Streptococcus mitis and Prevotella melaninogenica Influence Gene Expression Changes in Oral Mucosal Lesions in Periodontitis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Sample Collection
2.3. DNA Extraction and Microorganisms’ Detection
2.4. RNA Extraction, Reverse Transcription, and Real-Time PCR Relative Gene Expression Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical and Epidemiological Findings
3.2. Microbiological Findings
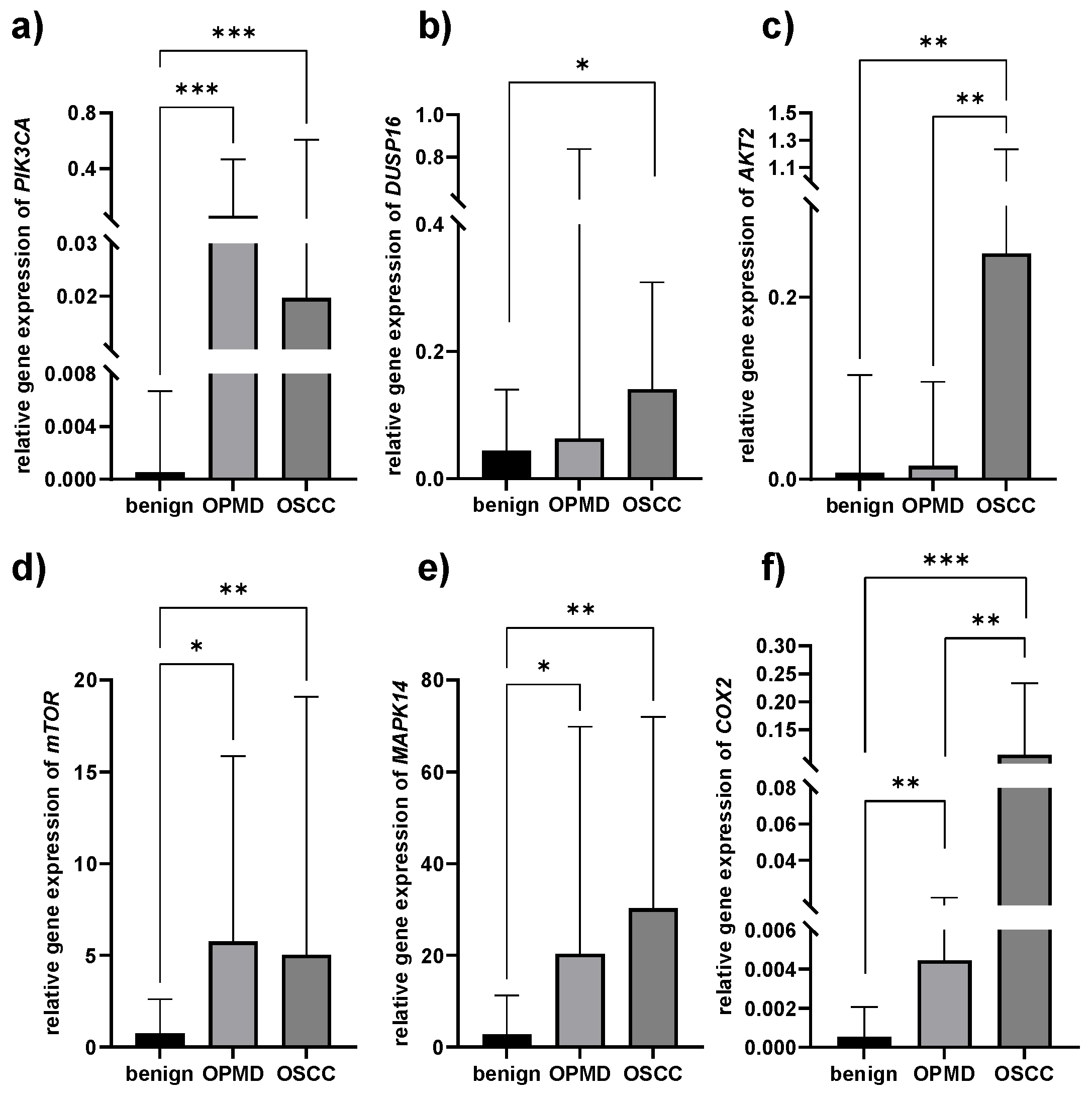
3.3. Gene Expression Patterns
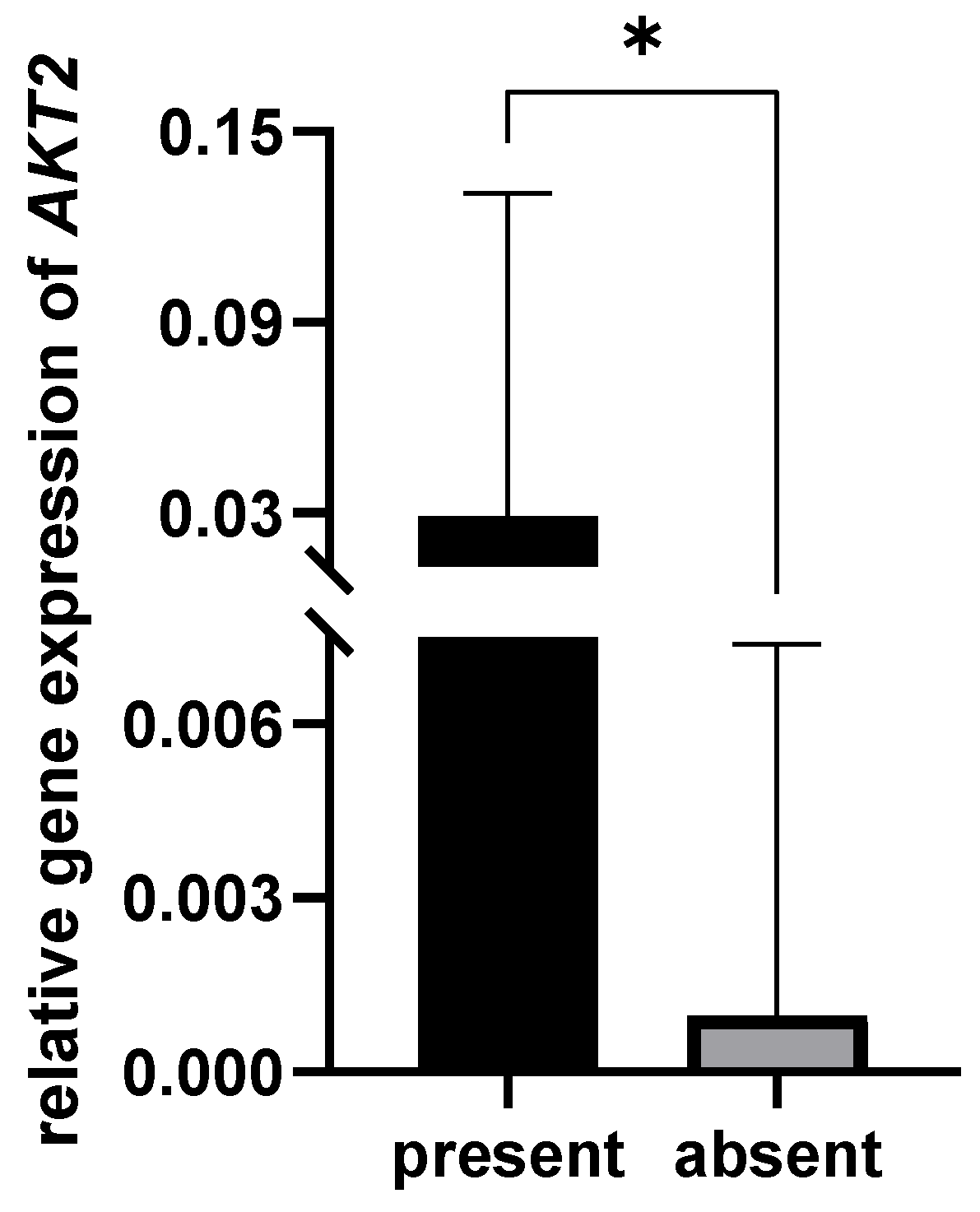
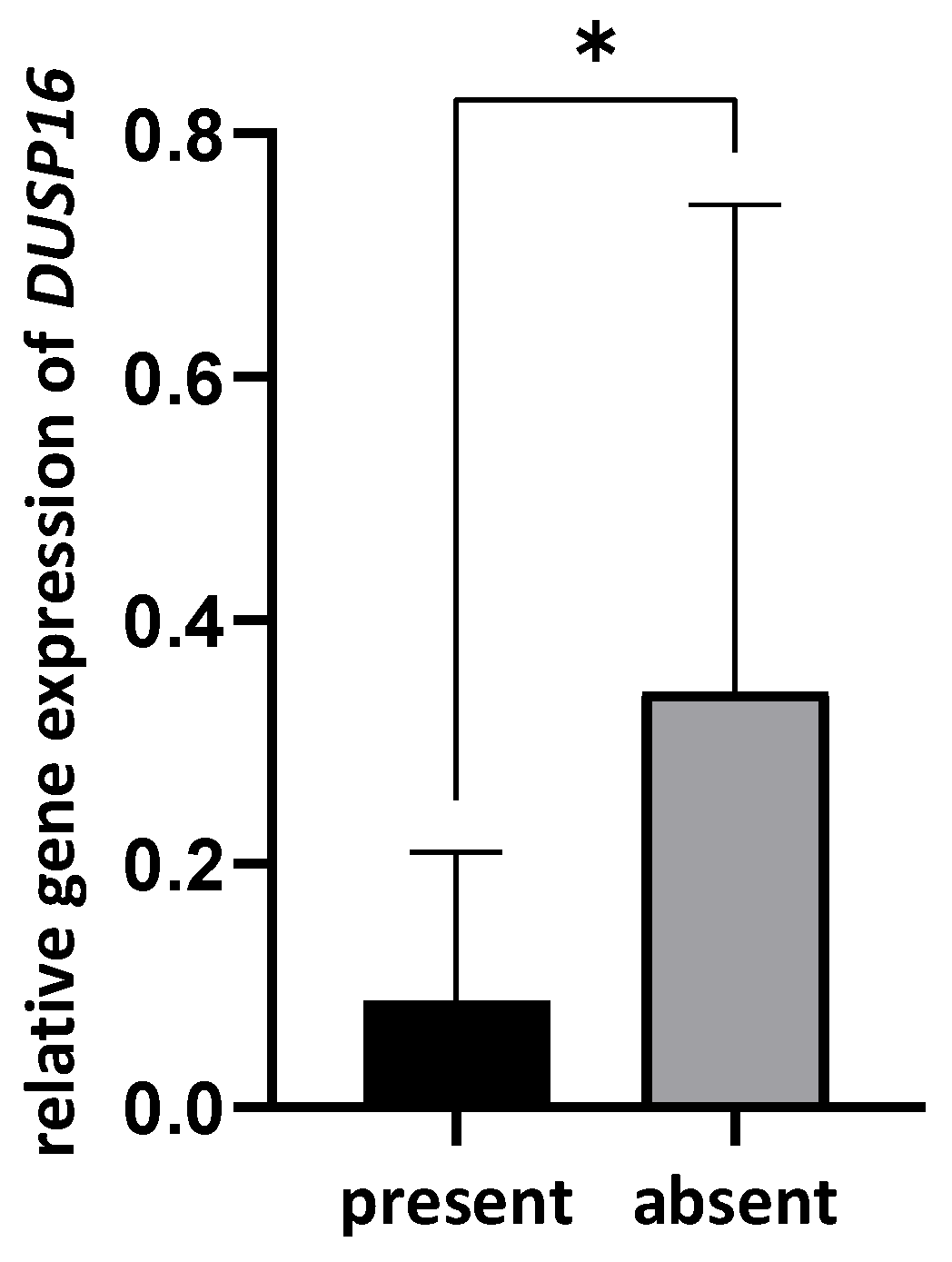
3.4. Correlation between Bacterial Presence and Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deo, P.N.; Deshmukh, R. Oral Microbiome: Unveiling the Fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Beyond the Red Complex and into More Complexity: The Polymicrobial Synergy and Dysbiosis (PSD) Model of Periodontal Disease Etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Yang, L.; Wen, L.; Lu, H.; Chen, Q.; Wang, Z. Crosstalk between the Oral Microbiota, Mucosal Immunity, and the Epithelial Barrier Regulates Oral Mucosal Disease Pathogenesis. Mucosal Immunol. 2021, 14, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Bhat, S.; Roberts-Thomson, K.; Do, L.G. Is Periodontitis Independently Associated with Potentially Malignant Disorders of the Oral Cavity? Asian Pac. J. Cancer Prev. 2019, 20, 283–287. [Google Scholar] [CrossRef]
- Elebyary, O.; Barbour, A.; Fine, N.; Tenenbaum, H.C.; Glogauer, M. The Crossroads of Periodontitis and Oral Squamous Cell Carcinoma: Immune Implications and Tumor Promoting Capacities. Front. Oral Health 2020, 1, 584705. [Google Scholar] [CrossRef] [PubMed]
- Allon, I.; Kaplan, I.; Gal, G.; Chaushu, G.; Allon, D.M. The Clinical Characteristics of Benign Oral Mucosal Tumors. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e438–e443. [Google Scholar] [CrossRef]
- Ghai, S.; Sharma, Y. Demographic Profile of Benign and Malignant Oral Tumors in Central India: A Retrospective Comparative Study. Cureus 2022, 14, e25345. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Mello, F.W.; Miguel, A.F.P.; Dutra, K.L.; Porporatti, A.L.; Warnakulasuriya, S.; Guerra, E.N.S.; Rivero, E.R.C. Prevalence of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis. J. Oral Pathol. Med. 2018, 47, 633–640. [Google Scholar] [CrossRef]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially Malignant Disorders of the Oral Cavity and Oral Dysplasia: A Systematic Review and Meta-Analysis of Malignant Transformation Rate by Subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- Woo, S.-B.; Grammer, R.L.; Lerman, M.A. Keratosis of Unknown Significance and Leukoplakia: A Preliminary Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global Epidemiology of Oral and Oropharyngeal Cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M.; Rams, T.E. Global Epidemiology of Periodontal Diseases: An Overview. Periodontology 2000 2002, 29, 7–10. [Google Scholar] [CrossRef]
- Hernández, M.; Dutzan, N.; García-Sesnich, J.; Abusleme, L.; Dezerega, A.; Silva, N.; González, F.E.; Vernal, R.; Sorsa, T.; Gamonal, J. Host-Pathogen Interactions in Progressive Chronic Periodontitis. J. Dent. Res. 2011, 90, 1164–1170. [Google Scholar] [CrossRef]
- Irani, S.; Barati, I.; Badiei, M. Periodontitis and Oral Cancer—Current Concepts of the Etiopathogenesis. Oncol. Rev. 2020, 14, 465. [Google Scholar] [CrossRef]
- Kavarthapu, A.; Gurumoorthy, K. Linking Chronic Periodontitis and Oral Cancer: A Review. Oral Oncol. 2021, 121, 105375. [Google Scholar] [CrossRef]
- Sami, A.; Elimairi, I.; Stanton, C.; Ross, R.P.; Ryan, C.A. The Role of the Microbiome in Oral Squamous Cell Carcinoma with Insight into the Microbiome–Treatment Axis. Int. J. Mol. Sci. 2020, 21, 8061. [Google Scholar] [CrossRef]
- Pietrobon, G.; Tagliabue, M.; Stringa, L.M.; De Berardinis, R.; Chu, F.; Zocchi, J.; Carlotto, E.; Chiocca, S.; Ansarin, M. Leukoplakia in the Oral Cavity and Oral Microbiota: A Comprehensive Review. Cancers 2021, 13, 4439. [Google Scholar] [CrossRef]
- Pignatelli, P.; Romei, F.M.; Bondi, D.; Giuliani, M.; Piattelli, A.; Curia, M.C. Microbiota and Oral Cancer as A Complex and Dynamic Microenvironment: A Narrative Review from Etiology to Prognosis. Int. J. Mol. Sci. 2022, 23, 8323. [Google Scholar] [CrossRef]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/mTOR Pathway in Breast Cancer: Targets, Trials and Biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Leelahavanichkul, K.; Amornphimoltham, P.; Molinolo, A.A.; Basile, J.R.; Koontongkaew, S.; Gutkind, J.S. A Role for P38 MAPK in Head and Neck Cancer Cell Growth and Tumor-Induced Angiogenesis and Lymphangiogenesis. Mol. Oncol. 2014, 8, 105–118. [Google Scholar] [CrossRef]
- Dempke, W.; Rie, C.; Grothey, A.; Schmoll, H.J. Cyclooxygenase-2: A Novel Target for Cancer Chemotherapy? J. Cancer Res. Clin. Oncol. 2001, 127, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.A.; Estefan, D.J. Assessing Oral Malignancies. Am. Fam. Physician 2002, 65, 1379–1384. [Google Scholar] [PubMed]
- Ainamo, J.; Bay, I. Problems and Proposals for Recording Gingivitis and Plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Tonetti, M.S.; Sanz, M. Implementation of the New Classification of Periodontal Diseases: Decision-Making Algorithms for Clinical Practice and Education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wade, W.G. The Oral Microbiome in Health and Disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Gopinath, D.; Kunnath Menon, R.; Veettil, S.K.; George Botelho, M.; Johnson, N.W. Periodontal Diseases as Putative Risk Factors for Head and Neck Cancer: Systematic Review and Meta-Analysis. Cancers 2020, 12, 1893. [Google Scholar] [CrossRef]
- Komlós, G.; Csurgay, K.; Horváth, F.; Pelyhe, L.; Németh, Z. Periodontitis as a Risk for Oral Cancer: A Case–Control Study. BMC Oral Health 2021, 21, 640. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Lemmer, J. Oral Squamous Cell Carcinoma: Epidemiology, Clinical Presentation and Treatment. J. Cancer Ther. 2012, 3, 263–268. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2019, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Tan, J.; Guo, G.; Li, Z.; Yang, L.; Lao, X.; Wang, D.; Ma, J.; Zhang, S.; Liao, G.; et al. The Oral Cancer Microbiome Contains Tumor Space–Specific and Clinicopathology-Specific Bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 942328. [Google Scholar] [CrossRef]
- Moghimi, M.; Bakhtiari, R.; Mehrabadi, J.F.; Jamshidi, N.; Jamshidi, N.; Siyadatpanah, A.; Mitsuwan, W.; Nissapatorn, V. Interaction of Human Oral Cancer and the Expression of Virulence Genes of Dental Pathogenic Bacteria. Microb. Pathog. 2020, 149, 104464. [Google Scholar] [CrossRef]
- Mager, D.; Haffajee, A.; Devlin, P.; Norris, C.; Posner, M.; Goodson, J. The Salivary Microbiota as a Diagnostic Indicator of Oral Cancer: A Descriptive, Non-Randomized Study of Cancer-Free and Oral Squamous Cell Carcinoma Subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef]
- Chocolatewala, N.; Chaturvedi, P.; Desale, R. The Role of Bacteria in Oral Cancer. Indian J. Med. Paediatr. Oncol. 2010, 31, 126–131. [Google Scholar] [CrossRef]
- Gopinath, D.; Menon, R.K.; Wie, C.C.; Banerjee, M.; Panda, S.; Mandal, D.; Behera, P.K.; Roychoudhury, S.; Kheur, S.; Botelho, M.G.; et al. Differences in the Bacteriome of Swab, Saliva, and Tissue Biopsies in Oral Cancer. Sci. Rep. 2021, 11, 1181. [Google Scholar] [CrossRef]
- Robayo, D.A.G.; Erira, H.A.T.; Jaimes, F.O.G.; Torres, A.M.; Galindo, A.I.C. Oropharyngeal Squamous Cell Carcinoma: Human Papilloma Virus Coinfection with Streptococcus Anginosus. Braz. Dent. J. 2019, 30, 626–633. [Google Scholar] [CrossRef]
- De Martin, A.; Lütge, M.; Stanossek, Y.; Engetschwiler, C.; Cupovic, J.; Brown, K.; Demmer, I.; Broglie, M.A.; Geuking, M.B.; Jochum, W.; et al. Distinct Microbial Communities Colonize Tonsillar Squamous Cell Carcinoma. OncoImmunology 2021, 10, 1945202. [Google Scholar] [CrossRef]
- Zheng, S.; Xu, P.; Cai, L.; Tan, Z.; Guo, Y.; Zhu, R.; He, Y. The Presence of Prevotella melaninogenica within Tissue and Preliminary Study on Its Role in the Pathogenesis of Oral Lichen Planus. Oral Dis. 2022, 28, 1580–1590. [Google Scholar] [CrossRef]
- Karpiński, T. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef]
- Roy, N.K.; Monisha, J.; Padmavathi, G.; Lalhruaitluanga, H.; Kumar, N.S.; Singh, A.K.; Bordoloi, D.; Baruah, M.N.; Ahmed, G.N.; Longkumar, I.; et al. Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma. Biomolecules 2019, 9, 253. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, N.; Ma, D.; Liu, L.; Jiang, L.; Zhou, Y.; Zeng, X.; Li, J.; Chen, Q. Receptor for Activated C Kinase 1 (RACK1) Promotes the Progression of OSCC via the AKT/MTOR Pathway. Int. J. Oncol. 2016, 49, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; Liu, D.D.; Lee, J.J.; El-Naggar, A.K.; Lo Muzio, L.; Staibano, S.; De Placido, S.; Myers, J.N.; Papadimitrakopoulou, V.A. Akt Activation Correlates with Adverse Outcome in Tongue Cancer. Cancer 2005, 104, 2430–2436. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Li, L.; Shao, S.; Wu, J.; Bian, L.; He, Y. Epithelial Mesenchymal Transition Induced by the CXCL9/CXCR3 Axis through AKT Activation Promotes Invasion and Metastasis in Tongue Squamous Cell Carcinoma. Oncol. Rep. 2018, 39, 1356–1368. [Google Scholar] [PubMed]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P.; et al. Targeting AKT/MTOR in Oral Cancer: Mechanisms and Advances in Clinical Trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Yamamoto, T.; Maeda, R.; Nishida, E. A Novel MAPK Phosphatase MKP-7 Acts Preferentially on JNK/SAPK and P38 Alpha and Beta MAPKs. J. Biol. Chem. 2001, 276, 26629–26639. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, J.; Shi, Y.; Fang, X.; Tang, Z. MAPK Signaling Pathway in Oral Squamous Cell Carcinoma: Biological Function and Targeted Therapy. Cancers 2022, 14, 4625. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, H.; Mu, W.; He, Z.; Yang, B.; Ji, Y.; Hui, L. DUSP16 Ablation Arrests the Cell Cycle and Induces Cellular Senescence. FEBS J. 2015, 282, 4580–4594. [Google Scholar] [CrossRef]
- Low, H.B.; Wong, Z.L.; Wu, B.; Kong, L.R.; Png, C.W.; Cho, Y.L.; Li, C.W.; Xiao, F.; Xin, X.; Yang, H.; et al. DUSP16 Promotes Cancer Chemoresistance through Regulation of Mitochondria-Mediated Cell Death. Nat. Commun. 2021, 12, 2284. [Google Scholar] [CrossRef]
- Narikiyo, M.; Tanabe, C.; Yamada, Y.; Igaki, H.; Tachimori, Y.; Kato, H.; Muto, M.; Montesano, R.; Sakamoto, H.; Nakajima, Y.; et al. Frequent and Preferential Infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in Esophageal Cancers. Cancer Sci. 2004, 95, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Al-Hebshi, N.N.; Nasher, A.T.; Idris, A.M.; Chen, T. Robust Species Taxonomy Assignment Algorithm for 16S RRNA NGS Reads: Application to Oral Carcinoma Samples. J. Oral Microbiol. 2015, 7, 28934. [Google Scholar] [CrossRef] [PubMed]
- Baraniya, D.; Jain, V.; Lucarelli, R.; Tam, V.; Vanderveer, L.; Puri, S.; Yang, M.; Al-hebshi, N.N. Screening of Health-Associated Oral Bacteria for Anticancer Properties In Vitro. Front. Cell. Infect. Microbiol. 2020, 10, 575656. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, F.; Lin, X.; Li, Z.; Mao, X.; Jiang, C. Cytotoxic T Cell Responses to Streptococcus Are Associated with Improved Prognosis of Oral Squamous Cell Carcinoma. Exp. Cell Res. 2018, 362, 203–208. [Google Scholar] [CrossRef]
- Castañeda-Corzo, G.J.; Infante-Rodríguez, L.F.; Villamil-Poveda, J.C.; Bustillo, J.; Cid-Arregui, A.; García-Robayo, D.A. Association of Prevotella intermedia with Oropharyngeal Cancer: A Patient-Control Study. Heliyon 2023, 9, e14293. [Google Scholar] [CrossRef]
- Chang, Y.C.; Huang, F.M.; Yang, S.F.; Liu, C.M.; Lai, C.C.; Chan, Y.; Hsieh, Y.S. Induction of Cyclooxygenase-2 MRNA and Protein Expression in Human Pulp Cells Stimulated with Black-Pigmented Bacteroides. J. Endod. 2003, 29, 240–243. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, E.Y.; Kim, E.G.; Shin, S.H.; Lee, J.Y.; Choi, J.I.; Choi, I.S. Prevotella intermedia Lipopolysaccharide Stimulates Release of Tumor Necrosis Factor-Alpha through Mitogen-Activated Protein Kinase Signaling Pathways in Monocyte-Derived Macrophages. FEMS Immunol. Med. Microbiol. 2007, 51, 407–413. [Google Scholar] [CrossRef]
- Guan, S.M.; Zhang, M.; He, J.J.; Wu, J.Z. Mitogen-Activated Protein Kinases and Phosphatidylinositol 3-Kinase Are Involved in Prevotella intermedia-Induced Proinflammatory Cytokines Expression in Human Periodontal Ligament Cells. Biochem. Biophys. Res. Commun. 2009, 386, 471–476. [Google Scholar] [CrossRef]
| Microorganism/Gene | Primer and/or Probe Sequence |
|---|---|
| Prevotella intermedia | Fwd: GACCCGAACGCAAAATACAT Rv: AGGGCGAAAAGAACGTTAGG Probe: FAM-AAAGAAGGAACACCCCGACT-TAMRA |
| Prevotella melaninogenica | Fwd: GTGGGATAACCTGCCGAAAG Rv: CCCATCCATTACCGATAAATCTTTA Probe: FAM-CAAATCTGATGCCGTCATCGAAGACTATGC-TAMRA |
| Streptococcus mitis | Fwd: TTTTGTCATCTAGCCTTGC Rv: GCAGTCATATCATCACCTTC Probe: FAM-ACTTGGGCAATCCCGACAGATTCTAAC-TAMRA |
| GAPDH | Fwd: GGGCTCTCCAGAACATCATCC Rv: GTCCACCACTGACACGTTGG Probe: FAM-CCTCTACTGGCGCTGCCAAGGCT-TAMRA |
| PIK3CA | Fwd: TTACCCTCTTCTGCCGGAGG Rv: AAGTGGATGCCCCACAGTTC |
| DUSP16 | Fwd: AGGTGGGTTTGCTGAGTTCTC Rv: CTCGGGGATAAAGTCAGGCTT |
| AKT2 | Fwd: GCAAAGCAGGAGTATAAGAAAGGAA Rv: GCAGAGAGGTAATCAGCACCAA |
| mTOR | Fwd: GCCGCGCGAATATTAAAGGAA Rv: TGGTTTCCTCATTCCGGCTC |
| MAPK14 | Fwd: ACTGGCTCGGCACACAGATG Rv: TCCCACTGACCAAATATCAACTG |
| COX-2 | Fwd: CAGCACTTCACGCATCAGTT Rv: CGCAGTTTACGCTGTCTAGC |
| GAPDH | Fwd: ATGGGGAAGGTGAAGGTCG Rv: GGGGTCATTGATGGCAACAATA |
| Benign (n = 25) | OPMD (n = 30) | OSCC (n = 35) | p Value | |
|---|---|---|---|---|
| age (mean ± SD; median) | 58.29 ± 2.79; 58 | 54.29 ± 3.82; 50 | 60.39 ± 2.41; 63 | 0.322 |
| sex (male/female) | 6/19 | 18/12 | 20/15 | 0.013 |
| smoking (yes/used to smoke/no) | 5/9/11 | 8/15/7 | 8/14/13 | 0.596 |
| alcohol (yes/used to drink/no) | 17/3/5 | 17/4/9 | 23/5/7 | 0.871 |
| periodontal stage (1/2/3/4/toothless) | 0/11/7/6/1 | 2/10/9/6/3 | 0/6/7/19/3 | 0.047 |
| periodontal probing depth (mm) (mean ± SD, median) | 3.55 ± 0.15; 3.5 | 3.05 ± 0.22; 3.2 | 3.47 ± 0.22; 3.2 | 0.295 |
| clinical attachment level (mm) (mean ± SD, median) | 4.29 ± 0.30; 3.8 | 3.91 ± 0.36; 3.77 | 4.73 ± 0.49; 4.1 | 0.720 |
| plaque index (mean ± SD, median) | 0.60 ± 0.06; 0.51 | 0.63 ± 0.08; 0.76 | 0.69 ± 0.08; 0.78 | 0.327 |
| bleeding on probing (mean ± SD, median) | 0.62 ± 0.06; 0.54 | 0.63 ± 0.07; 0.65 | 0.72 ± 0.07; 0.8 | 0.243 |
| duration of oral lesion (in months) (mean ± SD, median) | 20.52 ± 6.72; 12 | 14.43 ± 5.6; 6 | 12.30 ± 5.23; 4 | 0.111 |
| Bacteria | Benign (n = 25) | OPMD (n = 30) | OSCC (n = 35) | p Value | OR (95%CI) | |
|---|---|---|---|---|---|---|
| Streptococcus mitis (y/n) | 15/10 | 15/15 | 24/11 | benign vs. OPMD | 0.458 | 0.67 (0.23–1.95) |
| benign vs. OSCC | 0.493 | 1.45 (0.50–4.25) | ||||
| OPMD vs. OSCC | 0.128 | 2.18 (0.79–5.99) | ||||
| Prevotella melaninogenica (y/n) | 15/10 | 26/4 | 27/8 | benign vs. OPMD | 0.024 | 4.33 (1.15–16.26) |
| benign vs. OSCC | 0.153 | 2.25 (0.73–6.92) | ||||
| OPMD vs. OSCC | 0.325 | 0.519 (0.14–1.94) | ||||
| Prevotella intermedia (y/n) | 4/21 | 8/22 | 11/24 | benign vs. OPMD | 0.340 | 1.91 (0.50–7.30) |
| benign vs. OSCC | 0.174 | 2.41 (0.67–8.70) | ||||
| OPMD vs. OSCC | 0.671 | 1.26 (0.43–3.71) |
| Gene Expression Median (Range) | Benign (n = 25) | OPMD (n = 30) | OSCC (n = 35) | Comparison | p Value |
|---|---|---|---|---|---|
| PIK3CA | 0.0007 (0.00001–0.28) | 0.05 (0.00005–7.98) | 0.027 (0.00–57.65) | benign vs. OPMD | 0.0001 |
| benign vs. OSCC | 0.0001 | ||||
| OPMD vs. OSCC | 0.717 | ||||
| DUSP16 | 0.039 (0.0002–0.56) | 0.06 (0.00013–20.98) | 0.14 (0.02–2.48) | benign vs. OPMD | 0.161 |
| benign vs. OSCC | 0.050 | ||||
| OPMD vs. OSCC | 0.990 | ||||
| AKT2 | 0.009 (0.00002–0.66) | 0.009 (0.00001–2.66) | 0.25 (0.00005–15.61) | benign vs. OPMD | 0.859 |
| benign vs. OSCC | 0.002 | ||||
| OPMD vs. OSCC | 0.002 | ||||
| mTOR | 0.72 (0.009–23.6) | 4.43 (0.0022–171.65) | 5.03 (0.0006–181.9) | benign vs. OPMD | 0.016 |
| benign vs. OSCC | 0.003 | ||||
| OPMD vs. OSCC | 0.664 | ||||
| MAPK14 | 2.89 (0.03–98.38) | 19.38 (0.15–432) | 30.21 (0.0055–223.12) | benign vs. OPMD | 0.007 |
| benign vs. OSCC | 0.001 | ||||
| OPMD vs. OSCC | 0.580 | ||||
| COX2 | 0.0007 (0.00001–0.66) | 0.004 (0.00001–0.71) | 0.091 (0.00003–1.03) | benign vs. OPMD | 0.003 |
| benign vs. OSCC | 0.0001 | ||||
| OPMD vs. OSCC | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomic, U.; Nikolic, N.; Carkic, J.; Mihailovic, D.; Jelovac, D.; Milasin, J.; Pucar, A. Streptococcus mitis and Prevotella melaninogenica Influence Gene Expression Changes in Oral Mucosal Lesions in Periodontitis Patients. Pathogens 2023, 12, 1194. https://doi.org/10.3390/pathogens12101194
Tomic U, Nikolic N, Carkic J, Mihailovic D, Jelovac D, Milasin J, Pucar A. Streptococcus mitis and Prevotella melaninogenica Influence Gene Expression Changes in Oral Mucosal Lesions in Periodontitis Patients. Pathogens. 2023; 12(10):1194. https://doi.org/10.3390/pathogens12101194
Chicago/Turabian StyleTomic, Uros, Nadja Nikolic, Jelena Carkic, Djordje Mihailovic, Drago Jelovac, Jelena Milasin, and Ana Pucar. 2023. "Streptococcus mitis and Prevotella melaninogenica Influence Gene Expression Changes in Oral Mucosal Lesions in Periodontitis Patients" Pathogens 12, no. 10: 1194. https://doi.org/10.3390/pathogens12101194





