dos Santos, L.P.A.; Galdino, B.F.; Corte, L.S.; Silva, V.R.; Chang, A.Y.; et al. Recent Advances in the Rheumatic Fever and Rheumatic Heart Disease Continuum. Pathogens 2022, 11, 179. https://doi.org/10.3390/pathogens11020179
Rwebembera J, Nascimento BR, Minja NW, de Loizaga S, Aliku T, dos Santos LPA, Galdino BF, Corte LS, Silva VR, Chang AY, et al. Recent Advances in the Rheumatic Fever and Rheumatic Heart Disease Continuum. Pathogens. 2022; 11(2):179. https://doi.org/10.3390/pathogens11020179
Chicago/Turabian StyleRwebembera, Joselyn, Bruno Ramos Nascimento, Neema W. Minja, Sarah de Loizaga, Twalib Aliku, Luiza Pereira Afonso dos Santos, Bruno Fernandes Galdino, Luiza Silame Corte, Vicente Rezende Silva, Andrew Young Chang, and et al. 2022. "Recent Advances in the Rheumatic Fever and Rheumatic Heart Disease Continuum" Pathogens 11, no. 2: 179. https://doi.org/10.3390/pathogens11020179
APA StyleRwebembera, J., Nascimento, B. R., Minja, N. W., de Loizaga, S., Aliku, T., dos Santos, L. P. A., Galdino, B. F., Corte, L. S., Silva, V. R., Chang, A. Y., Dutra, W. O., Nunes, M. C. P., & Beaton, A. Z. (2022). Recent Advances in the Rheumatic Fever and Rheumatic Heart Disease Continuum. Pathogens, 11(2), 179. https://doi.org/10.3390/pathogens11020179







