Molecular Epidemiology and Genetic Diversity of the Enteric Protozoan Parasite Blastocystis sp. in the Northern Egypt Population
Abstract
:1. Introduction
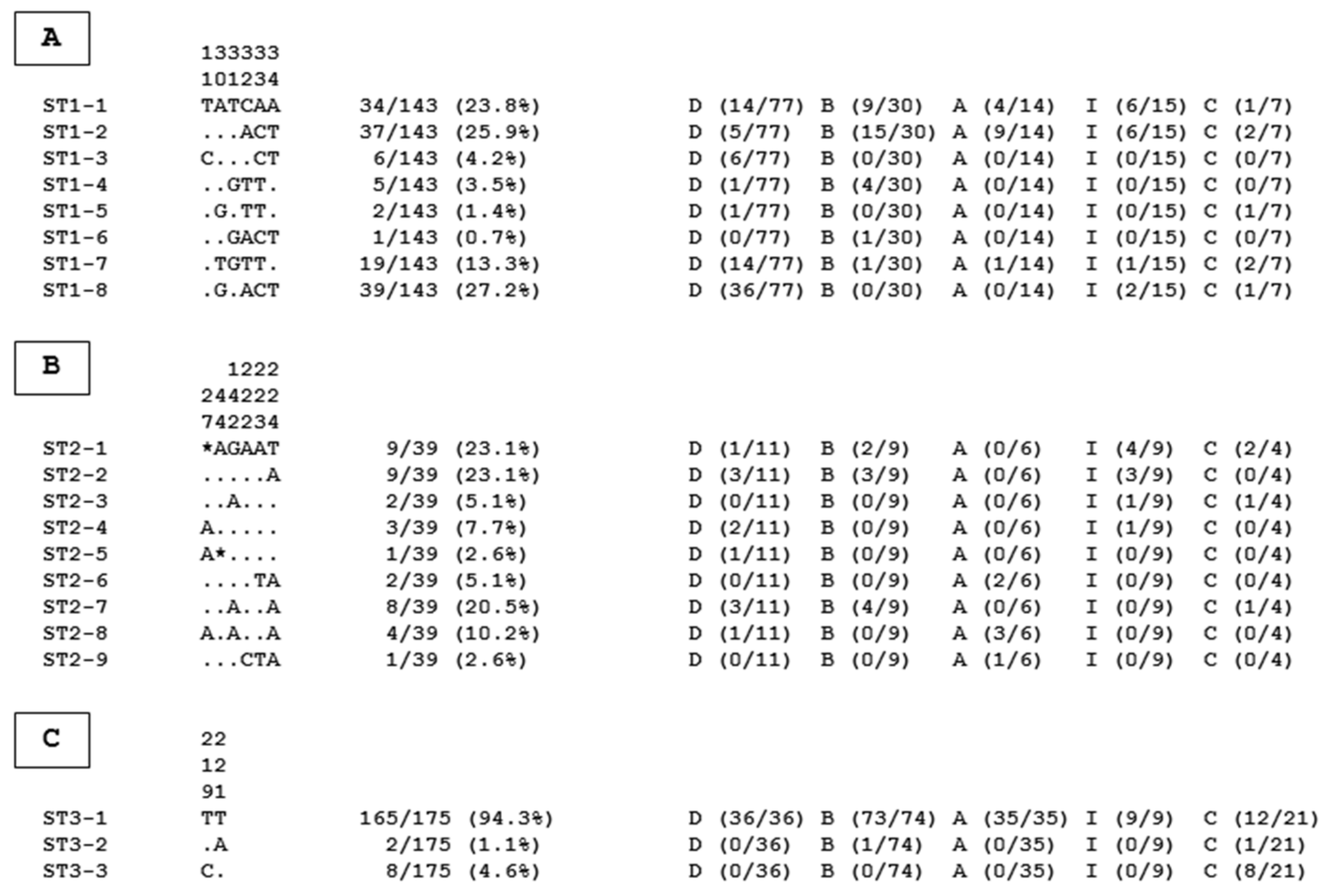
2. Results and Discussion
3. Materials and Methods
3.1. Study Area and Sample Collection
3.2. DNA Extraction, PCR Assay, and Molecular Subtyping of Isolates
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nemati, S.; Reza Zali, M.; Johnson, P.; Mirjalali, H.; Karanis, P. Molecular prevalence and subtype distribution of Blastocystis sp. in Asia and Australia. J. Water Health 2021, 19, 687. [Google Scholar] [CrossRef] [PubMed]
- Rauff-Adetotun, A.A.; Meor Termizi, F.H.; Shaari, N.; Lee, I.L. The coexistence of Blastocystis spp. in humans, animals and environmental sources from 2010–2021 in Asia. Biology 2021, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Munoz, M.; Ramirez, J.D. An update on the distribution of Blastocystis subtypes in the Americas. Heliyon 2023, 8, e12592. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.-Q.; Hu, Z.-H.; Chen, J.-H.; Tian, L.-G. Epidemiology of Blastocystis infection from 1990 to 2019 in China. Infect. Dis. Poverty 2020, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.; Gantois, N.; Tidjani Ly, A.; Senghor, S.; Even, G.; Dautel, E.; Dejager, R.; Sawant, M.; Baydoun, M.; Benamrouz-Vanneste, S.; et al. Prevalence and subtype distribution of Blastocystis sp. in Senegalese school children. Microorganisms 2020, 8, 1408. [Google Scholar] [CrossRef] [PubMed]
- Guilavogui, T.; Gantois, N.; Even, G.; Desramaut, J.; Dautel, E.; Denoyelle, C.; Cissé, F.I.; Touré, S.C.; Kourouma, B.L.; Sawant, M.; et al. Detection, molecular identification and transmission of the intestinal protozoa Blastocystis sp. in Guinea from a large-scale epidemiological study conducted in the Conakry area. Microorganisms 2022, 10, 446. [Google Scholar] [CrossRef]
- Andersen, L.O.; Stensvold, C.R. Blastocystis in health and disease: Are we moving from a clinical to a public health perspective? J. Clin. Microbiol. 2016, 54, 524–528. [Google Scholar] [CrossRef]
- Tan, K.S.W. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef]
- Hublin, J.S.Y.; Maloney, J.G.; Santin, M. Blastocystis in domesticated and wild mammals and birds. Res. Vet. Sci. 2021, 135, 260–282. [Google Scholar] [CrossRef]
- Sanggari, A.; Komala, T.; Rauff-Adetotun, A.A.; Awosolu, O.B.; Attah, O.A.; Farah Haziqah, M.T. Blastocystis in captivated and free-ranging wild animals worldwide: A review. Trop. Biomed. 2022, 39, 338–372. [Google Scholar] [PubMed]
- Gantois, N.; Lamot, A.; Seesao, Y.; Creusy, C.; Li, L.L.; Monchy, S.; Benamrouz-Vanneste, S.; Karpouzopoulos, J.; Bourgain, J.L.; Rault, C.; et al. First report on the prevalence and subtype distribution of Blastocystis sp. in edible marine fish and marine mammals: A large scale-study conducted in Atlantic Northeast and on the coasts of Northern France. Microorganisms 2020, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Cian, A.; El Safadi, D.; Osman, M.; Moriniere, R.; Gantois, N.; Benamrouz-Vanneste, S.; Delgado-Viscogliosi, P.; Guyot, K.; Li, L.L.; Monchy, S.; et al. Molecular epidemiology of Blastocystis sp. in various animal groups from two French zoos and evaluation of potential zoonotic risk. PLoS ONE 2017, 12, e0169659. [Google Scholar] [CrossRef]
- Wang, W.; Owen, H.; Traub, R.J.; Cuttell, L.; Inpankaew, T.; Bielefeldt-Ohmann, H. Molecular epidemiology of Blastocystis in pigs and their in-contact humans in Southeast Queensland, Australia, and Cambodia. Vet. Parasitol. 2014, 203, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Greige, S.; El Safadi, D.; Bécu, N.; Gantois, N.; Pereira, B.; Chabé, M.; Benamrouz-Vanneste, S.; Certad, G.; El Hage, R.; Chemaly, M.; et al. Prevalence and subtype distribution of Blastocystis sp. isolates from poultry in Lebanon and evidence of zoonotic potential. Parasit. Vectors 2018, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Alfellani, M.A.; Norskov-Lauritsen, S.; Prip, K.; Victory, E.L.; Maddox, C.; Nielsen, H.V.; Clark, C.G. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int. J. Parasitol. 2009, 39, 473–479. [Google Scholar] [CrossRef]
- Gentekaki, E.; Curtis, B.A.; Stairs, C.W.; Klimes, V.; Elias, M.; Salas-Leiva, D.E.; Herman, E.K.; Eme, L.; Arias, M.C.; Henrissat, B.; et al. Extreme genome diversity in the hyper-prevalent parasitic eukaryote Blastocystis. PLoS Biol. 2017, 15, e2003769. [Google Scholar] [CrossRef]
- Maloney, J.G.; Molokin, A.; Segui, R.; Maravilla, P.; Martinez-Hernandez, F.; Villalobos, G.; Tsaousis, A.D.; Gentekaki, E.; Munoz-Antoli, C.; Klisiowicz, D.R.; et al. Identification and molecular characterization of four new Blastocystis subtypes designated ST35-ST38. Microorganisms 2023, 11, 46. [Google Scholar] [CrossRef]
- Yu, M.; Yao, Y.; Xiao, H.; Xie, M.; Xiong, Y.; Yang, S.; Ni, Q.; Zhang, M.; Xu, H. Extensive prevalence and significant genetic differentiation of Blastocystis in high- and low-altitude populations of wild rhesus macaques in China. Parasit. Vectors 2023, 16, 107. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Berg, R.P.K.D.; Maloney, J.G.; Molokin, A.; Santin, M. Molecular characterization of Blastocystis and Entamoeba of muskoxen and sheep in Greenland. Int. J. Parasitol. 2023, 55, 673–685. [Google Scholar] [CrossRef]
- Hernández-Castro, C.; Maloney, J.G.; Agudelo-López, S.P.; Toro-Londoño, M.A.; Botero-Garcés, J.H.; Orozco, M.C.; Quintero-Quinchia, Y.C.; Correa-Cote, J.C.; Múnera-Duque, A.; Ricaurte-Ciro, J.C.; et al. Identification and validation of novel Blastocystis subtype ST41 in a Colombian patient undergoing colorectal cancer screening. J. Eukaryot. Microbiol. 2023, 17, e12978. [Google Scholar] [CrossRef]
- Santin, M.; Figueiredo, A.; Molokin, A.; George, N.S.; Köster, P.C.; Dashti, A.; Gonzalez-Barrio, D.; Carmena, D.; Maloney, J.G. Division of Blastocystis ST10 into three new subtypes: ST42–ST44. J. Eukaryot. Microbiol. 2023, e12998. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Clark, C.G. Pre-empting Pandora’s box: Blastocystis subtypes revisited. Trends Parasitol. 2020, 36, 229–232. [Google Scholar] [CrossRef]
- Alfellani, M.A.; Stensvold, C.R.; Vidal-Lapiedra, A.; Onuoha, E.S.; Fagbenro-Beyioku, A.F.; Clark, C.G. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 2013, 126, 11–18. [Google Scholar] [CrossRef]
- Ramírez, J.D.; Sánchez, A.; Hernández, C.; Florez, C.; Bernal, M.C.; Giraldo, J.C.; Reyes, P.; Lopez, M.C.; Garcia, L.; Cooper, P.J.; et al. Geographic distribution of human Blastocystis subtypes in South America. Infect. Genet. Evol. 2016, 41, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.; Gantois, N.; Ayoubi, A.; Even, G.; Sawant, M.; El Houmayraa, J.; Nabot, M.; Benamrouz-Vanneste, S.; Chabé, M.; Certad, G.; et al. Blastocystis sp. prevalence and subtypes distribution amongst Syrian refugee communities living in North Lebanon. Microorganisms 2021, 9, 184. [Google Scholar] [CrossRef]
- Osario-Pulgarin, M.I.; Higuera, A.; Beltran-Alzate, J.C.; Sanchez-Jimenez, M.; Ramirez, J.D. Epidemiological and molecular characterization of Blastocystis infection in children attending daycare centers in Medellin, Colombia. Biology 2021, 10, 669. [Google Scholar] [CrossRef]
- Jinatham, V.; Maxamhud, S.; Popluechai, S.; Tsaousis, A.D.; Gentekaki, E. Blastocystis One Health approach in a rural community of Northern Thailand: Prevalence, subtypes and novel transmission routes. Front. Microbiol. 2021, 12, 746340. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.D.N.; Gantois, N.; Hoang, T.T.; Do, B.T.; Desramaut, J.; Naguib, D.; Tran, T.N.; Truong, A.D.; Even, G.; Certad, G.; et al. First epidemiological survey on the prevalence and subtypes distribution of the enteric parasite Blastocystis sp. in Vietnam. Microorganisms 2023, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Greige, S.; El Safadi, D.; Khaled, S.; Gantois, N.; Baydoun, M.; Chemaly, M.; Benamrouz-Vanneste, S.; Chabe, M.; Osman, M.; Certad, G.; et al. First report on the prevalence and subtype distribution of Blastocystis sp. in dairy cattle in Lebanon and assessment of zoonotic transmission. Acta Trop. 2019, 194, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Naguib, D.; Gantois, N.; Desramaut, J.; Arafat, N.; Even, G.; Certad, G.; Chabe, M.; Viscogliosi, E. Prevalence, subtype distribution and zoonotic significance of Blastocystis sp. isolates from poultry, cattle and pets in Northern Egypt. Microorganisms 2022, 10, 2259. [Google Scholar] [CrossRef] [PubMed]
- Fréalle, E.; El Safadi, D.; Cian, A.; Aubry, E.; Certad, G.; Osman, M.; Wacrenier, A.; Dutoit, E.; Creusy, C.; Dubos, F.; et al. Acute Blastocystis-associated appendicular peritonitis in a child, Casablanca, Morocco. Emerg. Infect. Dis. 2015, 21, 91–94. [Google Scholar] [CrossRef]
- Ajjampur, S.S.; Tan, K.S.W. Pathogenic mechanisms in Blastocystis spp.—Interpreting results from in vitro and in vivo studies. Parasitol. Int. 2016, 65, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wojciech, L.; Gascoigne, N.R.J.; Peng, G.; Tan, K.S.W. New insights into the interactions between Blastocystis, the gut microbiota, and host immunity. PLoS Pathog. 2021, 17, e1009253. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Tan, K.S.W. Interactions between Blastocystis subtype ST4 and gut microbiota in vitro. Parasit. Vectors 2022, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wojciech, L.; Png, C.W.; Koh, E.Y.; Aung, T.T.; Kioh, D.Y.Q.; Chan, E.C.Y.; Malleret, B.; Zhang, Y.; Peng, G.; et al. Experimental colonization with Blastocystis ST4 is associated with protective immune responses and modulation of gut microbiome in a DSS-induced colitis mouse model. Cell. Mol. Life Sci. 2022, 79, 245. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Lee, J.W.J.; Tan, K.S.W. Infection with pathogenic Blastocystis ST7 is associated with decreased bacterial diversity and altered gut microbiome profiles in diarrheal patients. Parasit. Vectors 2022, 15, 312. [Google Scholar] [CrossRef]
- Aykur, M.; Camyar, A.; Türk, B.G.; Sin, A.Z.; Dagci, H. Evaluation of association with subtypes and alleles of Blastocystis with chronic spontaneous urticarial. Acta Trop. 2022, 231, 106455. [Google Scholar] [CrossRef]
- Siwila, J.; Mwaba, F.; Chidumayo, N.; Mubanga, C. Food and waterborne protozoan parasites: The African perspective. Food Waterborne Parasitol. 2020, 20, e00088. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, H.K.; Khodeer, S. Blastocystis spp.: Frequency and subtype distribution in iron deficiency anemic versus non-anemic subjects from Egypt. J. Parasitol. 2013, 99, 599–602. [Google Scholar] [CrossRef] [PubMed]
- El-Badry, A.A.; Abd El Wahab, W.M.; Hamdy, D.A.; Aboud, A. Blastocystis subtypes isolated from irritable bowel syndrome patients and co-infection with Helicobacter pylori. Parasitol. Res. 2018, 117, 127–137. [Google Scholar] [CrossRef]
- Mokhtar, A.; Youssef, A. Subtype analysis of Blastocystis spp. isolated from domestic mammals and poultry and its relation to transmission to their in-contact humans in Ismailia governorate, Egypt. Parasitol. United J. 2018, 11, 90–98. [Google Scholar] [CrossRef]
- El-Taweel, H.; Isaa, Y.; Shehata, G.; Gaballah, A.; Lotfy, W.; Tolba, M. Restriction fragment length polymorphism (RFLP) analysis of Blastocystis spp. in symptomatic and asymptomatic individuals from Alexandria, Egypt. Parasitol. United J. 2020, 13, 164–171. [Google Scholar] [CrossRef]
- Abdo, S.M.; El-Adawy, H.; Farag, H.F.; El-Taweel, H.A.; Elhadad, H.; El-Badry, A.A. Detection and molecular identification of Blastocystis isolates from humans and cattle in northern Egypt. J. Parasit. Dis. 2021, 45, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.M.; Hussein, A.M.; Eida, M.M.; Atwa, M.M. Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol. Res. 2008, 102, 853–860. [Google Scholar] [CrossRef]
- Ahmed, S.A.; El-Mahallawy, H.S.; Mohamed, S.F.; Angelici, M.C.; Hasapis, K.; Saber, T.; Karanis, P. Subtypes and phylogenetic analysis of Blastocystis sp. isolates from West Ismailia, Egypt. Sci. Rep. 2022, 12, 19084. [Google Scholar] [CrossRef] [PubMed]
- El Saftawy, E.A.; Amin, N.M.; Hamed, D.H.; Elkazazz, A.; Adel, S. The hidden impact of different Blastocystis genotypes on C-3 and IgE serum levels: A matter of debate in asthmatic Egyptian children. J. Parasit. Dis. 2019, 43, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, A.B.; Ahmed, S.A.; Eltamany, E.E.; Karanis, P. Anti-Blastocystis activity in vitro of Egyptian herbal extracts (Family: Asteraceae) with emphasis on Artemisia Judaica. Int. J. Environ. Res. Public Health 2019, 16, 1555. [Google Scholar] [CrossRef] [PubMed]
- Mossallam, S.F.; El-Mansoury, S.A.T.; Tolba, M.M.; Kohla, A.A.; Khedr, S.I. In vitro susceptibility of human Blastocystis subtypes to simeprevir. Saudi J. Biol. Sci. 2021, 28, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; Ismail, M.A.M.; El-Badry, A.A.; Abu-Sarea, E.Y.; Dewidar, A.M.; Hamdy, D.A. An association between Blastocystis subtypes and colorectal cancer patients: A significant different profile from non-cancer individuals. Acta Parasitol. 2022, 67, 752–763. [Google Scholar] [CrossRef]
- Hussein, E.M.; Muhammad, M.A.A.; Hussein, A.M.; Elzagawy, S.M.; Zaki, W.M.; Temsah, A.G.; Badr, M.S.; Alabbassy, M.M. Levels of genetic variants among symptomatic Blastocystis subtypes and their relationship to mucosal immune surveillance in the precancerous colons of experimentally infected rats. Acta Parasitol. 2023, 68, 70–83. [Google Scholar] [CrossRef]
- Shehab, A.Y.; Allam, A.F.; Farag, H.F.; Elhadad, H.; El Kotb, S.F.; El-Taweel, H.A. Intestinal parasites among humans and their livestock animals in a rural community in Gharbia governorate, Egypt. J. Parasit. Dis. 2021, 45, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Wawrzyniak, I.; Albert, A.; El Alaoui, H.; Delbac, F.; Livrelli, V. Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: Prospective study of patients with hematological malignancies. J. Clin. Microbiol. 2011, 49, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Padukone, S.; Mandal, J.; Rajkumari, N.; Bhat, B.V.; Swaminathan, R.P.; Parija, S.C. Detection of Blastocystis in clinical stool specimens using three different methods and morphological examination in Jones’ medium. Trop. Parasitol. 2018, 8, 33–40. [Google Scholar] [PubMed]
- El-Wakil, E.S.; Zalat, R.S.; El-Badry, A.A. Mapping gut parasitism patterns in a cohort of Egyptians. Sci. Rep. 2023, 13, 9961. [Google Scholar] [CrossRef] [PubMed]
- Shakra, M.Y.; Abu-Sheishaa, G.A.; Hafez, A.O.; El-Lessy, F.M. Molecular assay and in vitro culture for Blastocystis prevalence in Dakahlia governorate, Egypt. Egypt. J. Immunol. 2023, 30, 1–10. [Google Scholar] [CrossRef]
- El Safadi, D.; Gaayeb, L.; Meloni, D.; Cian, A.; Poirier, P.; Wawrzyniak, I.; Delbac, F.; Dabboussi, F.; Delhaes, L.; Seck, M.; et al. Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect. Dis. 2014, 14, 164. [Google Scholar] [CrossRef]
- Souppart, L.; Moussa, H.; Cian, A.; Sanciu, G.; Poirier, P.; El Alaoui, H.; Delbac, F.; Boorom, K.; Delhaes, L.; Dei-Cas, E.; et al. Subtype analysis of Blastocystis isolates from symptomatic patients in Egypt. Parasitol. Res. 2010, 106, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Fouad, S.A.; Basyoni, M.M.; Fahmy, R.A.; Kobaisi, M.H. The pathogenic role of different Blastocystis hominis genotypes isolated from patients with irritable bowel syndrome. Arab J. Gastroenterol. 2011, 12, 194–200. [Google Scholar] [CrossRef]
- Hameed, D.M.; Hassanin, O.M.; Zuel-Fakkar, N.M. Association of Blastocystis hominis genetic subtypes with urticaria. Parasitol. Res. 2011, 108, 553–560. [Google Scholar] [CrossRef]
- Abaza, S.M.; Rayan, H.Z.; Soliman, R.H.; Nemr, N.A.; Mokhtar, A.B. Subtype analysis of Blastocystis spp. isolates from symptomatic and asymptomatic patients in Suez Canal University Hospitals, Ismailia, Egypt. Parasitol. United J. 2014, 7, 56–67. [Google Scholar] [CrossRef]
- Abu El-Fetouh, N.I.; Abdelmegeed, E.S.; Attia, R.A.; El-Dosoky, I.; Azab, M.S. Genotyping of Blastocystis hominis symptomatic isolates and kinetics of associated local CD3 and CD20 cell infiltrate. Parasitol. United J. 2015, 8, 115–122. [Google Scholar]
- Ben Abda, I.; Maatoug, N.; Ben Romdhane, R.; Bouhelmi, N.; Zallegua, N.; Aoun, K.; Viscogliosi, E.; Bouratbine, A. Prevalence and subtype identification of Blastocystis sp. in healthy individuals in the Tunis area, Tunisia. Am. J. Trop. Med. Hyg. 2017, 96, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Bachi, F.; Abidat, F.; Ghaffor, Y.; Bellili, S.; Goura, S.; Belmadani, S.A. Molecular characterization of algerian strains of Blastocysts sp. Med. Trop. Sante Int. 2022, 2, mtsi.v2i1.2022.226. [Google Scholar]
- Boutellis, A.; Aissi, M.; Harhoura, K.; Drali, R.; Kernif, T.; Tazerouti, F. First molecular characterization of Blastocystis subtypes from animals and animal-keepers stool in Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2021, 78, 101695. [Google Scholar] [CrossRef] [PubMed]
- Abdulsalam, A.M.; Ithoi, I.; Al-Mekhlafi, H.M.; Khan, A.H.; Ahmed, A.; Surin, J.; Mak, J.W. Prevalence, predictors and clinical significance of Blastocystis sp. in Sebha, Libya. Parasit. Vectors 2013, 6, 86. [Google Scholar] [CrossRef]
- Elseadawy, R.; Abbas, I.; Al-Araby, M.; Abu-Elwafa, S. Occurrence and molecular characterization of Acanthamoeba, Naegleria fowleri and Blastocystis in water samples from various sources in Egypt. Acta Trop. 2023, 237, 106733. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, F.; Masoud, I.M.; Fekry, M.M.; Abdel-Latif, M.S.; Abdel-Salam, H.; Salem, M.; Shehata, A.I. Environmental health aspects and microbial infections of the recreational water. BMC Public Health 2023, 23, 302. [Google Scholar] [CrossRef] [PubMed]

| Blastocystis sp. STs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Governorate | Samples (n) | Positive Samples (n) | Prevalence (%) | ST1 | ST2 | ST3 | ST10 | ST14 | MI a |
| Alexandria | 86 | 86 | 100 | 14 | 6 | 35 | 0 | 0 | 31 |
| Beheira | 202 | 154 | 76.2 | 30 | 9 | 74 | 0 | 1 | 40 |
| Cairo | 93 | 67 | 72.0 | 7 | 4 | 21 | 0 | 1 | 34 |
| Dakahlia | 301 | 231 | 76.7 | 77 | 11 | 36 | 1 | 2 | 104 |
| Ismailia | 143 | 59 | 41.3 | 15 | 9 | 9 | 0 | 0 | 26 |
| Total | 825 | 597 | 72.4 | 143 | 39 | 175 | 1 | 4 | 235 |
| Countries | Prevalence | Identification Method | Number of Subtyped Isolates | Subtyping Method | Blastocystis sp. STs | MI a | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST1 | ST2 | ST3 | ST4 | ST5 | ST6 | ST7 | ST10 | ST14 | |||||||
| Egypt | 34.5% | DLM b | 36 | PCR-STS c | 6 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [39] |
| Egypt | 19.1% | XIVC d | 22 | Sequencing | 4 | 0 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [40] |
| Egypt | 35.7% | XIVC d | 53 | PCR-STS c | 16 | 4 | 30 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | [41] |
| Egypt | 63.0% | DLM b | 20 | RFLP e | 4 | 2 | 11 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | [42] |
| Egypt | 39.0% | DLM b + XIVC d | 6 | Sequencing | 2 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [43] |
| Egypt | 23.1% | XIVC d | 44 | PCR-STS c | 8 | 0 | 24 | 0 | 0 | 8 | 4 | 0 | 0 | 0 | [44] |
| Egypt | 15.4% | DLM b | 11 | Sequencing | 3 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [45] |
| Egypt | 33.0% | DLM b | 100 | RFLP e | 0 | 0 | 84 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | [46] |
| Egypt | 18.2% | XIVC d | 2 | PCR-STS c | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [47] |
| Egypt | 54.2% | DLM b | 51 | PCR-STS c | 9 | 2 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [48] |
| Egypt | 52.0% | XIVC d | 20 | Sequencing | 5 | 4 | 8 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | [49] |
| Egypt | 39.4% | DLM b | 63 | PCR-STS c | 10 | 0 | 29 | 15 | 0 | 0 | 0 | 0 | 0 | 9 | [50] |
| Egypt | 60.4% | PCR f | 10 | Sequencing | 3 | 3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [54] |
| Egypt | NA g | DLM b | 21 | Sequencing | 4 | 4 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [57] |
| Egypt | NA g | XIVC d | 110 | PCR-STS c | 15 | 0 | 49 | 0 | 0 | 33 | 13 | 0 | 0 | 0 | [58] |
| Egypt | NA g | XIVC d | 33 | RFLP e | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [59] |
| Egypt | NA g | XIVC d | 60 | PCR-STS c | 23 | 2 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [60] |
| Egypt | NA g | XIVC d | 102 | RFLP e | 20 | 15 | 57 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | [61] |
| Egypt | 72.4% | qPCR | 597 | Sequencing | 143 | 39 | 175 | 0 | 0 | 0 | 0 | 1 | 4 | 235 | Present study |
| Total | 1361 | 276 | 81 | 647 | 47 | 0 | 41 | 20 | 1 | 4 | 244 | ||||
| Tunisia | NA g | DLM b | 61 | Sequencing | 18 | 10 | 31 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | [62] |
| Algeria | 7.4% | DLM b | 30 | Sequencing | 10 | 4 | 15 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | [63] |
| Algeria | 11.6% | DLM b | 3 | Sequencing | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [64] |
| Libya | 28.0% | XIVC d | 38 | Sequencing | 19 | 3 | 15 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | [23] |
| Libya | 22.1% | XIVC d | 48 | Sequencing | 26 | 13 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | [65] |
| Grand total | 1541 | 349 | 113 | 718 | 49 | 0 | 41 | 22 | 1 | 4 | 244 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naguib, D.; Gantois, N.; Desramaut, J.; Arafat, N.; Mandour, M.; Abdelmaogood, A.K.K.; Mosa, A.F.; Denoyelle, C.; Even, G.; Certad, G.; et al. Molecular Epidemiology and Genetic Diversity of the Enteric Protozoan Parasite Blastocystis sp. in the Northern Egypt Population. Pathogens 2023, 12, 1359. https://doi.org/10.3390/pathogens12111359
Naguib D, Gantois N, Desramaut J, Arafat N, Mandour M, Abdelmaogood AKK, Mosa AF, Denoyelle C, Even G, Certad G, et al. Molecular Epidemiology and Genetic Diversity of the Enteric Protozoan Parasite Blastocystis sp. in the Northern Egypt Population. Pathogens. 2023; 12(11):1359. https://doi.org/10.3390/pathogens12111359
Chicago/Turabian StyleNaguib, Doaa, Nausicaa Gantois, Jeremy Desramaut, Nagah Arafat, Mohamed Mandour, Asmaa Kamal Kamal Abdelmaogood, Ashraf Fawzy Mosa, Constance Denoyelle, Gaël Even, Gabriela Certad, and et al. 2023. "Molecular Epidemiology and Genetic Diversity of the Enteric Protozoan Parasite Blastocystis sp. in the Northern Egypt Population" Pathogens 12, no. 11: 1359. https://doi.org/10.3390/pathogens12111359
APA StyleNaguib, D., Gantois, N., Desramaut, J., Arafat, N., Mandour, M., Abdelmaogood, A. K. K., Mosa, A. F., Denoyelle, C., Even, G., Certad, G., Chabé, M., & Viscogliosi, E. (2023). Molecular Epidemiology and Genetic Diversity of the Enteric Protozoan Parasite Blastocystis sp. in the Northern Egypt Population. Pathogens, 12(11), 1359. https://doi.org/10.3390/pathogens12111359








