Abstract
The protozoon Babesia is a blood parasite transmitted by hard ticks and commonly parasitizes ruminants such as cattle, buffaloes, goats, and sheep. Babesiosis, the disease caused by Babesia infection, has been considered a potential threat to ruminant production due to the grave and enormous impact it brings. About 125 million ruminants are at risk of babesiosis in Southeast Asia (SEA), a region composed of 11 countries. In recent decades, molecular-based diagnostic platforms, such as polymerase chain reaction (PCR) assays, have been a reliable and broadly employed tool in Babesia detection. In this article, the authors compiled and summarized the molecular studies conducted on ruminant babesiosis and mapped the species, including B. bovis, B. bigemina, B. ovata, Babesia sp. Mymensingh, Babesia sp. Hue, and B. ovis, and determined the host diversity of ruminant Babesia in SEA.
Keywords:
Babesia; molecular epidemiology; PCR; cattle; water buffalo; goat; sheep; Southeast Asia; tick-borne 1. Introduction
Babesia is a genus of apicomplexan parasites which parasitizes various hosts ranging from avian to domesticated and wild mammals and humans [1]. Since its first discovery over 130 years ago, greater than 100 species of Babesia have been reported and described, with several novel species documented only in recent years [2]. As Babesia is transmitted by ixodid hard ticks, its geographical distribution closely resembles that of its tick vectors, which more frequently exist in the tropical and subtropical regions of the world. Babesiosis refers to the disease caused by infection with Babesia parasites. Babesiosis causes hemolytic anemia, fever, inappetence, jaundice, and hemoglobinuria in animals with acute clinical disease, which could be fatal in severe cases. As one of the major tick-borne diseases (TBDs) in animals, babesiosis is a major concern due to its sizeable impact on farmers from the millions worth of direct and indirect losses to livestock production [3].
Breakthroughs in molecular biology have permitted the development of molecular tools which enable rapid and precise diagnosis of economically important diseases such as babesiosis. However, the lack of epidemiological data on babesiosis hinders the accurate evaluation of damages brought by this disease to ruminant farming, especially in countries where veterinary services and resources are either inaccessible or unavailable. More importantly, the scant information impedes the establishment of appropriate and adequate treatment and prevention measures against babesiosis. To this end, this mini-review aims to collate the molecular reports and map the Babesia species infecting cattle, water buffaloes, goats, and sheep in Southeast Asia, particularly those that utilized molecular detection tools (PCR-based assays), and to uncover insights into the available molecular information that may be useful in formulating disease control programs against babesiosis.
2. Livestock in Southeast Asia and Relevance of Babesiosis
Southeast Asia (SEA), a geographical region in Asia, is composed of two subregions: (a) the mainland or continental subregion, composed of Cambodia, Laos, Myanmar, Thailand, Vietnam, and Singapore, and (b) the maritime or insular subregion, composed of the archipelagos, Philippines and Indonesia, the Malaysian peninsula, Singapore, Brunei Darussalam, and Timor-Leste (Figure 1) [4]. SEA’s climate is monsoonal, characterized by wet and dry seasons, which brings plenty of rainfall to support the growing of crops and the raising of animals for food production. Agriculture accounts for a huge portion of the economy of the majority of countries in SEA, and the region is a key player in the world agro-food trade, shown by its continuously increasing agricultural exports [5].
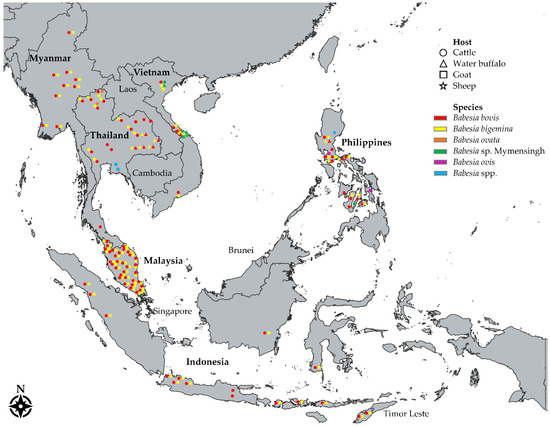
Figure 1.
A map showing the distribution of molecularly confirmed Babesia parasites in ruminants across Southeast Asia. Names of countries with documented Babesia molecular reports in cattle (○), water buffaloes (△), goats (□), and sheep (☆) are in bold font. Each color corresponds to a species —red: Babesia bovis; yellow: B. bigemina; orange: B. ovata; green: Babesia sp. Mymensingh; purple: B. ovis; blue: Babesia spp. Each shape indicates detection of a species in a particular province or area.
The traditional livestock raising in SEA is based on the mixed crop-livestock systems, where the majority of ruminant farms are owned by smallholder farmers who implement either extensive or semi-intensive management practices [6]. In 2019, there were a total of 125,358,751 ruminants in SEA [7]; 43.66%, 10.89%, 30.19%, and 15.43% of the total ruminant population were cattle, water buffaloes, goats, and sheep, respectively (Table 1). More than 70% of the ruminant population is raised in Indonesia and Myanmar, while 21% can be found in Vietnam, the Philippines, and Thailand (Table 1).

Table 1.
Ruminant population in Southeast Asian countries as of 2019.
Driven by the increasing population, expanding urbanization, and higher incomes in Southeast Asia, the demand for livestock produce will continue to rise in the coming decades [8]. People in SEA derive a huge portion of their protein intake from pork and poultry meat. However, with infectious diseases such as African swine fever [9] and avian influenza [10] drastically reducing the production of the more preferred pork and poultry meat, respectively, increased consumption of alternatives such as meat from cattle (beef), water buffalo (red beef and carabeef), goat (chevon), and sheep (mutton and lamb) is highly expected. For instance, the global demand for beef is expected to increase by 25 million tons in the year 2030, with half of the projected increased annual consumption coming from Asia [11]. Consequently, this amplified demand necessitates more efficient production practices, such as minimizing potential losses through the establishment of control of economically devastating infectious diseases such as TBDs.
Several TBDs have been confirmed to be present in SEA. The warm and humid climate of SEA supports the proliferation and dissemination of tick vectors and the tick-borne pathogens they carry [12]. Regardless of the threat to the livestock industry through losses and damages, TBDs continue to be neglected, especially in resource-constrained areas. The burden of livestock babesiosis is among the gravest of the TBDs, particularly in highly susceptible herds, i.e., exotic breeds and naïve older animals, in locations where the disease is not endemically stable [13]. Alongside the efforts of some Southeast Asian countries to boost production through the introduction of improved stocks [14,15,16], either by importing purebred exotic animals or crossbreeding native species with the imported breeds, is the risk of increased susceptibility to Babesia infection. Thus, surveillance and monitoring are crucial in ensuring healthy livestock herds and shielding them against the adverse impacts of babesiosis.
3. Applicability of PCR Assays for the Detection of Babesia in Ruminants
The past half-century has witnessed the influx of the development of novel molecular tools which revolutionized the diagnosis of parasitic diseases. As such, molecular diagnostics has become instrumental in uncovering the epidemiology of diseases that are important in the medical, veterinary, and economic sense [17]. Likewise, a multitude of previously unknown pathogens have been discovered through the application of molecular techniques.
Unlike tools that directly detect the presence of parasites (blood smears) or determine the exposure of an animal to the parasite (enzyme-linked immunosorbent assay, indirect fluorescent antibody test, and immunochromatographic test), nucleic-acid-based diagnostic assays provide highly accurate detection of the parasite DNA in samples collected from the field, addressing the various sensitivity and specificity issues of the formerly mentioned tools [18]. Among the nucleic-acid-detecting platforms, the polymerase chain reaction (PCR) assays, including variants such as conventional PCR (cPCR), nested PCR (nPCR), and multiplex PCR assays (mPCR), have been excellent in qualitatively confirming the presence of Babesia through the amplification of a DNA fragment in blood samples [19].
The earliest molecular survey of Babesia in SEA was performed in Vietnam two decades ago [20]. Since then, several PCR-based assays have been used in subsequent studies to confirm the presence of ruminant Babesia in SEA, the most common of which are listed in Table 2. All documented studies used cPCR, nPCR, or mPCR assays to confirm the presence of Babesia DNA in blood samples of cattle (Table 3), water buffaloes (Table 4), and small ruminants (Table 5). In addition, the molecular markers targeted to detect the Babesia parasites have been consistent across different SEA countries, attesting to the applicability of these assays in the field. The 18S rRNA gene, along with various genes of protein families of spherical body protein, apical membrane antigen, and rhoptry-associated protein, were the most frequently targeted markers for the PCR detection of ruminant Babesia in SEA (Table 2).

Table 2.
Commonly used PCR assays in detecting Babesia in ruminants in Southeast Asia.
Table 2.
Commonly used PCR assays in detecting Babesia in ruminants in Southeast Asia.
| Organism | Target Gene | PCR Assay Type | Target Size (bp) | Primers (5′→> 3′) | References |
|---|---|---|---|---|---|
| Babesia bigemina | Apical membrane antigen-1 (ama-1) | Nested PCR | 738 | GTATCAGCCGCCGACCTCCGTAAGT | [31] |
| GGCGTCAGACTCCAACGGGGAACCG | |||||
| 211 | TACTGTGACGAGGACGGATC | ||||
| CCTCAAAAGCAGATTCGAGT | |||||
| Rhoptry-associated protein-1a (rap-1a) | Nested PCR | 879 | GAGTCTGCCAAATCCTTAC | [34] | |
| TCCTCTACAGCTGCTTCG | |||||
| 412 | AGCTTGCTTTCACAACTCGCC | [35] | |||
| TTGGTGCTTTGACCGACGACAT | |||||
| 18S rRNA | Conventional PCR | 689 | TAGTTGTATTTCAGCCTCGCG | [36] | |
| AACATCCAAGCAGCTAHTTAG | |||||
| Babesia bovis | Rhoptry-associated protein-1 (rap-1) | Conventional PCR | 356 | CACGAGCAAGGAACTACCGATGTTGA | [27] |
| CCAAGGACCTTCAACGTACGAGGTCA | |||||
| Spherical body protein-2 (sbp-2) | Nested PCR | 1236 | CCGAATTCCTGGAAGTGGATCTCATGCAACC | [32] | |
| ATCTCGAGTCACGAGCACTCTACGGCTTTGCAG | |||||
| 580 | CGAATCTAGGCATATAAGGCAT | ||||
| ATCCCCTCCTAAGGTTGGCTAC | |||||
| Spherical body protein-4 (sbp-4) | Nested PCR | 907 | AGTTGTTGGAGGAGGCTAAT | [33] | |
| TCCTTCTCGGCGTCCTTTTC | |||||
| 503 | GAAATCCCTGTTCCAGAG | ||||
| TCGTTGATAACACTGCAA | |||||
| Variant erythrocyte surface antigen-1α (vesa-1α) | Conventional PCR | 166 | CAAGCATACAACCAGGTGG | [37] | |
| ACCCCAGGCACATCCAGCTA | |||||
| Babesia ovata | Apical membrane antigen-1 (ama-1) | Conventional PCR | 504 | GATACGAGGCTGTCGGTAGC | [38] |
| AGTATAGGTGAGCATCAGTG | |||||
| Babesia sp. Mymensingh | Apical membrane antigen-1 (ama-1) | Conventional PCR | 371 | TGGCGCCGACTTCCTGGAGCCCATCTCCAA | [39] |
| AGCTGGGGCCCTCCTTCGATGAACCGTCGG | |||||
| Babesia ovis | 18S rRNA | Conventional PCR | 549 | TGGGCAGGACCTTGGTTCTTCT | [40] |
| CCGCGTAGCGCCGGCTAAATA |
The 18S rRNA is an evolutionarily conserved gene and is the usual target gene for molecular detection due to its structural and functional stability, low substitution rates, and lack of horizontal gene transfer [21]. Its conserved region has been leveraged for developing PCR assays while its variable region has been used to differentiate Babesia species and resolve phylogenetic relationships among species [22].
Meanwhile, the apical complex, which includes secretory organelles rhoptries, micronemes, and dense granules (analogous to spherical bodies in Babesia and Theileria), is a defining structural characteristic in all apicomplexan parasites [23]. In Babesia, these three organelles secrete proteins that are involved in parasite attachment, invasion, and post-invasion host cell modifications [24]. Babesia rhoptry-associated protein 1 (RAP-1) is a variable multigene family which is characterized by very minimal intraspecies diversity and relatively high interspecies diversity [25]. Babesia bovis rap-1 sequences and B. bigemina rap-1a sequences were shown to be highly conserved, demonstrating the strongpoint of rap-1 gene as a diagnostic marker in epidemiological surveys [26,27]. In a similar manner, the apical membrane antigen (AMA-1) is an essential protein implicated in the erythrocyte invasion of the parasite [24]. In previous investigations, the ama-1 gene proved to be greatly conserved among various geographical isolates, making it an invaluable diagnostic target for parasite detection [26,28,29,30,31]. On the other hand, spherical body proteins (SBP) are involved in alterations and remodeling of the infected erythrocytes and are localized in the spherical body organelles post-invasion [24]. PCR assays developed based on the sbp-2 and sbp-4 genes have been widely used to detect B. bovis in field samples from different parts of the world [32,33].
4. Molecular Reports of Babesia in Ruminants in Southeast Asia
4.1. Bovine Babesiosis
The world cattle population stands at 1.5 billion heads, of which a little below one-third of the population is raised in the Asian continent [7]. Compared to other subregions, cattle in SEA account for a relatively minute portion of the total cattle population in Asia. Despite this, SEA’s total bovine production is still considered a significant contributor to meeting the exponentially rising demand for cattle produce. In 2019, Southeast Asian bovine production consisted of 1.74 million tons of beef, 5.57 million tons of cow milk, 237,000 tons of hide, and 55,000 tons of fat [7].

Table 3.
Molecular reports of Babesia in cattle in Southeast Asian countries.
Table 3.
Molecular reports of Babesia in cattle in Southeast Asian countries.
| Country | Pathogen | Conventional PCR | Nested PCR | ||||
|---|---|---|---|---|---|---|---|
| Detection Rate (%) * | Samples (n) | References | Detection Rate (%) * | Samples (n) | References | ||
| Vietnam | Babesia bigemina | 5.20–22.60 | 96–258 | [20,41,42,43] | 16.00 | 94 | [44] |
| Babesia bovis | 4.20–12.30 | 120–258 | [20,43,45] | 15.60–21.30 | 94–96 | [41,44] | |
| Babesia sp. Hue | 1.20 | 258 | [42] | n. r. | |||
| Babesia ovata | 0.00 | 184 | [29] | n. r. | |||
| Babesia sp. Mymensingh | 9.60 | 460 | [30] | n. r. | |||
| Philippines | Babesia bigemina | 15.40–61.70 | 339–408 | [46,47] | 0–10.80 | 48–412 | [48,49,50,51] |
| Babesia bovis | 10.00–45.40 | 339–408 | [46,47] | 0–11.50 | 48–412 | [48,49,50,51] | |
| Babesia ovata | 0.00 | 300 | [29] | n. r. | |||
| Babesia sp. Mymensingh | 11.30 | 408 | [30] | n. r. | |||
| Babesia spp. | 2.00 | 246 | [52] | n. r. | |||
| Thailand | Babesia bigemina | n. r. | 2.90–38.90 | 96–329 | [34,53,54,55,56] | ||
| Babesia bovis | n. r. | 1.40–24.50 | 53–1824 | [34,53,54,55,57] | |||
| Babesia ovata | 2.50 | 200 | [29] | n. r. | |||
| Indonesia | Babesia bigemina | 14.20 | 141 | [58] | 19.10 | 487 | [59] |
| Babesia bovis | 34.80 | 141 | [58] | 50.70 | 487 | [59] | |
| Myanmar | Babesia bigemina | 9.80 | 713 | [60] | n. r. | ||
| Babesia bovis | n. r. | 17.10 | 713 | [60] | |||
| Malaysia | Babesia bigemina | 30.50 | 1,045 | [61,62] | n. r. | ||
| Babesia bovis | 32.50 | 1045 | [62] | n. r. | |||
* Total detection rates from each study were used. n. r.: no report.
Among the domestic ruminants covered in this mini-review, babesiosis in cattle is more extensively studied in SEA, owing to the well-known susceptibility of cattle to the disease, specifically those of the taurine breed [63]. Twenty years ago, an economic assessment of the impact of cattle fever (babesiosis and anaplasmosis) estimated herd mortality rates of 0.5% in Indonesia, 0.1% in the Philippines, and 0.5% in Thailand [64]. Furthermore, bovine production losses amounting to USD 3.10 million and USD 0.60 million were calculated for Indonesia and the Philippines, respectively [64].
The Babesia species that are known to infect cattle are Babesia bovis, B. bigemina, B. major, B. divergens, B. ovata, B. occultans, B. jakimovi [65], and several undescribed taxa, namely Babesia sp. Oshima [66], Babesia sp. Kashi [67], Babesia sp. Hue [42], and Babesia sp. Mymensingh [68]. Babesia bovis and B. bigemina are the most commonly reported etiologic agents of bovine babesiosis worldwide and have the greatest impact on bovines [69]. These two species are widely present in tropical and subtropical regions where the tick vectors Rhipicephalus and Ixodes are present. Cattle infected with B. bovis can be severely ill compared to the milder B. bigemina infection [3]. On the other hand, the predominant bovine Babesia in Europe includes the zoonotic B. divergens [70] and the less pathogenic B. major [1]. Additionally, B. occultans and B. ovata were thought to have low pathogenicity in cattle [1,71], but clinical outbreaks [72,73] and cases of exacerbated anemia [38] have been attributed to each respective species. Of the undescribed species, only Babesia sp. Mymensingh has been proven to be of major clinical significance [39].
Hitherto, five Babesia species, specifically B. bigemina, B. bovis, B. ovata, Babesia sp. Hue, and Babesia sp. Mymensingh, have been identified in SEA after molecular screening of more than seven thousand individual samples as reported in 25 molecular studies conducted in cattle (Figure 1 and Table S1). Countries with the most numbers of cattle surveyed were Thailand (n = 2929), the Philippines (n = 1851), and Malaysia (n = 1045). The species B. bovis and B. bigemina were detected in bovine blood DNA samples collected from Indonesia, Malaysia, Myanmar, the Philippines, Thailand, and Vietnam (Table 3), with detection rates as high as 61.70% (cPCR) for B. bigemina [46] and 50.70% (nPCR) for B. bovis [59]. Meanwhile, B. ovata investigations were conducted in three countries, but its presence was detected only in Thailand [29] and Vietnam [42] (Table 3). Interestingly, sequences of B. ovata-positive cattle samples in Vietnam led to the discovery of a B. ovata-related benign species designated as Babesia sp. Hue [42]. In addition, upon the comprehensive description of the novel species Babesia sp. Mymensingh in cattle, a molecular survey detected the parasite in 11.30% (cPCR) and 9.57% (cPCR) of archived cattle DNA samples from the Philippines and Vietnam, respectively [30].
4.2. Bubaline Babesiosis
The majority of the world’s 198 million water buffaloes are found in Asia [7]. About 70% of the bubaline population of SEA is concentrated in Myanmar, the Philippines, and Vietnam [7], most of which are largely owned by small-scale farmers for draft work in unmechanized crop production systems and as means of transportation in the rural areas [74]. Besides these, buffalo raising can be a source of additional income in the form of milk and meat and breeding stock. Moreover, the water buffalo’s sturdiness and rusticity enable farmers to keep them with minimal sustenance costs based on low-quality fodder.
Similar to cattle, B. bovis and B. bigemina are the primary species affecting buffaloes. In contrast to the more obvious signs in cattle, clinical babesiosis in water buffaloes is rare and has been clinically documented only with B. bigemina infections [75]. This relatively stronger resistance of water buffaloes to developing clinical disease after B. bovis infection was also observed experimentally [76]. The prevailing hypothesis posed by Benitez et al. [76] is largely based on the probable co-evolutionary adaptation among B. bovis –buffalo–Rhipicephalus ticks, which could explain the resistance of water buffaloes to pathogenic B. bovis. Another species, B. orientalis, is known to be pathogenic in water buffalo and occurs only in the southeastern part of China [77].
Compared to cattle surveys, molecular studies in water buffaloes in SEA are notably fewer (Figure 1 and Table 4). A total of 1156 (n) individual water buffalo samples from Indonesia, the Philippines, Thailand, and Vietnam have been molecularly evaluated for various Babesia species (Table 4 and Table S2). The highest detection rate for bubaline B. bovis was 32.70% (cPCR) in Vietnam, 21.10% (cPCR) in Indonesia, 21.00% (nPCR) in the Philippines, and 11.20% (nPCR) in Thailand. In the case of B. bigemina, the highest detection rates were 17.50% (cPCR), 4.40% (cPCR), 4.10% (cPCR), and 3.60% (nPCR) in Indonesia, the Philippines, Vietnam, and Thailand, respectively. Finally, the detection of Babesia sp. Mymensingh in samples from Vietnam (Table 4) added water buffalo to the list of host ranges of this novel Babesia species [30].

Table 4.
Molecular reports of Babesia in water buffaloes in Southeast Asian countries.
Table 4.
Molecular reports of Babesia in water buffaloes in Southeast Asian countries.
| Country | Pathogen | Conventional PCR | Nested PCR | ||||
|---|---|---|---|---|---|---|---|
| Detection Rate (%) * | Samples (n) | References | Detection Rate (%) * | Samples (n) | References | ||
| Vietnam | Babesia bigemina | 0–4.10 | 43–49 | [41,42] | 0 | 43 | [44] |
| Babesia bovis | 32.70 | 49 | [45] | 9.30–23.30 | 43; 43 | [41,44] | |
| Babesia ovata | 0 | 49 | [42] | n. r. | |||
| Babesia sp. Mymensingh | 2.30–18.40 | 43–49 | [30] | n. r. | |||
| Philippines | Babesia bigemina | 4.40 | 272 | [78] | 0–3.00 | 65–114 | [49,50,51,79] |
| Babesia bovis | n. r. | 0–21.00 | 65–114 | [49,50,51,79] | |||
| Babesia ovata | 0 | 100 | [79] | n. r. | |||
| Thailand | Babesia bigemina | n. r. | 3.60 | 305 | [33] | ||
| Babesia bovis | n. r. | 11.20 | 305 | [33] | |||
| Indonesia | Babesia bigemina | 17.50 | 57 | [58] | n. r. | ||
| Babesia bovis | 21.10 | 57 | [58] | n. r. | |||
* Total detection rates from each study were used. n. r.: no report.
4.3. Caprine and Ovine Babesiosis
Sheep and goats are among the earliest domesticated animals by humans, preceding cattle domestication by thousands of years [80]. There are over 1.2 billion sheep and 1 billion goats in the world [7]. Sheep and goat production has a significant socioeconomic value for rural households and subsistence farming families, specifically as a supplement to farmers’ income and as means of additional food sources [81]. However, herd health is often neglected despite the high susceptibility of small ruminants to major infections such as those caused by parasitic helminths, arthropods, and protozoa [82,83], which include TBDs such as babesiosis and theileriosis.
Babesiosis causes huge economic losses in terms of lower production of milk, meat, and other livestock byproducts, combined with the indirect burden of additional cost for treatment of animals, control of the disease, and the opportunity cost of production [3]. Various species of Babesia are responsible for causing babesiosis in small ruminants, including B. ovis, B. motasi, B. crassa, B. motasi-like, and Babesia sp. Xinjiang [69,84]. Of these causative agents of babesiosis, B. ovis is the most severely pathogenic species and is responsible for causing fever, hemoglobinuria, anemia, and icterus, oftentimes leading to death [85]. In the field, mortality caused by B. ovis infection ranges from 30% to 50% in sheep [86], while natural infection in goats is subclinical [87]. Babesia motasi may have milder virulence in sheep but is more common in goats [88], whereas B. crassa seems to have low pathogenicity [89]. Ixodid ticks belonging to the genus Rhipicephalus and Hyalomma are the vectors of B. ovis, while B. motasi is transmitted by Haemaphysalis and Rhipicephalus ticks [90]. Babesia ovis is widely distributed globally, while other ovine and caprine Babesia species occur only in particular areas [88]. Although believed to be present, the distribution of B. ovis in SEA has been sporadic and its occurrence is generally unknown [90].
Despite goats and sheep ranking second (37 million) and third (19 million) in terms of the population of all ruminants in SEA, only a small number of goats and sheep have been molecularly evaluated for babesiosis (Table 5 and Table S3). So far, a total of six molecular investigations on small ruminant babesiosis in SEA have been conducted (Table 5). Babesia ovis was recently confirmed in Philippine goats [91], while Babesia sp. was molecularly detected in goats in Thailand [92]. In Vietnam, goats and sheep were positive for Babesia sp. Mymensingh, further expanding the host range of this species, whereas B. bigemina DNA was detected in a goat sample [41].

Table 5.
Molecular detection rates for Babesia in small ruminants in Southeast Asian countries.
Table 5.
Molecular detection rates for Babesia in small ruminants in Southeast Asian countries.
| Country | Host | Pathogen | Detection Rate (%) * | Samples (n) | References |
|---|---|---|---|---|---|
| Vietnam | goat | Babesia bigemina | 0.80 | 127 | [41] |
| sheep | Babesia bigemina | 0 | 51 | [41] | |
| goat | Babesia bovis | 0 | 127 | [41] | |
| sheep | Babesia bovis | 0 | 51 | [41] | |
| goat | Babesia sp. Mymensingh | 1.60 | 127 | [30] | |
| sheep | Babesia sp. Mymensingh | 2.00 | 51 | [30] | |
| Philippines | goat | Babesia ovis | 1.50 | 396 | [91] |
| goat | Babesia spp. | 0 | 100 | [93] | |
| Thailand | goat | Babesia spp. | 2.00 | 100 | [92] |
| goat | Babesia ovis | 0 | 262 | [94] |
* Total detection rates from each study were used.
5. Factors Associated with Ruminant Babesia Infection in SEA
Several factors have been associated with bovine babesiosis in SEA. Studies conducted in Thailand [53], Myanmar [60], and Malaysia [61] identified a higher number of young cattle that tested positive for bovine Babesia, whereas cattle age was negligible in bovine Babesia infections reported in the Philippines [48] and Indonesia [59]. In cattle, inverse age immunity, where the development of clinical disease is low, is an observed characteristic of bovine babesiosis and anaplasmosis in endemically stable areas. Young animals are exposed to the infection early in their life when they have a more robust immunity through maternal antibodies and strong innate immunity, enabling them to acquire natural protection against subsequent infections [13]. Likewise, higher B. bovis infection rates were recorded for taurine breeds and/or crossbreds compared with common indicine breeds (i.e., Zebu, Brahman) in Myanmar [60] and Indonesia [59]. A similar trend for B. bigemina infection was observed in cattle in Malaysia [61]. The impact of babesiosis on Bos indicus cattle is known to be milder compared with that on Bos taurus [63]. Notably, the indigenous breeds in Indonesia recorded higher molecular detection rates of B. bovis (Bali cattle) and of B. bigemina (Pesisir cattle) [59], suggesting that other cattle breeds may have variable susceptibility to Babesia infections. On the other hand, the sex of cattle was not associated with bovine Babesia positivity in surveys in the Philippines [48], Thailand [53], Myanmar [60], and Malaysia [61]. Additionally, the practice of grazing has been identified as a significant factor for bovine Babesia infections in Thailand [54], B. bovis infection in Myanmar [60], and B. bigemina infection in Malaysia [61]. The extensive management system may be directly linked to the increased exposure of the animals to the vectors that may carry the parasites.
Studies that evaluated significant factors for Babesia infection in water buffaloes in SEA are scarce. In Thailand, the age of the animal was associated with B. bovis or B. bigemina positivity [33], while the opposite was observed in the Philippines [79]. Furthermore, Babesia infections did not differ between sexes and among breeds in water buffaloes in Thailand and the Philippines, respectively [33,79]. Meanwhile, as Babesia detection studies in SEA small ruminants are in their infancy, risk factors related to such are virtually non-existent. Therefore, identifying significant factors that may increase the risk of water buffaloes, goats, and sheep to contract babesiosis may be a valuable topic to explore in future investigations.
6. Conclusions
In this mini-review, we compiled the existing molecular records and mapped the species diversity of Babesia in large and small ruminants in SEA. Molecularly confirmed Babesia species in Southeast Asian ruminants include B. bovis, B. bigemina, B. ovata, B. ovis, Babesia sp. Hue, and Babesia sp. Mymensingh. To date, molecular studies in cattle and water buffaloes have provided fundamental information on babesiosis, whereas studies on small ruminants are lacking and need more attention considering that small ruminant production is a common venture among many rural farming communities in SEA.
In an epidemiological context, molecular babesiosis research in some SEA countries has had significant success in confirming the presence of various Babesia species, albeit, it has been inadequate in truly uncovering the situation of ruminant babesiosis in the field. This calls for more extensive molecular surveillance, particularly in countries with denser ruminant populations. With various molecular diagnostic platforms becoming relatively more affordable and accessible, their utility in Babesia infection diagnosis in the field has been beneficial and shall play an important part in assessing the disease’s real impact on animal production and in formulating and implementing control programs for economically devastating diseases such as babesiosis and other TBDs.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens11080915/s1, Table S1. List of PCR-based Babesia reports on cattle in Southeast Asia. Table S2. List of PCR-based Babesia reports on water buffaloes in Southeast Asia. Table S3. List of PCR-based Babesia reports on small ruminants in Southeast Asia.
Author Contributions
Conceptualization, E.M.G. and X.X.; formal analysis, E.M.G.; writing—original draft preparation, E.M.G. and I.Z.; writing—review and editing, S.J., H.L., Z.M., and X.X.; visualization, E.M.G.; supervision, X.X.; project administration, X.X.; funding acquisition, X.X. All authors have read and agreed to the published version of the manuscript.
Funding
Eloiza May Galon is supported by a research fellowship from the Japan Society for the Promotion of Science (JSPS) for young scientists, Japan (20J20134). This project was funded by the Grant-in-Aid for Scientific Research (18H02336) and the JSPS Core-to-Core program, both from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and a grant from the Strategic International Collaborative Research Project (JPJ008837) provided by the Ministry of Agriculture, Forestry, and Fisheries of Japan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
New data were not generated in the current study; thus, statement of data availability is not applicable.
Acknowledgments
The authors would like to thank the editors and reviewers for the constructive comments which helped improve this mini-review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uilenberg, G. Babesia—A historical overview. Vet. Parasitol. 2006, 138, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A world emerging. Infect. Genet. Evol. 2012, 12, 1788–1809. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, O.J.B.; Giraldo-Ríos, C. Economic and health impact of the ticks in production animals. In Ticks and Tick-Borne Pathogens; Abubakar, M.K., Perera, P., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Leinbach, T.; Frederick, W. Southeast Asia. Encycl. Br. 2020. Available online: https://www.britannica.com/place/Southeast-Asia (accessed on 1 October 2021).
- OECD. Food and Agriculture Organization of the United Nations. In OECD-FAO Agricultural Outlook 2017–2026; OECD: Paris, France, 2017. [Google Scholar]
- Perera, B.; Oswin, M.A. Livestock production-current status in South and South-east Asia, future directions and priority areas for research. In Animal Production and Health Newsletter; Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Animal Production and Health Section: Vienna, Austria, 2014; Volume 59, p. 44. Available online: https://www.osti.gov/etdeweb/biblio/22190329 (accessed on 28 October 2021).
- Food and Agriculture Organization. Food and Agriculture Organization. Food and agriculture corporate statistical database 2019. In FAOSTAT Database; Food and Agriculture Organization: Rome, Italy, 2021; Available online: http://faostat.fao.org/ (accessed on 30 October 2021).
- Lee, T.; Hansen, J. Southeast Asia’s growing meat demand and its implications for feedstuffs imports. 2019. Available online: https://www.ers.usda.gov/amber-waves/2019/april/southeast-asia-s-growing-meat-demand-and-its-implications-for-feedstuffs-imports/ (accessed on 22 October 2021).
- Kedkovid, R.; Sirisereewan, C.; Thanawongnuwech, R. Major swine viral diseases: An Asian perspective after the African swine fever introduction. Porc. Health Manag. 2020, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Rushton, J.; Viscarra, R.; Guerne Bleich, E.; Mcleod, A. Impact of avian influenza outbreaks in the poultry sectors of five South East Asian countries (Cambodia, Indonesia, Lao PDR, Thailand, Viet Nam) outbreak costs, responses and potential long term control. Worlds Poult. Sci. J. 2005, 61, 491–514. [Google Scholar] [CrossRef]
- Park, C.-Y.; Kumar, U.; San Andres, E.A. Food Security in Asia and the Pacific; Asian Development Bank: Mandaluyong City, Philippines, 2013. [Google Scholar]
- Tan, L.P.; Hamdan, R.H.; Hassan, B.N.H.; Reduan, M.F.H.; Okene, I.A.-A.; Loong, S.K.; Khoo, J.J.; Samsuddin, A.S.; Lee, S.H. Rhipicephalus tick: A contextual review for Southeast Asia. Pathogens 2021, 10, 821. [Google Scholar] [CrossRef]
- Jonsson, N.N.; Bock, R.E.; Jorgensen, W.K.; Morton, J.M.; Stear, M.J. Is endemic stability of tick-borne disease in cattle a useful concept? Trends Parasitol. 2012, 28, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.D.S.; Mujitaba, M.A.; Xayalath, S.; Gutierrez, W.; Soriano, A.C.; Szabó, C. Perspectives of the livestock sector in the Philippines: A review. Acta Agrar. Debr. 2021, 1, 175–188. [Google Scholar] [CrossRef]
- Bunmee, T.; Chaiwang, N.; Kaewkot, C.; Jaturasitha, S. Current situation and future prospects for beef production in Thailand —A review. Asian-Australas. J. Anim. Sci. 2018, 31, 968–975. [Google Scholar] [CrossRef]
- Hostiou, N.; Duy, K.P.; Cesaro, J.-D.; Thanh, H.L.T.; Duteurtre, G.; Tien, D.N.; Bonnet, P.; Cournut, S. The Transition of Animal Farming in Vietnam: From Semi-Subsistence to Commercial Systems; Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD): Montpellier, France, 2016; Available online: https://agritrop.cirad.fr/583049/1/P31.pdf (accessed on 22 October 2021).
- Eybpoosh, S.; Haghdoost, A.A.; Mostafavi, E.; Bahrampour, A.; Azadmanesh, K.; Zolala, F. Molecular epidemiology of infectious diseases. Electron. Physician 2017, 9, 5149–5158. [Google Scholar] [CrossRef]
- Mosqueda, J.; Olvera-Ramirez, A.; Aguilar-Tipacamu, G.; Canto, G.J. Current advances in detection and treatment of babesiosis. Curr. Med. Chem. 2012, 19, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Rojas, C.; Figueroa, J.V. Diagnostic tools for the identification of Babesia sp. in persistently infected cattle. Pathogens 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Hau, N.V.; Thu, N.V.; Hanh, H.T.; Sat, L.M. A preliminary study on application of polymerase chain reaction in diagnosis of haemosporidiosis in cattle. Vet. Sci. Tech. 1999, 6, 48–52. [Google Scholar]
- Allsopp, M.T.E.P.; Allsopp, B.A. Molecular sequence evidence for the reclassification of some Babesia species. Ann. N. Y. Acad. Sci. 2006, 1081, 509–517. [Google Scholar] [CrossRef]
- Jalovecka, M.; Sojka, D.; Ascencio, M.; Schnittger, L. Babesia life cycle—When phylogeny meets biology. Trends Parasitol. 2019, 35, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Ruef, B.J.; Dowling, S.C.; Conley, P.G.; Perryman, L.E.; Brown, W.C.; Jasmer, D.P.; Rice-Ficht, A.C. A unique Babesia bovis spherical body protein is conserved among geographic isolates and localizes to the infected erythrocyte membrane. Mol. Biochem. Parasitol. 2000, 105, 1–12. [Google Scholar] [CrossRef]
- Gohil, S.; Kats, L.M.; Sturm, A.; Cooke, B.M. Recent insights into alteration of red blood cells by Babesia bovis: Moovin’ forward. Trends Parasitol. 2010, 26, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Allred, D. Variable and variant protein multigene families in Babesia bovis persistence. Pathogens 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Simas, P.V.M.; Bassetto, C.C.; Giglioti, R.; Okino, C.H.; de Oliveira, H.N.; de Sena Oliveira, M.C. Use of molecular markers can help to understand the genetic diversity of Babesia bovis. Infect. Genet. Evol. 2020, 79, 104161. [Google Scholar] [CrossRef]
- Figueroa, J.V.; Chieves, L.P.; Johnson, G.S.; Buening, G.M. Multiplex polymerase chain reaction based assay for the detection of Babesia bigemina, Babesia bovis and Anaplasma marginale DNA in bovine blood. Vet. Parasitol. 1993, 50, 69–81. [Google Scholar] [CrossRef]
- Rittipornlertrak, A.; Nambooppha, B.; Simking, P.; Punyapornwithaya, V.; Tiwananthagorn, S.; Jittapalapong, S.; Chung, Y.-T.; Sthitmatee, N. Low levels of genetic diversity associated with evidence of negative selection on the Babesia bovis apical membrane antigen 1 from parasite opulations in Thailand. Infect. Genet. Evol. 2017, 54, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, T.; Sivakumar, T.; Asada, M.; Battsetseg, B.; Huang, X.; Lan, D.T.B.; Inpankaew, T.; Ybañez, A.P.; Alhassan, A.; Thekisoe, O.M.M.; et al. A PCR based survey of Babesia ovata in cattle from various Asian, African and South American countries. J. Vet. Med. Sci. 2013, 75, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Tuvshintulga, B.; Kothalawala, H.; Silva, S.S.P.; Lan, D.T.B.; Long, P.T.; Ybañez, A.P.; Ybañez, R.H.D.; Francisco Benitez, D.; Tayebwa, D.S.; et al. Host range and geographical distribution of Babesia sp. Mymensingh. Transbound. Emerg. Dis. 2020, 67, 2233–2239. [Google Scholar] [CrossRef]
- Sivakumar, T.; Altangerel, K.; Battsetseg, B.; Battur, B.; AbouLaila, M.; Munkhjargal, T.; Yoshinari, T.; Yokoyama, N.; Igarashi, I. Genetic detection of Babesia bigemina from Mongolian cattle using apical membrane antigen-1 gene-based PCR assay. Vet. Parasitol. 2012, 187, 17–22. [Google Scholar] [CrossRef] [PubMed]
- AbouLaila, M.; Yokoyama, N.; Igarashi, I. Development and evaluation of a nested PCR based on spherical body protein 2 gene for the diagnosis of Babesia bovis infection. Vet. Parasitol. 2010, 169, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Huyen, N.X.; Shinuo, C.; Inpankaew, T.; Maklon, K.; Aboulaila, M.; Ueno, A.; Goo, Y.-K.; Yokoyama, N.; Jittapalapong, S.; et al. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet. Parasitol. 2011, 178, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Aboge, G.O.; Terkawi, M.A.; Yu, L.; Kamyingkird, K.; Luo, Y.; Li, Y.; Goo, Y.-K.; Yamagishi, J.; Nishikawa, Y.; et al. Molecular detection and identification of Babesia bovis and Babesia bigemina in cattle in northern Thailand. Parasitol. Res. 2012, 111, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Petrigh, R.; Ruybal, P.; Thompson, C.; Neumann, R.; Moretta, R.; Wilkowsky, S.; Draghi, G.; Echaide, I.; de Echaide, S.T.; Farber, M. Improved molecular tools for detection of Babesia bigemina. Ann. N. Y. Acad. Sci. 2008, 1149, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Hefford, C.; Baverstock, P.R.; Dalrymple, B.P.; Johnson, A.M. Ribosomal DNA sequence comparison of Babesia and Theileria. Mol. Biochem. Parasitol. 1992, 54, 87–95. [Google Scholar] [CrossRef]
- Bilgiç, H.B.; Karagenç, T.; Simuunza, M.; Shiels, B.; Tait, A.; Eren, H.; Weir, W. Development of a multiplex PCR assay for simultaneous detection of Theileria annulata, Babesia bovis and Anaplasma marginale in cattle. Exp. Parasitol. 2013, 133, 222–229. [Google Scholar] [CrossRef]
- Sivakumar, T.; Tagawa, M.; Yoshinari, T.; Ybañez, A.P.; Igarashi, I.; Ikehara, Y.; Hata, H.; Kondo, S.; Matsumoto, K.; Inokuma, H.; et al. PCR detection of Babesia ovata from cattle reared in Japan and clinical significance of coinfection with Theileria orientalis. J. Clin. Microbiol. 2012, 50, 2111–2113. [Google Scholar] [CrossRef]
- Sivakumar, T.; Tuvshintulga, B.; Zhyldyz, A.; Kothalawala, H.; Yapa, P.R.; Kanagaratnam, R.; Vimalakumar, S.C.; Abeysekera, T.S.; Weerasingha, A.S.; Yamagishi, J.; et al. Genetic analysis of Babesia isolates from cattle with clinical babesiosis in Sri Lanka. J. Clin. Microbiol. 2018, 56, e00895-18. [Google Scholar] [CrossRef]
- Aktaş, M.; Altay, K.; Dumanlı, N. Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet. Parasitol. 2005, 133, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Lan, D.T.B.; Long, P.T.; Yoshinari, T.; Tattiyapong, M.; Guswanto, A.; Okubo, K.; Igarashi, I.; Inoue, N.; Xuan, X.; et al. PCR detection and genetic diversity of bovine hemoprotozoan parasites in Vietnam. J. Vet. Med. Sci. 2013, 75, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Weerasooriya, G.; Sivakumar, T.; Lan, D.T.B.; Long, P.T.; Takemae, H.; Igarashi, I.; Inoue, N.; Yokoyama, N. Epidemiology of bovine hemoprotozoa parasites in cattle and water buffalo in Vietnam. J. Vet. Med. Sci. 2016, 78, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Lan, D.T.B.; Long, P.T.; Viet, L.Q.; Weerasooriya, G.; Kume, A.; Suganuma, K.; Igarashi, I.; Yokoyama, N. Serological and molecular surveys of Babesia bovis and Babesia bigemina among native cattle and cattle imported from Thailand in Hue, Vietnam. J. Vet. Med. Sci. 2018, 80, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, Y.; Cao, S.; Terkawi, M.A.; Lan, D.T.B.; Long, P.T.; Yu, L.; Zhou, M.; Gong, H.; Zhang, H.; et al. Molecular and seroepidemiological survey of Babesia bovis and Babesia bigemina infections in cattle and water buffaloes in the central region of Vietnam. Trop. Biomed. 2014, 31, 406–413. [Google Scholar] [PubMed]
- Yokoyama, N.; Sivakumar, T.; Tuvshintulga, B.; Hayashida, K.; Igarashi, I.; Inoue, N.; Long, P.T.; Lan, D.T.B. Genetic variations in merozoite surface antigen genes of Babesia bovis detected in Vietnamese cattle and water buffaloes. Infect. Genet. Evol. 2015, 30, 288–295. [Google Scholar] [CrossRef]
- Ochirkhuu, N.; Konnai, S.; Mingala, C.N.; Okagawa, T.; Villanueva, M.; Pilapil, F.M.I.R.; Murata, S.; Ohashi, K. Molecular epidemiological survey and genetic analysis of vector-borne infections of cattle in Luzon island, the Philippines. Vet. Parasitol. 2015, 212, 161–167. [Google Scholar] [CrossRef]
- Ybañez, A.P.; Sivakumar, T.; Ybañez, R.H.D.; Vincoy, M.R.B.; Tingson, J.A.; Perez, Z.O.; Gabotero, S.R.; Buchorno, L.P.; Inoue, N.; Matsumoto, K.; et al. Molecular survey of bovine vector-borne pathogens in Cebu, Philippines. Vet. Parasitol. 2013, 196, 13–20. [Google Scholar] [CrossRef]
- Yu, L.; Terkawi, M.A.; Cruz-Flores, M.J.; Claveria, F.G.; Aboge, G.O.; Yamagishi, J.; Goo, Y.-K.; Cao, S.; Masatani, T.; Nishikawa, Y.; et al. Epidemiological survey of Babesia bovis and Babesia bigemina infections of cattle in Philippines. J. Vet. Med. Sci. 2013, 75, 995–998. [Google Scholar] [CrossRef]
- Herrera, P.C.; Viloria, V.; Balbin, M.; Mingala, C. Prevalence of babesiosis (Babesia bovis and Babesia bigemina) in cattle and water buffalo in Nueva Ecija, Philippines using nested polymerase chain reaction. Ann. Parasitol. 2017, 63, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Galon, E.M.S.; Ybañez, R.H.D.; Adjou Moumouni, P.F.; Tumwebaze, M.A.; Fabon, R.J.A.; Callanta, M.R.R.; Labutong, K.J.E.; Salazar, G.B.; Liu, M.; Li, J.; et al. Molecular survey of tick-borne pathogens infecting backyard cattle and water buffaloes in Quezon province, Philippines. J. Vet. Med. Sci. 2020, 82, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Prado, I.C.B.; Capuno, L.X.B.; Collera, P.D.L.P.; Cabralda, A.P.D.; De Ramos, K.A.S.; Bernardo, J.M.G.; Divina, B.P.; Masatani, T.; Tanaka, T.; Galay, R.L. Molecular detection and characterization of Babesia and Theileria in cattle and water buffaloes from southern Luzon, Philippines. Microorganisms 2022, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Foronda, J.; Baticados, W.; Baticados, A. Molecular evidence of Babesia spp. in cattle in the Philippines. Online J. Vet. Res. 2010, 14, 188–193. [Google Scholar]
- Simking, P.; Yatbantoong, N.; Saetiew, N.; Saengow, S.; Yodsri, W.; Chaiyarat, R.; Wongnarkpet, S.; Jittapalapong, S. Prevalence and risk factors of Babesia infections in cattle trespassing natural forest areas in Salakpra wildlife sanctuary, Kanchanaburi province. J. Trop. Med. Parasitol. 2014, 37, 10–19. [Google Scholar]
- Jirapattharasate, C.; Adjou Moumouni, P.F.; Cao, S.; Iguchi, A.; Liu, M.; Wang, G.; Zhou, M.; Vudriko, P.; Changbunjong, T.; Sungpradit, S.; et al. Molecular epidemiology of bovine Babesia spp. and Theileria orientalis parasites in beef cattle from northern and northeastern Thailand. Parasitol. Internat. 2016, 65, 62–69. [Google Scholar] [CrossRef]
- Jirapattharasate, C.; Adjou Moumouni, P.F.; Cao, S.; Iguchi, A.; Liu, M.; Wang, G.; Zhou, M.; Vudriko, P.; Efstratiou, A.; Changbunjong, T.; et al. Molecular detection and genetic diversity of bovine Babesia spp., Theileria orientalis, and Anaplasma marginale in beef cattle in Thailand. Parasitol. Res. 2017, 116, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Rittisut, B.; Sarkaou, P.; Na-Lampang, K. Detection of blood parasites infection in native cattle in Chiang Mai under the participatory one health disease and detection project. J. Agric. Res. Ext. 2018, 35, 32–45. [Google Scholar]
- Simking, P.; Saengow, S.; Bangphoomi, K.; Sarataphan, N.; Wongnarkpet, S.; Inpankaew, T.; Jittapalapong, S.; Munkhjargal, T.; Sivakumar, T.; Yokoyama, N.; et al. The molecular prevalence and MSA-2b gene-based genetic dversity of Babesia bovis in dairy cattle in Thailand. Vet. Parasitol. 2013, 197, 642–648. [Google Scholar] [CrossRef]
- Sawitri, D.H.; Wardhana, A.H.; Ekawasti, F.; Dewi, D.A. Parasitological and molecular detection of babesiosis in cattle and buffalo in west and central Java. In Proceedings of the 9th International Seminar on Tropical Animal Production (ISTAP 2021), Yogyakarta, Indonesia, 21–22 September 2021; Atlantis Press: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Guswanto, A.; Allamanda, P.; Mariamah, E.S.; Sodirun, S.; Wibowo, P.E.; Indrayani, L.; Nugroho, R.H.; Wirata, I.K.; Jannah, N.; Dias, L.P.; et al. Molecular and serological detection of bovine babesiosis in Indonesia. Parasites Vectors 2017, 10, 550. [Google Scholar] [CrossRef]
- Bawm, S.; Htun, L.L.; Maw, N.N.; Ngwe, T.; Tosa, Y.; Kon, T.; Kaneko, C.; Nakao, R.; Sakurai, T.; Kato, H.; et al. Molecular survey of Babesia infections in cattle from different areas of Myanmar. Ticks Tick-Borne Dis. 2016, 7, 204–207. [Google Scholar] [CrossRef]
- Ola-Fadunsin, S.D.; Sharma, R.S.K.; Abdullah, D.A.; Gimba, F.I.; Abdullah, F.F.J.; Sani, R.A. The molecular prevalence, distribution and risk factors associated with Babesia bigemina infection in Peninsular Malaysia. Ticks Tick-Borne Dis. 2021, 12, 101653. [Google Scholar] [CrossRef] [PubMed]
- Ola-Fadunsin, S.D.; Maizatul, A.M.; Ibrahim, A.R.; Amlizawathy, A.; Chandrawathani, P.; Jesse, F.F.A.; Sani, R.A.; Sharma, R.S.K. Molecular prevalence and species co-Infection of bovine haemoparasites in Peninsular Malaysia. Malays. J. Vet. Res. 2017, 8, 13–22. [Google Scholar]
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef] [PubMed]
- McLeod, R.; Kristjanson, P. Final report of joint eSYS/ILRI/ACIAR Tick Cost project—Economic impact of ticks and tick-borne diseases to livestock in Africa, Asia and Australia. Int. Livest. Res. Inst. Nairobi. 1999, 3. [Google Scholar]
- Ganzinelli, S.; Rodriguez, A.; Schnittger, L.; Florin-Christensen, M. Babesia in domestic ruminants. In Parasitic Protozoa of Farm Animals and Pets; Florin-Christensen, M., Schnittger, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 215–239. [Google Scholar]
- Ohta, M.; Tsuji, M.; Tsuji, N.; Fujisaki, K. Morphological, serological and antigenic characteristics, and protein profile of newly isolated Japanese bovine Babesia parasite with particular reference to those of B. ovata. J. Vet. Med. Sci. 1995, 57, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yin, H.; Liu, Z.; Yang, D.; Guan, G.; Liu, A.; Ma, M.; Dang, S.; Lu, B.; Sun, C.; et al. Molecular phylogenetic studies on an unnamed bovine Babesia sp. based on small subunit ribosomal RNA gene sequences. Vet. Parasitol. 2005, 133, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.C.; Krücken, J.; Ahmed, J.S.; Majumder, S.; Baumann, M.P.; Clausen, P.-H.; Nijhof, A.M. Molecular identification of tick-borne pathogens infecting cattle in Mymensingh district of Bangladesh reveals emerging species of Anaplasma and Babesia. Transbound. Emerg. Dis. 2018, 65, e231–e242. [Google Scholar] [CrossRef]
- Schnittger, L.; Ganzinelli, S.; Bhoora, R.; Omondi, D.; Nijhof, A.M.; Florin-Christensen, M. The piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: Species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 2022, 121, 1207–1245. [Google Scholar] [CrossRef] [PubMed]
- Zintl, A.; Mulcahy, G.; Skerrett, H.E.; Taylor, S.M.; Gray, J.S. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin. Microbiol. Rev. 2003, 16, 622–636. [Google Scholar] [CrossRef]
- Sivakumar, T.; Igarashi, I.; Yokoyama, N. Babesia ovata: Taxonomy, phylogeny and epidemiology. Vet. Parasitol. 2016, 229, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Larocca, V.; Parisi, A.; Losurdo, M.; Lia, R.P.; Greco, M.F.; Miccolis, A.; Ventrella, G.; Otranto, D.; Buonavoglia, C. Clinical bovine piroplasmosis caused by Babesia occultans in Italy. J. Clin. Microbiol. 2013, 51, 2432–2434. [Google Scholar] [CrossRef] [PubMed]
- Noaman, V.; Ghadimipour, R.; Nabavi, R. First report of Babesia occultans in two symptomatic cows in Iran. Parasitol. Res. 2021, 120, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Borghese, A.; Mazzi, M. Buffalo population and strategies in the world. In Buffalo Production and Research; FAO regional office for Europe interregional cooperative research network on buffalo (ESCORENA): Rome, Italy, 2005; pp. 1–2. [Google Scholar]
- Singla, L.D.; Singh, J.; Aulakh, G.S. Babesiosis in an unusual case of Murrah buffalo with six functional teats. Buffalo Bull. 2002, 21, 55–58. [Google Scholar]
- Benitez, D.; Mesplet, M.; Echaide, I.; de Echaide, S.T.; Schnittger, L.; Florin-Christensen, M. Mitigated clinical disease in water buffaloes experimentally infected with Babesia bovis. Ticks Tick-borne Dis. 2018, 9, 1358–1363. [Google Scholar] [CrossRef]
- He, L.; Liu, Q.; Yao, B.; Zhou, Y.; Hu, M.; Fang, R.; Zhao, J. A historical overview of research on Babesia orientalis, a protozoan parasite infecting water buffalo. Front. Microbiol. 2017, 8, 1323. [Google Scholar] [CrossRef] [PubMed]
- Mingala, C.N.; Konnai, S.; Cruz, L.C.; Onuma, M.; Ohashi, K. Comparative moleculo-immunological analysis of swamp- and riverine-type water buffaloes responses. Cytokine 2009, 46, 273–282. [Google Scholar] [CrossRef]
- Galon, E.M.S.; Adjou Moumouni, P.F.; Ybañez, R.H.D.; Ringo, A.E.; Efstratiou, A.; Lee, S.-H.; Liu, M.; Guo, H.; Gao, Y.; Li, J.; et al. First molecular detection and characterization of tick-borne pathogens in water buffaloes in Bohol, Philippines. Ticks Tick-Borne Dis. 2019, 10, 815–821. [Google Scholar] [CrossRef]
- Mazinani, M.; Rude, B. Population, world production and quality of sheep and goat products. Am. J. Anim. Vet. Sci. 2020, 15, 291–299. [Google Scholar] [CrossRef]
- Kumar, S.; Roy, M.M. Small ruminant’s role in sustaining rural livelihoods in arid and semiarid regions and their potential for commercialization. In New Paradigms in Livestock Production from Traditional to Commercial Farming and Beyond; Agrotech Publishing Academy: Udaipur, India, 2013; pp. 57–80. [Google Scholar]
- Sargison, N. The critical importance of planned small ruminant livestock health and production in addressing global challenges surrounding food production and poverty alleviation. N. Z. Vet. J. 2020, 68, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Mcleod, R.S. Costs of major parasites to the Australian livestock industries. Int. J. Parasitol. 1995, 25, 1363–1367. [Google Scholar] [CrossRef]
- Lempereur, L.; Beck, R.; Fonseca, I.; Marques, C.; Duarte, A.; Santos, M.; Zúquete, S.; Gomes, J.; Walder, G.; Domingos, A.; et al. Guidelines for the detection of Babesia and Theileria parasites. Vector Borne Zoonotic Dis. 2017, 17, 51–65. [Google Scholar] [CrossRef]
- Yeruham, I.; Hadani, A.; Galker, F. Some epizootiological and clinical aspects of ovine babesiosis caused by Babesia ovis—A review. Vet. Parasitol. 1998, 74, 153–163. [Google Scholar] [CrossRef]
- Hashemi-Fesharki, R. Tick-borne diseases of sheep and goats and their related vectors in Iran. Parassitologia 1997, 39, 115–117. [Google Scholar] [PubMed]
- Stuen, S. Haemoparasites—Challenging and wasting infections in small ruminants: A review. Animals 2020, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Sherman, D.M. Goat Medicine; John Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Hasherni-Fesharki, R.; Uilenberg, G. Babesia crassa n. sp. (Sporozoa, Babesiidae) of domestic sheep in Iran. Vet. Q. 1981, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Friedhoff, K.T. Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 1997, 39, 99–109. [Google Scholar]
- National Research Center for Protozoan Diseases; Galon, E.M.; Ybañez, R.H.; Macalanda, A.M.; Liu, M.; Tumwebaze, M.A.; Byamukama, B.; Ji, S.; Zafar, I.; Li, J.; et al. First Molecular Confirmation of Caprine Piroplasma in the Philippine; Obihiro University of Agriculture and Veterinary Medicine: Obihiro, Japan, 2022; submitted. [Google Scholar]
- Tu, H.L.C.; Nugraheni, Y.R.; Tiawsirisup, S.; Saiwichai, T.; Thiptara, A.; Kaewthamasorn, M. Development of a novel multiplex PCR assay for the detection and differentiation of Plasmodium caprae from Theileria luwenshuni and Babesia spp. in goats. Acta Trop. 2021, 220, 105957. [Google Scholar] [CrossRef] [PubMed]
- Ybañez, A.P.; Arrabis, O.V.; Alvarez, D.J.M.; Galon, E.M.S.; Jayag, R.M.P.; Delan, E.S.; Ybañez, R.H.D.; Xuan, X. Evaluation on the presence of Anaplasma, Ehrlichia, and Babesia spp. in goats (Capra hircus) in Cebu, the Philippines. Vet. World 2019, 12, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Udonsom, R.; Mahittikorn, A.; Jirapattharasate, C. Molecular detection and genetic diversity of tick-borne pathogens in goats from the southern part of Thailand. Pathogens 2022, 11, 477. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).