Encephalitozoon hellem Infection Promotes Monocytes Extravasation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Pathogen
2.3. Experimental Design
2.4. Bone Marrow Isolation
2.5. Flow Cytometry Analysis
2.6. Peripheral Blood Collection
2.7. Detection of E. hellem
2.8. Reverse Transcription-Quantitative Polymerase Chain Reaction
2.9. Statistical Analysis
3. Results
3.1. Effects of E. hellem Infection on Monocyte Population in Bone Marrow
3.2. Effects of E. hellem Infection on Monocyte Population in Peripheral Blood
3.3. Monocytes and Derivatives Propagation in Small Intestine
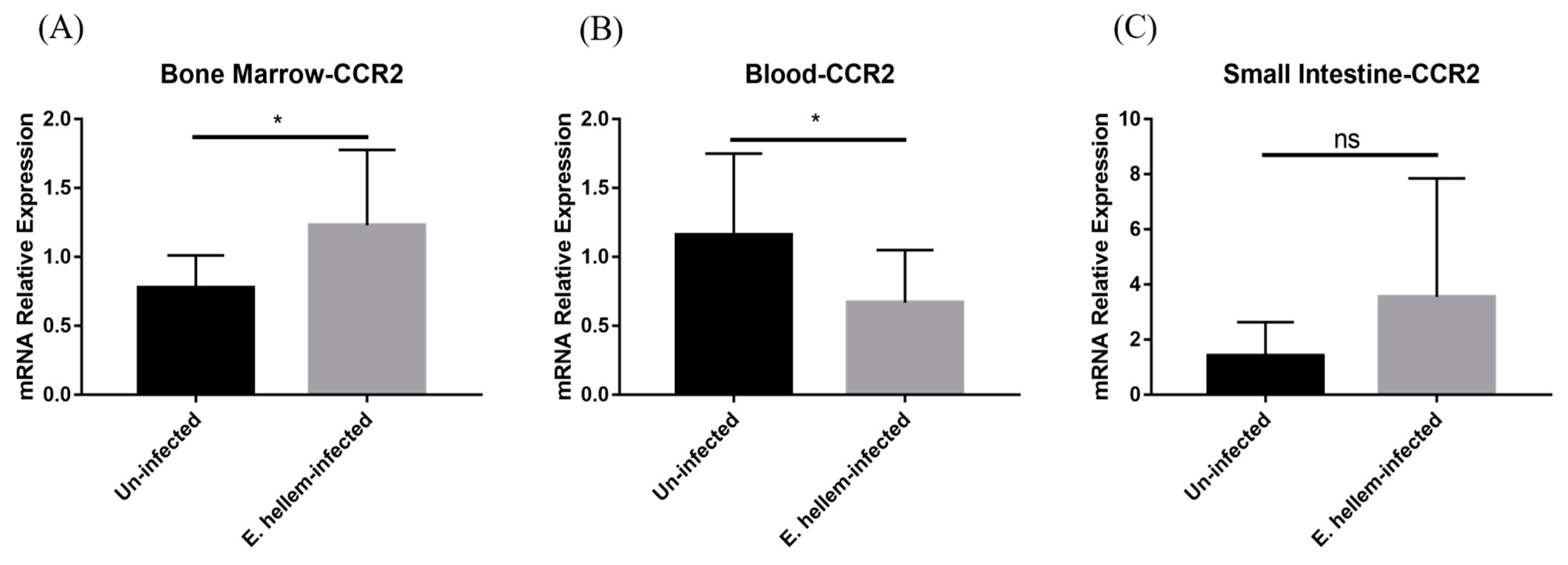
3.4. CCR2 Mediates Monocytes Egression and Extravasation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, L.M. Microsporidia: Emerging pathogenic protists. Acta Trop. 2001, 78, 89–102. [Google Scholar] [CrossRef]
- Didier, E.S.; Weiss, L.M. Microsporidiosis: Current status. Curr. Opin. Infect. Dis. 2006, 19, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Bryan, R.T.; Schwartz, D.A.; Owen, R.L. Human microsporidial infections. Clin. Microbiol. Rev. 1994, 7, 426–461. [Google Scholar] [CrossRef] [PubMed]
- Szumowski, S.C.; Troemel, E.R. Microsporidia-host interactions. Curr. Opin. Microbiol. 2015, 26, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Sancak, B.; Akyön, Y. Microsporidia: General characteristics, infections and laboratory diagnosis. Mikrobiyol. Bul. 2005, 39, 513–522. [Google Scholar]
- Janeway, C.A., Jr. How the immune system works to protect the host from infection: A personal view. Proc. Natl. Acad. Sci. USA 2001, 98, 7461–7468. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.; Hotard, A.; Hale-Donze, H. Innate immune responses to Encephalitozoon species infections. Microbes. Infect. 2009, 11, 905–911. [Google Scholar] [CrossRef]
- Aseeja, P.; Shaikh, Y.; Bajpai, A.; Sirsikar, P.; Kalra, S.K. Advancement in our understanding of immune response against Encephalitozoon infection. Parasite Immunol. 2021, 43, e12828. [Google Scholar] [CrossRef]
- Sokolova, Y.Y.; Bowers, L.C.; Alvarez, X.; Didier, E.S. Encephalitozoon cuniculi and Vittaforma corneae (Phylum Microsporidia) inhibit staurosporine-induced apoptosis in human THP-1 macrophages in vitro. Parasitology 2019, 146, 569–579. [Google Scholar] [CrossRef]
- Dalboni, L.C.; Alvares Saraiva, A.M.; Konno, F.T.C.; Perez, E.C.; Codeceira, J.F.; Spadacci-Morena, D.D.; Lallo, M.A. Encephalitozoon cuniculi takes advantage of efferocytosis to evade the immune response. PLoS ONE 2021, 16, e0247658. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gao, H.; Xu, J.; Luo, J.; Han, B.; Bao, J.; Pan, G.; Li, T.; Zhou, Z. Innate and Adaptive Immune Responses Against Microsporidia Infection in Mammals. Front. Microbiol. 2020, 11, 1468. [Google Scholar] [CrossRef]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; West, J.; Agochukwu, N.; Suire, C.; Hale-Donze, H. Induction of host chemotactic response by Encephalitozoon spp. Infect. Immun. 2007, 75, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Morias, Y.; Abels, C.; Laoui, D.; Van Overmeire, E.; Guilliams, M.; Schouppe, E.; Tacke, F.; Devries, C.J.; De Baetselier, P.; Beschin, A. Ly6C− Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C+ Monocytes into Macrophages. PLoS Pathog. 2015, 11, e1004873. [Google Scholar] [CrossRef] [PubMed]
- Palframan, R.T.; Jung, S.; Cheng, G.; Weninger, W.; Luo, Y.; Dorf, M.; Littman, D.R.; Rollins, B.J.; Zweerink, H.; Rot, A.; et al. Inflammatory chemokine transport and presentation in HEV: A remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 2001, 194, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.L.; Peters, W.; Si, Y.; Slaymaker, S.; Aslanian, A.M.; Weisberg, S.P.; Mack, M.; Charo, I.F. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Investig. 2007, 117, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Pariyakanok, L.; Satitpitakul, V.; Laksanaphuk, P.; Ratanawongphaibul, K.; Putaporntip, C.; Jongwutiwes, S. Stromal Keratitis with Endophthalmitis Caused by Vittaforma Corneae in an Immunocompetent Patient: A Case Report. Ocul. Immunol. Inflamm. 2019, 27, 826–828. [Google Scholar] [CrossRef]
- Chanthick, C.; Suttitheptumrong, A.; Rawarak, N.; Pattanakitsakul, S.N. Transcytosis Involvement in Transport System and Endothelial Permeability of Vascular Leakage during Dengue Virus Infection. Viruses 2018, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Sunderkötter, C.; Nikolic, T.; Dillon, M.J.; Van Rooijen, N.; Stehling, M.; Drevets, D.A.; Leenen, P.J. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004, 172, 4410–4417. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Tang, Y.; Mo, B.; Ran, M.; He, X.; Bao, J.; Zhou, Z. Characterization of a Murine Model for Encephalitozoon hellem Infection after Dexamethasone Immunosuppression. Microorganisms 2020, 8, 1891. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S.; Leitch, G.J.; Moura, H.; Wallace, S.; Weber, R.; Bryan, R.T. Culture, electron microscopy, and immunoblot studies on a microsporidian parasite isolated from the urine of a patient with AIDS. J. Protozool. 1991, 38, 105s–111s. [Google Scholar] [PubMed]
- Venugopalan, S.K.; Shanmugarajan, T.S.; Navaratnam, V.; Mansor, S.M.; Ramanathan, S. Dexamethasone provoked mitochondrial perturbations in thymus: Possible role of N-acetylglucosamine in restoration of mitochondrial function. Biomed. Pharmacother. 2016, 83, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Quan, N. Immune Cell Isolation from Mouse Femur Bone Marrow. Bio Protoc. 2015, 5, e1631. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, X.; Li, H.; Borger, D.K.; Wei, Q.; Yang, E.; Xu, C.; Pinho, S.; Frenette, P.S. The microbiota regulates hematopoietic stem cell fate decisions by controlling iron availability in bone marrow. Cell Stem Cell 2022, 29, 232–247.e237. [Google Scholar] [CrossRef]
- Bandeira Ferreira, F.L.; Séguin, O.; Descoteaux, A.; Heinonen, K.M. Persistent Cutaneous Leishmania major Infection Promotes Infection-Adapted Myelopoiesis. Microorganisms 2022, 10, 535. [Google Scholar] [CrossRef]
- de Bruijn, M.F.; van Vianen, W.; Ploemacher, R.E.; Bakker-Woudenberg, I.A.; Campbell, P.A.; van Ewijk, W.; Leenen, P.J. Bone marrow cellular composition in Listeria monocytogenes infected mice detected using ER-MP12 and ER-MP20 antibodies: A flow cytometric alternative to differential counting. J. Immunol. Methods 1998, 217, 27–39. [Google Scholar] [CrossRef]
- De Trez, C.; Magez, S.; Akira, S.; Ryffel, B.; Carlier, Y.; Muraille, E. iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS Pathog. 2009, 5, e1000494. [Google Scholar] [CrossRef]
- Pereira, A.; Alvares-Saraiva, A.M.; Konno, F.T.C.; Spadacci-Morena, D.D.; Perez, E.C.; Mariano, M.; Lallo, M.A. B-1 cell-mediated modulation of M1 macrophage profile ameliorates microbicidal functions and disrupt the evasion mechanisms of Encephalitozoon cuniculi. PLoS Negl. Trop. Dis. 2019, 13, e0007674. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.E.; Zorro, M.M.; Sierra, J.; Gilchrist, K.; Botero, J.H.; Baena, A.; Ramirez-Pineda, J.R. Encephalitozoon intestinalis Inhibits Dendritic Cell Differentiation through an IL-6-Dependent Mechanism. Front. Cell Infect. Microbiol. 2016, 6, 4. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; An, G.; Wang, X.; Tang, Y.; Jin, J.; Bao, J.; Zhou, Z. Encephalitozoon hellem Infection Promotes Monocytes Extravasation. Pathogens 2022, 11, 914. https://doi.org/10.3390/pathogens11080914
Lu Y, An G, Wang X, Tang Y, Jin J, Bao J, Zhou Z. Encephalitozoon hellem Infection Promotes Monocytes Extravasation. Pathogens. 2022; 11(8):914. https://doi.org/10.3390/pathogens11080914
Chicago/Turabian StyleLu, Yishan, Guozhen An, Xue Wang, Yunlin Tang, Jiangyan Jin, Jialing Bao, and Zeyang Zhou. 2022. "Encephalitozoon hellem Infection Promotes Monocytes Extravasation" Pathogens 11, no. 8: 914. https://doi.org/10.3390/pathogens11080914
APA StyleLu, Y., An, G., Wang, X., Tang, Y., Jin, J., Bao, J., & Zhou, Z. (2022). Encephalitozoon hellem Infection Promotes Monocytes Extravasation. Pathogens, 11(8), 914. https://doi.org/10.3390/pathogens11080914





