Use of Mpox Multiplex Serology in the Identification of Cases and Outbreak Investigations in the Democratic Republic of the Congo (DRC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Samples
2.2. PCR Detection, Differential Testing and Sequencing
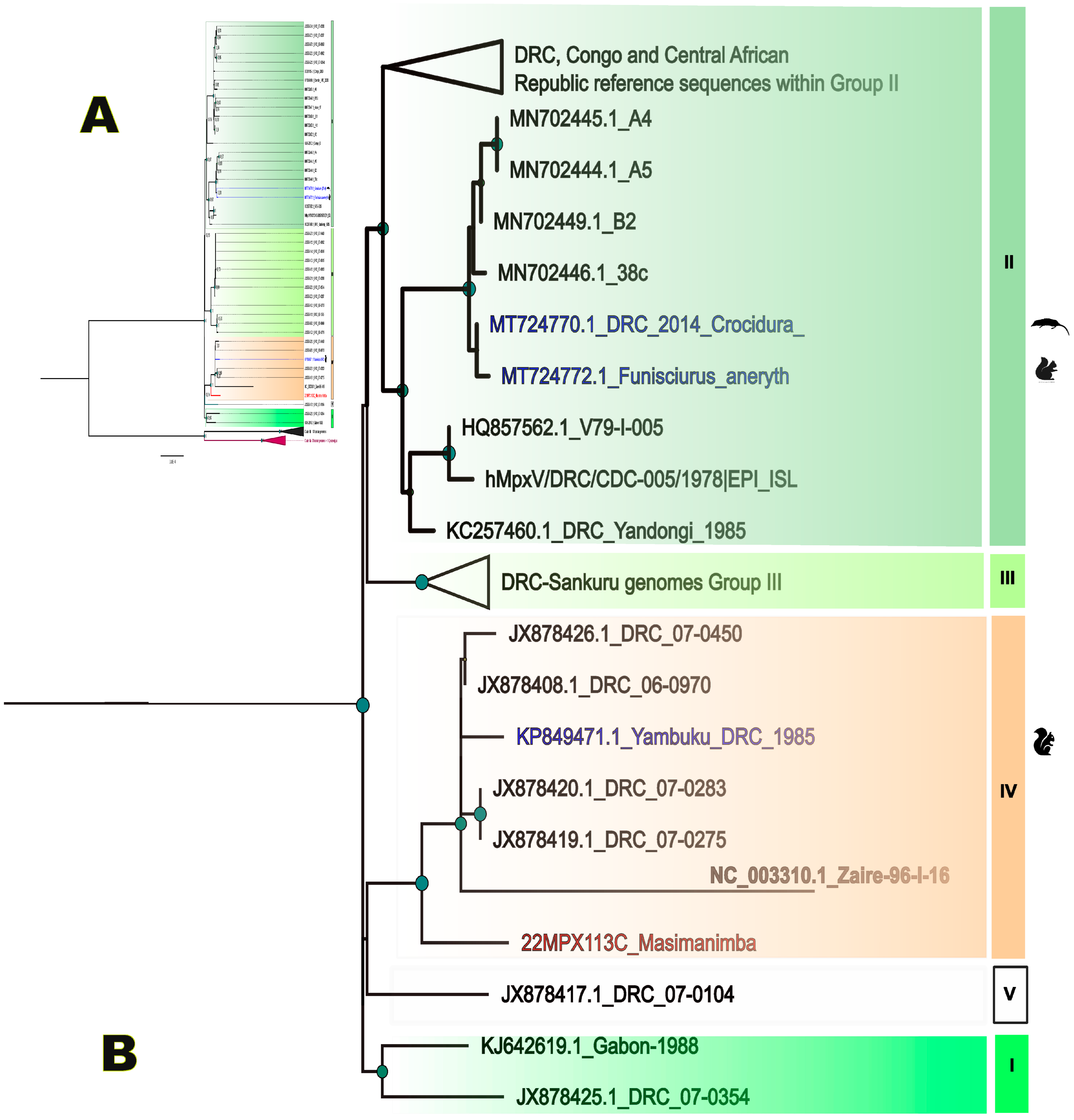
2.3. Bioinformatics and Phylogeny
2.4. Antibody Testing
2.5. Statistical Analysis
3. Results
3.1. Assay Optimization and Antibody Testing for Mpox Outbreak Confirmation
3.2. Antibody Testing during an Outbreak Investigation in Masimanimba Health Zone, Kwilu Province
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Hughes, C.M.; Kabamba, J.; Malekani, J.; et al. Extended Human-to-Human Transmission during a Monkeypox Outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis. 2016, 22, 1014–1021. [Google Scholar] [CrossRef]
- Sklenovská, N. Monkeypox Virus. In Animal-Origin Viral Zoonoses; Malik, Y.S., Singh, R.K., Dhama, K., Eds.; Livestock Diseases and Management; Springer: Singapore, 2020; pp. 39–68. ISBN 9789811526503. [Google Scholar]
- WHO. WHO Recommends New Name for Monkeypox Disease. Available online: https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease (accessed on 6 June 2023).
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Damon, I.K. Status of Human Monkeypox: Clinical Disease, Epidemiology and Research. Vaccine 2011, 29, D54–D59. [Google Scholar] [CrossRef]
- Doshi, R.H.; Guagliardo, S.A.J.; Doty, J.B.; Babeaux, A.D.; Matheny, A.; Burgado, J.; Townsend, M.B.; Morgan, C.N.; Satheshkumar, P.S.; Ndakala, N.; et al. Epidemiologic and Ecologic Investigations of Monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg. Infect. Dis. 2019, 25, 281–289. [Google Scholar] [CrossRef] [PubMed]
- WHO. Monkeypox. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 25 November 2022).
- Bryer, J.; Freeman, E.E.; Rosenbach, M. Monkeypox Emerges on a Global Scale: A Historical Review and Dermatologic Primer. J. Am. Acad. Dermatol. 2022, 87, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Doty, J.; Malekani, J.; Kalemba, L.; Stanley, W.; Monroe, B.; Nakazawa, Y.; Mauldin, M.; Bakambana, T.; Liyandja Dja Liyandja, T.; Braden, Z.; et al. Assessing Monkeypox Virus Prevalence in Small Mammals at the Human–Animal Interface in the Democratic Republic of the Congo. Viruses 2017, 9, 283. [Google Scholar] [CrossRef]
- Antunes, F.; Cordeiro, R.; Virgolino, A. Monkeypox: From A Neglected Tropical Disease to a Public Health Threat. Infect. Dis. Rep. 2022, 14, 772–783. [Google Scholar] [CrossRef]
- CDC (DHCPP). 2022 Monkeypox and Orthopoxvirus Outbreak Global Map. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 12 June 2022).
- Gessain, A.; Nakoune, E.; Yazdanpanah, Y. Monkeypox. N. Engl. J. Med. 2022, 387, 1783–1793. [Google Scholar] [CrossRef]
- WHO. Laboratory Testing for the Monkeypox Virus: Interim Guidance, 23 May 2022; World Health Organization: Geneva, Switzerland, 2022; p. 6. [Google Scholar]
- WHO. Monkeypox: Experts Give Virus Variants New Names. Available online: https://www.who.int/news/item/12-08-2022-monkeypox--experts-give-virus-variants-new-names (accessed on 21 December 2022).
- WHO. Integrated Disease Surveillance and Response Technical Guidelines: Booklet Two: Sections 1, 2, and 3, 3rd ed.; World Health Organization, Regional Office for Africa: Brazzaville, Congo, 2019; pp. xi, 119. [Google Scholar]
- Traité de Virologie Médicale, 2nd ed.; Société Française de Microbiologie Société Française de Virologie: Paris, France, 2019; ISBN 978-2-87805-036-3.
- Fine, P.E.M.; Jezek, Z.; Grab, B.; Dixon, H. The Transmission Potential of Monkeypox Virus in Human Populations. Int. J. Epidemiol. 1988, 17, 643–650. [Google Scholar] [CrossRef]
- MacNeil, A.; Reynolds, M.G.; Carroll, D.S.; Karem, K.; Braden, Z.; Lash, R.; Moundeli, A.; Mombouli, J.-V.; Jumaan, A.O.; Schmid, D.S.; et al. Monkeypox or Varicella? Lessons from a Rash Outbreak Investigation in the Republic of the Congo. Am. J. Trop. Med. Hyg. 2009, 80, 503–507. [Google Scholar] [CrossRef]
- Marin, M.; Leung, J.; Gershon, A.A. Transmission of Vaccine-Strain Varicella-Zoster Virus: A Systematic Review. Pediatrics 2019, 144, e20191305. [Google Scholar] [CrossRef]
- Meyer, H.; Perrichot, M.; Stemmler, M.; Emmerich, P.; Schmitz, H.; Varaine, F.; Shungu, R.; Tshioko, F.; Formenty, P. Outbreaks of Disease Suspected of Being Due to Human Monkeypox Virus Infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002, 40, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Olson, V.A.; Laue, T.; Laker, M.T.; Damon, I.K. Detection of Monkeypox Virus with Real-Time PCR Assays. J. Clin. Virol. 2006, 36, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.E.; Hammarlund, E.; Slifka, M.K. Optimization of Peptide-Based ELISA for Serological Diagnostics: A Retrospective Study of Human Monkeypox Infection. Vector-Borne Zoonotic Dis. 2012, 12, 400–409. [Google Scholar] [CrossRef]
- Kulesh, D.A.; Baker, R.O.; Loveless, B.M.; Norwood, D.; Zwiers, S.H.; Mucker, E.; Hartmann, C.; Herrera, R.; Miller, D.; Christensen, D.; et al. Smallpox and Pan-Orthopox Virus Detection by Real-Time 3′-Minor Groove Binder TaqMan Assays on the Roche LightCycler and the Cepheid Smart Cycler Platforms. J. Clin. Microbiol. 2004, 42, 601–609. [Google Scholar] [CrossRef]
- Twist Bioscience. Twist Pan-Viral Panel Confident Identification of over 1000 Viral Human Pathogens from a Single Sample; 2021. Available online: https://www.twistbioscience.com/sites/default/files/resources/2021-12/ProductSheet_NGS_PanViralPanel_30NOV21_Rev2.0.pdf (accessed on 14 June 2022).
- Kinganda-Lusamaki, E.; Black, A.; Mukadi, D.B.; Hadfield, J.; Mbala-Kingebeni, P.; Pratt, C.B.; Aziza, A.; Diagne, M.M.; White, B.; Bisento, N.; et al. Integration of Genomic Sequencing into the Response to the Ebola Virus Outbreak in Nord Kivu, Democratic Republic of the Congo. Nat. Med. 2021, 27, 710–716. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Ayouba, A.; Thaurignac, G.; Morquin, D.; Tuaillon, E.; Raulino, R.; Nkuba, A.; Lacroix, A.; Vidal, N.; Foulongne, V.; Le Moing, V.; et al. Multiplex Detection and Dynamics of IgG Antibodies to SARS-CoV2 and the Highly Pathogenic Human Coronaviruses SARS-CoV and MERS-CoV. J. Clin. Virol. 2020, 129, 104521. [Google Scholar] [CrossRef]
- Berthet, N.; Descorps-Declère, S.; Besombes, C.; Curaudeau, M.; Nkili Meyong, A.A.; Selekon, B.; Labouba, I.; Gonofio, E.C.; Ouilibona, R.S.; Tchetgna, H.D.S.; et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci. Rep. 2021, 1, 13085. [Google Scholar] [CrossRef] [PubMed]
- HCSP. Conduite à Tenir Autour d’un Cas Suspect, Probable Ou Confirmé d’infection à Monkeypox Virus; Haut Conseil de la Santé Publique: Paris, France, 2022. [Google Scholar]
- Besombes, C.; Gonofio, E.; Konamna, X.; Selekon, B.; Gessain, A.; Berthet, N.; Manuguerra, J.-C.; Fontanet, A.; Nakouné, E. Intrafamily Transmission of Monkeypox Virus, Central African Republic, 2018. Emerg. Infect. Dis. 2019, 25, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Mande, G.; Akonda, I.; De Weggheleire, A.; Brosius, I.; Liesenborghs, L.; Bottieau, E.; Ross, N.; Gembu, G.-C.; Colebunders, R.; Verheyen, E.; et al. Enhanced Surveillance of Monkeypox in Bas-Uélé, Democratic Republic of Congo: The Limitations of Symptom-Based Case Definitions. Int. J. Infect. Dis. 2022, 122, 647–655. [Google Scholar] [CrossRef] [PubMed]

| Variable | Mpox PCR Positive N = 157 * | Mpox PCR Negative N = 306 | Total N = 463 | p |
|---|---|---|---|---|
| Age 1, years | ||||
| median (IQR #) | 12 (5–25) | 13 (6–26) | 13 (5–25) | 0.523 |
| Sex 2, | 0.434 | |||
| Female | 70 (44.9%) | 145 (47.2%) | 214 (46.2%) | |
| Male | 83 (53.2%) | 146 (47.6%) | 229 (49.5%) | |
| Missing | 4 (2.6%) | 16 (5.2%) | 20 (4.3%) | |
| Delay symptoms and sampling 3 | ||||
| Days (IQR) | 6 (3–9) | 6 (4–11) | 6 (4–10) | 0.042 |
| Range | 0–28 | 0–95 | 0–95 | |
| Delay sample shipment 4 | ||||
| Days (IQR) | 13 (8–21) | 12 (7–20) | 12 (8–20) | 0.435 |
| Range | 2–58 | 0–66 | 0–66 | |
| Mpox IgG antibodies | 58 (36.9%) | 66(21.6%) | 124 (26.8%) | <0.001 ** |
| VZV PCR positive 5 | 0/20 (0.0%) | 28/305 (9.2%) | 28/325 (8.6%) | |
| Reactive Antigen(s) | Positive Samples (N = 463) | Median Days of Delay between Symptoms and Sample Collection (IQR) |
|---|---|---|
| B21R-179/180 only | 17 (3.7%) | 9.5 (5–18) |
| B21R-180/186 only | 5 (1.1% | 4 (2–6) |
| B22R-64/65 only | 70 (15.1%) | 8 (5.5–10) |
| Both B21R-179/180 and B21R-180/186 | 3 (0.6%) | 2 (0–13) |
| Both B21R-179/180 and B22R-64/65 | 24 (5.2%) | 9.5 (6–10.5) |
| Both B21R-180/186 and B22R-64/65 | 4 (0.9%) | 37.5 (23–52) |
| All 3 peptides | 1 (0.2%) | 8 |
| All positive to B21R-179/180 | 45 (9.7%) | 8.5 (5–11) |
| All positive to B21R-180/186 | 13 (2.8%) | 6 (2–13) |
| All positive to B22R-64/65 | 99 (21.4%) | 8 (6–10) |
| Positive to at least one antigen | 124 (26.8%) | 8 (5–11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinganda-Lusamaki, E.; Baketana, L.K.; Ndomba-Mukanya, E.; Bouillin, J.; Thaurignac, G.; Aziza, A.A.; Luakanda-Ndelemo, G.; Nuñez, N.F.; Kalonji-Mukendi, T.; Pukuta, E.S.; et al. Use of Mpox Multiplex Serology in the Identification of Cases and Outbreak Investigations in the Democratic Republic of the Congo (DRC). Pathogens 2023, 12, 916. https://doi.org/10.3390/pathogens12070916
Kinganda-Lusamaki E, Baketana LK, Ndomba-Mukanya E, Bouillin J, Thaurignac G, Aziza AA, Luakanda-Ndelemo G, Nuñez NF, Kalonji-Mukendi T, Pukuta ES, et al. Use of Mpox Multiplex Serology in the Identification of Cases and Outbreak Investigations in the Democratic Republic of the Congo (DRC). Pathogens. 2023; 12(7):916. https://doi.org/10.3390/pathogens12070916
Chicago/Turabian StyleKinganda-Lusamaki, Eddy, Lionel Kinzonzi Baketana, Etienne Ndomba-Mukanya, Julie Bouillin, Guillaume Thaurignac, Adrienne Amuri Aziza, Gradi Luakanda-Ndelemo, Nicolas Fernandez Nuñez, Thierry Kalonji-Mukendi, Elisabeth Simbu Pukuta, and et al. 2023. "Use of Mpox Multiplex Serology in the Identification of Cases and Outbreak Investigations in the Democratic Republic of the Congo (DRC)" Pathogens 12, no. 7: 916. https://doi.org/10.3390/pathogens12070916
APA StyleKinganda-Lusamaki, E., Baketana, L. K., Ndomba-Mukanya, E., Bouillin, J., Thaurignac, G., Aziza, A. A., Luakanda-Ndelemo, G., Nuñez, N. F., Kalonji-Mukendi, T., Pukuta, E. S., Nkuba-Ndaye, A., Lofiko, E. L., Kibungu, E. M., Lushima, R. S., Ayouba, A., Mbala-Kingebeni, P., Muyembe-Tamfum, J.-J., Delaporte, E., Peeters, M., & Ahuka-Mundeke, S. (2023). Use of Mpox Multiplex Serology in the Identification of Cases and Outbreak Investigations in the Democratic Republic of the Congo (DRC). Pathogens, 12(7), 916. https://doi.org/10.3390/pathogens12070916







