Detection of Merkel Cell Polyomavirus (MCPyV) DNA and Transcripts in Merkel Cell Carcinoma (MCC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples Collection
2.2. Histological Diagnosis
2.3. DNA Extraction
2.4. Real Time Polymerase Chain Reaction (qPCR)
2.5. MCPyV LTAg, NCCR and VP1 Standard PCR and Sequencing
2.6. LT and VP1 Gene Expression
2.7. Analysis of the MCPyV Integration Sites
2.8. Sequencing Analysis of the MCPyV LT Gene
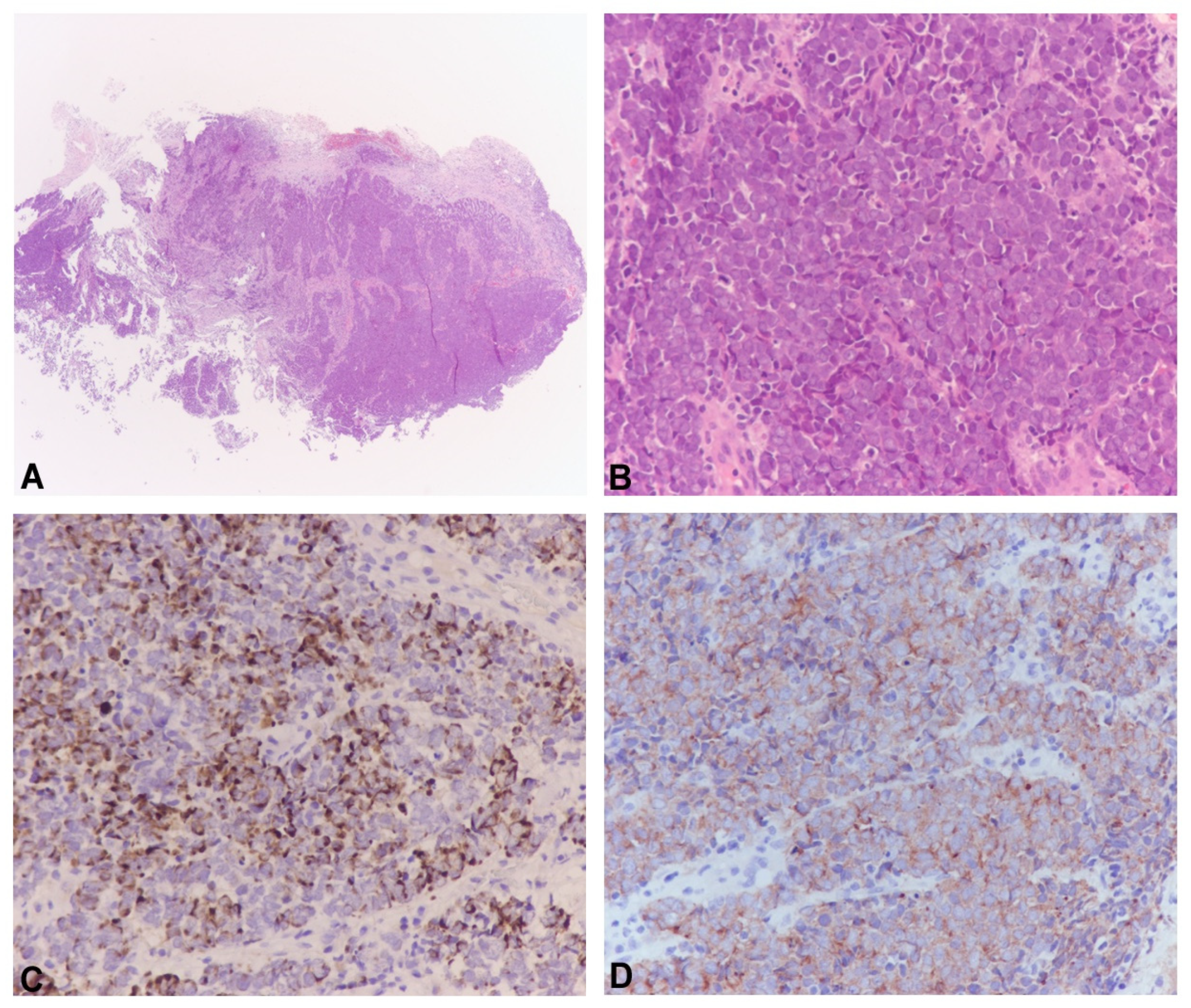
3. Results
3.1. Detection of MCPyV DNA by Real-Time PCR (qPCR) and Standard PCR
3.2. MCPyV NCCR and VP1 Sequence Analysis
3.3. Expression of LTAg and VP1 Transcripts
3.4. Integration Analysis of MCPyV
3.5. DNA Sequencing Analysis of the MCPyV LT Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pietropaolo, V.; Prezioso, C.; Moens, U. Merkel Cell Polyomavirus and Merkel Cell Carcinoma. Cancers 2020, 12, 1774. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.W.; Qazi, J.; Hippe, D.S.; Lachance, K.; Thomas, H.; Cook, M.M.; Juhlin, I.; Singh, N.; Thuesmunn, Z.; Takagishi, S.R.; et al. Patterns of distant metastases in 215 Merkel cell carcinoma patients: Implications for prognosis and surveillance. Cancer Med. 2020, 9, 1374–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwijnenburg, E.M.; Lubeek, S.F.K.; Werner, J.E.M.; Amir, A.L.; Weijs, W.L.J.; Takes, R.P.; Pegge, S.A.H.; van Herpen, C.M.L.; Adema, G.J.; Kaanders, J.H.A.M. Merkel Cell Carcinoma: New Trends. Cancers 2021, 13, 1614. [Google Scholar] [CrossRef] [PubMed]
- Pulitzer, M.P.; Amin, B.D.; Busam, K.J. Merkel cell carcinoma: Review. Adv. Anat. Pathol. 2009, 16, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbe, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17077. [Google Scholar] [CrossRef]
- Kervarrec, T.; Tallet, A.; Miquelestorena-Standley, E.; Houben, R.; Schrama, D.; Gambichler, T.; Berthon, P.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Aubin, F.; et al. Diagnostic accuracy of a panel of immunohistochemical and molecular markers to distinguish Merkel cell carcinoma from other neuroendocrine carcinomas. Mod. Pathol. 2019, 32, 499–510. [Google Scholar] [CrossRef]
- DeCaprio, J.A. Molecular Pathogenesis of Merkel Cell Carcinoma. Annu. Rev. Pathol. 2021, 16, 69–91. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; You, J. Molecular Mechanisms of Merkel Cell Polyomavirus Transformation and Replication. Annu. Rev. Virol. 2020, 7, 289–307. [Google Scholar] [CrossRef]
- Lee, S.; Paulson, K.G.; Murchison, E.P.; Afanasiev, O.K.; Alkan, C.; Leonard, J.H.; Byrd, D.R.; Hannon, G.J.; Nghiem, P. Identification and validation of a novel mature microRNA encoded by the Merkel cell polyomavirus in human Merkel cell carcinomas. J. Clin. Virol. 2011, 52, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Konstatinell, A.; Coucheron, D.H.; Sveinbjørnsson, B.; Moens, U. MicroRNAs as Potential Biomarkers in Merkel Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moens, U.; Prezioso, C.; Pietropaolo, V. Genetic Diversity of the Noncoding Control Region of the Novel Human Polyomaviruses. Viruses 2020, 12, 1406. [Google Scholar] [CrossRef] [PubMed]
- Csoboz, B.; Rasheed, K.; Sveinbjørnsson, B.; Moens, U. Merkel cell polyomavirus and non-Merkel cell carcinomas: Guilty or circumstantial evidence? APMIS 2020, 128, 104–120. [Google Scholar] [CrossRef] [Green Version]
- DeCoste, R.C.; Carter, M.D.; Ly, T.Y.; Gruchy, J.R.; Nicolela, A.P.; Pasternak, S. Merkel cell carcinoma: An update. Hum. Pathol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Feng, H.; Kwun, H.J.; Rosen, S.T.; Gjoerup, O.; Moore, P.S.; Chang, Y. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc. Natl. Acad. Sci. USA 2008, 105, 16272–16277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, X.; Diaz, J.; Tsang, S.H.; Buck, C.B.; You, J. Merkel cell polyomavirus large T antigen disrupts host genomic integrity and inhibits cellular proliferation. J. Virol. 2013, 87, 9173–9188. [Google Scholar] [CrossRef] [Green Version]
- Starrett, G.J.; Thakuria, M.; Chen, T.; Marcelus, C.; Cheng, J.; Nomburg, J.; Thorner, A.R.; Slevin, M.K.; Powers, W.; Burns, R.T.; et al. Clinical and molecular characterization of virus-positive and virus-negative Merkel cell carcinoma. Genome Med. 2020, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Böhling, T.; Joensuu, H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J. Natl. Cancer Inst. 2009, 101, 938–945. [Google Scholar] [CrossRef]
- Arora, R.; Gupta, K.; Vijaykumar, A.; Krishna, S. DETECTing Merkel Cell Polyomavirus in Merkel Tumors. Front. Mol. Biosci. 2020, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Saiki, R.K.; Bugawan, T.L.; Horn, G.T.; Mullis, K.B.; Erlich, H.A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature 1986, 324, 163–166. [Google Scholar] [CrossRef]
- Rodig, S.J.; Cheng, J.; Wardzala, J.; DoRosario, A.; Scanlon, J.J.; Laga, A.C.; Martinez-Fernandez, A.; Barletta, J.A.; Bellizzi, A.M.; Sadasivam, S.; et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J. Clin. Investig. 2012, 122, 4645–4653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashida, Y.; Imajoh, M.; Nemoto, Y.; Kamioka, M.; Taniguchi, A.; Taguchi, T.; Kume, M.; Orihashi, K.; Daibata, M. Detection of Merkel cell polyomavirus with a tumour-specific signature in non-small cell lung cancer. Br. J. Cancer. 2013, 108, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, C.; Bianchi, M.; Obregon, F.; Ciotti, M.; Sarmati, L.; Andreoni, M.; Palamara, A.T.; Pascarella, S.; Moens, U.; Pietropaolo, V. Structural Analysis of Merkel Cell Polyomavirus (MCPyV) Viral Capsid Protein 1 (VP1) in HIV-1 Infected Individuals. Int. J. Mol. Sci. 2020, 21, 7998. [Google Scholar] [CrossRef] [PubMed]
- ClustalW2–Multiple Sequence Alignment. Available online: http://www.ebi.ac.uk/Tools/msa/clustalw2/ (accessed on 12 May 2023).
- Sastre-Garau, X.; Peter, M.; Avril, M.F.; Laude, H.; Couturier, J.; Rozenberg, F.; Almeida, A.; Boitier, F.; Carlotti, A.; Couturaud, B.; et al. Merkel cell carcinoma of the skin: Pathological and molecular evidence for a causative role of MCV in oncogenesis. J. Pathol. 2009, 218, 48–56. [Google Scholar] [CrossRef]
- Luft, F.; Klaes, R.; Nees, M.; Dürst, M.; Heilmann, V.; Melsheimer, P.; von Knebel Doeberitz, M. Detection of integrated papillomavirus sequences by ligation-mediated PCR (DIPS-PCR) and molecular characterization in cervical cancer cells. Int. J. Cancer 2001, 92, 9–17. [Google Scholar] [CrossRef]
- zur Hausen, H. A specific signature of Merkel cell polyomavirus persistence in human cancer cells. Proc. Natl. Acad. Sci. USA 2008, 105, 16063–16064. [Google Scholar] [CrossRef] [Green Version]
- Martel-Jantin, C.; Filippone, C.; Cassar, O.; Peter, M.; Tomasic, G.; Vielh, P.; Brière, J.; Petrella, T.; Aubriot-Lorton, M.H.; Mortier, L.; et al. Genetic variability and integration of Merkel cell polyomavirus in Merkel cell carcinoma. Virology 2012, 426, 134–142. [Google Scholar] [CrossRef]
- Laude, H.C.; Jonchère, B.; Maubec, E.; Carlotti, A.; Marinho, E.; Couturaud, B.; Peter, M.; Sastre-Garau, X.; Avril, M.F.; Dupin, N.; et al. Distinct merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with merkel cell carcinoma. PLoS Pathog. 2010, 6, e1001076. [Google Scholar] [CrossRef]
- DeCoste, R.C.; Walsh, N.M.; Gaston, D.; Ly, T.Y.; Pasternak, S.; Cutler, S.; Nightingale, M.; Carter, M.D. RB1-deficient squamous cell carcinoma: The proposed source of combined Merkel cell carcinoma. Mod. Pathol. 2022, 35, 1829–1836. [Google Scholar] [CrossRef]
- Liu, C.Y.; Kang, N.W.; Takeuchi, K.; Chuang, S.S. Combined Merkel Cell Carcinoma with Nodal Presentation: Report of a Case Diagnosed with Excisional but Not Incisional Biopsy and Literature Review. Diagnostics 2023, 13, 449. [Google Scholar] [CrossRef]
- Navarrete, J.; Gugelmeier, N.; Mazzei, M.E.; González, S.; Barcia, J.J.; Magliano, J. Lymph Node Metastasis with Both Components of Combined Cutaneous Squamous Cell Carcinoma/Merkel Cell (Neuroendocrine) Carcinoma. Am. J. Dermatopathol. 2018, 40, 626–628. [Google Scholar] [CrossRef]
- Hayashi, D.; Kusutani, N.; Sowa-Osako, J.; Kamo, R.; Hayashi, E.; Ohsawa, M.; Goto, K.; Tsuruta, D. Combined Merkel cell carcinoma and sebaceous carcinoma in the eyelid with cervical lymph node metastasis of both components. J. Dermatol. 2021, 48, e175–e177. [Google Scholar] [CrossRef]
- Ríos-Viñuela, E.; Traves, V.; Cruz, J.; Machado, I.; López-Guerrero, J.A.; Requena, C.; Llombart, B. Combined Merkel cell carcinoma and cutaneous squamous cell carcinoma with lymph node metastases: Report of two cases. J. Cutan. Pathol. 2023, 50, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Loke, A.S.W.; Lambert, P.F.; Spurgeon, M.E. Current In Vitro and In Vivo Models to Study MCPyV-Associated MCC. Viruses 2022, 14, 2204. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, M.E.; Mangelberger, D.; Harms, P.W.; Eberl, M.; Wilbert, D.M.; Meireles, J.; Bichakjian, C.K.; Saunders, T.L.; Wong, S.Y.; Dlugosz, A.A. Merkel Cell Polyomavirus Small T Antigen Initiates Merkel Cell Carcinoma-like Tumor Development in Mice. Cancer Res. 2017, 77, 3151–3157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patient | Age, Gender | Site | β-Globin DNA | Viral Load (gEq/mL) | LT | NCCR | VP1 | β-Globin cDNA | LT | VP1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LT1 | LT3 | Transcript | Transcript | ||||||||
| 1 | 81, M | Left arm | + | 8.10 × 102 | + | + | canonical | canonical | + | + | - |
| Lymph node | + | - | - | - | - | - | + | - | - | ||
| 2 | 70, M | Right thigh | + | 7.50 × 102 | + | + | canonical | canonical | + | + | - |
| Lymph node | + | 5.35 × 102 | + | + | canonical | canonical | + | + | - | ||
| Patients | Viral Junction | Cellular Junction |
|---|---|---|
| 1 | 5′-2738 (LT) | 5q23.1 |
| 2 | 5′-2597 (LT) | 5q11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passerini, S.; Prezioso, C.; Babini, G.; Ferlosio, A.; Cosio, T.; Campione, E.; Moens, U.; Ciotti, M.; Pietropaolo, V. Detection of Merkel Cell Polyomavirus (MCPyV) DNA and Transcripts in Merkel Cell Carcinoma (MCC). Pathogens 2023, 12, 894. https://doi.org/10.3390/pathogens12070894
Passerini S, Prezioso C, Babini G, Ferlosio A, Cosio T, Campione E, Moens U, Ciotti M, Pietropaolo V. Detection of Merkel Cell Polyomavirus (MCPyV) DNA and Transcripts in Merkel Cell Carcinoma (MCC). Pathogens. 2023; 12(7):894. https://doi.org/10.3390/pathogens12070894
Chicago/Turabian StylePasserini, Sara, Carla Prezioso, Giulia Babini, Amedeo Ferlosio, Terenzio Cosio, Elena Campione, Ugo Moens, Marco Ciotti, and Valeria Pietropaolo. 2023. "Detection of Merkel Cell Polyomavirus (MCPyV) DNA and Transcripts in Merkel Cell Carcinoma (MCC)" Pathogens 12, no. 7: 894. https://doi.org/10.3390/pathogens12070894
APA StylePasserini, S., Prezioso, C., Babini, G., Ferlosio, A., Cosio, T., Campione, E., Moens, U., Ciotti, M., & Pietropaolo, V. (2023). Detection of Merkel Cell Polyomavirus (MCPyV) DNA and Transcripts in Merkel Cell Carcinoma (MCC). Pathogens, 12(7), 894. https://doi.org/10.3390/pathogens12070894









