Unusual Observations in Leishmaniasis—An Overview
Abstract
:1. Introduction
Leishmaniasis: Typical Manifestations and Leishmania spp.
2. Atypical Presentations of Leishmaniasis
2.1. Visceral Leishmaniasis
2.1.1. Immunocompromised Cases with Unusual Presentation of VL
2.1.2. Unusual Presentation in Immuno-Competent Individuals
2.1.3. Leishman Donovan Bodies (LDBs) Localized in Unusual Body Parts
2.1.4. Miscellaneous Atypical Manifestations of VL
2.2. Post-Kala-Azar Dermal Leishmaniasis
Atypical Presentations of PKDL
2.3. Cutaneous Leishmaniasis
2.3.1. Unusual Presentations Caused by Atypical Species
2.3.2. Unusual Sites and Number of Lesions
2.3.3. Unusual/Atypical Morphology/Characteristics
2.4. Mucocutaneous Leishmaniasis (MCL)
2.4.1. Unusual/Atypical Variants of Causative Species
2.4.2. Unusual Sites/Morphology/Characteristics

3. Coinfection and Unusual Presentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Fourteenth Meeting of the Strategic and Technical Advisory Group for Neglected Tropical Diseases, 22–24 June 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Dey, A.; Singh, S. Transfusion transmitted leishmaniasis: A case report and review of literature. Indian J. Med. Microbiol. 2006, 24, 165–170. [Google Scholar] [CrossRef]
- Meinecke, C.K.; Schottelius, J.; Oskam, L.; Fleischer, B. Congenital transmission of visceral leishmaniasis (Kala Azar) from an asymptomatic mother to her child. Pediatrics 1999, 104, e65. [Google Scholar] [CrossRef] [PubMed]
- Amela, C.; López-Gay, D.; Alberdig, J.; Castilla, J. Injecting drug use as risk factor for visceral leishmaniasis in AIDS patients. Eur. J. Epidemiol. 1996, 12, 91–92. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Leishmaniasis Factsheet; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Abadias-Granado, I.; Diago, A.; Cerro, P.A.; Palma-Ruiz, A.M.; Gilaberte, Y. Cutaneous and Mucocutaneous Leishmaniasis. Actas Dermosifiliogr. (Engl. Ed.) 2021, 6, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, O.A.; Serrano, M.G.; Camargo, E.P.; Teixeira, M.M.G.; Shaw, J.J. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology 2018, 145, 430–442. [Google Scholar] [CrossRef]
- Dostálová, A.; Volf, P. Leishmania development in sand flies: Parasite-vector interactions overview. Parasites Vectors 2012, 5, 276. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Leishmaniasis. Available online: https://www.cdc.gov/dpdx/leishmaniasis/index.html (accessed on 6 May 2022).
- Shin, J.Y.; Lee, Y.B.; Cho, B.K.; Park, H.J. New world cutaneous leishmaniasis treated with intralesional injection of pentavalent antimony. Ann. Dermatol. 2013, 25, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.Y.M.; Dewan, A.; Shogib, M.R.I.; Rahman, M.M.; Hossain, M.F. Environmental factors associated with the distribution of visceral leishmaniasis in endemic areas of Bangladesh: Modeling the ecological niche. Trop. Med. Health 2017, 45, 13. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host–pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, S.R.; Samaranayake, N.; Kariyawasam, U.L.; Siriwardana, Y.D.; Imamura, H.; Karunaweera, N.D. Genomic insights into virulence mechanisms of Leishmania donovani: Evidence from an atypical strain. BMC Genom. 2018, 19, 843. [Google Scholar] [CrossRef]
- Lipoldová, M.; Demant, P. Genetic susceptibility to infectious disease: Lessons from mouse models of leishmaniasis. Nat. Rev. Genet. 2006, 7, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Srivarasat, S.; Brownell, N.; Siriyasatien, P.; Noppakun, N.; Asawanonda, P.; Rattanakorn, K.; Preativatanyou, K.; Kumtornrut, C. Case Report: Autochthonous Disseminated Cutaneous, Mucocutaneous, and Visceral Leishmaniasis Caused by Leishmania martiniquensis in a Patient with HIV/AIDS from Northern Thailand and Literature Review. Am. J. Trop. Med. Hyg. 2022, 107, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Weiss, F.; Vogenthaler, N.; Franco-Paredes, C.; Parker, S.R. Leishmania tropica–induced cutaneous and presumptive concomitant viscerotropic Leishmaniasis with prolonged incubation. Arch. Dermatol. 2009, 145, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- McCall, L.-I.; Zhang, W.-W.; Matlashewski, G. Determinants for the development of visceral leishmaniasis disease. PLoS Pathog. 2013, 9, e1003053. [Google Scholar] [CrossRef]
- Lypaczewski, P.; Matlashewski, G. Leishmania donovani hybridisation and introgression in nature: A comparative genomic investigation. Lancet Microbe 2021, 2, e250–e258. [Google Scholar] [CrossRef]
- van Griensven, J.; Carrillo, E.; López-Vélez, R.; Lynen, L.; Moreno, J. Leishmaniasis in immunosuppressed individuals. Clin. Microbiol. Infect. 2014, 20, 286–299. [Google Scholar] [CrossRef]
- Kumar, R.; Nylén, S. Immunobiology of visceral leishmaniasis. Front. Immunol. 2012, 3, 251. [Google Scholar] [CrossRef]
- Ghazzawi, Y.; Absah, I. Visceral Leishmania as Unusual Cause of Splenic Peliosis in the United States. ACG Case Rep. J. 2013, 1, 61. [Google Scholar] [CrossRef]
- Trück, J.; Rampton, C.; Kelly, S.J.; Kelly, D. Visceral leishmaniasis in an infant following a holiday trip to Spain. Case Rep. 2015, 2015, bcr2015209484. [Google Scholar] [CrossRef]
- Rosenthal, E.; Marty, P.; del Giudice, P.; Pradier, C.; Ceppi, C.; Gastaut, J.-A.; Le Fichoux, Y.; Cassuto, J.-P. HIV and Leishmania coinfection: A review of 91 cases with focus on atypical locations of Leishmania. Clin. Infect. Dis. 2000, 31, 1093–1095. [Google Scholar] [CrossRef]
- Jawhar, N.M. Visceral leishmaniasis with an unusual presentation in an HIV positive patient. Sultan Qaboos Univ. Med. J. 2011, 11, 269. [Google Scholar] [PubMed]
- Agarwal, P.; Kumar, V.; Kaushal, M.; Kumari, M.; Chaudhary, A. Indian visceral leishmaniasis with extensive lymphadenopathy–An unusual presentation: A case report with literature review. Cytojournal 2017, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Pintado, V.; Martin-Rabadan, P.; Rivera, M.L.; Moreno, S.; Bouza, E. Visceral leishmaniasis in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients. A comparative study. Medicine 2001, 80, 54–73. [Google Scholar] [CrossRef] [PubMed]
- Prestes-Carneiro, L.E.; Spir, P.R.N.; Fontanesi, M.; Pereira Garcia, K.G.; Silva, F.A.D.; Flores, E.F.; Vasconcelos, D.M. Unusual manifestations of visceral leishmaniasis in children: A case series and its spatial dispersion in the western region of Sao Paulo state, Brazil. BMC Infect. Dis. 2019, 19, 70. [Google Scholar] [CrossRef]
- Ortiz, M.; Mon, C.; Herrero, J.C.; Oliet, A.; Rodriguez, I.; Ortega, O.; Gallar, P.; Hinostroza, J.; Cobo, G.; del Alamo, M.; et al. Glomerulonephritis and cryoglobulinemia: First manifestation of visceral leishmaniasis. Clin. Nephrol. 2015, 83, 370–377. [Google Scholar] [CrossRef]
- Cenderello, G.; Pontali, E.; Ruggeri, C.; Dusi, A.; De Maria, A. Unusual presentation of visceral leishmaniasis in an HIV-infected patient. AIDS Res. Hum. Retrovir. 2014, 30, 846–847. [Google Scholar] [CrossRef]
- Carnaúba Jr, D.; Konishi, C.T.; Petri, V.; Martinez, I.C.P.; Shimizu, L.; Pereira-Chioccola, V.L. Atypical disseminated leishmaniasis similar to post-kala-azar dermal leishmaniasis in a Brazilian AIDS patient infected with Leishmania (Leishmania) infantum chagasi: A case report. Int. J. Infect. Dis. 2009, 13, e504–e507. [Google Scholar] [CrossRef]
- Dolin, R.; Bennett, J.E.; Mandell, G.L. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005; pp. 1727–3662. [Google Scholar]
- Hicks, L.; Kant, P.; Tay, P.H.; Vincini, V.; Schuster, H.; Rotimi, O.; Maughan, N.; Jordan, C.; Moss, S.; Everett, S.; et al. Visceral Leishmaniasis presenting with intestinal failure: A case report and literature review. Eur J. Gastroenterol. Hepatol. 2009, 21, 117–122. [Google Scholar] [CrossRef]
- Alvarez-Nebreda, M.L.; Alvarez-Fernandez, E.; Rada, S.; Branas, F.; Maranon, E.; Vidan, M.T.; Serra-Rexach, J.A. Unusual duodenal presentation of leishmaniasis. J. Clin. Pathol. 2005, 58, 1321–1322. [Google Scholar] [CrossRef]
- Gibson, G.M.; Arnold, C.; Ravi Kumar, A.S. 18F-FDG uptake in multiple splenic foci on PET/CT: An unusual case of visceral leishmaniasis. Clin. Nucl. Med. 2014, 39, 828–830. [Google Scholar] [CrossRef]
- Brandonisio, O.; Fumarola, L.; Spinelli, R.; Gradoni, L. Unusual presentation of leishmaniasis as an adrenal cystic mass. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 682–683. [Google Scholar] [CrossRef] [PubMed]
- Cermano, J.R.; Caraballo, A.J.; Gonzalez, J. Acalculous cholecystitis in a patient with visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.-J.; Juliă, J.; Martinez-Lacasa, J.; Garau, J. Clinical microbiological case: Esophageal lesion in an AIDS patient. Clin. Microbiol. Infect. 2003, 9, 463–466. [Google Scholar] [CrossRef]
- Bajraktari, A.; Seccia, V.; Casani, A.P.; Franceschini, S.S. Isolated laryngeal leishmaniasis in an immunocompetent patient: A case report. B-ENT 2016, 12, 333–337. [Google Scholar]
- Tiseo, D.; Tosone, G.; Conte, M.C.; Scordino, F.; Mansueto, G.; Mesolella, M.; Parrella, G.; Pennone, R.; Orlando, R. Isolated laryngeal leishmaniasis in an immunocompetent patient: A case report. Infez. Med. 2008, 16, 233–235. [Google Scholar]
- Mishra, P.; Dixit, A.; Chatterjee, T.; Bhattacharya, M.; Bhattacharya, J.; Dutta, P.; Mahapatra, M.; Pati, H.P.; Choudhry, V.P.; Saxena, R. Disseminated intravascular coagulation as an unusual presentation of Kala-azar: Report of two cases. Scand. J. Infect. Dis. 2004, 36, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.M.; Tello, A.; Leon, R.; Merino, E. An unusual case of pancytopenia in a nonagenarian: Visceral leishmaniasis. Aging Clin. Exp. Res. 2014, 26, 671–672. [Google Scholar] [CrossRef]
- Prasad, R.; Muthusami, S.; Pandey, N.; Tilak, V.; Shukla, J.; Mishra, O.P. Unusual presentations of Visceral leishmaniasis. Indian J. Pediatr. 2009, 76, 843–845. [Google Scholar] [CrossRef]
- Zumrutdal, A.; Erken, E.; Turunc, T.; Colakoglu, S.; Demiroglu, Y.Z.; Ozelsancak, R.; Solmaz, S. Delayed and overlooked diagnosis of an unusual opportunistic infection in a renal transplant recipient: Visceral leishmaniasis. Turk. Parazitol. Derg. 2010, 34, 183–185. [Google Scholar] [CrossRef]
- Lagadinou, M.; Dimitropoulou, D.; Assimakopoulos, S.F.; Davoulos, G.; Marangos, M. Recurrent visceral leishmaniasis in an immunocompetent patient: A case report. J. Med. Case Rep. 2013, 7, 68. [Google Scholar] [CrossRef]
- Zijlstra, E.E.; Musa, A.M.; Khalil, E.A.; el-Hassan, I.M.; el-Hassan, A.M. Post-kala-azar dermal leishmaniasis. Lancet Infect. Dis. 2003, 3, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.; Islam, S.; Rahman, M.W.; Kenah, E.; Ghalib, C.M.; Zahid, M.M.; Maguire, J.; Rahman, M.; Haque, R.; Luby, S.P.; et al. Increasing incidence of post-kala-azar dermal leishmaniasis in a population-based study in Bangladesh. Clin. Infect. Dis. 2010, 50, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Salotra, P.; Singh, R. Challenges in the diagnosis of post kala-azar dermal leishmaniasis. Indian J. Med. Res. 2006, 123, 295–310. [Google Scholar] [PubMed]
- Zijlstra, E.E.; Khalil, E.A.; Kager, P.A.; El-Hassan, A.M. Post-kala-azar dermal leishmaniasis in the Sudan: Clinical presentation and differential diagnosis. Br. J. Derm. 2000, 143, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Topno, R.K.; Rabi Das, V.N.; Kumar, M.; Madhukar, M.; Pandey, K.; Verma, N.; Agrawal, K.; Lal, C.S.; Siddiqui, N.A.; Bimal, S.; et al. Advanced case of PKDL due to delayed treatment: A rare case report. PLoS Negl. Trop. Dis. 2020, 14, e0008052. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.; Khanna, N.; Pandhi, R.K. Post-kala-azar dermal leishmaniasis with atypical presentation. Indian J. Derm. Venereol. Leprol. 1995, 61, 323. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S. Post-kala-azar dermal leishmaniasis with an atypical presentation. J. Derm. 2001, 28, 341–342. [Google Scholar] [CrossRef]
- Rijal, A.; Agrawal, S.; Agarwalla, A.; Agrawal, A.; Rijal, S. Post-kala-azar dermal leishmaniasis with visceral leishmaniasis: A rare presentation. Int. J. Derm. 2005, 44, 494–496. [Google Scholar] [CrossRef]
- Khandpur, S.; Ramam, M.; Sharma, V.K.; Salotra, P.; Singh, M.K.; Malhotra, A. Nerve involvement in Indian post kala-azar dermal leishmaniasis. Acta Derm. Venereol. 2004, 84, 245–246. [Google Scholar] [CrossRef]
- Arora, S.; D’Souza, P.; Haroon, M.A.; Ramesh, V.; Kaur, O.; Chandoke, R.K. Post-kala-azar dermal leishmaniasis mimicking leprosy relapse: A diagnostic dilemma. Int. J. Dermatol. 2014, 53, 606–608. [Google Scholar] [CrossRef]
- Ramesh, V.; Singh, R.; Salotra, P. Short communication: Post-kala-azar dermal leishmaniasis—An appraisal. Trop. Med. Int. Health 2007, 12, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Siddiqui, M.A.; Nabi, S.G.; Bhaskar, K.R.; Mondal, D. Post-kala-azar dermal leishmaniasis with mucosal involvement: An unusual case presentation including successful treatment with miltefosine. J. Health Popul. Nutr. 2013, 31, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Misra, D.; Rai, S.; Khatri, M. Post-Kala-Azar Dermal Leishmaniasis Associated With Oral Lesions: A Rare Case Report. MAMC J. Med. Sci. 2017, 3, 87. [Google Scholar] [CrossRef]
- Arora, S.; Bal, A.S.; Baveja, S.; Sood, A.; Rathi, K.R.; Patil, P. Atypical post kala azar dermal leishmaniasis with “muzzle area” swelling. Indian J. Dermatol. 2015, 60, 88. [Google Scholar] [CrossRef]
- Lakhanpal, S.; Pandhi, R.K.; Khaitan, B.K. Post-kala-azar dermal leishmaniasis—An unusual presentation. Acta Derm. Venereol. 1998, 78, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Kumar, J.; Salotra, P. An unusual presentation of post-kala-azar dermal leishmaniasis. Trop. Doct. 2007, 37, 172–173. [Google Scholar] [CrossRef]
- Pathania, S.; Kumari, P.; Suvirya, S.; Chakravarty, J. An Unusual Presentation of Post kala-azar Dermal Leishmaniasis. Indian Derm. Online J. 2020, 11, 269–271. [Google Scholar] [CrossRef]
- Pradhan, A.; Tobgay, T.; Dorjee, S.; Wangdi, T.; Zhou, G.; Karunaweera, N.D. Atypical Presentation of Post-Kala-Azar Dermal Leishmaniasis in Bhutan. Case Rep. Derm. Med. 2020, 2020, 8899586. [Google Scholar] [CrossRef]
- Nandy, A.; Addy, M.; Banerjee, D.; Guha, S.K.; Maji, A.K.; Saha, A.M. Laryngeal involvement during post kala-azar dermal leishmaniasis in India. Trop. Med. Int. Health 1997, 2, 371–373. [Google Scholar] [CrossRef]
- Vinay, K.; De, D.; Saikia, U.N. Nonhealing Leg Ulcer in a Middle-aged Indian Man. JAMA Dermatol. 2018, 154, 207–208. [Google Scholar] [CrossRef]
- Bhandare, P.; Shukla, P.; Bhobe, M.; Pai, V.V. Post-kala-Azar dermal leishmaniasis: A diagnostic dilemma in a nonendemic area. Indian Dermatol. Online J. 2014, 5, S122. [Google Scholar] [CrossRef] [PubMed]
- Dechant, W.; Rees, P.H.; Kager, P.A.; Klauss, V.; Adala, H. Post kala-azar uveitis. Br. J. Ophthalmol. 1980, 64, 680–683. [Google Scholar] [CrossRef] [PubMed]
- el Hassan, A.M.; Ghalib, H.W.; Zijlstra, E.E.; Eltoum, I.A.; Satti, M.; Ali, M.S.; Ali, H.M. Post kala-azar dermal leishmaniasis in the Sudan: Clinical features, pathology and treatment. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Roizenblatt, J. Interstitial keratitis caused by American (mucocutaneous) leishmaniasis. Am. J. Ophthalmol. 1979, 87, 175–179. [Google Scholar] [CrossRef]
- Abdel-Hameed, A.A.; Hassan, M.; Abdalla, K.; el Basha, A.; Ahmed, B.; Mohammedani, A. Two cases of ocular leishmaniasis. Trop. Geogr. Med. 1991, 43, 91–93. [Google Scholar]
- el Hassan, A.M.; Khalil, E.A.; el Sheikh, E.A.; Zijlstra, E.E.; Osman, A.; Ibrahim, M.E. Post kala-azar ocular leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 177–179. [Google Scholar] [CrossRef]
- Khalil, E.A.; Musa, A.M.; Younis, B.M.; Elfaki, M.E.; Zijlstra, E.E.; Elhassan, A.M. Blindness following visceral leishmaniasis: A neglected post-kala-azar complication. Trop. Doct. 2011, 41, 139–140. [Google Scholar] [CrossRef]
- Couture, S.; Agrawal, R.; Woods, K.; Lockwood, D.; Pavesio, C.E.; Addison, P.K. A case of panuveitis with hypopyon due to presumed ocular leishmaniasis in a HIV patient. J. Ophthalmic Inflamm. Infect. 2014, 4, 21. [Google Scholar] [CrossRef]
- World Health Organization. Statement on Miltefosine—Potential Ocular Disorders in Patients Treated with Miltefosine for Post-kala-Azar Dermal Leishmaniasis (PKDL); World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Celesia, B.M.; Cacopardo, B.; Massimino, D.; Gussio, M.; Tosto, S.; Nunnari, G.; Pinzone, M.R. Atypical Presentation of PKDL due to Leishmania infantum in an HIV-Infected Patient with Relapsing Visceral Leishmaniasis. Case Rep. Infect. Dis. 2014, 2014, 370286. [Google Scholar] [CrossRef]
- Elliott, T.; Simpson, J.; Naresh, K.N.; Lockwood, D.; Bailey, A.C. An unusual case of post-kala-azar dermal leishmaniasis in a patient with HIV and visceral leishmaniasis co-infection. Int. J. STD AIDS 2019, 30, 1221–1223. [Google Scholar] [CrossRef]
- Thakur, L.; Singh, K.K.; Shanker, V.; Negi, A.; Jain, A.; Matlashewski, G.; Jain, M. Atypical leishmaniasis: A global perspective with emphasis on the Indian subcontinent. PLoS Negl. Trop. Dis. 2018, 12, e0006659. [Google Scholar] [CrossRef] [PubMed]
- Karunaweera, N.D. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: A wolf in sheep’s clothing? Trends Parasitol. 2009, 25, 458–463. [Google Scholar] [CrossRef]
- Siriwardana, H.Y.D.; Noyes, H.A.; Beeching, N.J.; Chance, M.L.; Karunaweera, N.D.; Bates, P.A. Leishmania donovani and cutaneous leishmaniasis, Sri Lanka. Emerg. Infect. Dis. 2007, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Siriwardana, Y.; Deepachandi, B.; Gunasekara, C.; Warnasooriya, W.; Karunaweera, N.D. Leishmania donovani induced cutaneous leishmaniasis: An insight into atypical clinical variants in Sri Lanka. J. Trop. Med. 2019, 2019, 4538597. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, U.L.; Selvapandiyan, A.; Rai, K.; Wani, T.H.; Ahuja, K.; Beg, M.A.; Premathilake, H.U.; Bhattarai, N.R.; Siriwardena, Y.D.; Zhong, D. Genetic diversity of Leishmania donovani that causes cutaneous leishmaniasis in Sri Lanka: A cross sectional study with regional comparisons. BMC Infect. Dis. 2017, 17, 791. [Google Scholar] [CrossRef] [PubMed]
- Karunaweera, N.D.; Pratlong, F.; Siriwardane, H.; Ihalamulla, R.; Dedet, J. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Özbilgin, A.; Harman, M.; Karakuş, M.; Bart, A.; Töz, S.; Kurt, Ö.; Çavuş, İ.; Polat, E.; Gündüz, C.; Van Gool, T. Leishmaniasis in Turkey: Visceral and cutaneous leishmaniasis caused by Leishmania donovani in Turkey. Acta Trop. 2017, 173, 90–96. [Google Scholar] [CrossRef]
- Sharma, N.L.; Mahajan, V.K.; Kanga, A.; Sood, A.; Katoch, V.M.; Mauricio, I.; Singh, C.D.; Parwan, U.C.; Sharma, V.K.; Sharma, R.C. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: Preliminary findings of the study of 161 new cases from a new endemic focus in himachal pradesh, India. Am. J. Trop. Med. Hyg. 2005, 72, 819–824. [Google Scholar] [CrossRef]
- Alam, M.Z.; Kuhls, K.; Schweynoch, C.; Sundar, S.; Rijal, S.; Shamsuzzaman, A.K.M.; Raju, B.V.S.; Salotra, P.; Dujardin, J.-C.; Schönian, G. Multilocus microsatellite typing (MLMT) reveals genetic homogeneity of Leishmania donovani strains in the Indian subcontinent. Infect. Genet. Evol. 2009, 9, 24–31. [Google Scholar] [CrossRef]
- Koliou, M.G.; Antoniou, Y.; Antoniou, M.; Christodoulou, V.; Mazeris, A.; Soteriades, E.S. A cluster of four cases of cutaneous leishmaniasis by Leishmania donovani in Cyprus: A case series. J. Med. Case Rep. 2014, 8, 354. [Google Scholar] [CrossRef]
- Özbilgin, A.; Töz, S.; Harman, M.; Topal, S.G.; Uzun, S.; Okudan, F.; Güngör, D.; Erat, A.; Ertabaklar, H.; Ertuğ, S. The current clinical and geographical situation of cutaneous leishmaniasis based on species identification in Turkey. Acta Trop. 2019, 190, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lypaczewski, P.; Thakur, L.; Jain, A.; Kumari, S.; Paulini, K.; Matlashewski, G.; Jain, M. An intraspecies Leishmania donovani hybrid from the Indian subcontinent is associated with an atypical phenotype of cutaneous disease. iScience 2022, 25, 103802. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Gramiccia, M.; Gradoni, L.; Amerio, P. Isolation of the agent causing cutaneous leishmaniasis in Italy and its visceralization in inbred hamsters. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 260–261. [Google Scholar] [CrossRef] [PubMed]
- del Giudice, P.; Marty, P.; Lacour, J.P.; Perrin, C.; Pratlong, F.; Haas, H.; Dellamonica, P.; Le Fichoux, Y. Cutaneous leishmaniasis due to Leishmania infantum: Case reports and literature review. Arch. Dermatol. 1998, 134, 193–198. [Google Scholar] [CrossRef]
- Rhajaoui, M.; Nasereddin, A.; Fellah, H.; Azmi, K.; Amarir, F.; Al-Jawabreh, A.; Ereqat, S.; Planer, J.; Abdeen, Z. New clinicoepidemiologic profile of cutaneous leishmaniasis, Morocco. Emerg. Infect. Dis. 2007, 13, 1358. [Google Scholar] [CrossRef]
- Elmazini, S.; Ejghal, R.; Bekhti, K.; Lemrani, M. The Sporadic cutaneous leishmaniasis due to Leishmania infantum in Morocco: A presumably trend towards endemicity. Acta Trop. 2021, 227, 106288. [Google Scholar] [CrossRef]
- Aoun, K.; Bouratbine, A. Cutaneous leishmaniasis in North Africa: A review. Parasite 2014, 21, 14. [Google Scholar] [CrossRef]
- BenSaid, M.; Guerbouj, S.; Saghrouni, F.; Fathallah-Mili, A.; Guizani, I. Occurrence of Leishmania infantum cutaneous leishmaniasis in central Tunisia. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 521–526. [Google Scholar] [CrossRef]
- Kallel, K.; Pratlong, F.; Haouas, N.; Kaouech, E.; Belhadj, S.; Anane, S.; Dedet, J.; Babba, H.; Chaker, E. Isoenzymatic variability of Leishmania infantum in Tunisia concerning 254 human strains. Acta Trop. 2008, 106, 132–136. [Google Scholar] [CrossRef]
- Boussoffara, T.; Boubaker, M.S.; Ahmed, M.B.; Mokni, M.; Guizani, I.; Salah, A.B.; Louzir, H. Histological and immunological differences between zoonotic cutaneous leishmaniasis due to Leishmania major and sporadic cutaneous leishmaniasis due to Leishmania infantum. Parasite 2019, 26, 9. [Google Scholar] [CrossRef]
- Hakimi, S.; Rivière, S.; Del Giudice, P.; Dereure, J.; Le Quellec, A. Localized cutaneous leishmaniasis due to Leishmania infantum in a patient treated with infliximab. Dermatology 2010, 220, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Paniz Mondolfi, A.; Stavropoulos, C.; Gelanew, T.; Loucas, E.; Perez Alvarez, A.; Benaim, G.; Polsky, B.; Schoenian, G.; Sordillo, E. Successful treatment of Old World cutaneous leishmaniasis caused by Leishmania infantum with posaconazole. Antimicrob. Agents Chemother. 2011, 55, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Ok, Ü.; Balcıoğlu, İ.; Özkan, A.T.; Özensoy, S.; Özbel, Y. Leishmaniasis in Turkey. Acta Trop. 2002, 84, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gürel, M.S.; Yesilova, Y.; Ölgen, M.K.; Özbel, Y. Cutaneous leishmaniasis in Turkey. Türk. Parazitolojii Derg. 2012, 36, 121. [Google Scholar] [CrossRef]
- Badirzadeh, A.; Mohebali, M.; Ghasemian, M.; Amini, H.; Zarei, Z.; Akhoundi, B.; Hajjaran, H.; Emdadi, D.; Molaei, S.; Kusha, A. Cutaneous and post kala-azar dermal leishmaniasis caused by Leishmania infantum in endemic areas of visceral leishmaniasis, northwestern Iran 2002–2011: A case series. Pathog. Glob. Health 2013, 107, 194–197. [Google Scholar] [CrossRef]
- Gállego, M.; Pratlong, F.; Riera, C.; Fisa, R.; Muñoz, C.; Dedet, J.P.; Portús, M. Cutaneous leishmaniasis due to Leishmania infantum in the northeast of Spain: The isoenzymatic analysis of parasites. Arch. Dermatol. 2001, 137, 667–668. [Google Scholar]
- Kroidl, A.; Kroidl, I.; Bretzel, G.; Löscher, T. Non-healing old world cutaneous leishmaniasis caused by L. infantumin a patient from Spain. BMC Infect. Dis. 2014, 14, 206. [Google Scholar] [CrossRef]
- Alcover, M.M.; Rocamora, V.; Guillén, M.C.; Berenguer, D.; Cuadrado, M.; Riera, C.; Fisa, R. Case report: Diffuse cutaneous leishmaniasis by Leishmania infantum in a patient undergoing immunosuppressive therapy: Risk status in an endemic Mediterranean area. Am. J. Trop. Med. Hyg. 2018, 98, 1313. [Google Scholar] [CrossRef]
- Lyra, M.R.; Pimentel, M.I.F.; Madeira, M.d.F.; Antonio, L.d.F.; Lyra, J.P.D.M.; Fagundes, A.; Schubach, A.d.O. First report of cutaneous leishmaniasis caused by Leishmania (Leishmania) infantum chagasi in an urban area of Rio de Janeiro, Brazil. Rev. Inst. Med. Trop. São Paulo 2015, 57, 451–454. [Google Scholar] [CrossRef]
- Castro, L.S.; Franca, A.d.O.; Ferreira, E.d.C.; Hans Filho, G.; Higa Junior, M.G.; Gontijo, C.M.F.; Pereira, A.A.S.; Dorval, M.E.M.C. Leishmania infantum as a causative agent of cutaneous leishmaniasis in the state of Mato Grosso do Sul, Brazil. Rev. Inst. Med. Trop. São Paulo 2016, 58, 23. [Google Scholar] [CrossRef]
- Khatri, M.L.; Di Muccio, T.; Gramiccia, M. Cutaneous leishmaniasis in North-Western Yemen: A clinicoepidemiologic study and Leishmania species identification by polymerase chain reaction–restriction fragment length polymorphism analysis. J. Am. Acad. Dermatol. 2009, 61, e15–e21. [Google Scholar] [CrossRef] [PubMed]
- Knio, K.; Baydoun, E.; Tawk, R.; Nuwayri-Salti, N. Isoenzyme characterization of Leishmania isolates from Lebanon and Syria. Am. J. Trop. Med. Hyg. 2000, 63, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, M.; Alten, B.; Zídková, L.; Dvořák, V.; Hlavačková, J.; Myšková, J.; Šeblová, V.; Kasap, O.E.; Belen, A.; Votýpka, J. Cutaneous leishmaniasis caused by Leishmania infantum transmitted by Phlebotomus tobbi. Int. J. Parasitol. 2009, 39, 251–256. [Google Scholar] [CrossRef]
- Ben-Shimol, S.; Sagi, O.; Horev, A.; Avni, Y.S.; Ziv, M.; Riesenberg, K. Cutaneous leishmaniasis caused by Leishmania infantum in Southern Israel. Acta Parasitol. 2016, 61, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Anugulruengkitt, S.; Songtaweesin, W.N.; Thepnarong, N.; Tangthanapalakul, A.; Sitthisan, M.; Chatproedprai, S.; Wititsuwannakul, J.; Likitnukul, S.; Jariyapan, N.; Weedall, G.D.; et al. Case Report: Simple Nodular Cutaneous Leishmaniasis Caused by Autochthonous Leishmania (Mundinia) orientalis in an 18-Month-Old Girl: The First Pediatric Case in Thailand and Literature Review. Am. J. Trop. Med. Hyg. 2022, 108, 44–50 tpmd220385. [Google Scholar] [CrossRef] [PubMed]
- Jariyapan, N.; Daroontum, T.; Jaiwong, K.; Chanmol, W.; Intakhan, N.; Sor-Suwan, S.; Siriyasatien, P.; Somboon, P.; Bates, M.D.; Bates, P.A. Leishmania (Mundinia) orientalis n. sp. (Trypanosomatidae), a parasite from Thailand responsible for localised cutaneous leishmaniasis. Parasit Vectors 2018, 11, 351. [Google Scholar] [CrossRef]
- Crowe, A.; Slavin, J.; Stark, D.; Aboltins, C. A case of imported Leishmania infantum cutaneous leishmaniasis; an unusual presentation occurring 19 years after travel. BMC Infect. Dis. 2014, 14, 597. [Google Scholar] [CrossRef]
- Antón, E.; López, A.; Martí, J. An unusual presentation of cutaneous leishmaniasis. Arch. Dermatol. 2005, 141, 109–110. [Google Scholar] [CrossRef]
- Sharma, A.; Gulati, A.; Kaushik, R. Cutaneous leishmaniasis presenting as a submandibular nodule-a case report. J. Cytol. 2007, 24, 149. [Google Scholar] [CrossRef]
- Kumari, S.; Garg, A. Lip leishmaniasis: A new emerging clinical form of cutaneous leishmaniasis from sub-Himalayan Region. J. Med. Sci. Clin. Res. 2018, 6, 62–69. [Google Scholar] [CrossRef]
- El-Hoshy, K. Lip leishmaniasis. J. Am. Acad. Derm. 1993, 28, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Gurel, M.S.; Ulukanligil, M.; Ozbilge, H. Cutaneous leishmaniasis in Sanliurfa: Epidemiologic and clinical features of the last four years (1997–2000). Int. J. Dermatol. 2002, 41, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Roundy, S.; Almony, J.; Zislis, T. Cutaneous Leishmaniasis of the lower Lip in a united states soldier. J. Oral Maxillofac. Surg. 2008, 66, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, S.; Mozafari, N.; Abdollahimajd, F. Unusual presentation of cutaneous leishmaniasis: Lower lip ulcer. Arch. Clin. Infect. Dis. 2012, 7, 66–68. [Google Scholar] [CrossRef]
- Mohammadpour, I.; Motazedian, M.H.; Handjani, F.; Hatam, G.R. Lip leishmaniasis: A case series with molecular identification and literature review. BMC Infect. Dis. 2017, 17, 96. [Google Scholar] [CrossRef]
- Parajuli, N.; Manandhar, K.D.; Shrestha, S.; Bastola, A. Cutaneous leishmaniasis of lip and role of polymerase chain reaction: A case report. JNMA J. Nepal Med. Assoc. 2020, 58, 494. [Google Scholar] [CrossRef]
- Goldin, H.; Kohen, S.; Taxy, J.; Libman, M.; Cibull, T.; Billick, K. Leishmania tropica infection of the ear treated with photodynamic therapy. JAAD Case Rep. 2020, 6, 514. [Google Scholar] [CrossRef]
- Doroodgar, M.; Doroodgar, M.; Doroodgar, A. Unusual presentation of cutaneous leishmaniasis: Ocular leishmaniasis. Case Rep. Infect. Dis. 2017, 2017, 3198547. [Google Scholar] [CrossRef]
- Sajad, P.; Rather, S.; Hassan, I.; Qureshi, W. Primary lip leishmaniasis-report of 4 cases from a non-endemic region of Kashmir valley of north India and review of literature. J. Adv. Med. Med. Res. 2017, 24, JAMMR.36072. [Google Scholar] [CrossRef]
- Ekiz, Ö.; Rifaioǧlu, E.N.; Şen, B.B.; Çulha, G.; Özgür, T.; Doǧramaci, A.Ç. Leishmaniasis recidiva cutis of the lips mimicking granulomatous cheilitis. Indian J. Dermatol. 2015, 60, 216. [Google Scholar] [CrossRef]
- Robati, R.M.; Qeisari, M.; Saeedi, M.; Karimi, M. Auricular enlargement: An atypical presentation of old world cutaneous leishmaniasis. Indian J. Dermatol. 2011, 56, 428. [Google Scholar] [CrossRef]
- Tarkan, Ö.; Cetik, F.; Uzun, S. Auricular cutaneous leishmaniasis mimicking neoplastic disease. J. Laryngol. Otol. 2012, 126, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi-Ashtiani, M.; Hasibi, M.; Yazdani, N.; Paydarfar, J.; Sadri, F.; Mirashrafi, F.; Kouhi, A. Auricular leishmaniasis mimicking squamous cell carcinoma. J. Laryngol. Otol. 2009, 123, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.; Khatib, L.; Kanj-Sharara, S.; Husseini, S.T.; Nuwayri-Salti, N.; Semaan, R.; Rameh, C. Leishmaniasis of the auricle mimicking carcinoma. Am. J. Otolaryngol. 2009, 30, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Oetken, T.; Hiscox, B.; Orengo, I.; Rosen, T. Cutaneous leishmaniasis mimicking squamous cell carcinoma. Dermatol. Online J. 2017, 23, 16. [Google Scholar] [CrossRef]
- Youssef, A.; Yaseer, S.; Harfouch, R.; Marouf, M.; Hasan, F. Chiclero’s ulcer: An unusual presentation of Leishmania tropica in Syria. Avicenna J. Med. 2018, 8, 117–119. [Google Scholar] [CrossRef]
- Karamian, M.; Motazedian, M.; Fakhar, M.; Pakshir, K.; Jowkar, F.; Rezanezhad, H. Atypical presentation of Old-World cutaneous leishmaniasis, diagnosis and species identification by PCR. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 958–962. [Google Scholar] [CrossRef]
- Zadeh, M.M.; Manshai, K.; Shaddel, M.; Oormazdi, H. Ocular leishmaniasis review article. Iran. J. Ophthalmol. 2006, 12, 1–5. [Google Scholar]
- Tabbara, K.; El-Sheikh, H. Ocular Complications Associated with Cutaneous Leishmaniasis. Investig. Ophthalmol. Vis. Sci. 2002, 43, 4288. [Google Scholar]
- Oliveira-Neto, M.P.; Martins, V.J.; Mattos, M.S.; Pirmez, C.; Brahin, L.R.; Benchimol, E. South American cutaneous leishmaniasis of the eyelids: Report of five cases in Rio de Janeiro State, Brazil. Ophthalmology 2000, 107, 169–172. [Google Scholar] [CrossRef]
- Mencía-Gutiérrez, E.; Gutiérrez-Díaz, E.; Rodríguez-Peralto, J.L.; Monsalve-Córdova, J. Old World eyelid cutaneous leishmaniasis: A case report. Dermatol. Online J. 2005, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, J.; Ayatollahi, A.; Shahcheraghi, S.H. Cutaneous leishmaniasis of the eyelid: A case report. Case Rep. Infect. Dis. 2013, 2013, 214297. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, G.; Nilfroushzadeh, M.A.; Moradi, S.H.; Hanjani, S.H. Ocular leishmaniasis: A case report. Derm. Online J. 2005, 11, 19. [Google Scholar] [CrossRef]
- Abrishami, M.; Soheilian, M.; Farahi, A.; Dowlati, Y. Successful treatment of ocular leishmaniasis. Eur. J. Dermatol. 2002, 12, 88–89. [Google Scholar] [PubMed]
- Jafari, A.K.; Akhyani, M.; Valikhani, M.; Ghodsi, Z.S.; Barikbin, B.; Toosi, S. Bilateral cutaneous leishmaniasis of upper eyelids: A case report. Dermatol. Online J. 2006, 12, 20. [Google Scholar] [CrossRef]
- Veraldi, S.; Bottini, S.; Currò, N.; Gianotti, R. Leishmaniasis of the eyelid mimicking an infundibular cyst and review of the literature on ocular leishmaniasis. Int. J. Infect. Dis. 2010, 14, e230–e232. [Google Scholar] [CrossRef]
- Nikandish, M.; Goyonlo, V.M.; Taheri, A.R.; Kiafar, B. Ocular leishmaniasis treated by intralesional amphotericin B. Middle East. Afr. J. Ophthalmol. 2016, 23, 153. [Google Scholar] [CrossRef]
- Gupta, M. Cutaneous leishmaniasis of the eyelid: An uncommon presentation of a common entity. Indian J. Paediatr. Dermatol. 2017, 18, 116. [Google Scholar] [CrossRef]
- Satici, A.; Gurler, B.; Aslan, G.; Ozturk, I. Ocular involvement in cutaneous leishmaniasis four cases with blepharoconjunctivitis. Eur. J. Epidemiol. 2004, 19, 263–266. [Google Scholar] [CrossRef]
- Ayele, F.A.; Wolde, Y.A.; Hagos, T.; Diro, E. Ocular leishmaniasis presenting as chronic ulcerative blepharoconjunctivitis: A case report. J. Clin. Exp. Ophthalmol. 2015, 6, e1000395. [Google Scholar] [CrossRef]
- Chefchaouni, C.; Lamrani, R.; Benjelloune, A.; El Lyacoubi, M.; Berraho, A. Cutaneous leishmaniasis of the lid. J. Fr. D’ophtalmol. 2002, 25, 522–526. [Google Scholar]
- Mignot, G.; Bhattacharya, Y.; Reddy, A. Ocular Leishmaniasis-A systematic review. Indian J. Ophthalmol. 2021, 69, 1052. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Noaimi, A.A.; Saleh, B.A. Cutaneous leishmaniasis as imitator of skin diseases and a diagnostic challenge. J. Cosmet. Dermatol. Sci. Appl. 2018, 8, 158–177. [Google Scholar] [CrossRef]
- Cárdenas, C.D.S.; Luna, Y.J.Á.; Hernández, Y.B.; Sigall, D.A.; Guzmán, R.A. Cutaneous Leishmaniasis: A Case Involving the Scalp-Clinical and Videodermoscopic Findings. Ski. Appendage Disord. 2018, 4, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Cabello, I.; Caraballo, A.; Millan, Y. Leishmaniasis in the genital area. Rev. Inst. Med. Trop. São Paulo 2002, 44, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Yesilova, Y.; Turan, E.; Sürücü, H.; Kocarslan, S.; Tanrikulu, O.; Eroglu, N. Ulcerative penile leishmaniasis in a child. Indian J. Dermatol. Venereol. Leprol. 2014, 80, 247. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi, M.; Mohebali, M.; Farazi, A.; Shirzadi, M.R.; Akhlaghi, D.; Hajhossein, R.; Elikaee, S. Leishmaniasis Caused by Leishmania major on the Glans Penis: A Case Report. Iran. J. Parasitol. 2019, 14, 472. [Google Scholar] [PubMed]
- Handjani, F.; Taghipour, K.; Miri, A. Cutaneous Leishmaniasis on the Glans Penis: A Case Report. J. Med. Microbiol. Infect. Dis. 2019, 7, 87–88. [Google Scholar] [CrossRef]
- Lyra, M.R.; da Silva, A.B.; Valete-Rosalino, C.M.; Pimentel, M.I.F. Clinical and epidemiological aspects of American cutaneous leishmaniasis with genital involvement. An. Bras. Dermatol. 2020, 95, 641–644. [Google Scholar] [CrossRef]
- Reis, L.C.; Lindoso, J.A.L.; Celeste, B.J.; Braz, L.M.A.; Ramos-Sanchez, E.M.; Yamashiro-Kanashiro, E.H.; Goto, H.; Oyafuso, L.K.M. Unusual manifestation of genital cutaneous leishmaniasis in an immunocompetent patient from São Paulo, Brazil: A case report. Rev. Soc. Bras. Med. Trop. 2021, 54, e0514-2020. [Google Scholar] [CrossRef]
- Couppie, P.; Clyti, E.; Sainte-Marie, D.; Dedet, J.; Carme, B.; Pradinaud, R. Disseminated cutaneous leishmaniasis due to Leishmania guyanensis: Case of a patient with 425 lesions. Am. J. Trop. Med. Hyg. 2004, 71, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Calvopina, M.; Gomez, E.A.; Uezato, H.; Kato, H.; Nonaka, S.; Hashiguchi, Y. Atypical clinical variants in New World cutaneous leishmaniasis: Disseminated, erysipeloid, and recidiva cutis due to Leishmania (V.) panamensis. Am. J. Trop. Med. Hyg. 2005, 73, 281–284. [Google Scholar] [CrossRef] [PubMed]
- An, I.; Aksoy, M.; Ozturk, M.; Ayhan, E.; Erat, T.; Yentur Doni, N.; Guldur, M.E. Atypical and unusual morphological variants of cutaneous leishmaniasis. Int. J. Clin. Pract. 2021, 75, e13730. [Google Scholar] [CrossRef]
- Di Altobrando, A.; Misciali, C.; Raone, B.; Attard, L.; Gaspari, V. Case Report: Cutaneous Leishmaniasis Misdiagnosed as Pyoderma Gangrenosum. Am. J. Trop. Med. Hyg. 2021, 104, 640. [Google Scholar] [CrossRef]
- Ceyhan, A.M.; Yildirim, M.; Basak, P.Y.; Akkaya, V.B.; Erturan, I. A case of erysipeloid cutaneous leishmaniasis: Atypical and unusual clinical variant. Am. J. Trop. Med. Hyg. 2008, 78, 406–408. [Google Scholar] [CrossRef]
- Bouomrani, S.; Houchet, M.B. Zosteriform Cutaneous Leishmaniasis of the Elbow. Am. J. Med. Case Rep. 2020, 8, 483–485. [Google Scholar] [CrossRef]
- Firooz, A.; Mortazavi, H.; Khamesipour, A.; Ghiasi, M.; Abedini, R.; Balighi, K.; Esmaili, N.; Nassiri-Kashani, M.; Eskandari, S.E.; Mohebali, M. Old world cutaneous leishmaniasis in Iran: Clinical variants and treatments. J. Dermatol. Treat. 2021, 32, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.M.; Rahman, B. Unusual clinical variants of cutaneous leishmaniasis in Pakistan. Br. J. Dermatol. 1998, 139, 111–113. [Google Scholar] [CrossRef]
- Nepal, S. Case Report: An Atypical Erysipeloid Presentation of Cutaneous Leishmaniasis from the Hilly Region of Nepal. Am. J. Trop. Med. Hyg. 2021, 105, 134–137. [Google Scholar] [CrossRef]
- Momeni, A.; Aminjavaheri, M. Clinical picture of cutaneous leishmaniasis in Isfahan, Iran. Int. J. Dermatol. 1994, 33, 260–265. [Google Scholar] [CrossRef]
- Karincaoglu, Y.; Esrefoglu, M.; Ozcan, H. Atypical clinical form of cutaneous leishmaniasis: Erysipeloid form. Int. J. Dermatol. 2004, 43, 827–829. [Google Scholar] [CrossRef]
- Zerehsaz, F.; Beheshti, S.; Rezaian, G.R.; Joubeh, S. Erysipeloid cutaneous leishmaniasis: Treatment with a new, topical, pure herbal extract. Eur. J. Dermatol. 2003, 13, 145–148. [Google Scholar] [PubMed]
- Robati, R.M.; Abdollahimajd, F. Cutaneous leishmaniasis: Report of two atypical cases. J. Clin. Med. Res. Updat. 2015, 2, 1–3. [Google Scholar]
- Salmanpour, R.; Handjani, F.; Zerehsaz, F.; Ardehali, S.; Panjehshahin, M. Erysipeloid leishmaniasis: An unusual clinical presentation. Eur. J. Dermatol. 1999, 9, 458–459. [Google Scholar]
- Guimaraes, L.H.; Queiroz, A.; Silva, J.A.; Silva, S.C.; Magalhaes, V.; Lago, E.L.; Machado, P.R.L.; Bacellar, O.; Wilson, M.E.; Beverley, S.M. Atypical manifestations of cutaneous leishmaniasis in a region endemic for Leishmania braziliensis: Clinical, immunological and parasitological aspects. PLoS Negl. Trop. Dis. 2016, 10, e0005100. [Google Scholar] [CrossRef]
- Bongiorno, M.R.; Pistone, G.; Aricò, M. Unusual clinical variants of cutaneous leishmaniasis in Sicily. Int. J. Dermatol. 2009, 48, 286–289. [Google Scholar] [CrossRef]
- Omidian, M.; Mapar, M. Chronic zosteriform cutaneous leishmaniasis. Indian J. Dermatol. Venereol. Leprol. 2006, 72, 41. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.M.V.d.; Pimentel, M.I.F.; Conceição-Silva, F.; Vasconcellos, É.d.C.F.; Valete-Rosalino, C.M.; Lyra, M.R.; Salgueiro, M.d.M.; Saheki, M.N.; Madeira, M.d.F.; Mouta-Confort, E. Sporotrichoid leishmaniasis: A cross-sectional clinical, epidemiological and laboratory study in Rio de Janeiro State, Brazil. Rev. Inst. Med. Trop. São Paulo 2017, 59, e33. [Google Scholar] [CrossRef]
- Iftikhar, N.; Bari, I.; Ejaz, A. Rare variants of cutaneous leishmaniasis: Whitlow, paronychia, and sporotrichoid. Int. J. Dermatol. 2003, 42, 807–809. [Google Scholar] [CrossRef]
- López-Escobar, M.; Drake-Monfort, M.; Salesa-Gutiérrez de Rozas, R.; Hermana-Ramírez, S. Sporotrichoid cutaneous leishmaniasis. Actas Dermo-Sifiliogr. 2007, 98, 444–445. [Google Scholar] [CrossRef]
- Kafaie, P.; Akaberi, A.A.; Amini, S.; Noorbala, M.T.; Moghimi, M. Multidermatomal zosteriform lupoid cutaneous leishmaniasis: A case report. J. Pak. Assoc. Dermatol. 2010, 20, 243–245. [Google Scholar]
- Ferahbas, A.; Mistik, S.; Utas, S.; Yaman, O.; Canoz, O.; Doganay, M.; Asçioǧlu, O. Cutaneous lupoid leishmaniasis: A case report. CUTIS N. Y. 2006, 77, 25. [Google Scholar]
- Pazoki, H.; Fakhar, M.; Rasooli, A.; Karamian, M.; Nazar, E. Lupoid leishmaniasis among the known cases of cutaneous leishmaniasis in Herat Province, western Afghanistan. J. Infect. Public Health 2016, 9, 557–563. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Control of the Leishmaniases: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, Switzerland, 22–26 March 2010; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Bari, A.U.; Raza, N. Lupoid cutaneous leishmaniasis: A report of 16 cases. Indian J. Derm. Venereol. Leprol. 2010, 76, 100. [Google Scholar] [CrossRef]
- Khaled, A.; Goucha, S.; Trabelsi, S.; Zermani, R.; Fazaa, B. Lupoid cutaneous leishmaniasis: A case report. Dermatol. Ther. 2011, 1, 36–41. [Google Scholar] [CrossRef]
- Gupta, L.K.; Meena, S.; Khare, A.K.; Balai, M.; Mittal, A.; Mehta, S. Lupoid cutaneous leishmaniasis: A report of three cases from nonendemic area. Indian J. Dermatol. 2017, 62, 548. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Vaccaro, M.; Guarneri, F. Leishmaniasis recidiva cutis. Int. J. Dermatol. 2000, 39, 205–206. [Google Scholar] [CrossRef]
- Bittencourt, A.L.; Costa, J.M.; Carvalho, E.M.; Barral, A. Leishmaniasis recidiva cutis in American cutaneous leishmaniasis. Int. J. Dermatol. 1993, 32, 802–805. [Google Scholar] [CrossRef]
- Oliveira-Neto, M.P.; Mattos, M.; da Silva, C.; de Souza, F.; Fernandes, O.; Pirmez, C. Leishmaniasis recidiva cutis in New World cutaneous leishmaniasis. Int. J. Dermatol. 1998, 37, 846–849. [Google Scholar] [CrossRef]
- Marovich, M.A.; Rosalia, L.; Marc, S.; Fuchs, G.H.; Kruetzer, R.; Nutman, T.B.; Franklin, A.N. Leishmaniasis recidivans recurrence after 43 years: A clinical and immunologic report after successful treatment. Clin. Infect. Dis. 2001, 33, 1076–1079. [Google Scholar] [CrossRef]
- Calza, L.; D’Antuono, A.; Marinacci, G.; Manfredi, R.; Colangeli, V.; Passarini, B.; Orioli, R.; Varoli, O.; Chiodo, F. Disseminated cutaneous leishmaniasis after visceral disease in a patient with AIDS. J. Am. Acad. Dermatol. 2004, 50, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Azeredo-Coutinho, R.; Conceição-Silva, F.; Schubach, A.; Cupolillo, E.; Quintella, L.; Madeira, M.; Pacheco, R.; Valete-Rosalino, C.; Mendonça, S. First report of diffuse cutaneous leishmaniasis and Leishmania amazonensis infection in Rio de Janeiro State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Chiewchanvit, S.; Tovanabutra, N.; Jariyapan, N.; Bates, M.D.; Mahanupab, P.; Chuamanochan, M.; Tantiworawit, A.; Bates, P.A. Chronic generalized fibrotic skin lesions from disseminated leishmaniasis caused by Leishmania martiniquensis in two patients from northern Thailand infected with HIV. Br. J. Derm. 2015, 173, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Noppakun, N.; Kraivichian, K.; Siriyasatien, P. Disseminated dermal leishmaniasis caused by Leishmania siamensis in a systemic steroid therapy patient. Am. J. Trop. Med. Hyg. 2014, 91, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, A.; Cocuzza, S.; Pinzone, M.R.; Postorino, M.C.; Cosentino, S.; Serra, A.; Cacopardo, B.; Nunnari, G. Mucosal leishmaniasis: An underestimated presentation of a neglected disease. BioMed Res. Int. 2013, 2013, 805108. [Google Scholar] [CrossRef]
- Aliaga, L.; Cobo, F.; Mediavilla, J.D.; Bravo, J.; Osuna, A.; Amador, J.M.; Martín-Sánchez, J.; Cordero, E.; Navarro, J.M. Localized mucosal leishmaniasis due to Leishmania (Leishmania) infantum: Clinical and microbiologic findings in 31 patients. Medicine 2003, 82, 147–158. [Google Scholar] [CrossRef]
- Mahdi, M.; Elamin, E.M.; Melville, S.E.; Musa, A.M.; Blackwell, J.M.; Mukhtar, M.M.; Elhassan, A.M.; Ibrahim, M.E. Sudanese mucosal leishmaniasis: Isolation of a parasite within the Leishmania donovani complex that differs genotypically from L. donovani causing classical visceral leishmaniasis. Infect. Genet. Evol. 2005, 5, 29–33. [Google Scholar] [CrossRef]
- Abbas, K.; Musatafa, M.A.; Abass, S.; Kheir, M.M.; Mukhtar, M.; Elamin, E.M.; Elhassan, A.M. Mucosal leishmaniasis in a Sudanese patient. Am. J. Trop. Med. Hyg. 2009, 80, 935–938. [Google Scholar] [CrossRef]
- Sethuraman, G.; Sharma, V.K.; Salotra, P. Indian mucosal leishmaniasis due to Leishmania donovani infection. N. Engl. J. Med. 2008, 358, 313–315. [Google Scholar] [CrossRef]
- Pulimood, S.; Rupali, P.; Ajjampur, S.; Thomas, M.; Mehrotra, S.; Sundar, S. Atypical mucocutaneous involvement with Leishmania donovani. Natl. Med. J. India 2012, 25, 148–150. [Google Scholar]
- Rathnayake, D.; Ranawake, R.R.; Sirimanna, G.; Siriwardhane, Y.; Karunaweera, N.; De Silva, R. Co-infection of mucosal leishmaniasis and extra pulmonary tuberculosis in a patient with inherent immune deficiency. Int. J. Dermatol. 2010, 49, 549–551. [Google Scholar] [CrossRef]
- Grech, P.; Vella, S.M.; Piscopo, T. Leishmania donovani mucosal leishmaniasis in Malta. BMJ Case Rep. CP 2020, 13, e237687. [Google Scholar] [CrossRef]
- Varghese, L.; Laxmanan, S.; Varghese, G.M. Mucosal leishmaniasis due to Leishmania donovani—A rare presentation. Ear Nose Throat J. 2022, 101, 226–227. [Google Scholar] [CrossRef]
- Kharfi, M.; Fazaa, B.; Chaker, E.; Kamoun, M. Mucosal localization of leishmaniasis in Tunisia: 5 cases. Ann. Dermatol. Vénéréol. 2003, 130 Pt 1, 27–30. [Google Scholar]
- Faucher, B.; Pomares, C.; Fourcade, S.; Benyamine, A.; Marty, P.; Pratlong, L.; Faraut, F.; Mary, C.; Piarroux, R.; Dedet, J.-P. Mucosal Leishmania infantum leishmaniasis: Specific pattern in a multicentre survey and historical cases. J. Infect. 2011, 63, 76–82. [Google Scholar] [CrossRef]
- Cocuzza, S.; Strazzulla, A.; Pinzone, M.R.; Cosentino, S.; Serra, A.; Caltabiano, R.; Lanzafame, S.; Cacopardo, B.; Nunnari, G. Isolated laryngeal leishmaniasis in immunocompetent patients: An underdiagnosed disease. Case Rep. Infect. Dis. 2013, 2013, 165409. [Google Scholar] [CrossRef] [PubMed]
- Casolari, C.; Guaraldi, G.; Pecorari, M.; Tamassia, G.; Cappi, C.; Fabio, G.; Cesinaro, A.M.; Piolini, R.; Rumpianesi, F.; Presutti, L. A rare case of localized mucosal leishmaniasis due to Leishmania infantum in an immunocompetent Italian host. Eur. J. Epidemiol. 2005, 20, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Shirian, S.; Oryan, A.; Hatam, G.R.; Daneshbod, Y. Three Leishmania/L. species–L. infantum, L. major, L. tropica–as causative agents of mucosal leishmaniasis in Iran. Pathog. Glob. Health 2013, 107, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Shirian, S.; Oryan, A.; Hatam, G.R.; Daneshbod, Y. Mixed mucosal leishmaniasis infection caused by Leishmania tropica and Leishmania major. J. Clin. Microbiol. 2012, 50, 3805–3808. [Google Scholar] [CrossRef] [PubMed]
- ul Bari, A.; Yusuf, R.; Bangash, T.; Ejaz, A. Mucocutaneous leishmaniasis in central Punjab and Azad Kashmir regions of Pakistan. J. Pak. Assoc. Dermatol. 2012, 22, 191–196. [Google Scholar]
- Habibzadeh, F.; Sajedianfard, J.; Yadollahie, M. Isolated lingual leishmaniasis. J. Postgrad. Med. 2005, 51, 218. [Google Scholar] [PubMed]
- Reinecke, P.; Gabbert, H.; Strunk, W.; Lösche, C. Ocular scleromalacia caused by leishmaniasis: A rare cause of scleral perforation. Br. J. Ophthalmol. 2001, 85, 238. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Lopes, M.; Ito, F.; Carlos-Bregni, R.; De Almeida, O.; Roselino, A. Oral leishmaniasis: A clinicopathological study of 11 cases. Oral Dis. 2007, 13, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Benmously-Mlika, R.; Fenniche, S.; Kerkeni, N.; Aoun, K.; Khedim, A.; Mokhtar, I. Primary Leishmania infantum MON-80 endonasal leishmaniasis in Tunisia. Ann. Dermatol. Vénéréol. 2008, 135, 389–392. [Google Scholar] [CrossRef] [PubMed]
- García de Marcos, J.A.; Dean Ferrer, A.; Alamillos Granados, F.; Ruiz Masera, J.J.; Cortés Rodríguez, B.; Vidal Jiménez, A.; García Lainez, A.; Lozano Rodríguez-Mancheño, A. Localized leishmaniasis of the oral mucosa: A report of three cases. Med. Oral Patol. Oral Cir. Bucal (Internet) 2007, 12, 281–286. [Google Scholar]
- Bains, A.; Vedant, D.; Gupta, P.; Tegta, G. Unusual presentation of mucocutaneous leishmaniasis in HIV-infected patient. Indian J. Sex. Transm. Dis. AIDS 2016, 37, 193. [Google Scholar] [CrossRef]
- Passi, D.; Sharma, S.; Dutta, S.; Gupta, C. Localised leishmaniasis of oral mucosa: Report of an unusual clinicopathological entity. Case Rep. Dent. 2014, 2014, 753149. [Google Scholar] [CrossRef]
- Almeida, T.F.A.; da Silveira, E.M.; Dos Santos, C.R.R.; León, J.E.; Mesquita, A.T.M. Exclusive primary lesion of oral leishmaniasis with immunohistochemical diagnosis. Head Neck Pathol. 2016, 10, 533–537. [Google Scholar] [CrossRef]
- Yosef, T.; Harris, R.M.; Girma, S.; Dotevall, L.; Issa, A.; Ayele, A.; Bradley, M. Mucocutaneous Leishmaniasis with A Typical Clinical Manifestation: A Case Report. Ann. Clin. Med. Microbio 2018, 3, 1014. [Google Scholar]
- Falcão, G.G.V.S.C.; Lins-Kusterer, L.; Leite-Ribeiro, P.M.; Sarmento, V.A. Orofacial manifestations of mucocutaneous leishmaniasis: A case series from Brazil. F1000Research 2019, 8, 756. [Google Scholar] [CrossRef]
- Casalle, N.; de Barros Pinto Grifoni, L.; Bosco Mendes, A.C.; Delort, S.; Massucato, E.M.S. Mucocutaneous leishmaniasis with rare manifestation in the nasal mucosa and cartilage bone septal. Case Rep. Infect. Dis. 2020, 2020, 8876020. [Google Scholar] [CrossRef]
- Tegegne, B.; Alemu, G. Progress of mucocutaneous leishmaniasis to drug nonresponsive diffuse cutaneous leishmaniasis in Ethiopia. A case report. Int. Med. Case Rep. J. 2020, 13, 551. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.A.R.; Sugui, D.; Nunes, R.F.; de Azevedo, K.; de Azevedo, M.; Marques, A.; Martins, C.; Ferry, F.R.d.A. Mucocutaneous leishmaniasis/HIV coinfection presented as a diffuse desquamative rash. Case Rep. Infect. Dis. 2014, 2014, 293761. [Google Scholar] [CrossRef] [PubMed]
- Sujanitha, V.; Kumanan, T.; Felicia, S.; Suganthan, N. Cheilitis: An unusual presentation of mucocutaneous leishmaniasis. Clin. Case Rep. 2018, 6, 1383. [Google Scholar] [CrossRef] [PubMed]
- Tejura, N.; Kim, E.; Dever, L.L.; Chew, D. Case report: Mucocutaneous leishmaniasis masquerading as idiopathic midline granulomatous disease. Am. J. Trop. Med. Hyg. 2019, 101, 1107. [Google Scholar] [CrossRef]
- Figueiredo, L.P.; do Carmo Almeida, L.; Magalhães, A.; Arruda, S.; Lessa, M.M.; Carvalho, E.M. Case Report: Unusual Presentation of Pharyngeal Mucosal Leishmaniasis due to Leishmania (Viannia) braziliensis. Am. J. Trop. Med. Hyg. 2020, 103, 1493. [Google Scholar] [CrossRef]
- Hocar, O.; Aboudourib, M.; Akhdari, N.; Hamdaoui, A.; Mouttaki, T.; Soussi, M.; Chiheb, S.; Amal, S.; Riyad, M. Leishmaniose sublinguale pseudotumorale à Leishmania infantum. Ann. Dermatol. Vénéréol. 2020, 147, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Kassam, K.; Davidson, R.; Tadrous, P.; Kumar, M. Lingual leishmaniasis presenting to maxillofacial surgery in UK with successful treatment with miltefosine. Case Rep. Med. 2013, 2013, 975131. [Google Scholar] [CrossRef]
- Lopez-Carvajal, L.; Cardona-Arias, J.A.; Zapata-Cardona, M.I.; Sanchez-Giraldo, V.; Velez, I.D. Efficacy of cryotherapy for the treatment of cutaneous leishmaniasis: Meta-analyses of clinical trials. BMC Infect. Dis. 2016, 16, 360. [Google Scholar] [CrossRef]
- Ferede, G.; Diro, E.; Getie, S.; Getnet, G.; Takele, Y.; Amsalu, A.; Wondimeneh, Y. Visceral leishmaniasis-malaria coinfection and their associated factors in patients attending Metema Hospital, Northwest Ethiopia: Suggestion for integrated vector management. Malar. Res. Treat. 2017, 2017, 6816913. [Google Scholar] [CrossRef]
- Gregorio, A.; Vasconcellos, M.; Enokihara, M.; Guerra, J.; Nonogaki, S.; Tomimori, J. Cutaneous schistosomiasis and leishmaniasis coinfection: A case report. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1781–1783. [Google Scholar] [CrossRef] [PubMed]
- Selvapandiyan, A.; Ahuja, K.; Puri, N.; Krishnan, A. Implications of co-infection of Leptomonas in visceral leishmaniasis in India. Parasitology 2015, 142, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Thakur, L.; Kushwaha, H.R.; Negi, A.; Jain, A.; Jain, M. Leptomonas seymouri co-infection in cutaneous leishmaniasis cases caused by Leishmania donovani from Himachal Pradesh, India. Front. Cell. Infect. Microbiol. 2020, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Heirwegh, E.; MacLean, E.; He, J.; Kamhawi, S.; Sagan, S.M.; Olivier, M. Sandfly Fever Sicilian Virus-Leishmania major co-infection modulates innate inflammatory response favoring myeloid cell infections and skin hyperinflammation. PLoS Negl. Trop. Dis. 2021, 15, e0009638. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, M.; Boussaa, S.; Echchakery, M.; Boumezzough, A. Risk mapping of human HIV-Leishmaniasis co-infection in Morocco. Heliyon 2019, 5, e02419. [Google Scholar] [CrossRef] [PubMed]
- Kassardjian, A.A.; Yim, K.M.; Rabi, S.; Liang, T.Z.; Kim, G.H.; Ochoa, M.T.; Sattah, M.V.; Ahronowitz, I.Z. Diffuse Cutaneous Leishmaniasis and HIV Co-infection: A Case Report and Review of the Literature. J. Cutan. Pathol. 2021, 48, 802–806. [Google Scholar] [CrossRef]
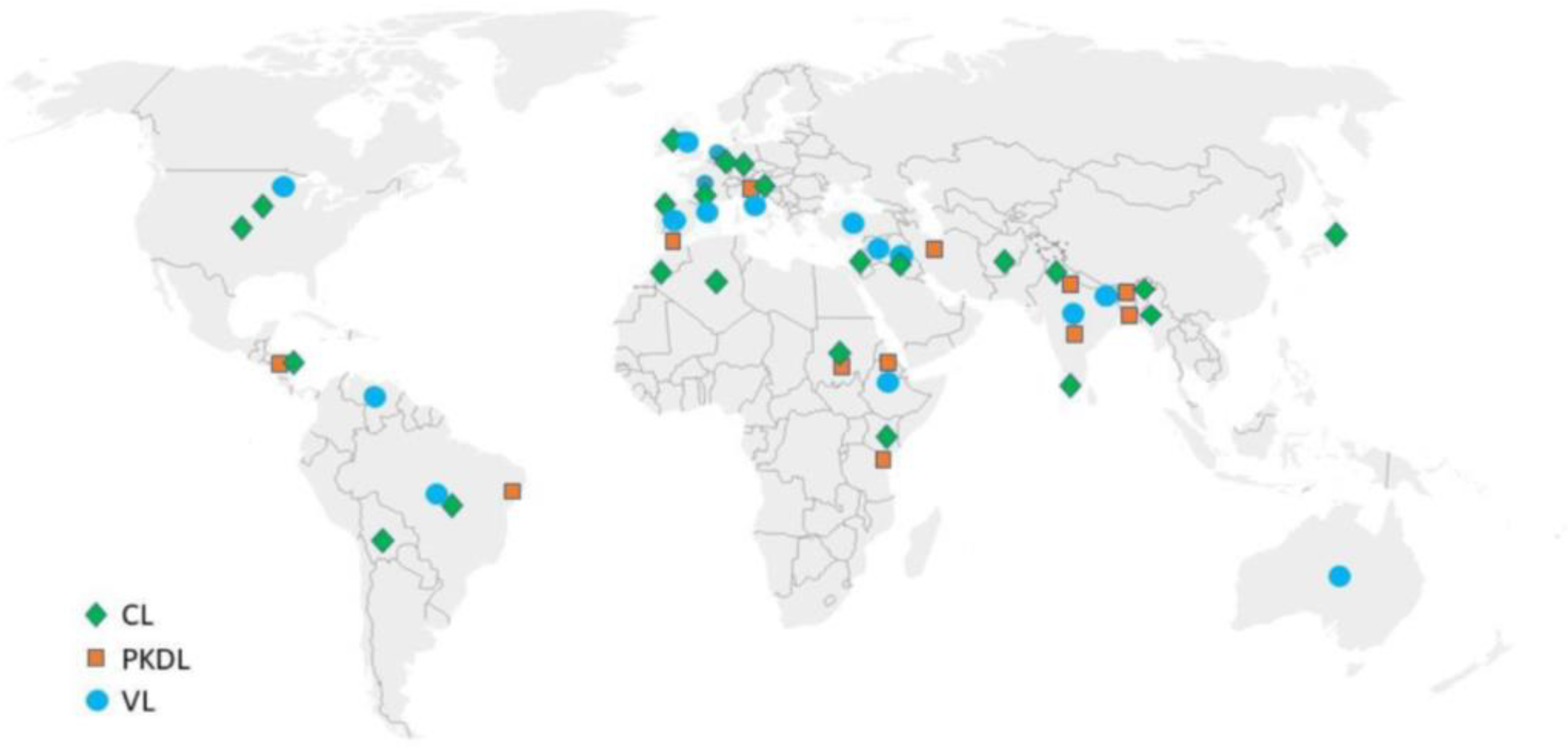
| Manifestation | Presentation | Species |
|---|---|---|
| Visceral leishmaniasis | Typical | L. donovani |
| L. infantum | ||
| L. chagasi L. (Mundinia) martiniquensis | ||
| Atypical | L. tropica | |
| Cutaneous leishmaniasis | Typical | L. major |
| L. tropica | ||
| L. mexicana | ||
| L. amazonensis | ||
| L. braziliensis L. (Mundinia) orientalis | ||
| Atypical | L. donovani | |
| L. infantum | ||
| Mucocutaneous leishmaniasis | Typical | L. amazonensis |
| L. braziliensis | ||
| L. panamensis | ||
| L. guyanensis | ||
| Atypical | L. donovani | |
| L. infantum | ||
| L. aethiopica | ||
| L. major | ||
| L. tropica |
| Disease Manifestations | Typical Manifestation | Unusual Observations | Plausible Mechanisms |
|---|---|---|---|
| Visceral leishmaniasis (VL) | Irregular bouts of fever, weight loss, anaemia, enlargement of spleen and liver LDB (amastigotes) spread to internal organs such as bone marrow, liver, spleen and lymph nodes through systemic circulation | Gastrointestinal tract, pulmonary system, larynx and skin are involved in addition to liver, spleen and bone marrow in in both immunocompromised and immune-competent cases LDB (amastigotes) seen in myelocytes, plasma cells and adrenal gland | Unusual presentation of an outcome of compromised immune response due to intrinsic poor immune system, co-infection with HIV or other pathogens Immune senescence leads to VL in geriatric population resulting in multiple relapses |
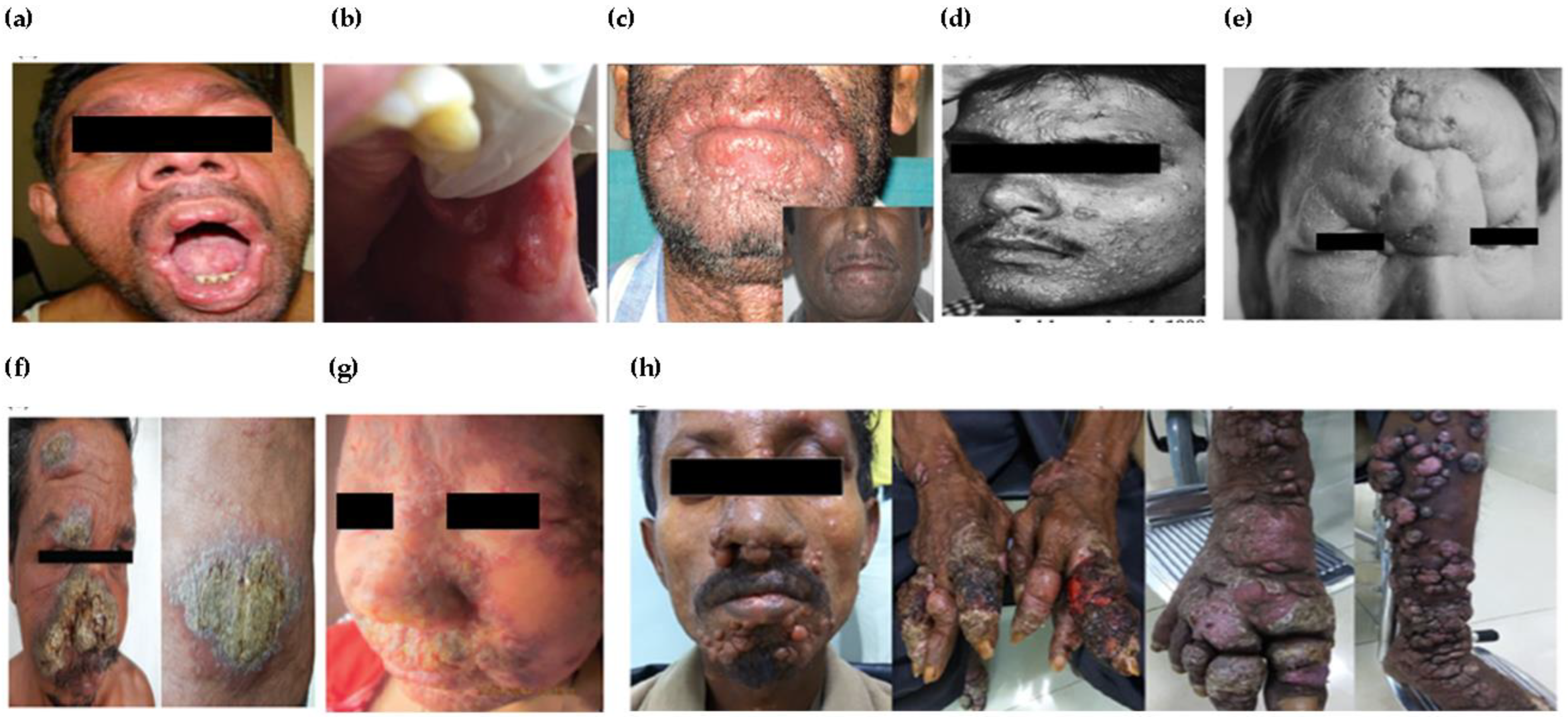
| Post-kala-azar dermal leishmaniasis (PKDL) | Dermal sequela of VL usually caused by L. donovani as a polymorphic presentation of macular, papular or nodular rash on face, upper arms, trunks and other parts of the body | Monomorphic presentation including macular, papular or papulonodular forms Localized or disseminated including mucosal, xanthomatous, verrucous, papillomatous, hypertrophic, fibroid, atrophic and extensive tumorous forms Lymph node and nerve involvement without impaired sensation Mucosa of genitalia, anus, lingual, perioral and oral cavity involved Indurated annular plaques with central clearing, irregular in shape, soft and non-tender juicy-looking papules, ulcerated lesions as seen in the tumorous, eroded and non-tender plaque Ocular leishmaniasis caused by L. donovani or dermotropic spp. causing permanent damage to the eyes, developed as post-kala-azar leishmanial conjunctivitis and blepharitis or post-kala-azar anterior uveitis | Ulcerations possibly due to repeated trauma Change in immune response from Th2 to a combined Th1/Th2 pattern underlines ocular leishmaniasis Antiretroviral therapy induced immune reconstitution syndrome among HIV–VL co-infected patients |
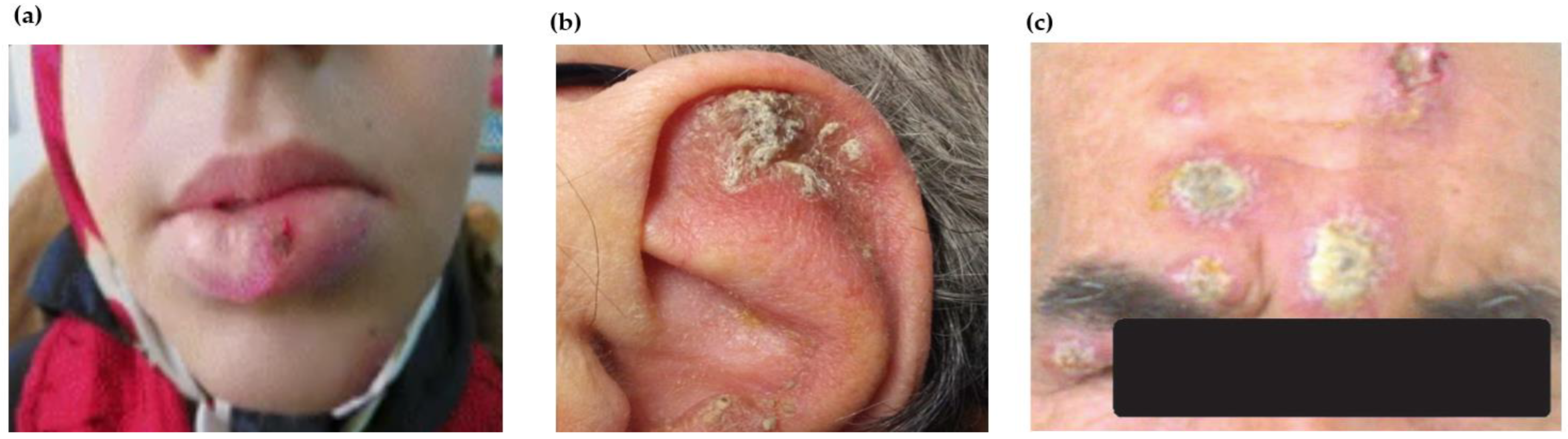
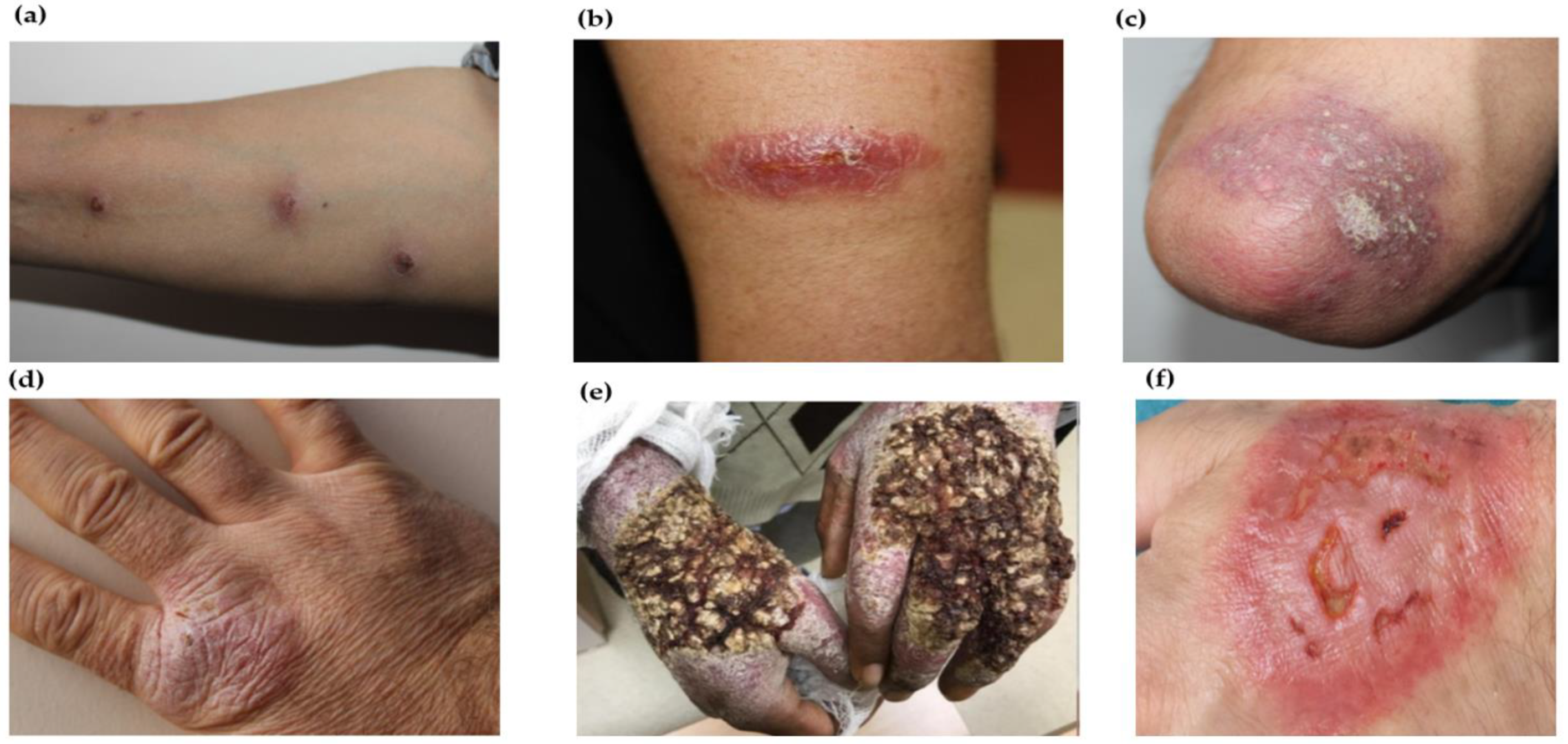
| Cutaneous leishmaniasis (CL) | Localized lesions at site of bite with changing appearance and size with course of time Mostly painless, however, may be painful pertaining to their presence near joints or due to bacterial infection | Atypical in terms of infecting species, e.g., VL-causing leishmania species causing cutaneous manifestations and vice versa CL at unusual sites including lesions in submandibular region mimic parotid neoplasm, auricle of ear, eyelids, haired and bald scalp, palm or lips, genitals (glans penis, scrotum) Morphological variants of CL lesions including erysipeloid form; chronic zosteriform CL in covered body parts; sporotrichoid form predominantly in upper limbs; lupoid form; leishmaniasis recidivans in the Old World regions or leishmaniasis recidiva cutis in New World regions Disseminated maculopapular rashes post relapse of VL | Genetic variations including gene polymorphisms or intra-species hybridization Alterations in immune response Treatment responses |
| Mucocutaneous leishmaniasis (MCL) | Metastatic sequela of New World cutaneous infection Dissemination of parasites from the skin to the naso- oropharyngeal mucosa causing degeneration and ultimately leading to destruction of the nasal septum | Oral leishmaniasis with the primary lesion (erythematous and oedematous) without involvement of cutaneous tissue Lesions on perioral mucosa, oro-facial mucosa, nasal mucosa, pharyngeal mucosa and cartilage bone septum, uvula, gingiva, soft palate, tonsils and epiglottis Recurrent epistaxis or nasal obstruction Primary endonasal leishmaniasis, focal hard whitish lesions on true vocal cords Ulcer with punched-out appearance extending to lip, smooth and erythematous swelling of lips (chelitis), granulomatous disease of endolarynx and oedema of oral mucosa resembling neoplasm Sublingual leishmaniasis with pseudotumoral morphology, lingual leishmaniasis with lymphoid-like tissue swelling Ocular scleromalacia, lesions on the conjunctiva of upper and lower eyelid, Disseminated MCL | Inadequate treatment of CL lesions Co-infection with interspecies strains |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, P.; Azam, M.; Ramesh, V.; Singh, R. Unusual Observations in Leishmaniasis—An Overview. Pathogens 2023, 12, 297. https://doi.org/10.3390/pathogens12020297
Yadav P, Azam M, Ramesh V, Singh R. Unusual Observations in Leishmaniasis—An Overview. Pathogens. 2023; 12(2):297. https://doi.org/10.3390/pathogens12020297
Chicago/Turabian StyleYadav, Priya, Mudsser Azam, V Ramesh, and Ruchi Singh. 2023. "Unusual Observations in Leishmaniasis—An Overview" Pathogens 12, no. 2: 297. https://doi.org/10.3390/pathogens12020297
APA StyleYadav, P., Azam, M., Ramesh, V., & Singh, R. (2023). Unusual Observations in Leishmaniasis—An Overview. Pathogens, 12(2), 297. https://doi.org/10.3390/pathogens12020297






