Evolution of the Ace-1 and Gste2 Mutations and Their Potential Impact on the Use of Carbamate and Organophosphates in IRS for Controlling Anopheles gambiae s.l., the Major Malaria Mosquito in Senegal
Abstract
1. Introduction
2. Results
2.1. Ace-1 and Gste2 Resistant Allele Frequencies in Anopheles gambiae s.l. from Senegal
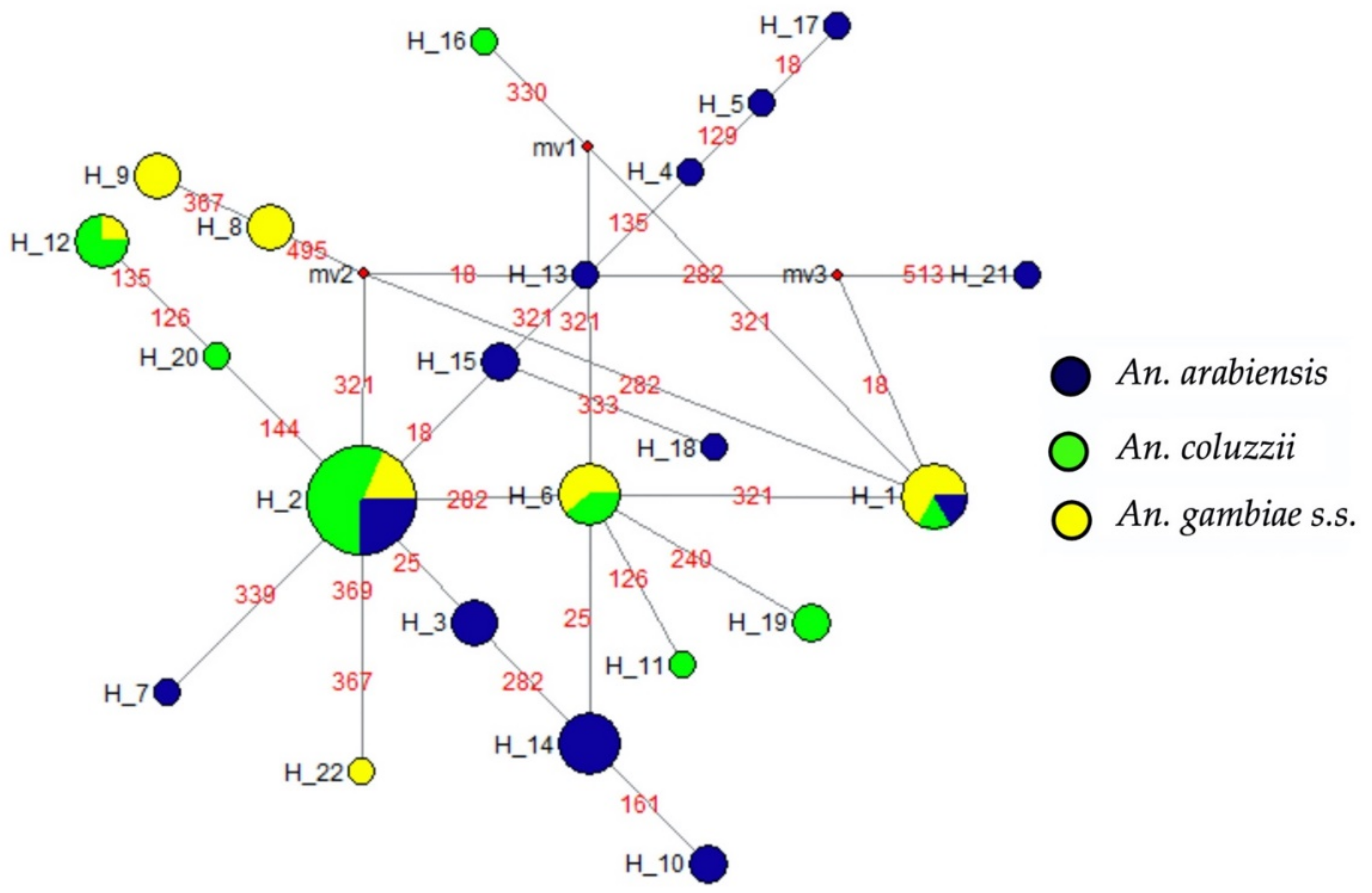
2.2. High Genetic and Haplotype Diversities at the Ace-1 Locus in Anopheles gambiae s.l. from Senegal
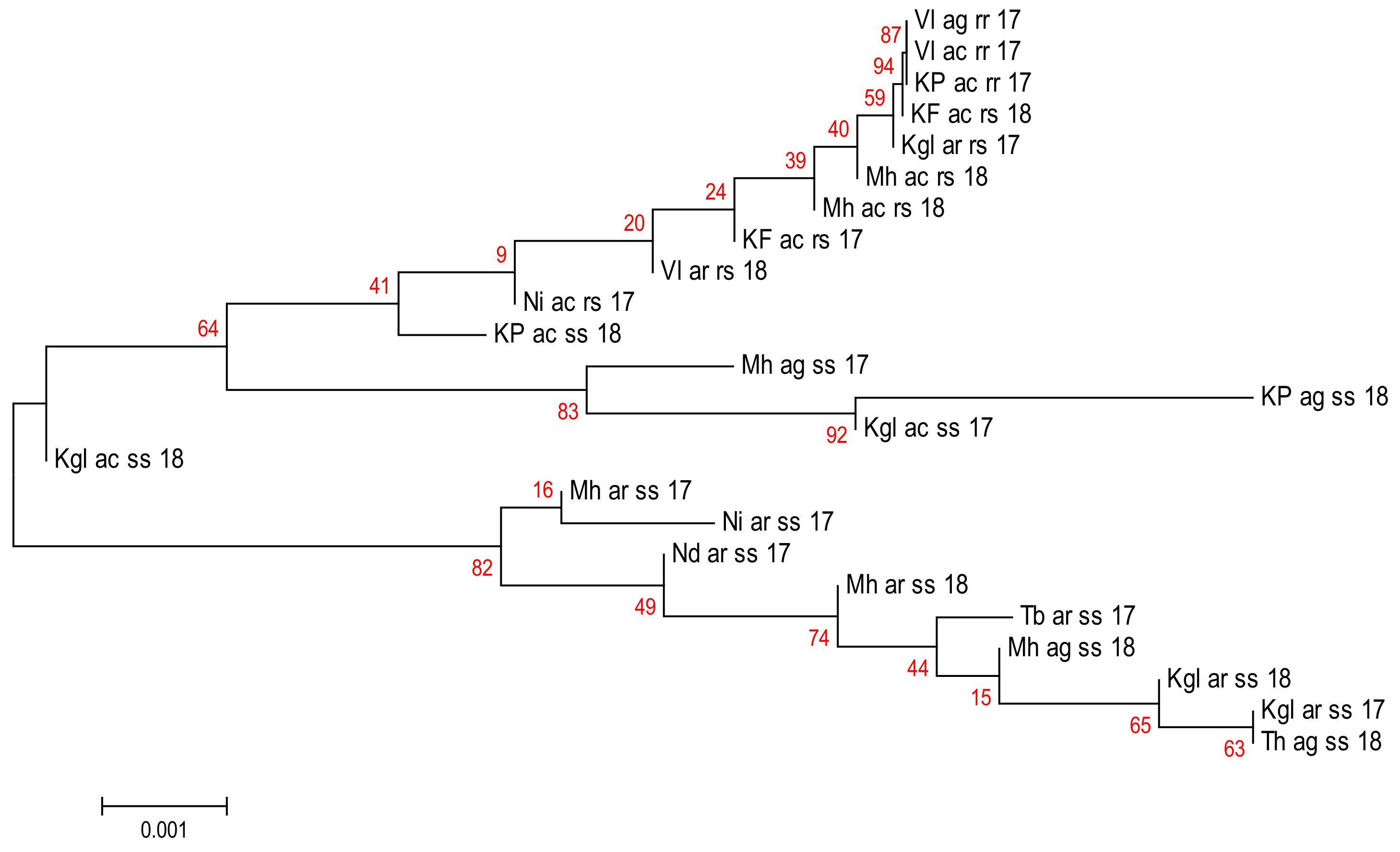
2.3. High Genetic and Haplotype Diversities at Gste2 Locus in Anopheles gambiae s.l. from Senegal
3. Discussion
4. Material and Methods
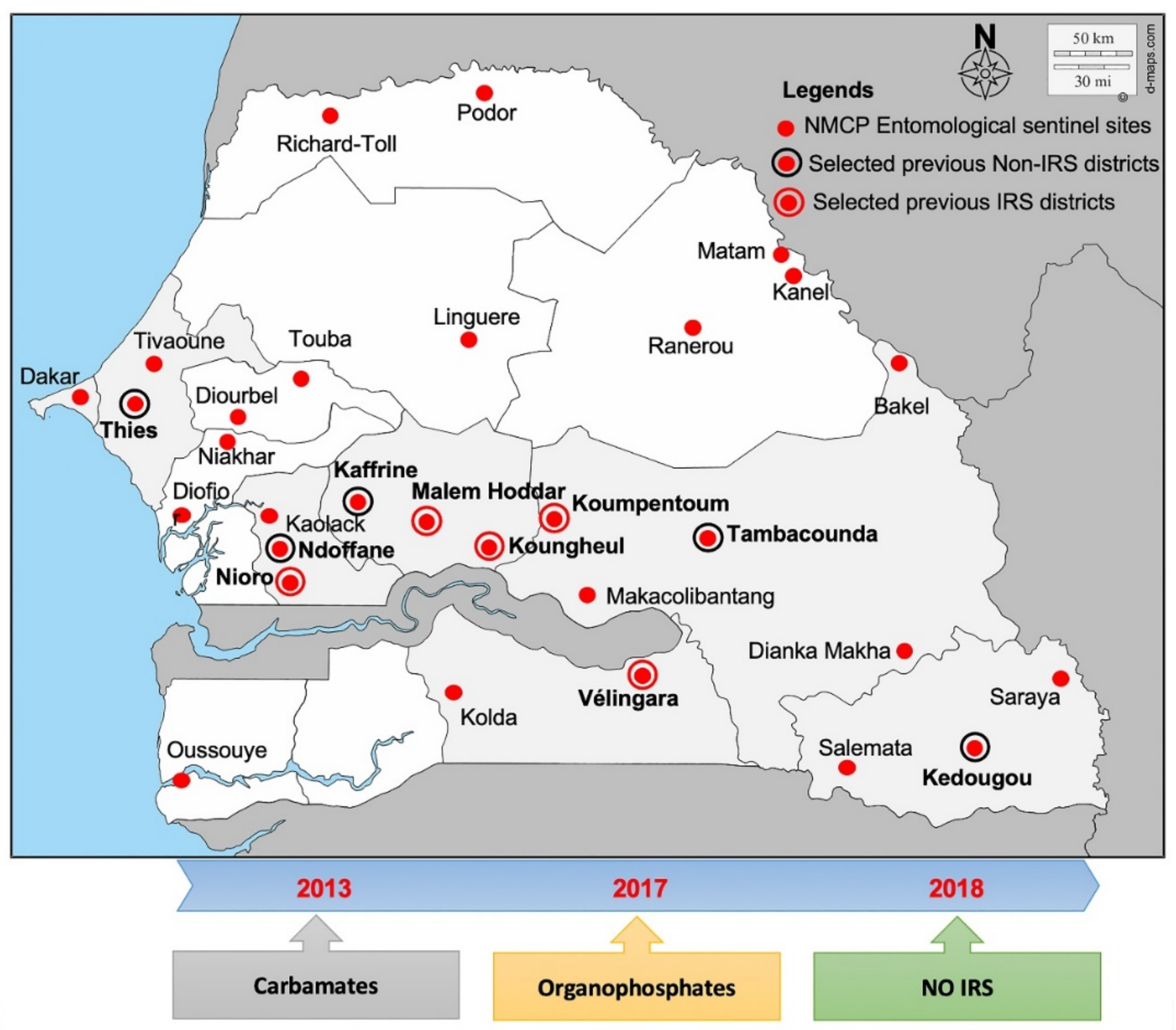
4.1. Study Area and Samples Collections
4.2. Molecular Identification of An. gambiae s.l. Species and Genotyping of the Ace-1 and Gste2 Mutations
4.3. Partial Sequences of Ace-1 and Gste2 Genes
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The Effect of Malaria Control on Plasmodium Falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Indoor Residual Spraying: An Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Fournier, D.; Bride, J.M.; Poirie, M.; Bergé, J.B.; Plapp, F.W. Insect Glutathione S-Transferases. Biochemical Characteristics of the Major Forms from Houseflies Susceptible and Resistant to Insecticides. J. Biol. Chem. 1992, 267, 1840–1845. [Google Scholar] [CrossRef]
- Djègbè, I.; Agossa, F.R.; Jones, C.M.; Poupardin, R.; Cornelie, S.; Akogbéto, M.; Ranson, H.; Corbel, V. Molecular Characterization of DDT Resistance in Anopheles gambiae from Benin. Parasit. Vectors 2014, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Yunta, C.; Ibrahim, S.S.; Djouaka, R.; Irving, H.; Menze, B.D.; Ismail, H.M.; Hemingway, J.; Ranson, H.; Albert, A.; et al. A Single Mutation in the GSTe2 Gene Allows Tracking of Metabolically Based Insecticide Resistance in a Major Malaria Vector. Genome Biol. 2014, 15, R27. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Rossiter, L.; Ortelli, F.; Jensen, B.; Wang, X.; Roth, C.W.; Collins, F.H.; Hemingway, J. Identification of a Novel Class of Insect Glutathione S-Transferases Involved in Resistance to DDT in the Malaria Vector Anopheles gambiae. Biochem. J. 2001, 359, 295–304. [Google Scholar] [CrossRef]
- Ding, Y.; Hawkes, N.; Meredith, J.; Eggleston, P.; Hemingway, J.; Ranson, H. Characterization of the Promoters of Epsilon Glutathione Transferases in the Mosquito Anopheles gambiae and Their Response to Oxidative Stress. Biochem. J. 2005, 387, 879–888. [Google Scholar] [CrossRef]
- Mitchell, S.N.; Rigden, D.J.; Dowd, A.J.; Lu, F.; Wilding, C.S.; Weetman, D.; Dadzie, S.; Jenkins, A.M.; Regna, K.; Boko, P.; et al. Metabolic and Target-Site Mechanisms Combine to Confer Strong DDT Resistance in Anopheles gambiae. PLoS ONE 2014, 9, e92662. [Google Scholar] [CrossRef]
- Hamid-Adiamoh, M.; Amambua-Ngwa, A.; Nwakanma, D.; D’Alessandro, U.; Awandare, G.A.; Afrane, Y.A. Insecticide Resistance in Indoor and Outdoor-Resting Anopheles gambiae in Northern Ghana. Malar. J. 2020, 19, 314. [Google Scholar] [CrossRef]
- Hamid-Adiamoh, M.; Nwakanma, D.; Assogba, B.S.; Ndiath, M.O.; D’Alessandro, U.; Afrane, Y.A.; Amambua-Ngwa, A. Influence of Insecticide Resistance on the Biting and Resting Preferences of Malaria Vectors in the Gambia. PLoS ONE 2021, 16, e0241023. [Google Scholar] [CrossRef]
- Weill, M.; Malcolm, C.; Chandre, F.; Mogensen, K.; Berthomieu, A.; Marquine, M.; Raymond, M. The Unique Mutation in Ace-1 Giving High Insecticide Resistance Is Easily Detectable in Mosquito Vectors. Insect Mol. Biol. 2004, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aïzoun, N.; Aïkpon, R.; Gnanguenon, V.; Oussou, O.; Agossa, F.; Padonou, G.G.; Akogbéto, M. Status of Organophosphate and Carbamate Resistance in Anopheles gambiae Sensu Lato from the South and North Benin, West Africa. Parasit. Vectors 2013, 6, 274. [Google Scholar] [CrossRef] [PubMed]
- Dabiré, R.K.; Namountougou, M.; Diabaté, A.; Soma, D.D.; Bado, J.; Toé, H.K.; Bass, C.; Combary, P. Distribution and Frequency of Kdr Mutations within Anopheles gambiae s.l. Populations and First Report of the Ace.1G119S Mutation in Anopheles arabiensis from Burkina Faso (West Africa). PLoS ONE 2014, 9, e101484. [Google Scholar] [CrossRef] [PubMed]
- Oumbouke, W.A.; Pignatelli, P.; Barreaux, A.M.G.; Tia, I.Z.; Koffi, A.A.; Ahoua Alou, L.P.; Sternberg, E.D.; Thomas, M.B.; Weetman, D.; N’Guessan, R. Fine Scale Spatial Investigation of Multiple Insecticide Resistance and Underlying Target-Site and Metabolic Mechanisms in Anopheles gambiae in Central Côte d’Ivoire. Sci. Rep. 2020, 10, 15066. [Google Scholar] [CrossRef] [PubMed]
- Sy, O.; Sarr, P.C.; Assogba, B.S.; Ndiaye, M.; Dia, A.K.; Ndiaye, A.; Nourdine, M.A.; Guèye, O.K.; Konaté, L.; Gaye, O.; et al. Detection of Kdr and Ace-1 Mutations in Wild Populations of Anopheles arabiensis and An. melas in a Residual Malaria Transmission Area of Senegal. Pestic. Biochem. Physiol. 2021, 173, 104783. [Google Scholar] [CrossRef] [PubMed]
- Assogba, B.S.; Milesi, P.; Djogbénou, L.S.; Berthomieu, A.; Makoundou, P.; Baba-Moussa, L.S.; Fiston-Lavier, A.-S.; Belkhir, K.; Labbé, P.; Weill, M. The Ace-1 Locus Is Amplified in All Resistant Anopheles gambiae Mosquitoes: Fitness Consequences of Homogeneous and Heterogeneous Duplications. PLoS Biol. 2016, 14, e2000618. [Google Scholar] [CrossRef]
- Akogbéto, M.C.; Padonou, G.G.; Gbénou, D.; Irish, S.; Yadouleton, A. Bendiocarb, a Potential Alternative against Pyrethroid Resistant Anopheles gambiae in Benin, West Africa. Malar. J. 2010, 9, 204. [Google Scholar] [CrossRef]
- Yewhalaw, D.; Balkew, M.; Shililu, J.; Suleman, S.; Getachew, A.; Ashenbo, G.; Chibsa, S.; Dissanayake, G.; George, K.; Dengela, D.; et al. Determination of the Residual Efficacy of Carbamate and Organophosphate Insecticides Used for Indoor Residual Spraying for Malaria Control in Ethiopia. Malar. J. 2017, 16, 471. [Google Scholar] [CrossRef]
- Agossa, F.R.; Aikpon, R.; Azondekon, R.; Govoetchan, R.; Padonou, G.G.; Oussou, O.; Oke-Agbo, F.; Akogbeto, M.C. Efficacy of Various Insecticides Recommended for Indoor Residual Spraying: Pirimiphos Methyl, Potential Alternative to Bendiocarb for Pyrethroid Resistance Management in Benin, West Africa. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 84–91. [Google Scholar] [CrossRef]
- N’Guessan, R.; Boko, P.; Odjo, A.; Chabi, J.; Akogbeto, M.; Rowland, M. Control of Pyrethroid and DDT-Resistant Anopheles gambiae by Application of Indoor Residual Spraying or Mosquito Nets Treated with a Long-Lasting Organophosphate Insecticide, Chlorpyrifos-Methyl. Malar. J. 2010, 9, 44. [Google Scholar] [CrossRef]
- Sharp, B.L.; Ridl, F.C.; Govender, D.; Kuklinski, J.; Kleinschmidt, I. Malaria Vector Control by Indoor Residual Insecticide Spraying on the Tropical Island of Bioko, Equatorial Guinea. Malar. J. 2007, 6, 52. [Google Scholar] [CrossRef]
- Akogbeto, M.; Gbedjissi, G.L.; Padonou, G.G.; Gazard, D.K.; Bankole, H.S. Dramatic Decrease in Malaria Transmission after Large-Scale Indoor Residual Spraying with Bendiocarb in Benin, an Area of High Resistance of Anopheles gambiae to Pyrethroids. Am. J. Trop. Med. Hyg. 2011, 85, 586–593. [Google Scholar] [CrossRef][Green Version]
- Yeebiyo, Y.; Dengela, D.; Tesfaye, A.G.; Anshebo, G.Y.; Kolyada, L.; Wirtz, R.; Chibsa, S.; Fornadel, C.; George, K.; Belemvire, A.; et al. Short Persistence of Bendiocarb Sprayed on Pervious Walls and Its Implication for the Indoor Residual Spray Program in Ethiopia. Parasit. Vectors 2016, 9, 266. [Google Scholar] [CrossRef]
- Tangena, J.-A.A.; Hendriks, C.M.J.; Devine, M.; Tammaro, M.; Trett, A.E.; Williams, I.; DePina, A.J.; Sisay, A.; Herizo, R.; Kafy, H.T.; et al. Indoor Residual Spraying for Malaria Control in Sub-Saharan Africa 1997 to 2017: An Adjusted Retrospective Analysis. Malar. J. 2020, 19, 150. [Google Scholar] [CrossRef]
- Templeton, A.R.; Crandall, K.A.; Sing, C.F. A Cladistic Analysis of Phenotypic AssociationsWith Haplotypes Inferred From Restriction Endonuclease Mapping and DNA Sequence Data. III. Cladogram Estimation. Genet. Soc. Am. 1992, 132, 619–633. [Google Scholar]
- World Health Organization. Regional Office for the Western Pacific. Strategy for Malaria Elimination in the Greater Mekong Subregion: 2015–2030; WHO Regional Office for the Western Pacific: Manila, Philippines, 2015. [Google Scholar]
- Essandoh, J.; Yawson, A.E.; Weetman, D. Acetylcholinesterase (Ace-1) Target Site Mutation 119S Is Strongly Diagnostic of Carbamate and Organophosphate Resistance in Anopheles gambiae s.s. and Anopheles coluzzii across Southern Ghana. Malar. J. 2013, 12, 404. [Google Scholar] [CrossRef]
- Aïkpon, R.; Agossa, F.; Ossè, R.; Oussou, O.; Aïzoun, N.; Oké-Agbo, F.; Akogbéto, M. Bendiocarb Resistance in Anopheles gambiae s.l. Populations from Atacora Department in Benin, West Africa: A Threat for Malaria Vector Control. Parasit. Vectors 2013, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Diouf, E.H.; Niang, E.H.A.; Samb, B.; Diagne, C.T.; Diouf, M.; Konaté, A.; Dia, I.; Faye, O.; Konaté, L. Multiple Insecticide Resistance Target Sites in Adult Field Strains of An. gambiae (s.l.) from Southeastern Senegal. Parasit. Vectors 2020, 13, 567. [Google Scholar] [CrossRef] [PubMed]
- Djogbénou, L.; Noel, V.; Agnew, P. Costs of Insensitive Acetylcholinesterase Insecticide Resistance for the Malaria Vector Anopheles gambiae Homozygous for the G119S Mutation. Malar. J. 2010, 9, 12. [Google Scholar] [CrossRef]
- Opondo, K.O.; Weetman, D.; Jawara, M.; Diatta, M.; Fofana, A.; Crombe, F.; Mwesigwa, J.; D’Alessandro, U.; Donnelly, M.J. Does Insecticide Resistance Contribute to Heterogeneities in Malaria Transmission in The Gambia? Malar. J. 2016, 15, 166. [Google Scholar] [CrossRef]
- Alou, L.P.A.; Koffi, A.A.; Adja, M.A.; Tia, E.; Kouassi, P.K.; Koné, M.; Chandre, F. Distribution of Ace-1R and resistance to carbamates and organophosphates in Anopheles gambiae s.s. populations from Côte d’Ivoire. Malar. J. 2010, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Dabiré, K.R.; Diabaté, A.; Namontougou, M.; Djogbenou, L.; Kengne, P.; Simard, F.; Bass, C.; Baldet, T. Distribution of Insensitive Acetylcholinesterase (Ace-1R) in Anopheles gambiae s.l. Populations from Burkina Faso (West Africa). Trop. Med. Int. Health 2009, 14, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Elanga-Ndille, E.; Nouage, L.; Ndo, C.; Binyang, A.; Assatse, T.; Nguiffo-Nguete, D.; Djonabaye, D.; Irwing, H.; Tene-Fossog, B.; Wondji, C.S. The G119S Acetylcholinesterase (Ace-1) Target Site Mutation Confers Carbamate Resistance in the Major Malaria Vector Anopheles gambiae from Cameroon: A Challenge for the Coming IRS Implementation. Genes 2019, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Diop, A.; Diop, Y.M.; Sarr, S.O.; Ndiaye, B.; Gueye, R.; Thiam, K.; Cazier, F.; Delattre, F. Pesticide Contamination of Soil and Groundwater in the Vulnerable Agricultural Zone of the Niayes (Dakar, Senegal). Anal. Chem. Lett. 2019, 9, 168–181. [Google Scholar] [CrossRef]
- Lo, C.; Dia, A.K.; Dia, I.; Niang, E.H.A.; Konaté, L.; Faye, O. Evaluation of the Residual Efficacy of Indoor Residual Spraying with Bendiocarb (FICAM WP 80) in Six Health Districts in Senegal. Malar. J. 2019, 18, 198. [Google Scholar] [CrossRef] [PubMed]
- Sy, O.; Niang, E.H.A.; Ndiaye, M.; Konaté, L.; Diallo, A.; Ba, E.C.C.; Tairou, F.; Diouf, E.; Cissé, B.; Gaye, O.; et al. Entomological Impact of Indoor Residual Spraying with Pirimiphos-Methyl: A Pilot Study in an Area of Low Malaria Transmission in Senegal. Malar. J. 2018, 17, 64. [Google Scholar] [CrossRef]
- Gimonneau, G.; Pombi, M.; Choisy, M.; Morand, S.; Dabiré, R.K.; Simard, F. Larval Habitat Segregation between the Molecular Forms of the Mosquito Anopheles gambiae in a Rice Field Area of Burkina Faso, West Africa. Med. Vet. Entomol. 2012, 26, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Chiang, M.-C.; Ndo, C.; Kuicheu, C.K.; Amvongo-Adjia, N.; Wondji, M.J.; Tchoupo, M.; Kusimo, M.O.; Riveron, J.M.; Wondji, C.S. A Marker of Glutathione S-Transferase-Mediated Resistance to Insecticides Is Associated with Higher Plasmodium Infection in the African Malaria Vector Anopheles funestus. Sci. Rep. 2019, 9, 5772. [Google Scholar] [CrossRef]
- Djouaka, R.; Riveron, J.M.; Yessoufou, A.; Tchigossou, G.; Akoton, R.; Irving, H.; Djegbe, I.; Moutairou, K.; Adeoti, R.; Tamò, M.; et al. Multiple Insecticide Resistance in an Infected Population of the Malaria Vector Anopheles funestus in Benin. Parasit. Vectors 2016, 9, 453. [Google Scholar] [CrossRef]
- Tchakounte, A.; Tchouakui, M.; Mu-Chun, C.; Tchapga, W.; Kopia, E.; Soh, P.T.; Njiokou, F.; Riveron, J.M.; Wondji, C.S. Exposure to the Insecticide-Treated Bednet PermaNet 2.0 Reduces the Longevity of the Wild African Malaria Vector Anopheles funestus but GSTe2-Resistant Mosquitoes Live Longer. PLoS ONE 2019, 14, e0213949. [Google Scholar]
- Tchigossou, G.M.; Atoyebi, S.M.; Akoton, R.; Tossou, E.; Innocent, D.; Riveron, J.; Irving, H.; Yessoufou, A.; Wondji, C.; Djouaka, R. Investigation of DDT Resistance Mechanisms in Anopheles funestus Populations from Northern and Southern Benin Reveals a Key Role of the GSTe2 Gene. Malar. J. 2020, 19, 456. [Google Scholar] [CrossRef]
- Williams, J.; Ingham, V.A.; Morris, M.; Toé, K.H.; Hien, A.S.; Morgan, J.C.; Dabiré, R.K.; Guelbéogo, W.M.; Sagnon, N.; Ranson, H. Sympatric Populations of the Anopheles gambiae Complex in Southwest Burkina Faso Evolve Multiple Diverse Resistance Mechanisms in Response to Intense Selection Pressure with Pyrethroids. Insects 2022, 13, 247. [Google Scholar] [CrossRef]
- Amvongo-Adjia, N.; Riveron, J.M.; Njiokou, F.; Wanji, S.; Wondji, C.S. Influence of a Major Mountainous Landscape Barrier (Mount Cameroon) on the Spread of Metabolic (GSTe2) and Target-Site (Rdl) Resistance Alleles in the African Malaria Vector Anopheles funestus. Genes 2020, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Kinya, F.; Mutero, C.M.; Sang, R.; Owino, E.A.; Rotich, G.; Ogola, E.O.; Wondji, C.S.; Torto, B.; Tchouassi, D.P. Outdoor Malaria Vector Species Profile in Dryland Ecosystems of Kenya. Sci. Rep. 2022, 12, 7131. [Google Scholar] [CrossRef]
- Corbel, V.; N’Guessan, R.; Brengues, C.; Chandre, F.; Djogbenou, L.; Martin, T.; Akogbéto, M.; Hougard, J.M.; Rowland, M. Multiple Insecticide Resistance Mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007, 101, 207–216. [Google Scholar] [CrossRef]
- Antonio-Nkondjio, C.; Sonhafouo-Chiana, N.; Ngadjeu, C.S.; Doumbe-Belisse, P.; Talipouo, A.; Djamouko-Djonkam, L.; Kopya, E.; Bamou, R.; Awono-Ambene, P.; Wondji, C.S. Review of the Evolution of Insecticide Resistance in Main Malaria Vectors in Cameroon from 1990 to 2017. Parasit. Vectors 2017, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.R.; Rockett, K.A.; Lynd, A.; Essandoh, J.; Grisales, N.; Kemei, B.; Njoroge, H.; Hubbart, C.; Rippon, E.J.; Morgan, J.; et al. A High Throughput Multi-Locus Insecticide Resistance Marker Panel for Tracking Resistance Emergence and Spread in Anopheles gambiae. Sci. Rep. 2019, 9, 13335. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.; Hamid-Adiamoh, M.; Sy, O.; Sarr, P.C.; Manneh, J.; Ndiath, M.O.; Gaye, O.; Faye, O.; Konaté, L.; Sesay, A.K.; et al. Evolution of the Pyrethroids Target-Site Resistance Mechanisms in Senegal: Early Stage of the Vgsc-1014F and Vgsc-1014S Allelic Frequencies Shift. Genes 2021, 12, 1948. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Brogdon, W.G.; Collins, F.H. Identification of Single Specimens of the Anopheles gambiae Complex by the Polymerase Chain Reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Fanello, C.; Santolamazza, F.D.; Della Torre, A. Simultaneous Identification of Species and Molecular Forms of the Anopheles gambiae Complex by PCR-RFLP. Med. Vet. Entomol. 2002, 16, 461–464. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Vontas, J.; Williamson, M.S.; Field, L.M. Development of High-Throughput Real-Time PCR Assays for the Identification of Insensitive Acetylcholinesterase (Ace-1R) in Anopheles gambiae. Pestic. Biochem. Physiol. 2010, 96, 80–85. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999, 41, 95–98. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Bandelt, H.-J.; Dress, A.W. Split Decomposition: A New and Useful Approach to Phylogenetic Analysis of Distance Data. Mol. Phylogenet. Evol. 1992, 1, 242–252. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolution Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2015, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]


| Genotypes | Frequency (S) | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | Locality | N | GG | GS | SS | G | S | |
| AR | Kaffrine | 87 | 87 | 0 | 0 | 1 | 0 | |
| Kedougou | 4 | 4 | 0 | 0 | 1 | 0 | ||
| Koumpentoum | 89 | 89 | 0 | 0 | 1 | 0 | ||
| Koungheul | 65 | 65 | 0 | 0 | 1 | 0 | ||
| Malem_Hodar | 103 | 103 | 0 | 0 | 1 | 0 | ||
| Ndoffane | 99 | 99 | 0 | 0 | 1 | 0 | ||
| Nioro | 70 | 70 | 0 | 0 | 1 | 0 | ||
| Tambacounda | 64 | 64 | 0 | 0 | 1 | 0 | ||
| Thies | 113 | 113 | 0 | 0 | 1 | 0 | ||
| Velingara | 54 | 54 | 0 | 0 | 1 | 0 | ||
| Total | 748 | 748 | 0 | 0 | 1 | 0 | ||
| AC | Kaffrine | 16 | 16 | 0 | 0 | 1 | 0 | |
| Kedougou | 4 | 4 | 0 | 0 | 1 | 0 | ||
| Koumpentoum | 14 | 14 | 0 | 0 | 1 | 0 | ||
| Koungheul | 38 | 38 | 0 | 0 | 1 | 0 | ||
| Malem_Hodar | 6 | 6 | 0 | 0 | 1 | 0 | ||
| Ndoffane | 5 | 5 | 0 | 0 | 1 | 0 | ||
| Nioro | 12 | 12 | 0 | 0 | 1 | 0 | ||
| Tambacounda | 4 | 4 | 0 | 0 | 1 | 0 | ||
| Thies | 3 | 3 | 0 | 0 | 1 | 0 | ||
| Velingara | 11 | 11 | 0 | 0 | 1 | 0 | ||
| Total | 113 | 113 | 0 | 0 | 1 | 0 | ||
| AG | Kedougou | 44 | 40 | 3 | 1 | 0.94 | 0.06 | |
| Koumpentoum | 6 | 6 | 0 | 0 | 1 | 0 | ||
| Malem_Hodar | 1 | 1 | 0 | 0 | 1 | 0 | ||
| Nioro | 19 | 19 | 0 | 0 | 1 | 0 | ||
| Tambacounda | 35 | 33 | 2 | 0 | 0.97 | 0.03 | ||
| Thies | 14 | 14 | 0 | 0 | 1 | 0 | ||
| Velingara | 22 | 21 | 1 | 0 | 0.98 | 0.02 | ||
| Total | 141 | 134 | 6 | 1 | 0.97 | 0.03 | ||
| AC-AG | Nioro | 2 | 2 | 0 | 0 | 1 | 0 | |
| Tambacounda | 5 | 4 | 1 | 0 | 0.9 | 0.1 | ||
| Thies | 1 | 1 | 0 | 0 | 1 | 0 | ||
| Velingara | 2 | 2 | 0 | 0 | 1 | 0 | ||
| Total | 10 | 9 | 1 | 0 | 0.95 | 0.05 | ||
| Genotypes | Frequency (S) | |||||||
|---|---|---|---|---|---|---|---|---|
| Years | Species | N | GG | GS | SS | G | S | |
| 2013 | AR | 35 | 35 | 0 | 0 | 1 | 0 | |
| AC | 4 | 4 | 0 | 0 | 1 | 0 | ||
| AG | 24 | 22 | 2 | 0 | 0.96 | 0.042 | ||
| AC-AG | 4 | 3 | 1 | 0 | 0.88 | 0.125 | ||
| Total | 67 | 64 | 3 | 0 | 0.98 | 0.022 | ||
| 2017 | AR | 348 | 348 | 0 | 0 | 1 | 0 | |
| AC | 43 | 43 | 0 | 0 | 1 | 0 | ||
| AG | 37 | 36 | 1 | 0 | 0.99 | 0.01 | ||
| AC-AG | 4 | 4 | 0 | 0 | 1 | 0 | ||
| Total | 432 | 431 | 1 | 0 | 0.999 | 0.001 | ||
| 2018 | AR | 365 | 365 | 0 | 0 | 1 | 0 | |
| AC | 66 | 66 | 0 | 0 | 1 | 0 | ||
| AG | 80 | 76 | 3 | 1 | 0.97 | 0.03 | ||
| AC-AG | 2 | 2 | 0 | 0 | 1 | 0 | ||
| Total | 513 | 509 | 3 | 1 | 0.995 | 0.005 | ||
| Species | Locality | Genotypes | Frequency (S) | |||||
|---|---|---|---|---|---|---|---|---|
| N | RR | RS | SS | R | S | |||
| AR | Kaffrine | 49 | 0 | 0 | 49 | 0 | 1 | |
| Kedougou | 3 | 0 | 0 | 3 | 0 | 1 | ||
| Koumpentoum | 58 | 0 | 0 | 58 | 0 | 1 | ||
| Koungheul | 40 | 0 | 0 | 40 | 0 | 1 | ||
| Malem_Hodar | 58 | 0 | 0 | 58 | 0 | 1 | ||
| Ndoffane | 37 | 1 | 0 | 36 | 0.03 | 0.97 | ||
| Nioro | 32 | 0 | 0 | 32 | 0 | 1 | ||
| Tambacounda | 40 | 1 | 0 | 39 | 0.03 | 0.97 | ||
| Thies | 34 | 0 | 0 | 34 | 0 | 1 | ||
| Velingara | 34 | 5 | 2 | 27 | 0.18 | 0.82 | ||
| Total | 385 | 7 | 2 | 376 | 0.02 | 0.98 | ||
| AC | Kaffrine | 15 | 2 | 3 | 10 | 0.23 | 0.77 | |
| Kedougou | 4 | 0 | 2 | 2 | 0.25 | 0.75 | ||
| Koumpentoum | 13 | 5 | 0 | 8 | 0.38 | 0.62 | ||
| Koungheul | 36 | 5 | 0 | 31 | 0.14 | 0.86 | ||
| Malem_Hodar | 7 | 1 | 1 | 5 | 0.21 | 0.79 | ||
| Ndoffane | 2 | 0 | 0 | 2 | 0 | 1 | ||
| Nioro | 11 | 3 | 0 | 8 | 0.27 | 0.73 | ||
| Tambacounda | 3 | 0 | 0 | 3 | 0 | 1 | ||
| Thies | 2 | 0 | 1 | 1 | 0.25 | 0.75 | ||
| Velingara | 13 | 7 | 1 | 5 | 0.58 | 0.42 | ||
| Total | 106 | 23 | 8 | 75 | 0.25 | 0.75 | ||
| AG | Kedougou | 53 | 7 | 3 | 43 | 0.16 | 0.84 | |
| Koumpentoum | 5 | 0 | 0 | 5 | 0 | 1 | ||
| Malem_Hodar | 1 | 0 | 0 | 1 | 0 | 1 | ||
| Nioro | 19 | 0 | 0 | 19 | 0 | 1 | ||
| Tambacounda | 35 | 7 | 1 | 27 | 0.21 | 0.79 | ||
| Thies | 7 | 0 | 0 | 7 | 0 | 1 | ||
| Velingara | 14 | 1 | 1 | 12 | 0.11 | 0.89 | ||
| Total | 134 | 15 | 5 | 114 | 0.13 | 0.87 | ||
| AC-AG | Nioro | 2 | 1 | 0 | 1 | 0.5 | 0.5 | |
| Tambacounda | 3 | 0 | 0 | 3 | 0 | 1 | ||
| Velingara | 5 | 0 | 2 | 3 | 0.2 | 0.8 | ||
| Total | 10 | 1 | 2 | 7 | 0.2 | 0.8 | ||
| Genotypes | Frequency (S) | |||||||
|---|---|---|---|---|---|---|---|---|
| Years | Species | N | RR | RS | SS | R | S | |
| 2013 | AR | 17 | 0 | 0 | 17 | 0 | 1 | |
| AC | 4 | 0 | 1 | 3 | 0.13 | 0.88 | ||
| AG | 23 | 5 | 1 | 17 | 0.24 | 0.76 | ||
| AC-AG | 2 | 0 | 0 | 2 | 0 | 1 | ||
| Total | 46 | 5 | 2 | 39 | 0.13 | 0.87 | ||
| 2017 | AR | 188 | 0 | 1 | 187 | 0.003 | 0.997 | |
| AC | 44 | 11 | 4 | 29 | 0.30 | 0.70 | ||
| AG | 37 | 2 | 1 | 34 | 0.07 | 0.93 | ||
| AC-AG | 7 | 0 | 2 | 5 | 0.14 | 0.86 | ||
| Total | 276 | 13 | 8 | 255 | 0.06 | 0.94 | ||
| 2018 | AR | 180 | 7 | 1 | 172 | 0.04 | 0.96 | |
| AC | 58 | 12 | 3 | 43 | 0.23 | 0.77 | ||
| AG | 74 | 8 | 3 | 63 | 0.13 | 0.87 | ||
| AC-AG | 1 | 1 | 0 | 1 | 0 | |||
| Total | 313 | 28 | 7 | 278 | 0.10 | 0.90 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diallo, M.; Kolley, E.S.; Dia, A.K.; Oboh, M.A.; Seck, F.; Manneh, J.; Sesay, A.K.; Diédhiou, S.M.; Sarr, P.C.; Sy, O.; et al. Evolution of the Ace-1 and Gste2 Mutations and Their Potential Impact on the Use of Carbamate and Organophosphates in IRS for Controlling Anopheles gambiae s.l., the Major Malaria Mosquito in Senegal. Pathogens 2022, 11, 1021. https://doi.org/10.3390/pathogens11091021
Diallo M, Kolley ES, Dia AK, Oboh MA, Seck F, Manneh J, Sesay AK, Diédhiou SM, Sarr PC, Sy O, et al. Evolution of the Ace-1 and Gste2 Mutations and Their Potential Impact on the Use of Carbamate and Organophosphates in IRS for Controlling Anopheles gambiae s.l., the Major Malaria Mosquito in Senegal. Pathogens. 2022; 11(9):1021. https://doi.org/10.3390/pathogens11091021
Chicago/Turabian StyleDiallo, Moussa, Ebrima SM Kolley, Abdoulaye Kane Dia, Mary Aigbiremo Oboh, Fatoumata Seck, Jarra Manneh, Abdul Karim Sesay, Seynabou Macote Diédhiou, Pape Cheikh Sarr, Ousmane Sy, and et al. 2022. "Evolution of the Ace-1 and Gste2 Mutations and Their Potential Impact on the Use of Carbamate and Organophosphates in IRS for Controlling Anopheles gambiae s.l., the Major Malaria Mosquito in Senegal" Pathogens 11, no. 9: 1021. https://doi.org/10.3390/pathogens11091021
APA StyleDiallo, M., Kolley, E. S., Dia, A. K., Oboh, M. A., Seck, F., Manneh, J., Sesay, A. K., Diédhiou, S. M., Sarr, P. C., Sy, O., Samb, B., Gaye, O., Faye, O., Konaté, L., Assogba, B. S., & Niang, E. H. A. (2022). Evolution of the Ace-1 and Gste2 Mutations and Their Potential Impact on the Use of Carbamate and Organophosphates in IRS for Controlling Anopheles gambiae s.l., the Major Malaria Mosquito in Senegal. Pathogens, 11(9), 1021. https://doi.org/10.3390/pathogens11091021







