Prevention and Treatment Strategies for Respiratory Syncytial Virus (RSV)
Abstract
:1. Introduction
2. Specific Respiratory Syncytial Virus Therapy
3. Non-Specific RSV Treatments
The Lung in RSV Infection
4. RSV Prevention
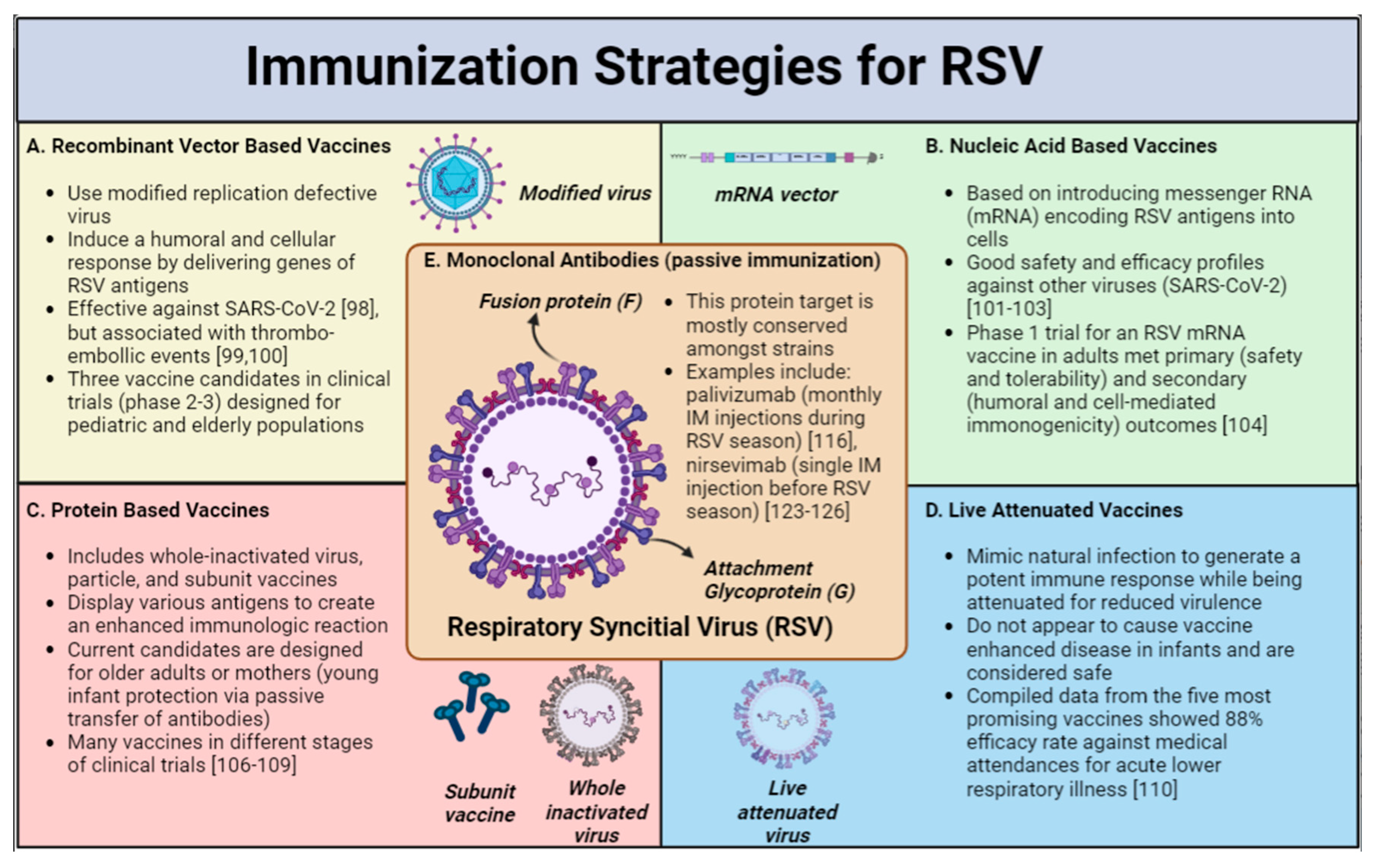
Vaccines
5. Expert Commentary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meissner, H.C.; Rennels, M.B.; Pickering, L.K.; Hall, C.B. Risk of severe respiratory syncytial virus disease, identification of high risk infants and recommendations for prophylaxis with palivizumab. Pediatr. Infect. Dis. J. 2004, 23, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Movva, N.; Jiang, X.; Reichert, H.; Bylsma, L.C.; Fryzek, J.P.; Nelson, C.B. Respiratory Syncytial Virus Burden and Healthcare Utilization in United States Infants <1 Year of Age: Study of Nationally Representative Databases, 2011–2019. J. Infect. Dis. 2022, 226, S184–S194. [Google Scholar] [CrossRef] [PubMed]
- Pisesky, A.; Benchimol, E.I.; Wong, C.A.; Hui, C.; Crowe, M.; Belair, M.A.; Pojsupap, S.; Karnauchow, T.; O’Hearn, K.; Yasseen, A.S., 3rd; et al. Incidence of Hospitalization for Respiratory Syncytial Virus Infection amongst Children in Ontario, Canada: A Population-Based Study Using Validated Health Administrative Data. PLoS ONE 2016, 11, e0150416. [Google Scholar] [CrossRef] [PubMed]
- Thampi, N.; Knight, B.D.; Thavorn, K.; Webster, R.J.; Lanctot, K.; Hawken, S.; McNally, J.D. Health care costs of hospitalization of young children for respiratory syncytial virus infections: A population-based matched cohort study. CMAJ Open 2021, 9, E948–E956. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simoes, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Boyapalle, S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin. Microbiol. Rev. 2008, 21, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Blanken, M.O.; Rovers, M.M.; Molenaar, J.M.; Winkler-Seinstra, P.L.; Meijer, A.; Kimpen, J.L.; Bont, L. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N. Engl. J. Med. 2013, 368, 1791–1799. [Google Scholar] [CrossRef] [Green Version]
- Binns, E.; Tuckerman, J.; Licciardi, P.V.; Wurzel, D. Respiratory syncytial virus, recurrent wheeze and asthma: A narrative review of pathophysiology, prevention and future directions. J. Paediatr. Child. Health 2022, 58, 1741–1746. [Google Scholar] [CrossRef]
- Ventre, K.; Randolph, A.G. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst. Rev. 2007, 1, CD000181. [Google Scholar] [CrossRef]
- Hoover, J.; Eades, S.; Lam, W.M. Pediatric Antiviral Stewardship: Defining the Potential Role of Ribavirin in Respiratory Syncytial Virus-Associated Lower Respiratory Illness. J. Pediatr. Pharmacol. Ther. 2018, 23, 372–378. [Google Scholar] [CrossRef]
- Boeckh, M.; Englund, J.; Li, Y.; Miller, C.; Cross, A.; Fernandez, H.; Kuypers, J.; Kim, H.; Gnann, J.; Whitley, R.; et al. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin. Infect. Dis. 2007, 44, 245–249. [Google Scholar] [CrossRef]
- Sinclair, S.M.; Jones, J.K.; Miller, R.K.; Greene, M.F.; Kwo, P.Y.; Maddrey, W.C. The Ribavirin Pregnancy Registry: An Interim Analysis of Potential Teratogenicity at the Mid-Point of Enrollment. Drug Saf. 2017, 40, 1205–1218. [Google Scholar] [CrossRef] [Green Version]
- Shults, R.A.; Baron, S.; Decker, J.; Deitchman, S.D.; Connor, J.D. Health care worker exposure to aerosolized ribavirin: Biological and air monitoring. J. Occup. Environ. Med. 1996, 38, 257–263. [Google Scholar] [CrossRef]
- Burrows, F.S.; Carlos, L.M.; Marriott, D.; Havryk, A.; Plit, M.; Glanville, A.R. Oral Ribavirin Is a Cost-Effective Alternative to Intravenous Ribavirin for Respiratory Syncytial Virus (RSV) Infection after Lung Transplantation (LTx). J. Heart Lung Transplant. 2013, 32, S109. [Google Scholar] [CrossRef]
- Tejada, S.; Martinez-Reviejo, R.; Karakoc, H.N.; Pena-Lopez, Y.; Manuel, O.; Rello, J. Ribavirin for Treatment of Subjects with Respiratory Syncytial Virus-Related Infection: A Systematic Review and Meta-Analysis. Adv. Ther. 2022, 39, 4037–4051. [Google Scholar] [CrossRef]
- Med., B.N.L. Identifier: NCT04225897. A Study in Infants With RSV LRTI to Evaluate RV521 (REVIRAL 1). 2020. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04225897 (accessed on 29 November 2022).
- Med., B.N.L. Identifier: NCT04231968. A Study of AK0529 in Chinese Infants Hospitalized With RSV (AirFLO). 2020. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04231968 (accessed on 8 April 2022).
- Alansari, K.; Toaimah, F.H.; Almatar, D.H.; El Tatawy, L.A.; Davidson, B.L.; Qusad, M.I.M. Monoclonal Antibody Treatment of RSV Bronchiolitis in Young Infants: A Randomized Trial. Pediatrics 2019, 143, e20182308. [Google Scholar] [CrossRef] [Green Version]
- Saez-Llorens, X.; Moreno, M.T.; Ramilo, O.; Sanchez, P.J.; Top, F.H., Jr.; Connor, E.M.; Group, M.-S. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2004, 23, 707–712. [Google Scholar] [CrossRef]
- Ramilo, O.; Lagos, R.; Sáez-Llorens, X.; Suzich, J.; Wang, C.K.; Jensen, K.M.; Harris, B.S.; Losonsky, G.A.; Griffin, M.P.; Group, M.S. Motavizumab Treatment of Infants Hospitalized With Respiratory Syncytial Virus Infection Does Not Decrease Viral Load or Severity of Illness. Pediatr. Infect. Dis. J. 2014, 33, 703–709. [Google Scholar] [CrossRef]
- Prince, G.A.; Hemming, V.G.; Horswood, R.L.; Chanock, R.M. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985, 3, 193–206. [Google Scholar] [CrossRef]
- Sanders, S.L.; Agwan, S.; Hassan, M.; van Driel, M.L.; Del Mar, C.B. Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection. Cochrane Database Syst. Rev. 2019, 8, CD009417. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Anderson, E.J.; Goldstein, M. The Future of Respiratory Syncytial Virus Disease Prevention and Treatment. Infect. Dis. Ther. 2021, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Hislop, A.A.; Haworth, S.G. Airway size and structure in the normal fetal and infant lung and the effect of premature delivery and artificial ventilation. Am. Rev. Respir. Dis. 1989, 140, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Aherne, W.; Bird, T.; Court, S.D.; Gardner, P.S.; McQuillin, J. Pathological changes in virus infections of the lower respiratory tract in children. J. Clin. Pathol. 1970, 23, 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickles, R.J.; DeVincenzo, J.P. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J. Pathol. 2015, 235, 266–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.E.; Gonzales, R.A.; Olson, S.J.; Wright, P.F.; Graham, B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007, 20, 108–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, E.K.; Hartert, T.V. Genes associated with RSV lower respiratory tract infection and asthma: The application of genetic epidemiological methods to understand causality. Future Virol. 2015, 10, 883–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilovskiy, I.P.; Yumashev, K.V.; Nikolsky, A.A.; Vishnyakova, L.I.; Khaitov, M.R. Molecular and Cellular Mechanisms of Respiratory Syncytial Viral Infection: Using Murine Models to Understand Human Pathology. Biochemistry 2021, 86, 290–306. [Google Scholar] [CrossRef]
- Stier, M.T.; Peebles, R.S., Jr. Host and Viral Determinants of Respiratory Syncytial Virus-induced Airway Mucus. Ann. Am. Thorac. Soc. 2018, 15 (Suppl. S3), S205–S209. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Moore, M.L.; Rudd, B.D.; Berlin, A.A.; Collins, R.D.; Olson, S.J.; Ho, S.B.; Peebles, R.S., Jr. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am. J. Pathol. 2006, 169, 977–986. [Google Scholar] [CrossRef] [Green Version]
- Linssen, R.S.N.; Ma, J.; Bem, R.A.; Rubin, B.K. Rational use of mucoactive medications to treat pediatric airway disease. Paediatr. Respir. Rev. 2020, 36, 8–14. [Google Scholar] [CrossRef]
- Gadomski, A.M.; Scribani, M.B. Bronchodilators for bronchiolitis. Cochrane Database Syst. Rev. 2014, 6, CD001266. [Google Scholar] [CrossRef]
- Krilov, L.R. Respiratory syncytial virus disease: Update on treatment and prevention. Expert Rev. Anti Infect. Ther. 2011, 9, 27–32. [Google Scholar] [CrossRef]
- Enriquez, A.; Chu, I.W.; Mellis, C.; Lin, W.Y. Nebulised deoxyribonuclease for viral bronchiolitis in children younger than 24 months. Cochrane Database Syst. Rev. 2012, 11, CD008395. [Google Scholar] [CrossRef]
- Merkus, P.J.; de Hoog, M.; van Gent, R.; de Jongste, J.C. DNase treatment for atelectasis in infants with severe respiratory syncytial virus bronchiolitis. Eur. Respir. J. 2001, 18, 734–737. [Google Scholar] [CrossRef]
- Balsamo, R.; Lanata, L.; Egan, C.G. Mucoactive drugs. Eur. Respir. Rev. 2010, 19, 127–133. [Google Scholar] [CrossRef]
- Banerjee, S.; McCormack, M. Acetylcysteine for Patients Requiring Mucous Secretion Clearance: A Review of Clinical Effectiveness and Safety. Canadian Agency for Drugs and Technologies in Health. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546019/ (accessed on 16 November 2022).
- Mandelberg, A.; Amirav, I. Hypertonic saline or high volume normal saline for viral bronchiolitis: Mechanisms and rationale. Pediatr. Pulmonol. 2010, 45, 36–40. [Google Scholar] [CrossRef]
- Zhang, L.; Gunther, C.B.; Franco, O.S.; Klassen, T.P. Impact of hypertonic saline on hospitalization rate in infants with acute bronchiolitis: A meta-analysis. Pediatr. Pulmonol. 2018, 53, 1089–1095. [Google Scholar] [CrossRef]
- Zhang, L.; Mendoza-Sassi, R.A.; Wainwright, C.; Klassen, T.P. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst. Rev. 2017, 12, CD006458. [Google Scholar] [CrossRef]
- Hartling, L.; Bialy, L.M.; Vandermeer, B.; Tjosvold, L.; Johnson, D.W.; Plint, A.C.; Klassen, T.P.; Patel, H.; Fernandes, R.M. Epinephrine for bronchiolitis. Cochrane Database Syst. Rev. 2011, 6, CD003123. [Google Scholar] [CrossRef]
- Elliott, S.A.; Gaudet, L.A.; Fernandes, R.M.; Vandermeer, B.; Freedman, S.B.; Johnson, D.W.; Plint, A.C.; Klassen, T.P.; Allain, D.; Hartling, L. Comparative Efficacy of Bronchiolitis Interventions in Acute Care: A Network Meta-analysis. Pediatrics 2021, 147, e2020040816. [Google Scholar] [CrossRef]
- Zomer-Kooijker, K.; van der Ent, C.K.; Ermers, M.J.; Rovers, M.M.; Bont, L.J.; Group, R.S.V.C.S. Lack of long-term effects of high-dose inhaled beclomethasone for respiratory syncytial virus bronchiolitis: A randomized placebo-controlled trial. Pediatr. Infect. Dis. J. 2014, 33, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, R.M.; Bialy, L.M.; Vandermeer, B.; Tjosvold, L.; Plint, A.C.; Patel, H.; Johnson, D.W.; Klassen, T.P.; Hartling, L. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst. Rev. 2013, 10, CD004878. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, S.R. Experimental and Clinical Pharmacology: Leukotrienes-biosynthesis and mechanisms of action. Aust. Prescr. 1999, 22, 55–57. [Google Scholar] [CrossRef]
- Liu, F.; Ouyang, J.; Sharma, A.N.; Liu, S.; Yang, B.; Xiong, W.; Xu, R. Leukotriene inhibitors for bronchiolitis in infants and young children. Cochrane Database Syst. Rev. 2015, 3, CD010636. [Google Scholar] [CrossRef] [PubMed]
- Roque i Figuls, M.; Gine-Garriga, M.; Granados Rugeles, C.; Perrotta, C.; Vilaro, J. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database Syst. Rev. 2016, 2, CD004873. [Google Scholar] [CrossRef]
- Florin, T.A.; Plint, A.C.; Zorc, J.J. Viral bronchiolitis. Lancet 2017, 389, 211–224. [Google Scholar] [CrossRef]
- Nasr, S.Z.; Strouse, P.J.; Soskolne, E.; Maxvold, N.J.; Garver, K.A.; Rubin, B.K.; Moler, F.W. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest 2001, 120, 203–208. [Google Scholar] [CrossRef]
- Thornby, K.A.; Johnson, A.; Axtell, S. Dornase Alfa for Non-Cystic Fibrosis Pediatric Pulmonary Atelectasis. Ann. Pharm. 2014, 48, 1040–1049. [Google Scholar] [CrossRef]
- Boogaard, R.; Hulsmann, A.R.; van Veen, L.; Vaessen-Verberne, A.; Yap, Y.N.; Sprij, A.J.; Brinkhorst, G.; Sibbles, B.; Hendriks, T.; Feith, S.W.W.; et al. Recombinant human deoxyribonuclease in infants with respiratory syncytial virus bronchiolitis. Chest 2007, 131, 788–795. [Google Scholar] [CrossRef]
- Sathe, N.A.; Krishnaswami, S.; Andrews, J.; Ficzere, C.; McPheeters, M.L. Pharmacologic Agents That Promote Airway Clearance in Hospitalized Subjects: A Systematic Review. Respir. Care 2015, 60, 1061–1070. [Google Scholar] [CrossRef]
- Mata, M.; Morcillo, E.; Gimeno, C.; Cortijo, J. N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV). Biochem. Pharm. 2011, 82, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Naz, F.; Raza, A.B.; Ijaz, I.; Kazi, M.Y. Effectiveness of nebulized N-acetylcysteine solution in children with acute bronchiolitis. J. Coll. Physicians Surg. Pak. 2014, 24, 408–411. [Google Scholar]
- Daviskas, E.; Anderson, S.D.; Gonda, I.; Eberl, S.; Meikle, S.; Seale, J.P.; Bautovich, G. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. Eur. Respir. J. 1996, 9, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Kellett, F.; Redfern, J.; Niven, R.M. Evaluation of nebulised hypertonic saline (7%) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respir. Med. 2005, 99, 27–31. [Google Scholar] [CrossRef]
- Wark, P.; McDonald, V.M. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst. Rev. 2018, 9, CD001506. [Google Scholar] [CrossRef]
- Wohl, M.E.; Chernick, V. State of the art: Bronchiolitis. Am. Rev. Respir. Dis. 1978, 118, 759–781. [Google Scholar] [CrossRef]
- Mandelberg, A.; Tal, G.; Witzling, M.; Someck, E.; Houri, S.; Balin, A.; Priel, I.E. Nebulized 3% hypertonic saline solution treatment in hospitalized infants with viral bronchiolitis. Chest 2003, 123, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Faber, T.E.; Kamps, A.W.; Sjoerdsma, M.H.; Vermeulen, S.; Veeger, N.J.; Bont, L.J. Computerized Assessment of Wheezing in Children With Respiratory Syncytial Virus Bronchiolitis Before and After Hypertonic Saline Nebulization. Respir. Care 2015, 60, 1252–1256. [Google Scholar] [CrossRef] [Green Version]
- Sarrell, E.M.; Tal, G.; Witzling, M.; Someck, E.; Houri, S.; Cohen, H.A.; Mandelberg, A. Nebulized 3% hypertonic saline solution treatment in ambulatory children with viral bronchiolitis decreases symptoms. Chest 2002, 122, 2015–2020. [Google Scholar] [CrossRef]
- Morikawa, Y.; Miura, M.; Furuhata, M.Y.; Morino, S.; Omori, T.; Otsuka, M.; Chiga, M.; Obonai, T.; Hataya, H.; Kaneko, T.; et al. Nebulized hypertonic saline in infants hospitalized with moderately severe bronchiolitis due to RSV infection: A multicenter randomized controlled trial. Pediatr. Pulmonol. 2018, 53, 358–365. [Google Scholar] [CrossRef]
- Jaquet-Pilloud, R.; Verga, M.E.; Russo, M.; Gehri, M.; Pauchard, J.Y. Nebulised hypertonic saline in moderate-to-severe bronchiolitis: A randomised clinical trial. Arch. Dis. Child. 2020, 105, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Kellner, J.D.; Ohlsson, A.; Gadomski, A.M.; Wang, E.E. Efficacy of bronchodilator therapy in bronchiolitis. A meta-analysis. Arch. Pediatr. Adolesc. Med. 1996, 150, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, C.E.; Nino, G.; Castro-Rodriguez, J.A.; Acuna-Cordero, R.; Sossa-Briceno, M.P.; Midulla, F. For which infants with viral bronchiolitis could it be deemed appropriate to use albuterol, at least on a therapeutic trial basis? Allergol. Immunopathol. 2021, 49, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, I.; Koster, J.D.; Powell, R.E.; Wolstein, R.; Chernick, V. Effect of racemic epinephrine and salbutamol on clinical score and pulmonary mechanics in infants with bronchiolitis. J. Pediatr. 1993, 122, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.C.; Jafri, H.S.; Bush, A.J.; Carubelli, C.M.; Sheeran, P.; Hardy, R.D.; Ottolini, M.G.; Ramilo, O.; DeVincenzo, J.P. A randomized, double-blind, placebo-controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: Effects on RSV quantity and clinical outcome. J. Infect. Dis. 2002, 185, 1222–1228. [Google Scholar] [CrossRef]
- Somers, C.C.; Ahmad, N.; Mejias, A.; Buckingham, S.C.; Carubelli, C.; Katz, K.; Leos, N.; Gomez, A.M.; DeVincenzo, J.P.; Ramilo, O.; et al. Effect of dexamethasone on respiratory syncytial virus-induced lung inflammation in children: Results of a randomized, placebo controlled clinical trial. Pediatr. Allergy Immunol. 2009, 20, 477–485. [Google Scholar] [CrossRef]
- Everard, M.L.; Swarbrick, A.; Wrightham, M.; McIntyre, J.; Dunkley, C.; James, P.D.; Sewell, H.F.; Milner, A.D. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch. Dis. Child. 1994, 71, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Alansari, K.; Sakran, M.; Davidson, B.L.; Ibrahim, K.; Alrefai, M.; Zakaria, I. Oral dexamethasone for bronchiolitis: A randomized trial. Pediatrics 2013, 132, e810–e816. [Google Scholar] [CrossRef] [Green Version]
- Pinto, L.A.; Pitrez, P.M.; Luisi, F.; de Mello, P.P.; Gerhardt, M.; Ferlini, R.; Barbosa, D.C.; Daros, I.; Jones, M.H.; Stein, R.T.; et al. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: A randomized, double-blinded, and placebo-controlled clinical trial. J. Pediatr. 2012, 161, 1104–1108. [Google Scholar] [CrossRef]
- Tal, G.; Cesar, K.; Oron, A.; Houri, S.; Ballin, A.; Mandelberg, A. Hypertonic saline/epinephrine treatment in hospitalized infants with viral bronchiolitis reduces hospitalization stay: 2 years experience. Isr. Med. Assoc. J. 2006, 8, 169–173. [Google Scholar]
- Flores-Gonzalez, J.C.; Matamala-Morillo, M.A.; Rodriguez-Campoy, P.; Perez-Guerrero, J.J.; Serrano-Moyano, B.; Comino-Vazquez, P.; Palma-Zambrano, E.; Bulo-Concellon, R.; Santos-Sanchez, V.; Lechuga-Sancho, A.M.; et al. Epinephrine Improves the Efficacy of Nebulized Hypertonic Saline in Moderate Bronchiolitis: A Randomised Clinical Trial. PLoS ONE 2015, 10, e0142847. [Google Scholar] [CrossRef]
- Goldbart, A.; Lavie, M.; Lubetzky, R.; Pillar, G.; Landau, D.; Schlesinger, Y.; Spiegel, R.; Golan-Tripto, I.; Nahum, A.; Greenberg, D.; et al. Inhaled Nitric Oxide for the Treatment of Acute Bronchiolitis: A Multicenter Randomized Controlled Trial to Evaluate Dose Response. Ann. Am. Thorac. Soc. 2022. [Google Scholar] [CrossRef]
- Hasegawa, K.; Dumas, O.; Hartert, T.V.; Camargo, C.A., Jr. Advancing our understanding of infant bronchiolitis through phenotyping and endotyping: Clinical and molecular approaches. Expert Rev. Respir. Med. 2016, 10, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.M.; Bloom, H.H.; Mufson, M.A.; Chanock, R.M. Natural reinfection of adults by respiratory syncytial virus. Possible relation to mild upper respiratory disease. N. Engl. J. Med. 1962, 267, 68–72. [Google Scholar] [CrossRef]
- Kutsaya, A.; Teros-Jaakkola, T.; Kakkola, L.; Toivonen, L.; Peltola, V.; Waris, M.; Julkunen, I. Prospective clinical and serological follow-up in early childhood reveals a high rate of subclinical RSV infection and a relatively high reinfection rate within the first 3 years of life. Epidemiol. Infect. 2016, 144, 1622–1633. [Google Scholar] [CrossRef]
- Hall, C.B.; Walsh, E.E.; Long, C.E.; Schnabel, K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 1991, 163, 693–698. [Google Scholar] [CrossRef]
- Bont, L.; Versteegh, J.; Swelsen, W.T.; Heijnen, C.J.; Kavelaars, A.; Brus, F.; Draaisma, J.M.; Pekelharing-Berghuis, M.; van Diemen-Steenvoorde, R.A.; Kimpen, J.L. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr. Res. 2002, 52, 363–367. [Google Scholar] [CrossRef]
- Chanock, R.; Roizman, B.; Myers, R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am. J. Hyg. 1957, 66, 281–290. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Castilow, E.M.; Olson, M.R.; Varga, S.M. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol. Res. 2007, 39, 225–239. [Google Scholar] [CrossRef]
- Openshaw, P.J.; Culley, F.J.; Olszewska, W. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine 2001, 20 (Suppl. S1), S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Terstappen, J.; Baral, R.; Bardaji, A.; Beutels, P.; Buchholz, U.J.; Cohen, C.; Crowe, J.E., Jr.; Cutland, C.L.; Eckert, L.; et al. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 2022, 23, e2–S21. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Higgins, D.; Nunes, M.C.; Melero, J.A.; Langedijk, A.C.; Horsley, N.; Buchholz, U.J.; Openshaw, P.J.; McLellan, J.S.; Englund, J.A.; et al. The respiratory syncytial virus vaccine landscape: Lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018, 18, e295–e311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejias, A.; Rodriguez-Fernandez, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef] [PubMed]
- PATH. RSV Vaccine and mAb Snapshot. Available online: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot (accessed on 16 November 2022).
- Rodriguez-Fernandez, R.; Mejias, A.; Ramilo, O. Monoclonal Antibodies for Prevention of Respiratory Syncytial Virus Infection. Pediatr. Infect. Dis. J. 2021, 40, S35–S39. [Google Scholar] [CrossRef]
- Anderson, L.J. Respiratory syncytial virus vaccine development. Semin. Immunol. 2013, 25, 160–171. [Google Scholar] [CrossRef]
- Mufson, M.A.; Orvell, C.; Rafnar, B.; Norrby, E. Two distinct subtypes of human respiratory syncytial virus. J. Gen. Virol. 1985, 66 (Pt 10), 2111–2124. [Google Scholar] [CrossRef]
- Hall, C.B. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 2001, 344, 1917–1928. [Google Scholar] [CrossRef] [Green Version]
- McLellan, J.S.; Chen, M.; Leung, S.; Graepel, K.W.; Du, X.; Yang, Y.; Zhou, T.; Baxa, U.; Yasuda, E.; Beaumont, T.; et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013, 340, 1113–1117. [Google Scholar] [CrossRef] [Green Version]
- Krarup, A.; Truan, D.; Furmanova-Hollenstein, P.; Bogaert, L.; Bouchier, P.; Bisschop, I.J.M.; Widjojoatmodjo, M.N.; Zahn, R.; Schuitemaker, H.; McLellan, J.S.; et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 2015, 6, 8143. [Google Scholar] [CrossRef] [Green Version]
- Madhi, S.A.; Polack, F.P.; Piedra, P.A.; Munoz, F.M.; Trenholme, A.A.; Simoes, E.A.F.; Swamy, G.K.; Agrawal, S.; Ahmed, K.; August, A.; et al. Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N. Engl. J. Med. 2020, 383, 426–439. [Google Scholar] [CrossRef]
- Samy, N.; Reichhardt, D.; Schmidt, D.; Chen, L.M.; Silbernagl, G.; Vidojkovic, S.; Meyer, T.P.; Jordan, E.; Adams, T.; Weidenthaler, H.; et al. Safety and immunogenicity of novel modified vaccinia Ankara-vectored RSV vaccine: A randomized phase I clinical trial. Vaccine 2020, 38, 2608–2619. [Google Scholar] [CrossRef]
- Williams, K.; Bastian, A.R.; Feldman, R.A.; Omoruyi, E.; de Paepe, E.; Hendriks, J.; van Zeeburg, H.; Godeaux, O.; Langedijk, J.P.M.; Schuitemaker, H.; et al. Phase 1 Safety and Immunogenicity Study of a Respiratory Syncytial Virus Vaccine With an Adenovirus 26 Vector Encoding Prefusion F (Ad26.RSV.preF) in Adults Aged >/=60 Years. J. Infect. Dis. 2020, 222, 979–988. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022, 9, e73–e80. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schafer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of Covid-19 mRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef]
- Aliprantis, A.O.; Shaw, C.A.; Griffin, P.; Farinola, N.; Railkar, R.A.; Cao, X.; Liu, W.; Sachs, J.R.; Swenson, C.J.; Lee, H.; et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum. Vaccin. Immunother. 2021, 17, 1248–1261. [Google Scholar] [CrossRef]
- Higgins, D.; Trujillo, C.; Keech, C. Advances in RSV vaccine research and development—A global agenda. Vaccine 2016, 34, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Crank, M.C.; Ruckwardt, T.J.; Chen, M.; Morabito, K.M.; Phung, E.; Costner, P.J.; Holman, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J.; et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019, 365, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Med., B.N.L. Identifier: NCT04071158. A study of a RSV vaccine when given together with TDap in healthy nonpregnant women aged between 18 to 49 years. Available online: https://clinicaltrials.gov/ct2/show/NCT04071158 (accessed on 15 February 2021).
- Med., B.N.L. Identifier: NCT04785612. Study of RSVpre-F Vaccination and RSV Challenge in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04785612 (accessed on 2 September 2021).
- Med., B.N.L. Identifier: NCT04681833. Safety and efficacy of BARS13 in the elderly. Available online: https://clinicaltrials.gov/ct2/show/NCT04681833 (accessed on 22 September 2022).
- Karron, R.A.; Atwell, J.E.; McFarland, E.J.; Cunningham, C.K.; Muresan, P.; Perlowski, C.; Libous, J.; Spector, S.A.; Yogev, R.; Aziz, M.; et al. Live-attenuated Vaccines Prevent Respiratory Syncytial Virus-associated Illness in Young Children. Am. J. Respir. Crit. Care Med. 2021, 203, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Murphy, B.R. New generation live vaccines against human respiratory syncytial virus designed by reverse genetics. Proc. Am. Thorac. Soc. 2005, 2, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abarca, K.; Rey-Jurado, E.; Munoz-Durango, N.; Vazquez, Y.; Soto, J.A.; Galvez, N.M.S.; Valdes-Ferrada, J.; Iturriaga, C.; Urzua, M.; Borzutzky, A.; et al. Safety and immunogenicity evaluation of recombinant BCG vaccine against respiratory syncytial virus in a randomized, double-blind, placebo-controlled phase I clinical trial. EClinicalMedicine 2020, 27, 100517. [Google Scholar] [CrossRef]
- Johnson, S.; Oliver, C.; Prince, G.A.; Hemming, V.G.; Pfarr, D.S.; Wang, S.C.; Dormitzer, M.; O’Grady, J.; Koenig, S.; Tamura, J.K.; et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997, 176, 1215–1224. [Google Scholar] [CrossRef] [Green Version]
- COMMITTEE ON INFECTIOUS DISEASES AND BRONCHIOLITIS GUIDELINES COMMITTEE; Brady, M.T.; Byington, C.L.; Davies, H.D.; Edwards, K.M.; Jackson, M.A.; Maldonado, Y.A.; Murray, D.L.; Orenstein, W.A.; Rathore, M.H. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014, 134, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Soto, J.A.; Galvez, N.M.S.; Pacheco, G.A.; Bueno, S.M.; Kalergis, A.M. Antibody development for preventing the human respiratory syncytial virus pathology. Mol. Med. 2020, 26, 35. [Google Scholar] [CrossRef] [Green Version]
- Garegnani, L.; Styrmisdottir, L.; Roson Rodriguez, P.; Escobar Liquitay, C.M.; Esteban, I.; Franco, J.V. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst. Rev. 2021, 11, CD013757. [Google Scholar] [CrossRef]
- Ananworanich, J.; Heaton, P.M. Bringing Preventive RSV Monoclonal Antibodies to Infants in Low- and Middle-Income Countries: Challenges and Opportunities. Vaccines 2021, 9, 961. [Google Scholar] [CrossRef]
- Meissner, H.C.; Kimberlin, D.W. RSV immunoprophylaxis: Does the benefit justify the cost? Pediatrics 2013, 132, 915–918. [Google Scholar] [CrossRef] [Green Version]
- Feltes, T.F.; Sondheimer, H.M.; Tulloh, R.M.; Harris, B.S.; Jensen, K.M.; Losonsky, G.A.; Griffin, M.P.; Motavizumab Cardiac Study, G. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatr. Res. 2011, 70, 186–191. [Google Scholar] [CrossRef]
- Carbonell-Estrany, X.; Simoes, E.A.; Dagan, R.; Hall, C.B.; Harris, B.; Hultquist, M.; Connor, E.M.; Losonsky, G.A.; Motavizumab Study, G. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: A noninferiority trial. Pediatrics 2010, 125, e35–e51. [Google Scholar] [CrossRef] [Green Version]
- Simoes, E.A.F.; Forleo-Neto, E.; Geba, G.P.; Kamal, M.; Yang, F.; Cicirello, H.; Houghton, M.R.; Rideman, R.; Zhao, Q.; Benvin, S.L.; et al. Suptavumab for the Prevention of Medically Attended Respiratory Syncytial Virus Infection in Preterm Infants. Clin. Infect. Dis. 2021, 73, e4400–e4408. [Google Scholar] [CrossRef]
- Venkatesan, P. Nirsevimab: A promising therapy for RSV. Lancet Microbe 2022, 3, e335. [Google Scholar] [CrossRef]
- Griffin, M.P.; Yuan, Y.; Takas, T.; Domachowske, J.B.; Madhi, S.A.; Manzoni, P.; Simoes, E.A.F.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Engl. J. Med. 2020, 383, 415–425. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Domachowske, J.; Madhi, S.A.; Simoes, E.A.F.; Atanasova, V.; Cabanas, F.; Furuno, K.; Garcia-Garcia, M.L.; Grantina, I.; Nguyen, K.A.; Brooks, D.; et al. Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. N. Engl. J. Med. 2022, 386, 892–894. [Google Scholar] [CrossRef]
- Agency, E.M. Beyfortus (nirsevimab) for the treatment of serious lower respiratory tract infection caused by RSV in infants and children during their first RSV season. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/beyfortus#authorisation-details-section (accessed on 15 November 2022).
- Med., B.N.L. Identifier: NCT05118386. Safety, tolerability, and pharmacokinetics of RSV monoclonal antibody RSM01 in healthy adults. Available online: https://clinicaltrials.gov/ct2/show/NCT05118386 (accessed on 12 November 2022).
- Jones, R.G.; Martino, A. Targeted localized use of therapeutic antibodies: A review of non-systemic, topical and oral applications. Crit. Rev. Biotechnol. 2016, 36, 506–520. [Google Scholar] [CrossRef]
- Groves, H.E.; Piche-Renaud, P.P.; Peci, A.; Farrar, D.S.; Buckrell, S.; Bancej, C.; Sevenhuysen, C.; Campigotto, A.; Gubbay, J.B.; Morris, S.K. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg. Health Am. 2021, 1, 100015. [Google Scholar] [CrossRef]
- DeVincenzo, J.P.; Whitley, R.J.; Mackman, R.L.; Scaglioni-Weinlich, C.; Harrison, L.; Farrell, E.; McBride, S.; Lambkin-Williams, R.; Jordan, R.; Xin, Y.; et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N. Engl. J. Med. 2014, 371, 711–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marty, F.M.; Chemaly, R.F.; Mullane, K.M.; Lee, D.G.; Hirsch, H.H.; Small, C.B.; Bergeron, A.; Shoham, S.; Ljungman, P.; Waghmare, A.; et al. A Phase 2b, Randomized, Double-blind, Placebo-Controlled Multicenter Study Evaluating Antiviral Effects, Pharmacokinetics, Safety, and Tolerability of Presatovir in Hematopoietic Cell Transplant Recipients with Respiratory Syncytial Virus Infection of the Lower Respiratory Tract. Clin. Infect. Dis. 2020, 71, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
| Product Name | Mode of Administration/Mechanism of Action | Recommendation |
|---|---|---|
| Mucus therapies | ||
| Deoxyribonuclease (hrDNase) | Nebulized solution Mucolytic compound Cleaves extracellular DNA | Not recommended [35] May be considered as a therapeutic option for atelectasis in severe cases, when conventional therapy is unsuccessful [36] |
| N-acetylcysteine | Nebulized solution Poor bioavailability of oral preparation [37] Mucolytic compound Hydrolyzes disulfide bonds of mucus proteins Antioxidant properties [38] | Insufficient data—further studies needed Not recommended |
| 3% hypertonic saline | Nebulized solution Creates osmotic gradient and pulls water into the mucus layer Improves ciliary activity Stimulates cough May reduce airway edema [39] | May reduce the risk of hospitalization in the ED setting [40] Not recommended for inpatient management May modestly reduce the duration of admission for infants admitted >72 h—further studies needed [40,41] |
| Bronchodilators | ||
| Salbutamol, albuterol, etc. | Nebulized solution β-2 adrenergic receptor agonist, relaxes smooth muscle and opens airways | Not recommended [33] Can be trialled to assess the response in certain cases and given where a benefit is seen |
| Epinephrine | Nebulized solution Some β-2 adrenergic effects Vasoconstriction due to α-1 adrenergic receptor effects also decrease airway edema | May reduce risk of hospitalization in the ED setting [42] Not recommended [43] |
| Therapies targeting inflammation | ||
| Glucocorticoids (dexamethasone, prednisolone, budesonide, etc.) | Oral solution, inhaled or nebulized preparations Broad spectrum anti-inflammatory Repress the expression of pro-inflammatory cytokines | Not recommended [44,45] Could be considered where reactive airway disease is strongly suspected (asthma, bronchopulmonary dysplasia, etc.), but no good evidence |
| Leukotriene inhibitors (montelukast, etc.) | Oral solution Inhibit leukotrienes, which are endogenous mediators of inflammation [46] | Poor evidence, not recommended [47] |
| Manual therapies | ||
| Chest physiotherapy | Chest percussion, suction Aids in the clearance of secretions Thought to decrease ventilatory effort for infants on the severe end of the disease spectrum | Not routinely recommended [48] Can be considered when relevant comorbidities are present (neuromuscular conditions, etc.) [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatt, D.; Martin, I.; AlFouzan, R.; Moraes, T.J. Prevention and Treatment Strategies for Respiratory Syncytial Virus (RSV). Pathogens 2023, 12, 154. https://doi.org/10.3390/pathogens12020154
Gatt D, Martin I, AlFouzan R, Moraes TJ. Prevention and Treatment Strategies for Respiratory Syncytial Virus (RSV). Pathogens. 2023; 12(2):154. https://doi.org/10.3390/pathogens12020154
Chicago/Turabian StyleGatt, Dvir, Isaac Martin, Rawan AlFouzan, and Theo J. Moraes. 2023. "Prevention and Treatment Strategies for Respiratory Syncytial Virus (RSV)" Pathogens 12, no. 2: 154. https://doi.org/10.3390/pathogens12020154
APA StyleGatt, D., Martin, I., AlFouzan, R., & Moraes, T. J. (2023). Prevention and Treatment Strategies for Respiratory Syncytial Virus (RSV). Pathogens, 12(2), 154. https://doi.org/10.3390/pathogens12020154








