Antimicrobial-Loaded Polyacrylamide Hydrogels Supported on Titanium as Reservoir for Local Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Silver Nanoparticles Preparation
2.3. Polyacrylamide Hydrogels Synthesis
2.4. Antimicrobial-Loaded Polyacrylamide Hydrogel Preparation
2.4.1. AMP-Loaded Polyacrylamide Hydrogel (AMP–PAAG)
2.4.2. AgNPs (AgNPs–PAAG)-Loaded Polyacrylamide Hydrogel
2.4.3. Polyacrylamide Hydrogel with AgNPs plus AMP (AgNPs+AMP–PAAG)
2.5. Antimicrobials Release
2.5.1. Silver Quantification
2.5.2. AMP Quantification
2.6. Hydrogel Swelling Measurements
2.7. Titanium Surface Preparation
2.8. Titanium Surfaces Coated with PAAG Film (Spin Coating Technique)
2.9. Contact Angle Measurements
2.10. Spectroscopic Measurements
2.11. Atomic Force Microscopy (AFM) Imaging
2.12. Bacterial Culture
2.12.1. Kirby–Bauer Tests and Bacterial Quantification (Unsupported Hydrogels)
2.12.2. Bacterial Attachment on Modified Titanium Surfaces
Viable Sessile Bacteria
Live/Dead BacLight Bacterial Assay
2.13. Cell Culture Assays
3. Results and Discussion
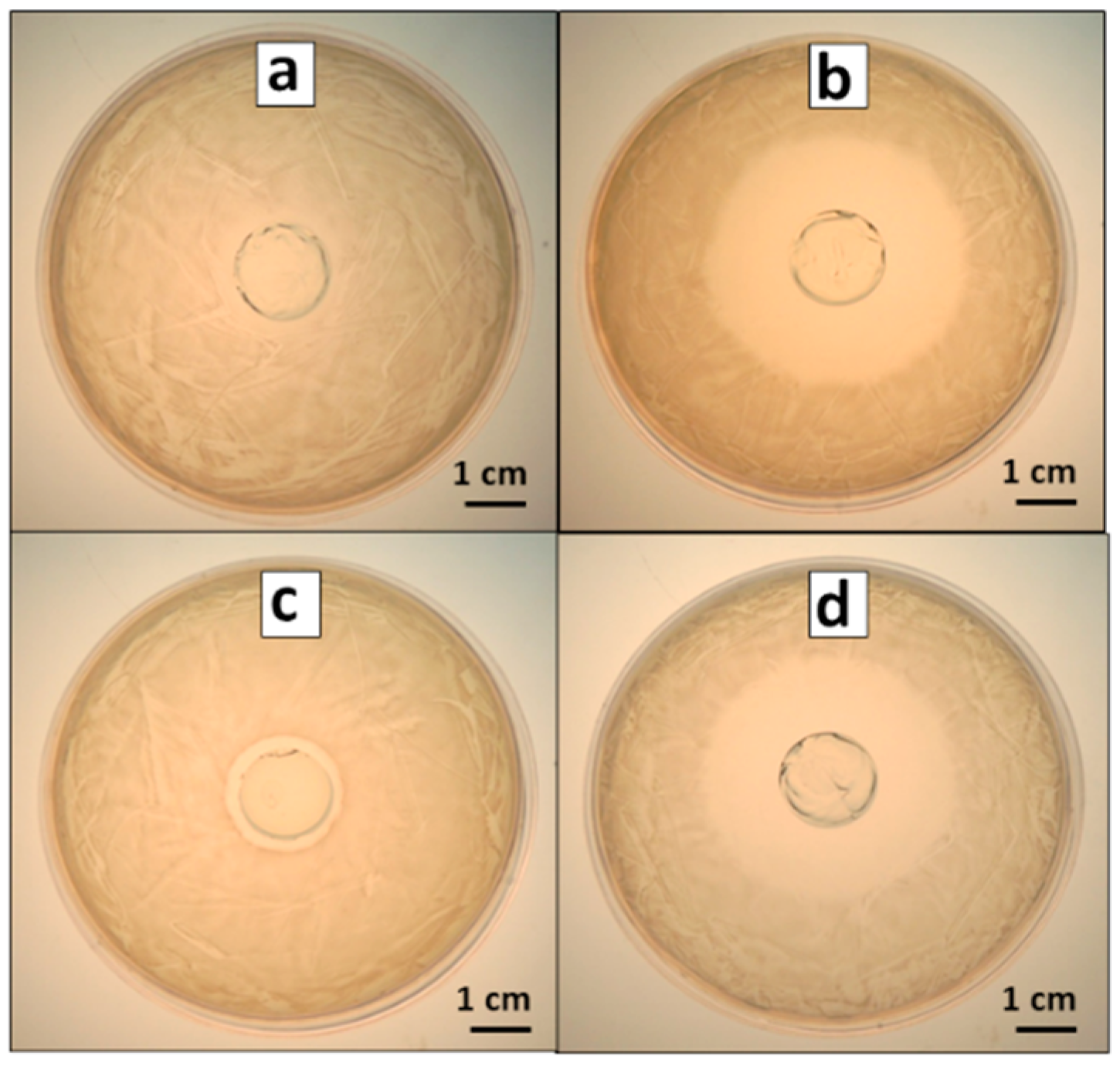
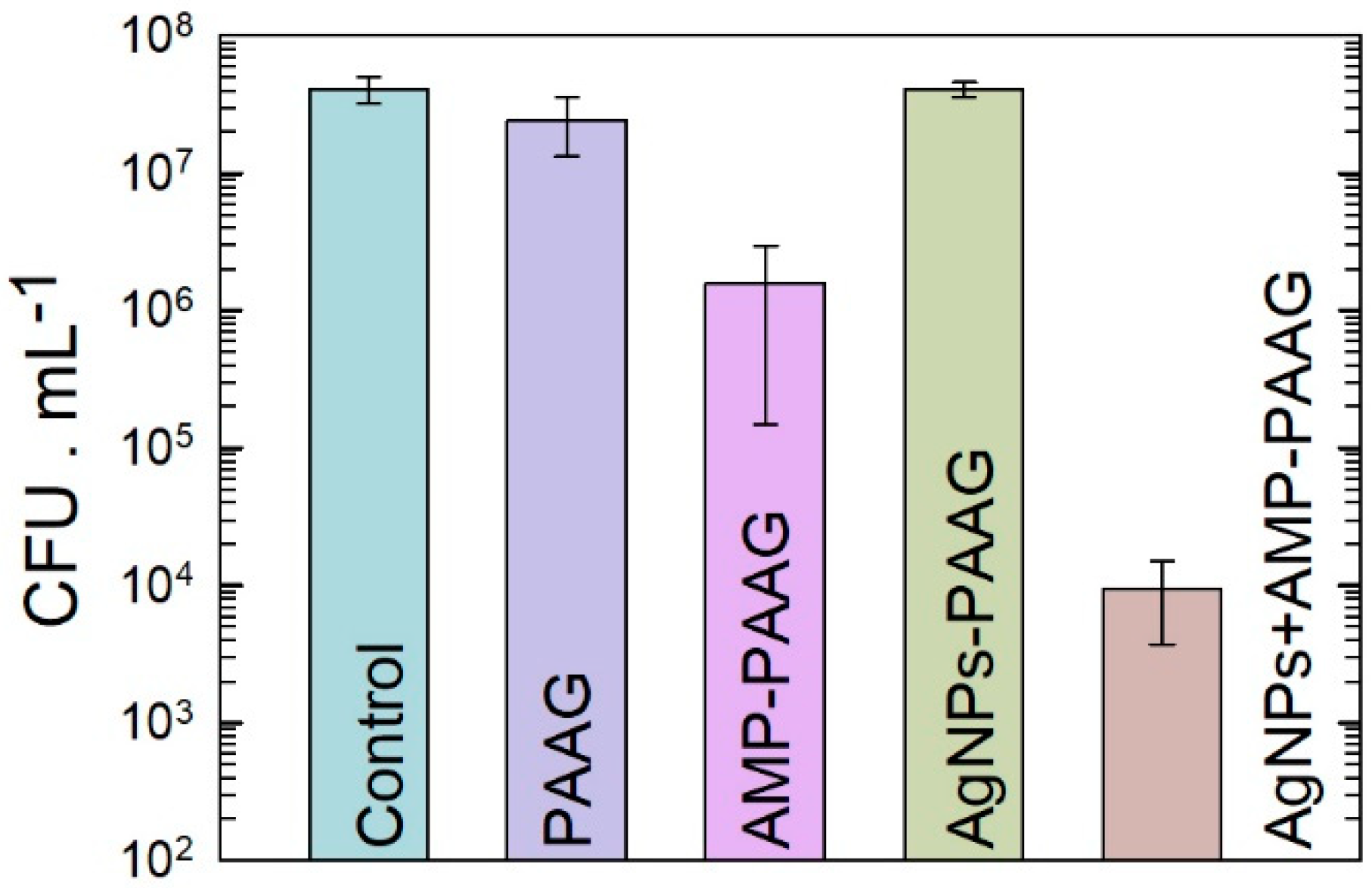
3.1. Antibacterial Effect of Antimicrobial-Loaded PAAG
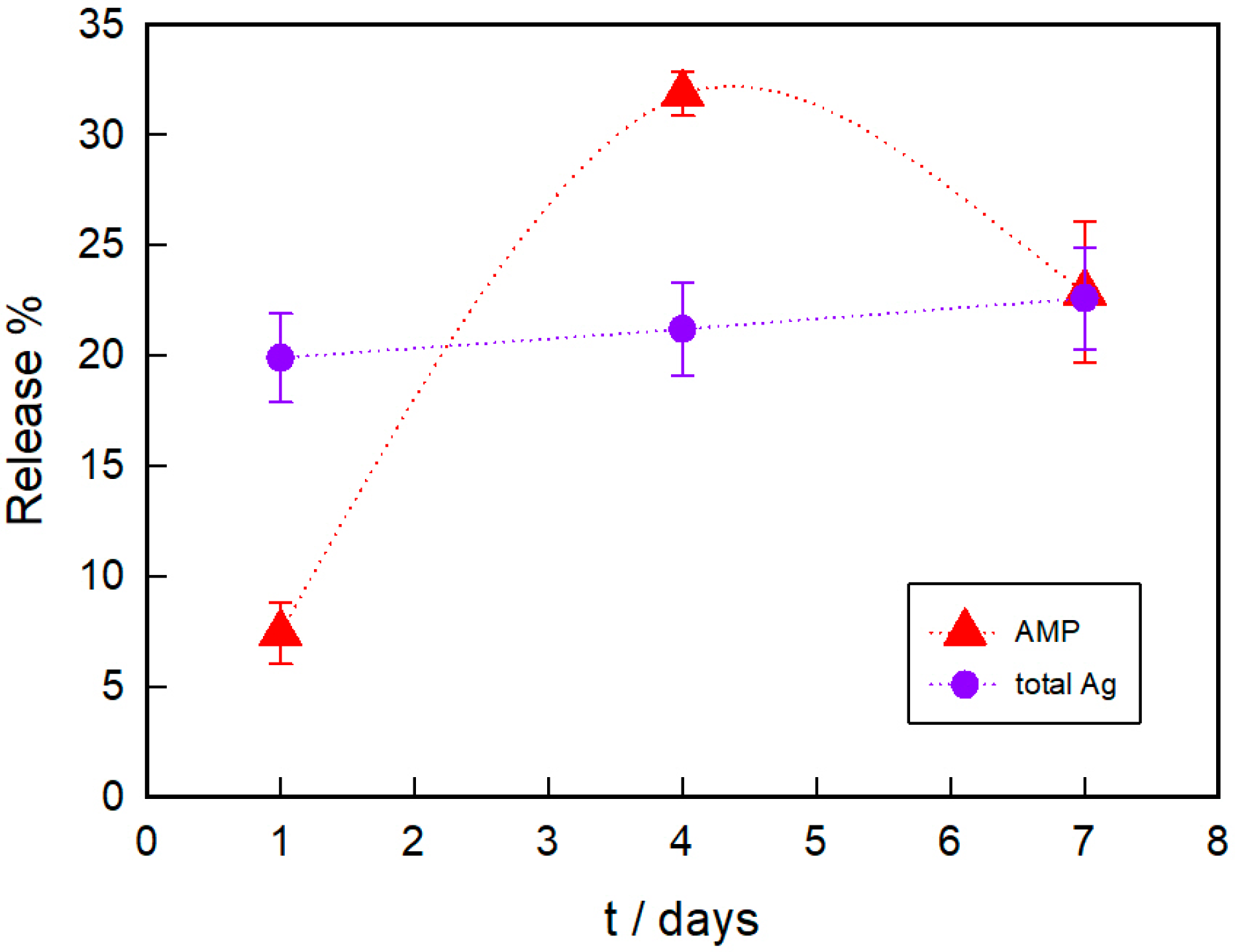
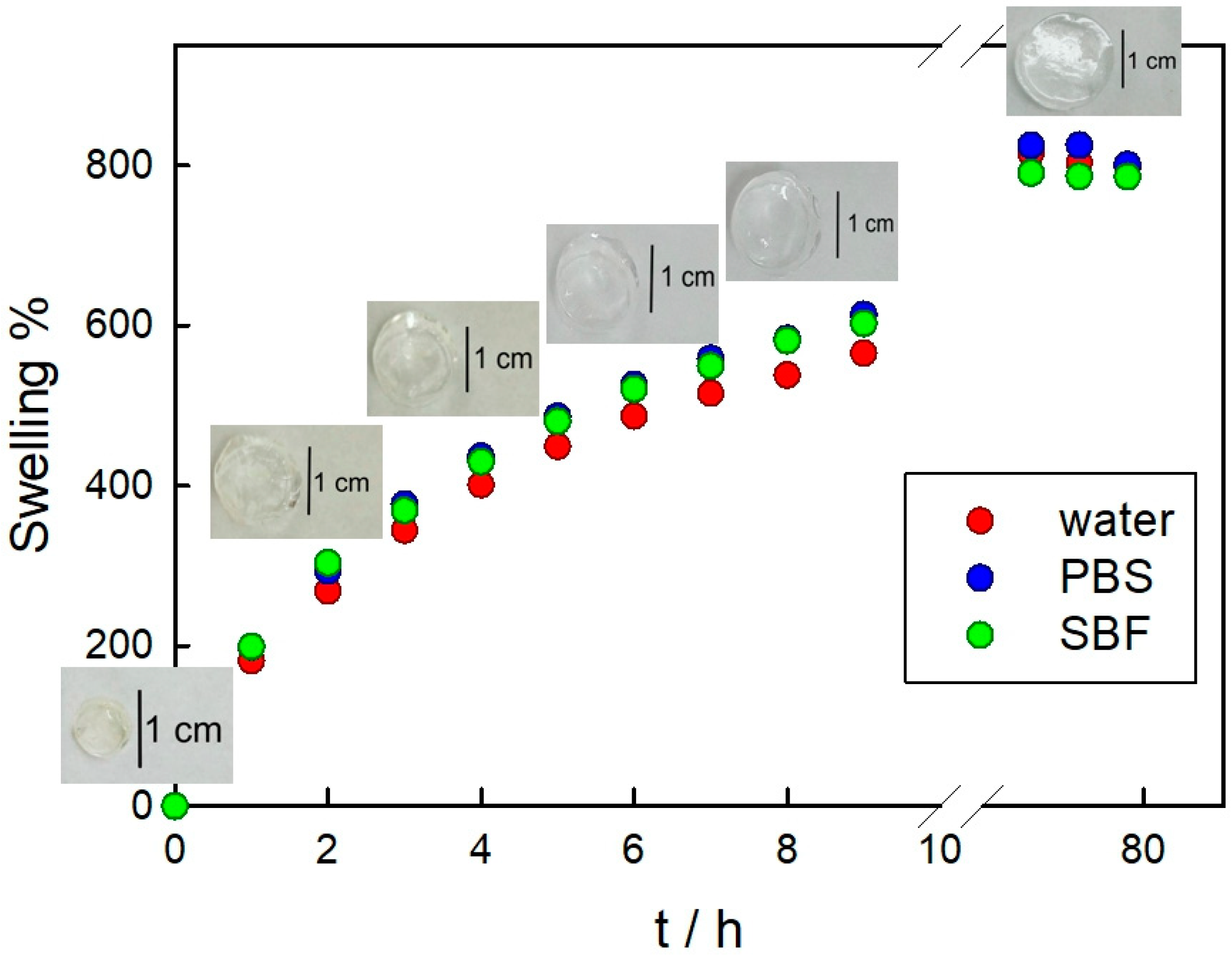
3.2. PAAG Swelling and AMP and Ag Release from AMP–PAAG and AgNPs–PAAG
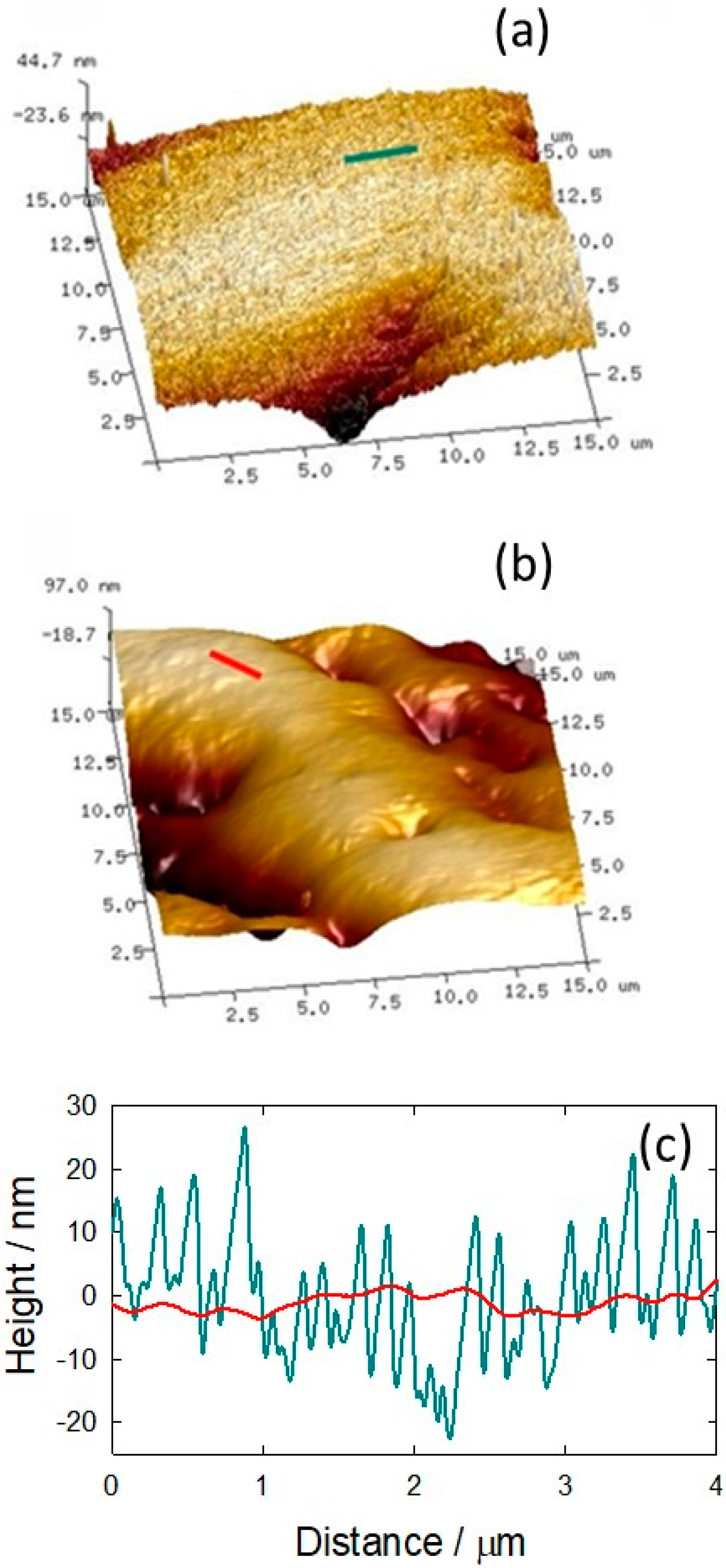
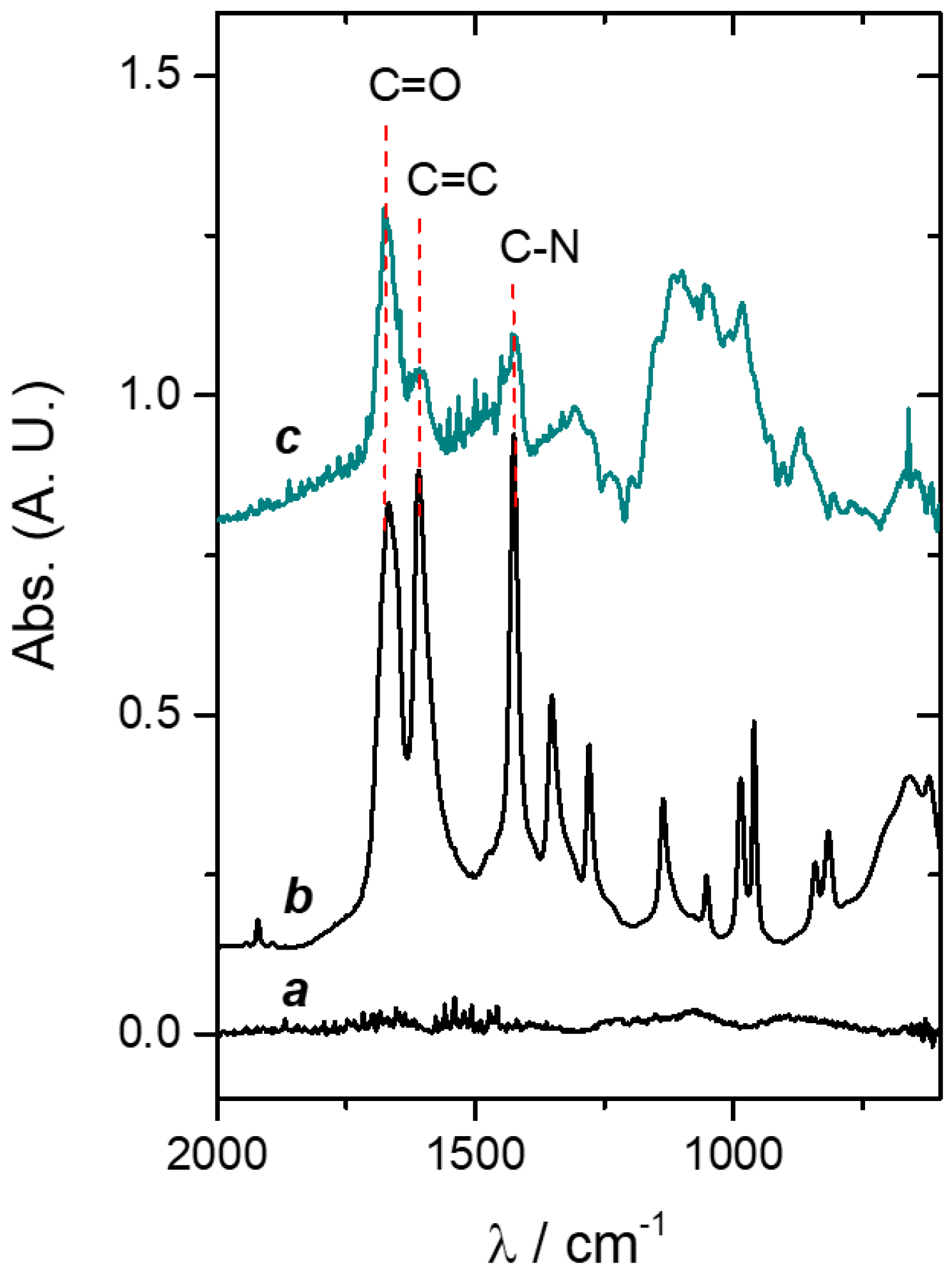
3.3. Hydrogel Film on Titanium Physicochemical Characterization
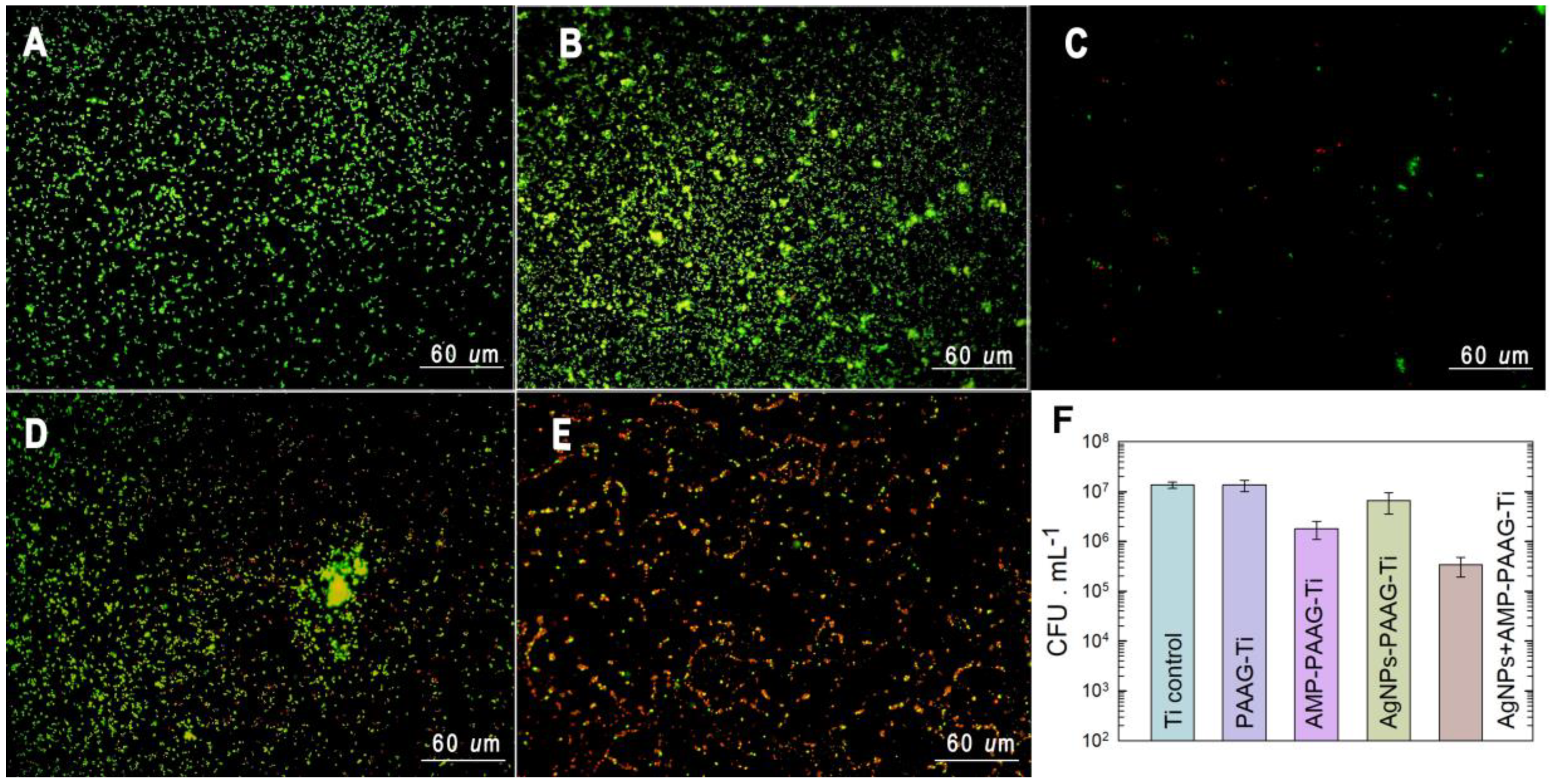
3.4. Antibacterial Effect of Functionalized Surfaces
3.5. Cytotoxicity Assays
3.5.1. Pre-Osteoblast Adhesion
3.5.2. Macrophage Adhesion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [Green Version]
- Tande, A.J.; Gomez-Urena, E.O.; Berbari, E.F.; Osmon, D.R. Management of Prosthetic Joint Infection. Infect. Dis. Clin. North Am. 2017, 31, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Urena, E.O.; Tande, A.J.; Osmon, D.R.; Berbari, E.F. Diagnosis of Prosthetic Joint Infection Prosthetic Joint Infection Arthroplasty Diagnosis. Infect. Dis. Clin. 2017, 31, 219–235. [Google Scholar] [CrossRef]
- Huotari, K.; Peltola, M.; Jämsen, E. The Incidence of Late Prosthetic Joint Infections. Acta Orthop. 2015, 86, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic Joint Infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The Role of Microbial Biofilms in Prosthetic Joint Infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Ehrlich, G.D.; Sedghizadeh, P.P.; Hall-Stoodley, L.; Baratz, M.E.; Altman, D.T.; Sotereanos, N.G.; William Costerton, J.; DeMeo, P. Orthopaedic Biofilm Infections. Curr. Orthop. Pract. 2011, 22, 558–563. [Google Scholar] [CrossRef]
- Rochford, E.T.J.; Richards, R.G.; Moriarty, T.F. Influence of Material on the Development of Device-Associated Infections The Effect of Implant Material. Clin. Microbiol. Infect. 2012, 18, 1162–1167. [Google Scholar] [CrossRef] [Green Version]
- Ghilini, F.; Pissinis, D.E.; Miñán, A.; Schilardi, P.L.; Diaz, C. How Functionalized Surfaces Can Inhibit Bacterial Adhesion and Viability. ACS Biomater. Sci. Eng. 2019, 5, 4920–4936. [Google Scholar] [CrossRef]
- Truong, V.K.; Pham, V.T.H.; Medvedev, A.; Lapovok, R.; Estrin, Y.; Lowe, T.C.; Baulin, V.; Boshkovikj, V.; Fluke, C.J.; Crawford, R.J.; et al. Self-Organised Nanoarchitecture of Titanium Surfaces Influences the Attachment of Staphylococcus Aureus and Pseudomonas Aeruginosa Bacteria. Appl. Microbiol. Biotechnol. 2015, 99, 6831–6840. [Google Scholar] [CrossRef]
- Vishnu, J.; Manivasagam, V.K.; Gopal, V.; Bartomeu Garcia, C.; Hameed, P.; Manivasagam, G.; Webster, T.J. Hydrothermal Treatment of Etched Titanium: A Potential Surface Nano-Modification Technique for Enhanced Biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102016. [Google Scholar] [CrossRef]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Pogodin, S.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; Crawford, R.J.; Ivanova, E.P. Selective Bactericidal Activity of Nanopatterned Superhydrophobic Cicada Psaltoda Claripennis Wing Surfaces. Appl. Microbiol. Biotechnol. 2012, 97, 9257–9262. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Li, X.; Zhao, Y.; Ren, L.; Yuan, X. Antifogging/Antibacterial Coatings Constructed by N-Hydroxyethylacrylamide and Quaternary Ammonium-Containing Copolymers. ACS Appl. Mater. Interfaces 2020, 12, 12305–12316. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Chaabane, A.; Bleicher, V.; Rau, S.; Al-Ahmad, A.; Lienkamp, K. Stimulus-Responsive Polyzwitterionic Surfaces Made from Itaconic Acid: Self-Triggered Antimicrobial Activity, Protein Repellency, and Cell Compatibility. ACS Appl. Mater. Interfaces 2020, 12, 21242–21253. [Google Scholar] [CrossRef] [PubMed]
- Poverenov, E.; Shemesh, M.; Gulino, A.; Cristaldi, D.A.; Zakin, V.; Yefremov, T.; Granit, R. Durable Contact Active Antimicrobial Materials Formed by a One-Step Covalent Modification of Polyvinyl Alcohol, Cellulose and Glass Surfaces. Colloids Surf. B. Biointerfaces 2013, 112, 356–361. [Google Scholar] [CrossRef]
- Ji, M.K.; Oh, G.; Kim, J.W.; Park, S.; Yun, K.D.; Bae, J.C.; Lim, H.P. Effects on Antibacterial Activity and Osteoblast Viability of Non-Thermal Atmospheric Pressure Plasma and Heat Treatments of TiO2 Nanotubes. J. Nanosci. Nanotechnol. 2017, 17, 2312–2315. [Google Scholar] [CrossRef] [PubMed]
- Gerits, E.; Kucharíková, S.; Van Dijck, P.; Erdtmann, M.; Krona, A.; Lövenklev, M.; Fröhlich, M.; Dovgan, B.; Impellizzeri, F.; Braem, A.; et al. Antibacterial Activity of a New Broad-Spectrum Antibiotic Covalently Bound to Titanium Surfaces. J. Orthop. Res. 2016, 34, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Kazek-Kęsik, A.; Nosol, A.; Płonka, J.; Śmiga-Matuszowicz, M.; Gołda-Cępa, M.; Krok-Borkowicz, M.; Brzychczy-Włoch, M.; Pamuła, E.; Simka, W. PLGA-Amoxicillin-Loaded Layer Formed on Anodized Ti Alloy as a Hybrid Material for Dental Implant Applications. Mater. Sci. Eng. C 2019, 94, 998–1008. [Google Scholar] [CrossRef]
- Ghimire, A.; Skelly, J.D.; Song, J. Micrococcal-Nuclease-Triggered On-Demand Release of Vancomycin from Intramedullary Implant Coating Eradicates Staphylococcus Aureus Infection in Mouse Femoral Canals. ACS Cent. Sci. 2019, 5, 1929–1936. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 5 January 2023).
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Duran, N.; Duran, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Trivedi, J.S.; Kumar, S.B.; Chandel, A.K.S.; Haldar, S.; Jewrajka, S.K. Anti-Organic Fouling and Anti-Biofouling Poly(Piperazineamide) Thin Film Nanocomposite Membranes for Low Pressure Removal of Heavy Metal Ions. J. Hazard. Mater. 2018, 343, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Mitsuhashi, K.; Chandel, A.K.S.; Qi, P.; Nakamura, N.; Nakamichi, A.; Yoshida, H.; Yamaguchi, G.; Hara, Y.; Sasaki, R.; et al. Silver-Loaded Carboxymethyl Cellulose Nonwoven Sheet with Controlled Counterions for Infected Wound Healing. Carbohydr. Polym. 2022, 286, 119289. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.Y.; Diaz, C.; Rubert, A.; Benítez, G.A.; Moreno, M.S.; de Mele, M.A.F.L.; Salvarezza, R.C.; Schilardi, P.L.; Vericat, C. Spontaneous Adsorption of Silver Nanoparticles on Ti/TiO2 Surfaces. Antibacterial Effect on Pseudomonas Aeruginosa. J. Colloid Interface Sci. 2010, 350, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Qian, S.; Guo, G.; Wang, Q.; Tang, J.; Shen, H.; Liu, X.; Zhang, X.; Chu, P.K. Antibacterial Surface Design of Titanium-Based Biomaterials for Enhanced Bacteria-Killing and Cell-Assisting Functions Against Periprosthetic Joint Infection. ACS Appl. Mater. Interfaces 2016, 8, 11162–11178. [Google Scholar] [CrossRef] [PubMed]
- Ghilini, F.; Rodriguez Gonzalez, M.C.; Miñán, A.G.; Pissinis, D.E.; Creus, A.H.; Salvarezza, R.C.; Schilardi, P.L. Highly-Stabilized Nanoparticles on Poly-L-Lysine-Coated Oxidized Metals: A Versatile Platform with Enhanced Antimicrobial Activity. ACS Appl. Mater. Interfaces 2018, 10, 23657–23666. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.; Valkov, A.; Kossenko, A.; Wolicki, I.; Zinigrad, M.; Borodianskiy, K. Bioactive Coating on Ti Alloy with High Osseointegration and Antibacterial Ag Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39534–39544. [Google Scholar] [CrossRef]
- Pissinis, D.E.; Benítez, G.A.; Schilardi, P.L. Two-Step Biocompatible Surface Functionalization for Two-Pathway Antimicrobial Action against Gram-Positive Bacteria. Colloids Surf. B Biointerfaces 2018, 164, 262–271. [Google Scholar] [CrossRef]
- Brown, A.N.; Smith, K.; Samuels, T.A.; Lu, J.; Obare, S.O.; Scott, M.E. Nanoparticles Functionalized with Ampicillin Destroy Multiple-Antibiotic-Resistant Isolates of Pseudomonas Aeruginosa and Enterobacter Aerogenes and Methicillin-Resistant Staphylococcus Aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar] [CrossRef]
- Martínez-Higuera, A.; Rodríguez-Beas, C.; Villalobos-Noriega, J.M.A.; Arizmendi-Grijalva, A.; Ochoa-Sánchez, C.; Larios-Rodríguez, E.; Martínez-Soto, J.M.; Rodríguez-León, E.; Ibarra-Zazueta, C.; Mora-Monroy, R.; et al. Hydrogel with Silver Nanoparticles Synthesized by Mimosa Tenuiflora for Second-Degree Burns Treatment. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Effect of Polyethylene Glycol on Properties and Drug Encapsulation-Release Performance of Biodegradable/Cytocompatible Agarose-Polyethylene Glycol-Polycaprolactone Amphiphilic Co-Network Gels. ACS Appl. Mater. Interfaces 2016, 8, 3182–3192. [Google Scholar] [CrossRef] [PubMed]
- Santhamoorthy, M.; Vy Phan, T.T.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.C. Thermo-Sensitive Poly (N-Isopropylacrylamide-Co-Polyacrylamide) Hydrogel for PH-Responsive Therapeutic Delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Han, X.; Zou, D.; Luo, X.; Liu, L.; Wang, J.; Maitz, M.F.; Yang, P.; Huang, N.; Zhao, A. Catechol-Chitosan/Polyacrylamide Hydrogel Wound Dressing for Regulating Local Inflammation. Mater. Today Bio 2022, 16, 100392. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Narula, P. Role of Molecularly Imprinted Hydrogels in Drug Delivery—A Current Perspective. Int. J. Pharm. 2022, 625, 121883. [Google Scholar] [CrossRef]
- Kumai, J.; Sasagawa, S.; Horie, M.; Yui, Y. A Novel Method for Polyacrylamide Gel Preparation Using N-Hydroxysuccinimide-Acrylamide Ester to Study Cell-Extracellular Matrix Mechanical Interactions. Front. Mater. 2021, 8, 20. [Google Scholar] [CrossRef]
- Samavedi, S.; Poindexter, L.K.; Van Dyke, M.; Goldstein, A.S. Synthetic Biomaterials for Regenerative Medicine Applications. In Regenerative Medicine Applications in Organ Transplantation; Elsevier: Amsterdam, The Netherlands, 2014; pp. 81–99. [Google Scholar] [CrossRef]
- Charrier, E.E.; Pogoda, K.; Li, R.; Park, C.Y.; Fredberg, J.J.; Janmey, P.A. A Novel Method to Make Viscoelastic Polyacrylamide Gels for Cell Culture and Traction Force Microscopy. APL Bioeng. 2020, 4, 036104. [Google Scholar] [CrossRef]
- Yamauchi, P.S. Emerging Permanent Filler Technologies: Focus on Aquamid. Clin. Cosmet. Investig. Dermatol. 2014, 7, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahabudeen, H.; Machatschek, R.; Lendlein, A. Multifunctionality as Design Principle for Contact Lens Materials. Multifunct. Mater. 2021, 4, 042001. [Google Scholar] [CrossRef]
- Bal, T.; Swain, S. Microwave Assisted Synthesis of Polyacrylamide Grafted Polymeric Blend of Fenugreek Seed Mucilage-Polyvinyl Alcohol (FSM-PVA-g-PAM) and Its Characterizations as Tissue Engineered Scaffold and as a Drug Delivery Device. DARU J. Pharm. Sci. 2020, 28, 33. [Google Scholar] [CrossRef]
- Wang, Y.; Nian, G.; Kim, J.; Suo, Z. Polyacrylamide Hydrogels. VI. Synthesis-Property Relation. J. Mech. Phys. Solids 2023, 170, 105099. [Google Scholar] [CrossRef]
- Frank, A.J.; Cathcart, N.; Maly, K.E.; Kitaev, V. Synthesis of Silver Nanoprisms with Variable Size and Investigation of Their Optical Properties: A First-Year Undergraduate Experiment Exploring Plasmonic Nanoparticles. J. Chem. Educ. 2010, 87, 1098–1101. [Google Scholar] [CrossRef]
- Das, A.L.; Mukherjee, R.; Katiyer, V.; Kulkarni, M.; Ghatak, A.; Sharma, A. Generation of Sub-Micrometer-Scale Patterns by Successive Miniaturization Using Hydrogels. Adv. Mater. 2007, 19, 1943–1946. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. Simulated Body Fluid (SBF) as a Standard Tool to Test the Bioactivity of Implants. In Handbook of Biomineralization; Bäuerlein, E., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 97–109. [Google Scholar]
- Díaz, C.; Miñán, A.; Schilardi, P.L.; de Mele, M.F.L. Synergistic Antimicrobial Effect against Early Biofilm Formation: Micropatterned Surface plus Antibiotic Treatment. Int. J. Antimicrob. Agents 2012, 40, 221–226. [Google Scholar] [CrossRef]
- Flores, C.Y.; Miñán, A.G.; Grillo, C.A.; Salvarezza, R.C.; Vericat, C.; Schilardi, P.L. Citrate-Capped Silver Nanoparticles Showing Good Bactericidal Effect against Both Planktonic and Sessile Bacteria and a Low Cytotoxicity to Osteoblastic Cells. ACS Appl. Mater. Interfaces 2013, 5, 3149–3159. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of Silver(I) Ions with the Respiratory Chain of Escherichia Coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia Coli and Staphylococcus Aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Dong, F.; Mohd Zaidi, N.F.; Valsami-Jones, E.; Kreft, J.-U. Time-Resolved Toxicity Study Reveals the Dynamic Interactions between Uncoated Silver Nanoparticles and Bacteria. Nanotoxicology 2017, 11, 637–646. [Google Scholar] [CrossRef]
- Kang, M.A.; Kang, J.-S.; Kang, M.A.; Kang, J.-S. Stability Test of Ampicillin Sodium Solutions in the Accufuser® Elastomeric Infusion Device Using HPLC: UV Method. Pharmacol. Pharm. 2012, 3, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Penkavova, V.; Spalova, A.; Tomas, J.; Tihon, J. Polyacrylamide Hydrogels Prepared by Varying Water Content during Polymerization: Material Characterization, Reswelling Ability, and Aging Resistance. Polym. Eng. Sci. 2022, 62, 901–916. [Google Scholar] [CrossRef]
- Sivanantham, M.; Tata, B.V.R. Swelling/Deswelling of Polyacrylamide Gels in Aqueous NaCl Solution: Light Scattering and Macroscopic Swelling Study. Pramana J. Phys. 2012, 79, 457–469. [Google Scholar] [CrossRef]
- van Hardeveld, R.M.; Gunter, P.L.J.; van IJzendoorn, L.J.; Wieldraaijer, W.; Kuipers, E.W.; Niemantsverdriet, J.W. Deposition of Inorganic Salts from Solution on Flat Substrates by Spin-Coating: Theory, Quantification and Application to Model Catalysts. Appl. Surf. Sci. 1995, 84, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Chou, K.S.; Huang, K.C.; Lee, H.H. Fabrication and Sintering Effect on the Morphologies and Conductivity of Nano-Ag Particle Films by the Spin Coating Method. Nanotechnology 2005, 16, 779–784. [Google Scholar] [CrossRef]
- Grigorov, K.G.; Oliveira, I.C.; MacIel, H.S.; Massi, M.; Oliveira, M.S.; Amorim, J.; Cunha, C.A. Optical and Morphological Properties of N-Doped TiO2thin Films. Surf. Sci. 2011, 605, 775–782. [Google Scholar] [CrossRef]
- He, B.; Patankar, N.A.; Lee, J. Multiple Equilibrium Droplet Shapes and Design Criterion for Rough Hydrophobic Surfaces. Langmuir 2003, 19, 4999–5003. [Google Scholar] [CrossRef]
- Patankar, N.A. On the Modeling of Hydrophobic Contact Angles on Rough Surfaces On the Modeling of Hydrophobic Contact Angles on Rough. Society 2003, 19, 1249–1253. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, X.; Feng, X.; Ma, Y.; Zou, H. Fabrication of Super-Hydrophobic Film from PMMA with Intrinsic Water Contact Angle below 90°. Polymer 2007, 48, 7455–7460. [Google Scholar] [CrossRef]
- Murugan, R.; Mohan, S.; Bigotto, A. FTIR and Polarized Raman Spectra of Acrylamide and Polyacrylamide. J. Kor. Phys. Soc. 1998, 32, 505–512. [Google Scholar]
- Ullah, R.; Khan, S.U.D.; Aamir, M.; Ullah, R. Terahertz Time Domain, Raman and Fourier Transform Infrared Spectroscopy of Acrylamide, and the Application of Density Functional Theory. J. Spectrosc. 2013, 1, 148903. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Che, L.; Zheng, J.; Huang, G.; Wu, X.; Chen, P.; Zhang, L.; Hu, Q. Synthesis and Thermal Degradation Property Study of N-Vinylpyrrolidone and Acrylamide Copolymer. RSC Adv. 2014, 4, 33269–33278. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, 25247. [Google Scholar] [CrossRef] [PubMed]
- Beadle, B.M.; Nicholas, R.A.; Shoichet, B.K. Interaction energies between β-lactam antibiotics and E. coli penicillin-binding protein 5 by reversible thermal denaturation. Protein Sci. 2001, 10, 1254–1259. [Google Scholar] [CrossRef]
- Cho, H.; Uehara, T.; Bernhardt, T.G. Beta-Lactam Antibiotics Induce a Lethal Malfunctioning of the Bacterial Cell Wall Synthesis Machinery. Cell 2014, 159, 1310–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Shao, D.; Wang, P.; Chen, D.; Zhang, Y.; Li, M.; Zhao, J.; Zhou, Y. Enhanced Osteoblast Adhesion on Amino- Functionalized Titanium Surfaces through Combined Plasma Enhanced Chemical Vapor Deposition. RSC Adv. 2016, 6, 82688–82697. [Google Scholar] [CrossRef]
- Li, Y.; Wong, C.; Xiong, J.; Hodgson, P.; Wen, C. Cytotoxicity of Titanium and Titanium Alloying Elements. J. Dent. Res. 2010, 89, 493–497. [Google Scholar] [CrossRef]
- Ghilini, F.; Fagali, N.; Pissinis, D.E.; Benítez, G.; Schilardi, P.L. Multifunctional Titanium Surfaces for Orthopedic Implants: Antimicrobial Activity and Enhanced Osseointegration. ACS Appl. Bio Mater. 2021, 4, 6451–6461. [Google Scholar] [CrossRef]
- Zinger, O.; Anselme, K.; Denzer, A.; Habersetzer, P.; Wieland, M.; Jeanfils, J.; Hardouin, P.; Landolt, D. Time-Dependent Morphology and Adhesion of Osteoblastic Cells on Titanium Model Surfaces Featuring Scale-Resolved Topography. Biomaterials 2004, 25, 2695–2711. [Google Scholar] [CrossRef]
- Nardo, T.; Carmagnola, I.; Ruini, F.; Caddeo, S.; Calzone, S.; Chiono, V.; Ciardelli, G. Synthetic Biomaterial for Regenerative Medicine Applications. In Kidney Transplantation in the Regenerative Medicine Era; Academic Press: Cambridge, MA, USA, 2017; pp. 901–921. [Google Scholar] [CrossRef]
- Zahedi, E.; Ansari, S.; Wu, B.M.; Bencharit, S.; Moshaverinia, A. Hydrogels in craniofacial tissue engineering. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 47–64. [Google Scholar] [CrossRef]
- Damle, A.; Sundaresan, R.; Rajwade, J.M.; Srivastava, P.; Naik, A. A Concise Review on Implications of Silver Nanoparticles in Bone Tissue Engineering. Biomater. Adv. 2022, 141, 213099. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Triffitt, J.T. A Review on Macrophage Responses to Biomaterials. Biomed. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sille, I.E.; Pissinis, D.E.; Fagali, N.S.; Ghilini, F.; Urrutia, M.N.; Schilardi, P.L. Antimicrobial-Loaded Polyacrylamide Hydrogels Supported on Titanium as Reservoir for Local Drug Delivery. Pathogens 2023, 12, 202. https://doi.org/10.3390/pathogens12020202
Sille IE, Pissinis DE, Fagali NS, Ghilini F, Urrutia MN, Schilardi PL. Antimicrobial-Loaded Polyacrylamide Hydrogels Supported on Titanium as Reservoir for Local Drug Delivery. Pathogens. 2023; 12(2):202. https://doi.org/10.3390/pathogens12020202
Chicago/Turabian StyleSille, Irene E., Diego E. Pissinis, Natalia S. Fagali, Fiorela Ghilini, María Noel Urrutia, and Patricia L. Schilardi. 2023. "Antimicrobial-Loaded Polyacrylamide Hydrogels Supported on Titanium as Reservoir for Local Drug Delivery" Pathogens 12, no. 2: 202. https://doi.org/10.3390/pathogens12020202




