Does Experimental Reduction of Blacklegged Tick (Ixodes scapularis) Abundance Reduce Lyme Disease Incidence?
Abstract
:1. Introduction
2. Experimental Tick Reduction and Epidemiological Outcomes
2.1. Tick Reduction
| Reference | Location | Acaricidal Product | Efficacy | Placebo Control? | Non-placebo Control? | Masking (=Blinding)? | Randomization? | Sample Size (expt:cntl) |
|---|---|---|---|---|---|---|---|---|
| [23] | CT | carbaryl | 90–100% reduction in host-seeking nymphal I. scapularis | N | N | N | N | 5:0 |
| [23] | CT | permethrin (“Damminix”) | 31–91% reduction in immature I. scapularis on mice, no effect on host-seeking nymphs | N | Y | N | N | 5:5 |
| [22] | NY | carbaryl | 68–78% reduction in host-seeking nymphal I. scapularis | N | Y | N | N | ~30:24 * |
| [22] | NY | chlorpyrifos | 87–97% reduction in host-seeking nymphal I. scapularis | N | Y | N | N | ~30:24 * |
| [22] | NY | cyfluthrin | 92% reduction in host-seeking nymphal I. scapularis | N | Y | N | N | ~30:24 * |
| [40] | NY | permethrin (“Damminix”) | <65% reduction in immature I. scapularis on mice, no effect on host-seeking nymphs | N | Y | N | N | 3:3 |
| [41] | MA | permethrin (“Damminix”) | ~100% reduction in immature I. scapularis on mice, ~100% reduction in host-seeking nymphs | N | Y | N | N | 1:2 |
| [26] | CT | fipronil (bait box) | 68-84% reduction in immature I. scapularis on mice, 77% reduction in host-seeking adults, 64–67% reduction in infection prevalence in nymphs | N | Y | N | N | 154:6 |
| [34] | NJ | Amitraz (4-poster), deltamethrin, fipronil (bait box) | >80% reduction in immature I. scapularis on mice, 56–94% reduction in host-seeking nymphs | N | Y | N | N | 1:1 (4-poster) 13:1 (deltametrhin, bait box) |
| [35] | NJ | deltamethrin | 80-100% reduction in host-seeking nymphs and adults | N | Y | N | N | 2:2 |
| [30] | CT, MD, NJ, NY | Amitraz (4-poster) | 71% reduction in host-seeking nymphs after 6 years (meta-analysis) | N | Y | N | N | 1:1 (each state) 4:4 (altogether) |
| [42] | ME | bifenthrin | 100% reduction in host-seeking larvae, nymphs, adults | N | Y | N | N | 3:3 |
| [33] | ME | “Eco-exempt IC2” botanical extract, bifenthin | 100% reduction in host-seeking larvae, nymphs, adults | N | Y | N | N | 3:3 |
| [6] | CT, MD, NY | bifenthrin | 63% reduction in host-seeking nymphs, no effect on tick encounters or cases of tick-borne disease | Y | N | Y | Y | 1362:1365 |
| [28] | NJ | fipronil (bait box) | 90–97% reduction in host-seeking nymphal I. scapularis | N | Y | N | N | 12:1 |
| [38] | CT | fungal biocide “Met52” plus fipronil (bait box) | 17-fold reduction in infected immature I. scapularis on mice | N | Y | N | N | 6:6 (2013) 12:13 (2014–2015) |
| [29] | CT | fungal biocide “Met52” plus fipronil (bait box) | 53% reduction in encounters with host-seeking I. scapularis nymphs, 77–97% reduction in host-seeking I. scapularis | N | Y | N | N | 6:6 (2013) 12:13 (2014–2015) |
| [37] | CT | fungal biocide “Met52” plus fipronil (bait box) | 93% reduction in probability of encountering infected I. scapularis nymph, 52% reduction in host-seeking nymphs | N | Y | N | N | 13:12 |
| [36] | CT | fipronil (bait box) | no effect, host-seeking nymphs, no effect infection prevalence, no effect on tick encounters or cases of tick-borne disease | Y | N | Y | Y | 51:49 |
| [43] | QU | fluralaner | 68–74% reduction in immature I. scapularis on mice | N | Y | N | N | 7:4 |
| [39] | NY | fipronil (bait box) | ~50% reduction in host-seeking I. scapularis nymphs, no effect on tick encounters or cases of tick-borne disease | Y | N | Y | Y | 12:12 |
| [39] | NY | fungal biocide “Met52” | No effect on host-seeking I. scapularis nymphs, no effect on tick encounters or cases of tick-borne disease | Y | N | Y | Y | 12:12 |
| [44] | NY | fipronil (bait box) and fungal biocide “Met52” | No effect on infection prevalence of I. scapularis with tick-borne pathogens | Y | N | Y | Y | 12:12 |
2.2. Effects on Disease Incidence
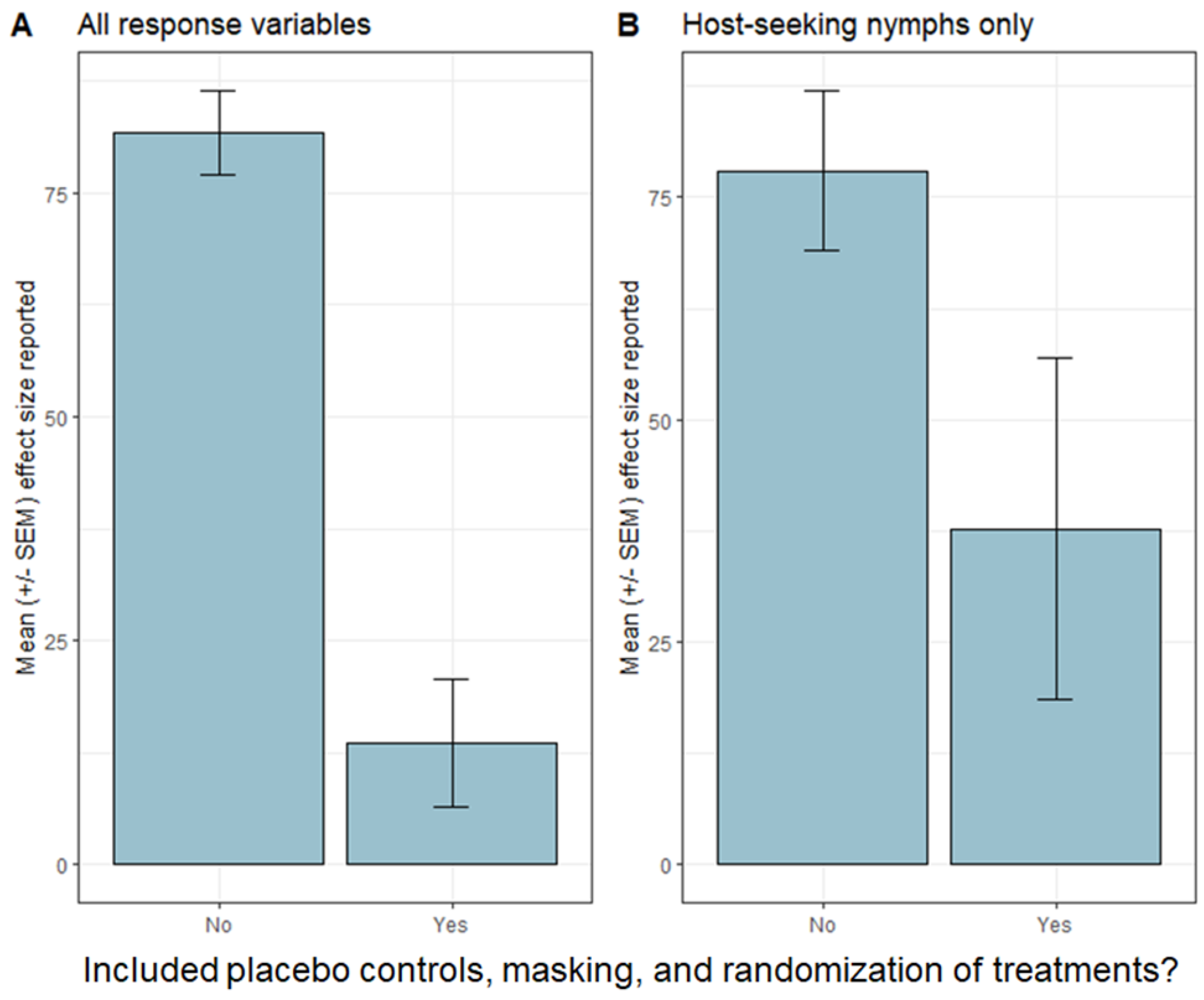
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostfeld, R.S. Lyme Disease: The Ecology of a Complex System; Oxford University Press: New York, NY, USA, 2012; p. 232. [Google Scholar]
- Eisen, R.J.; Eisen, L. The blacklegged tick, Ixodes scapularis: An increasing public health concern. Trends Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef]
- Mead, P.; Hook, S.; Niesobecki, S.; Ray, J.; Meek, J.; Delorey, M.; Prue, C.; Hinckley, A. Risk factors for tick exposure in suburban settings in the Northeastern United States. Ticks Tick-borne Dis. 2018, 9, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Fischhoff, I.R.; Keesing, F.; Ostfeld, R.S. Risk factors for bites and diseases associated with blacklegged ticks: A meta-analysis. Am. J. Epidemiol. 2019, 188, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.B.; Piesman, J. How can we prevent Lyme disease? N. Engl. J. Med. 2003, 348, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, A.F.; Meek, J.I.; Ray, J.A.E.; Niesobecki, S.A.; Connally, N.P.; Feldman, K.A.; Jones, E.H.; Backenson, P.B.; White, J.L.; Lukacik, G.; et al. Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J. Infect. Dis. 2016, 214, 182–188. [Google Scholar] [CrossRef]
- Eisen, L. Stemming the rising tide of human-biting ticks and tickborne diseases, United States. Emerg. Inf. Dis. 2020, 26, 641–647. [Google Scholar] [CrossRef]
- Eisen, L.; Stafford, K.C. Barriers to effective tick management and tick-bite prevention in the United States (Acari: Ixodidae). J. Med. Entomol. 2021, 58, 1588–1600. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Price, A.; Hornbostel, V.L.; Benjamin, M.A.; Keesing, F. Controlling ticks and tick-borne zoonoses with biological and chemical agents. Bioscience 2006, 56, 383–394. [Google Scholar] [CrossRef]
- Keesing, F.; Brunner, J.; Duerr, S.; Killilea, M.; LoGiudice, K.; Schmidt, K.; Vuong, H.; Ostfeld, R.S. Hosts as ecological traps for the vector of Lyme disease. Proc. R. Soc. B Biol. Sci. 2009, 276, 3911–3919. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J. Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme disease cases. J. Med. Entomol. 2016, 53, 1050–1062. [Google Scholar] [CrossRef]
- Ginsberg, H.S. Transmission risk of Lyme disease and implications for tick management. Am. J. Epidemiol. 1993, 138, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A. Prevention of Lyme disease: A review of the evidence. Mayo Clin. Proc. 2001, 76, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Piesman, J.; Zielinski-Gutierrez, E.; Eisen, L. What do we need to know about disease ecology to prevent Lyme disease in the northeastern United States? J. Med. Entomol. 2012, 49, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.A.; Nawrocki, C.C.; Meek, J.I.; Feldman, K.A.; White, J.L.; Connally, N.P.; Hinckley, A.F. Human-tick encounters as a measure of tickborne disease risk in Lyme disease endemic areas. Zoonoses Public Health 2021, 68, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.J.; Falco, R.C.; McHugh, E.E.; Vellozzi, J.; Boccia, T.; Denicola, A.J.; Pound, J.M.; Miller, J.A.; George, J.E.; Fish, D. Acaricidal treatment of white-tailed deer to control Ixodes scapularis (Acari: Ixodidae) in a New York Lyme disease-endemic community. Vector-Borne Zoonotic Dis. 2009, 4, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Stafford, K.C., III; Denicola, A.J.; Pound, J.M.; Miller, J.A.; George, J.E. Topical treatment of white-tailed deer with an acaricide for the control of Ixodes scapularis (Acari: Ixodidae) in a Connecticut Lyme borreliosis hyperendemic community. Vector-Borne Zoonotic Dis. 2009, 4, 371–379. [Google Scholar] [CrossRef]
- Stafford, K.C., III; Williams, S.C.; Molaei, G. Integrated pest management in controlling ticks and tick-associated diseases. J. Integrated Pest Manag. 2017, 8, 12–28. [Google Scholar] [CrossRef]
- Jordan, R.A.; Schulze, T.L. Availability and nature of commercial tick control services in three Lyme disease endemic states. J. Med. Entomol. 2020, 57, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Falco, R.; Fish, D. Prevalence of Ixodes dammini near the homes of Lyme disease patients in Westchester County, New York. Am. J. Epidemiol. 1988, 127, 826–830. [Google Scholar] [CrossRef]
- Keesing, F.; Ostfeld, R.S. The Tick Project: Testing environmental methods of preventing tick-borne diseases. Trends Parasitol. 2018, 34, 447–450. [Google Scholar] [CrossRef]
- Curran, K.L.; Fish, D.; Piesman, J. Reduction of nymphal Ixodes dammini (Acari: Ixodidae) in a residential suburban landscape by area application of insecticides. J. Med. Entomol. 1993, 30, 107–113. [Google Scholar] [CrossRef]
- Stafford, K.C., III. Effectiveness of host-targeted permethrin in the control of Ixodes dammini (Acari: Ixodidae). J. Med. Ent. 1991, 28, 611–617. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Hung, R.W.; Taylor, R.C.; Markowski, D.; Chomsky, M.S. Efficacy of granular deltamethrin against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J. Med. Ent. 2001, 38, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.A.; Schulze, T.L.; Eisen, L.; Dolan, M.C. Ability of three general-use pesticides to suppress nymphal Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Am. Mosq. Contro Assoc. 2017, 33, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Dolan, M.C.; Maupin, G.O.; Schneider, B.S.; Denatale, C.; Hamon, N.; Cole, C.; Zeidner, N.S.; Stafford, K.C. Control of immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of Southeastern Connecticut. J. Med. Entomol. 2004, 41, 1043–1054. [Google Scholar] [CrossRef]
- Jordan, R.A.; Schulze, T.L. Ability of two commercially available host-targeted technologies to reduce abundance of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J. Med. Entomol. 2019, 56, 1095–1101. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Williams, M.; Dolan, M.C. Evaluation of the SELECT tick control system (TCS), a host-targeted bait box, to reduce exposure to Ixodes scapularis (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey. J. Med. Entomol. 2017, 54, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C.; Stafford, K.C.; Molaei, G.; Linske, M.A. Integrated control of nymphal Ixodes scapularis: Effectiveness of white-tailed deer reduction, the entomopathogenic fungus Metarhizium anisopliae, and fipronil-based rodent bait boxes. Vector-Borne Zoonot. Dis. 2018, 18, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Brei, B.; Brownstein, J.S.; George, J.E.; Pound, J.M.; Miller, J.A.; Daniels, T.J.; Falco, R.C.; Stafford, K.C., 3rd; Schulze, T.L.; Mather, T.N.; et al. Evaluation of the United States Department of Agriculture northeast area-wide tick control project by meta-analysis. Vector-Borne Zoonotic Dis. 2009, 9, 423–430. [Google Scholar] [CrossRef]
- Grimes, D.A.; Schulz, K.F. Bias and causal associations in observational research. Lancet 2002, 359, 248–252. [Google Scholar] [CrossRef]
- Kaptchuk, T.J. The double-blind, randomized, placebo-controlled trial. J. Clin. Epidemiol. 2001, 54, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.P.; Lubelczyk, C.B.; Rand, P.W.; Staples, J.K.; St. Amand, T.W.; Stubbs, C.S.; Lacombe, E.H.; Smith, L.B.; Smith, R.P., Jr. Effect of a botanical acaricide on Ixodes scapularis (Acari: Ixodidae) and nontarget arthropods. J. Med. Entomol. 2013, 50, 126–136. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Schulze, C.J.; Healy, S.P.; Jahn, M.B.; Piesman, J. Integrated use of 4-poster passive topical treatment devices for deer, targeted acaricide applications, and Maxforce TMS bait boxes to rapidly suppress populations of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J. Med. Entomol. 2007, 44, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A.; Schulze, C.J.; Healy, S.P. Suppression of Ixodes scapularis (Acari: Ixodidae) following annual habitat-targeted acaricide applications against fall populations of adults. J. Mosq. Control Assoc. 2008, 24, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, A.F.; Niesobecki, S.A.; Connally, N.P.; Hook, S.A.; Biggerstaff, B.J.; Horiuchi, K.A.; Hojgaard, A.; Mead, P.S.; Meek, J.I. Prevention of Lyme and other tickborne diseases using a rodent-targeted approach: A randomized controlled trial in Connecticut. Zoonoses Public Health 2021, 68, 578–587. [Google Scholar] [CrossRef]
- Little, E.A.H.; Williams, S.C.; Stafford, K.C.; Linske, M.A.; Molaei, G. Evaluating the effectiveness of an integrated tick management approach on multiple pathogen infection in Ixodes scapularis questing nymphs and larvae parasitizing white-footed mice. Exp. Appl. Acarol. 2020, 80, 127–136. [Google Scholar] [CrossRef]
- Williams, S.C.; Little, E.A.H.; Stafford, K.C.; Molaei, G.; Linske, M.A. Integrated control of juvenile Ixodes scapularis parasitizing Peromyscus leucopus in residential settings in Connecticut, United States. Ticks Tick-borne. Dis. 2018, 9, 1310–1316. [Google Scholar] [CrossRef]
- Keesing, F.; Mowry, S.; Bremer, W.; Duerr, S.; Evans, A.S.; Fischhoff, I.R.; Hinckley, A.F.; Hook, S.A.; Keating, F.; Pendleton, J.; et al. Effects of tick-control interventions on tick abundance, human encounters with ticks, and incidence of tickborne diseases in residential neighborhoods, New York, USA. Emerg. Infect. Dis. 2022, 28, 957–966. [Google Scholar] [CrossRef]
- Daniels, T.J.; Fish, D.; Falco, R.D. Evaluation of host-targeted acaricide for reducing risk of Lyme disease in southern New York State. J. Med. Entomol. 1991, 28, 537–543. [Google Scholar] [CrossRef]
- Deblinger, R.D.; Rimmer, D.W. Efficacy of a permethrin-based acaricide to reduce the abundance of Ixodes dammini. J. Med. Entomol. 1991, 28, 708–711. [Google Scholar] [CrossRef]
- Rand, P.W.; Lacombe, E.H.; Elias, S.P.; Lubelczyk, C.B.; St. Amand, T.; Smith, R.P. Trial of a minimal-risk botanical compound to control the vector tick of Lyme disease. J. Med. Entomol. 2010, 47, 695–698. [Google Scholar] [CrossRef]
- Pelletier, J.; Rocheleau, J.-P.; Aenishaenslin, C.; Dimitri Masson, G.; Lindsay, R.; Ogden, N.; Bouchard, C.; Leighton, P. Fluralaner baits reduce the infestation of Peromyscus spp. mice (Rodentia: Cricetidae) by Ixodes scapularis (Acari: Ixodidae) larvae and nymphs in a natural environment. J. Med. Entomol. 2022, 59, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.S.; Mowry, S.; Bremer, W.; Duerr, S.; Evans, A.S., Jr.; Fischhoff, I.R.; Hinckley, A.F.; Hook, S.A.; Keating, F.; Pendleton, J.; et al. Impacts over time of neighborhood-scale interventions to control ticks and tick-borne disease incidence. Vector-Borne Zoonot. Dis. 2023, 23, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Connally, N.P.; Rowe, A.; Kaufman, A.; Meek, J.I.; Niesobecki, S.A.; Hansen, A.P.; White, J.; Nawrocki, C.; Foster, K.; Hinckley, A.F.; et al. Designing an intervention trial of human-tick encounters and tick-borne diseases in residential settings using 4-poster devices to control Ixodes scapularis (Acari: Ixodidae): Challenges for site selection and device placement. J. Med. Entomol. 2022, 59, 911–921. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostfeld, R.S.; Keesing, F. Does Experimental Reduction of Blacklegged Tick (Ixodes scapularis) Abundance Reduce Lyme Disease Incidence? Pathogens 2023, 12, 714. https://doi.org/10.3390/pathogens12050714
Ostfeld RS, Keesing F. Does Experimental Reduction of Blacklegged Tick (Ixodes scapularis) Abundance Reduce Lyme Disease Incidence? Pathogens. 2023; 12(5):714. https://doi.org/10.3390/pathogens12050714
Chicago/Turabian StyleOstfeld, Richard S., and Felicia Keesing. 2023. "Does Experimental Reduction of Blacklegged Tick (Ixodes scapularis) Abundance Reduce Lyme Disease Incidence?" Pathogens 12, no. 5: 714. https://doi.org/10.3390/pathogens12050714
APA StyleOstfeld, R. S., & Keesing, F. (2023). Does Experimental Reduction of Blacklegged Tick (Ixodes scapularis) Abundance Reduce Lyme Disease Incidence? Pathogens, 12(5), 714. https://doi.org/10.3390/pathogens12050714





