The Ubiquitous Wilt-Inducing Pathogen Fusarium oxysporum—A Review of Genes Studied with Mutant Analysis
Abstract
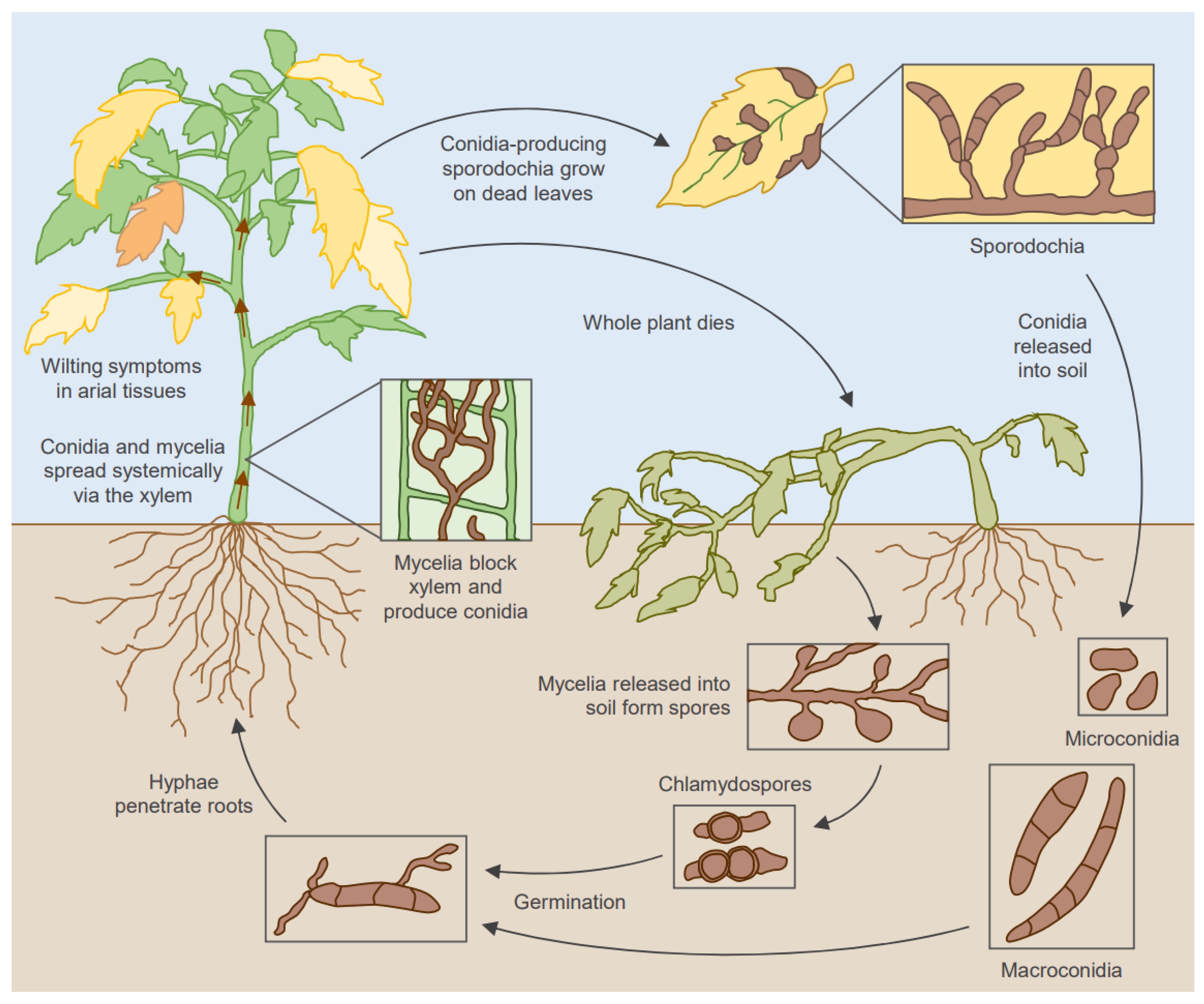
:1. Introduction
2. The Features of the F. oxysporum Genome
2.1. Genome Sequences
2.2. Transcriptomic and Secretomic Analyses
3. Molecular Dissection of F. oxysporum Biology
3.1. Central Signaling Pathways and Transcription Factors
3.1.1. MAP Kinase Signaling
3.1.2. TOR Signaling
3.1.3. GTPase Signaling
3.1.4. Ubiquitination
3.1.5. Other Signaling Components
3.1.6. Shared Transcription Factors and Gene Regulation Components
3.2. Genes Involved in Vegetative Hyphal Growth and Stress Tolerance
3.2.1. Protein Post-Translational Modifications (PTMs)
3.2.2. Vesicle Trafficking
3.2.3. Autophagy
3.2.4. Metabolism and Nutrient Acquisition
3.2.5. Cell Wall Biogenesis, Integrity, and Remodeling
3.2.6. Stress Tolerance and Defense
3.3. Genes Involved in Reproduction
3.3.1. Cell Division
3.3.2. Transcriptional Regulators of Conidiation
3.3.3. Post-Transcriptional Regulators
3.3.4. Nutrient Metabolism
3.3.5. Cell Wall Production and Stability
3.3.6. Virulence Genes Affecting Conidiation
3.4. Genes Involved in Virulence
3.4.1. Chemotrophic Growth
3.4.2. Fusaric Acid (FA) and Nitric Oxide (NO) Production
3.4.3. Cell Wall Degrading Enzymes (CWDEs)
3.4.4. Xylem Effectors: Secreted in Xylem (SIX) Proteins
3.4.5. Effectors in Pathogenicity and Suppression of Host Defenses
3.4.6. Virulence-Specific Transcription Factors and Small RNA Regulation
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current Status of Fusarium oxysporum Formae Speciales and Races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Snyder, W.C.; Hansen, H.N. The Species Concept in Fusarium. Am. J. Bot. 1940, 27, 64–67. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Baayen, R.P.; O’Donnell, K.; Bonants, P.J.; Cigelnik, E.; Kroon, L.P.; Roebroeck, E.J.; Waalwijk, C. Gene Genealogies and AFLP Analyses in the Fusarium oxysporum Complex Identify Monophyletic and Nonmonophyletic Formae Speciales Causing Wilt and Rot Disease. Phytopathology 2000, 90, 891–900. [Google Scholar] [CrossRef]
- Skovgaard, K.; Nirenberg, H.I.; O’Donnell, K.; Rosendahl, S. Evolution of Fusarium oxysporum f. sp. vasinfectum Races Inferred from Multigene Genealogies. Phytopathology 2001, 91, 1231–1237. [Google Scholar] [CrossRef]
- Kistler, H.C.; Alabouvette, C.; Baayen, R.P.; Bentley, S.; Brayford, D.; Coddington, A.; Correll, J.; Daboussi, M.J.; Elias, K.; Fernandez, D.; et al. Systematic Numbering of Vegetative Compatibility Groups in the Plant Pathogenic Fungus Fusarium oxysporum. Phytopathology 1998, 88, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Beckman, C.H. The Nature of Wilt Diseases of Plants; APS Press: St. Paul, MN, USA, 1987. [Google Scholar]
- Covey, P.A.; Kuwitzky, B.; Hanson, M.; Webb, K.M. Multilocus analysis using putative fungal effectors to describe a population of Fusarium oxysporum from sugar beet. Phytopathology 2014, 104, 886–896. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium Species and Their Associated Mycotoxins. Methods Mol. Biol. 2017, 1542, 51–106. [Google Scholar] [CrossRef]
- Houterman, P.M.; Cornelissen, B.J.; Rep, M. Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog. 2008, 4, e1000061. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Kew, UK, 1971. [Google Scholar]
- Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: Pennsylvania, PA, USA, 1983. [Google Scholar]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.D.; Cooper, R.M. An ultrastructural study of root invasion in three vascular wilt diseases. Physiol. Plant Pathol. 1983, 22, 15–27. [Google Scholar] [CrossRef]
- Katan, T.; Shlevin, E.; Katan, J. Sporulation of Fusarium oxysporum f. sp. lycopersici on Stem Surfaces of Tomato Plants and Aerial Dissemination of Inoculum. Phytopathology 1997, 87, 712–719. [Google Scholar] [CrossRef]
- Rekah, Y.; Shtienberg, D.; Katan, J. Disease Development Following Infection of Tomato and Basil Foliage by Airborne Conidia of the Soilborne Pathogens Fusarium oxysporum f. sp. radicis-lycopersici and F. oxysporum f. sp. basilici. Phytopathology 2000, 90, 1322–1329. [Google Scholar] [CrossRef]
- Rowe, R.C.; Farley, J.D.; Coplin, D.L. Airborne spore dispersal and recolonization of steamed soil by Fusarium oxysporum in tomato greenhouses. Phytopathology 1977, 67, 1513–1517. [Google Scholar] [CrossRef]
- Scarlett, K.; Tesoriero, L.; Daniel, R.; Guest, D. Sciarid and shore flies as aerial vectors of Fusarium oxysporum f. sp. cucumerinum in greenhouse cucumbers. J. Appl. Entomol. 2013, 138, 368–377. [Google Scholar] [CrossRef]
- Sampaio, A.M.; Araújo, S.d.S.; Rubiales, D.; Vaz Patto, M.C. Fusarium Wilt Management in Legume Crops. Agronomy 2020, 10, 1073. [Google Scholar] [CrossRef]
- Jiménez-Díaz, R.M.; Castillo, P.; del Mar Jiménez-Gasco, M.; Landa, B.B.; Navas-Cortés, J.A. Fusarium wilt of chickpeas: Biology, ecology and management. Crop Prot. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Ma, L.J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef]
- Cuomo, C.A.; Güldener, U.; Xu, J.R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef]
- Ayhan, D.H.; López-Díaz, C.; Di Pietro, A.; Ma, L.J. Improved Assembly of Reference Genome Fusarium oxysporum f. sp. lycopersici Strain Fol4287. Microbiol. Resour. Announc. 2018, 7. [Google Scholar] [CrossRef]
- Dvorianinova, E.M.; Pushkova, E.N.; Novakovskiy, R.O.; Povkhova, L.V.; Bolsheva, N.L.; Kudryavtseva, L.P.; Rozhmina, T.A.; Melnikova, N.V.; Dmitriev, A.A. Nanopore and illumina genome sequencing of Fusarium oxysporum f. sp. lini strains of different virulence. Front. Genet. 2021, 12, 662928. [Google Scholar] [CrossRef]
- Yang, J.; Mao, A.; Zhang, J.; Zhang, X.; Xia, C.; Zhao, H.; Wang, Y.; Wen, C.; Liu, H.; Wang, Q. Whole-genome sequencing of Fusarium oxysporum f. sp. cucumerinum strain race-4 infecting cucumber in China. Plant Dis. 2023, 107, 1210–1213. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, W.; Wang, S.; Wang, H.; Yu, L.; Zeng, X.; Fei, Z.; Li, J. Genome sequence of Fusarium oxysporum f. sp. conglutinans, the etiological agent of cabbage Fusarium wilt. Mol. Plant Microbe Interact. 2021, 34, 210–213. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Y.; Ping, X.; Yang, Q.; Mao, Z.; Zhao, J.; Lu, X.; Xie, B.; Yang, Y.; Ling, J. The genome of Fusarium oxysporum f. sp. phaseoli provides insight into the evolution of genomes and effectors of Fusarium oxysporum species. Int. J. Mol. Sci. 2023, 24, 963. [Google Scholar] [CrossRef]
- Schmidt, S.M.; Lukasiewicz, J.; Farrer, R.; van Dam, P.; Bertoldo, C.; Rep, M. Comparative genomics of Fusarium oxysporum f. sp. melonis reveals the secreted protein recognized by the Fom-2 resistance gene in melon. New Phytol. 2016, 209, 307–318. [Google Scholar] [CrossRef]
- Meng, T.; Jiao, H.; Zhang, Y.; Zhou, Y.; Chen, S.; Wang, X.; Yang, B.; Sun, J.; Geng, X.; Ayhan, D.H.; et al. FoPGDB: A pangenome database of Fusarium oxysporum, a cross-kingdom fungal pathogen. Database 2024, 2024. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, A.; Tan, K.; Yang, S.; Ma, X.; Bai, X.; Hou, Y.; Bai, J. Study on the interaction mechanism between Crocus sativus and Fusarium oxysporum based on dual RNA-seq. Plant Cell Rep. 2023, 42, 91–106. [Google Scholar] [CrossRef]
- Chang, H.X.; Noel, Z.A.; Chilvers, M.I. A β-lactamase gene of Fusarium oxysporum alters the rhizosphere microbiota of soybean. Plant J. 2021, 106, 1588–1604. [Google Scholar] [CrossRef]
- Wang, D.; Peng, C.; Zheng, X.; Chang, L.; Xu, B.; Tong, Z. Secretome analysis of the banana Fusarium wilt fungi Foc R1 and Foc TR4 reveals a new effector OASTL required for full pathogenicity of Foc TR4 in banana. Biomolecules 2020, 10, 1430. [Google Scholar] [CrossRef]
- Li, J.; Gao, M.; Gabriel, D.W.; Liang, W.; Song, L. Secretome-wide analysis of lysine acetylation in fusarium oxysporum f. sp. lycopersici provides novel insights into infection-related proteins. Front. Microbiol. 2020, 11, 559440. [Google Scholar] [CrossRef]
- Di Pietro, A.; García-MacEira, F.I.; Méglecz, E.; Roncero, M.I. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 2001, 39, 1140–1152. [Google Scholar] [CrossRef]
- Prados Rosales, R.C.; Di Pietro, A. Vegetative hyphal fusion is not essential for plant infection by Fusarium oxysporum. Eukaryot. Cell 2008, 7, 162–171. [Google Scholar] [CrossRef]
- Pareek, M.; Rajam, M.V. RNAi-mediated silencing of MAP kinase signalling genes (Fmk1, Hog1, and Pbs2) in Fusarium oxysporum reduces pathogenesis on tomato plants. Fungal Biol. 2017, 121, 775–784. [Google Scholar] [CrossRef]
- Segorbe, D.; Di Pietro, A.; Pérez-Nadales, E.; Turrà, D. Three Fusarium oxysporum mitogen-activated protein kinases (MAPKs) have distinct and complementary roles in stress adaptation and cross-kingdom pathogenicity. Mol. Plant Pathol. 2016, 18, 912–924. [Google Scholar] [CrossRef]
- Pérez-Nadales, E.; Di Pietro, A. The membrane mucin Msb2 regulates invasive growth and plant infection in Fusarium oxysporum. Plant Cell 2011, 23, 1171–1185. [Google Scholar] [CrossRef]
- Perez-Nadales, E.; Di Pietro, A. The transmembrane protein Sho1 cooperates with the mucin Msb2 to regulate invasive growth and plant infection in Fusarium oxysporum. Mol. Plant Pathol. 2015, 16, 593–603. [Google Scholar] [CrossRef]
- Turrà, D.; El Ghalid, M.; Rossi, F.; Di Pietro, A. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature 2015, 527, 521–524. [Google Scholar] [CrossRef]
- Rispail, N.; Di Pietro, A. The two-component histidine kinase Fhk1 controls stress adaptation and virulence of Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 395–407. [Google Scholar] [CrossRef]
- Ding, Z.; Li, M.; Sun, F.; Xi, P.; Sun, L.; Zhang, L.; Jiang, Z. Mitogen-activated protein kinases are associated with the regulation of physiological traits and virulence in Fusarium oxysporum f. sp. cubense. PLoS ONE 2015, 10, e0122634. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Yang, K.; Tang, Y.; Wei, L.; Liu, E.; Liang, Z. Protein kinase Ime2 is associated with mycelial growth, conidiation, osmoregulation, and pathogenicity in Fusarium oxysporum. Arch. Microbiol. 2022, 204, 455. [Google Scholar] [CrossRef]
- Nunez-Rodriguez, J.C.; Ruiz-Roldán, C.; Lemos, P.; Membrives, S.; Hera, C. The phosphatase Ptc6 is involved in virulence and MAPK signalling in Fusarium oxysporum. Mol. Plant Pathol. 2020, 21, 206–217. [Google Scholar] [CrossRef]
- Fernandes, T.R.; Sánchez Salvador, E.; Tapia, Á.; Di Pietro, A. Dual-specificity protein phosphatase Msg5 controls cell wall integrity and virulence in Fusarium oxysporum. Fungal Genet. Biol. 2021, 146, 103486. [Google Scholar] [CrossRef]
- De Virgilio, C.; Loewith, R. Cell growth control: Little eukaryotes make big contributions. Oncogene 2006, 25, 6392–6415. [Google Scholar] [CrossRef]
- Yuan, H.X.; Xiong, Y.; Guan, K.L. Nutrient sensing, metabolism, and cell growth control. Mol. Cell 2013, 49, 379–387. [Google Scholar] [CrossRef]
- Li, L.; Zhu, T.; Song, Y.; Feng, L.; Kear, P.J.; Riseh, R.S.; Sitohy, M.; Datla, R.; Ren, M. Salicylic acid fights against Fusarium wilt by inhibiting target of rapamycin signaling pathway in Fusarium oxysporum. J. Adv. Res. 2022, 39, 1–13. [Google Scholar] [CrossRef]
- López-Berges, M.S.; Rispail, N.; Prados-Rosales, R.C.; Di Pietro, A. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. Plant Cell 2010, 22, 2459–2475. [Google Scholar] [CrossRef]
- Li, L.; Zhu, T.; Song, Y.; Luo, X.; Datla, R.; Ren, M. Target of rapamycin controls hyphal growth and pathogenicity through FoTIP4 in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 1239–1255. [Google Scholar] [CrossRef]
- Qiu, M.; Deng, Y.; Deng, Q.; Sun, L.; Fang, Z.; Wang, Y.; Huang, X.; Zhao, J. Cysteine Inhibits the Growth of Fusarium oxysporum and Promotes T-2 Toxin Synthesis through the Gtr/Tap42 Pathway. Microbiol. Spectr. 2022, 10, e0368222. [Google Scholar] [CrossRef]
- Ospina-Giraldo, M.D.; Mullins, E.; Kang, S. Loss of function of the Fusarium oxysporum SNF1 gene reduces virulence on cabbage and Arabidopsis. Curr. Genet. 2003, 44, 49–57. [Google Scholar] [CrossRef]
- Neer, E.J. Heterotrimeric G proteins: Organizers of transmembrane signals. Cell 1995, 80, 249–257. [Google Scholar] [CrossRef]
- Jain, S.; Akiyama, K.; Mae, K.; Ohguchi, T.; Takata, R. Targeted disruption of a G protein alpha subunit gene results in reduced pathogenicity in Fusarium oxysporum. Curr. Genet. 2002, 41, 407–413. [Google Scholar] [CrossRef]
- Jain, S.; Akiyama, K.; Kan, T.; Ohguchi, T.; Takata, R. The G protein beta subunit FGB1 regulates development and pathogenicity in Fusarium oxysporum. Curr. Genet. 2003, 43, 79–86. [Google Scholar] [CrossRef]
- Jain, S.; Akiyama, K.; Takata, R.; Ohguchi, T. Signaling via the G protein alpha subunit FGA2 is necessary for pathogenesis in Fusarium oxysporum. FEMS Microbiol. Lett. 2005, 243, 165–172. [Google Scholar] [CrossRef]
- Delgado-Jarana, J.; Martínez-Rocha, A.L.; Roldán-Rodriguez, R.; Roncero, M.I.; Di Pietro, A. Fusarium oxysporum G-protein beta subunit Fgb1 regulates hyphal growth, development, and virulence through multiple signalling pathways. Fungal Genet. Biol. 2005, 42, 61–72. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, S.Y.; Lee, S.; Adams, E.L.; Czymmek, K.; Kang, S. Loss of cAMP-dependent protein kinase A affects multiple traits important for root pathogenesis by Fusarium oxysporum. Mol. Plant Microbe Interact. 2011, 24, 719–732. [Google Scholar] [CrossRef]
- Yang, C.; Sun, J.; Wu, Z.; Jiang, M.; Li, D.; Wang, X.; Zhou, C.; Liu, X.; Ren, Z.; Wang, J.; et al. FoRSR1 is important for conidiation, fusaric acid production, and pathogenicity in Fusarium oxysporum. Phytopathology 2023, 113, 1244–1253. [Google Scholar] [CrossRef]
- Li, B.; Mao, H.Y.; Zhang, Z.Y.; Chen, X.J.; Ouyang, S.Q. FolVps9, a Guanine Nucleotide Exchange Factor for FolVps21, Is Essential for Fungal Development and Pathogenicity in. Front. Microbiol. 2019, 10, 2658. [Google Scholar] [CrossRef]
- Navarro-Velasco, G.Y.; Di Pietro, A.; López-Berges, M.S. Constitutive activation of TORC1 signalling attenuates virulence in the cross-kingdom fungal pathogen Fusarium oxysporum. Mol. Plant Pathol. 2023, 24, 289–301. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef]
- Miguel-Rojas, C.; Hera, C. The F-box protein Fbp1 functions in the invasive growth and cell wall integrity mitogen-activated protein kinase (MAPK) pathways in Fusarium oxysporum. Mol. Plant Pathol. 2016, 17, 55–64. [Google Scholar] [CrossRef]
- Duyvesteijn, R.G.; van Wijk, R.; Boer, Y.; Rep, M.; Cornelissen, B.J.; Haring, M.A. Frp1 is a Fusarium oxysporum F-box protein required for pathogenicity on tomato. Mol. Microbiol. 2005, 57, 1051–1063. [Google Scholar] [CrossRef]
- Jonkers, W.; Rodrigues, C.D.; Rep, M. Impaired colonization and infection of tomato roots by the Δfrp1 mutant of Fusarium oxysporum correlates with reduced CWDE gene expression. Mol. Plant Microbe Interact. 2009, 22, 507–518. [Google Scholar] [CrossRef]
- Mullally, J.E.; Chernova, T.; Wilkinson, K.D. Doa1 is a Cdc48 adapter that possesses a novel ubiquitin binding domain. Mol. Cell Biol. 2006, 26, 822–830. [Google Scholar] [CrossRef]
- Noman, M.; Azizullah; Ahmed, T.; Gao, Y.; Wang, H.; Xiong, X.; Wang, J.; Lou, J.; Li, D.; Song, F. Degradation of α-Subunits, Doa1 and Doa4, are Critical for Growth, Development, Programmed Cell Death Events, Stress Responses, and Pathogenicity in the Watermelon Fusarium Wilt Fungus Fusarium oxysporum f. sp. niveum. J. Agric. Food Chem. 2023, 71, 11667–11679. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.E.; Frailey, D.; Nohe, A.; Duncan, R.; Czymmek, K.J.; Kang, S. Roles of three Fusarium oxysporum calcium ion (Ca(2+)) channels in generating Ca(2+) signatures and controlling growth. Fungal Genet. Biol. 2015, 82, 145–157. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, S.C.; Cardenas, M.E.; Heitman, J. Calcium-Calmodulin-Calcineurin Signaling: A Globally Conserved Virulence Cascade in Eukaryotic Microbial Pathogens. Cell Host Microbe 2019, 26, 453–462. [Google Scholar] [CrossRef]
- Hou, Y.H.; Hsu, L.H.; Wang, H.F.; Lai, Y.H.; Chen, Y.L. Calcineurin regulates conidiation, chlamydospore formation and virulence in Fusarium oxysporum f. sp. lycopersici. Front. Microbiol. 2020, 11, 539702. [Google Scholar] [CrossRef]
- Mariscal, M.; Miguel-Rojas, C.; Hera, C.; Fernandes, T.R.; Di Pietro, A. Fusarium oxysporum casein kinase 1, a negative regulator of the plasma membrane H+-ATPase Pma1, is required for development and pathogenicity. J. Fungi 2022, 8, 1300. [Google Scholar] [CrossRef] [PubMed]
- Iida, Y.; Fujiwara, K.; Yoshioka, Y.; Tsuge, T. Mutation of FVS1, encoding a protein with a sterile alpha motif domain, affects asexual reproduction in the fungal plant pathogen Fusarium oxysporum. FEMS Microbiol. Lett. 2014, 351, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Inoue, I.; Namiki, F.; Tsuge, T. Plant colonization by the vascular wilt fungus Fusarium oxysporum requires FOW1, a gene encoding a mitochondrial protein. Plant Cell 2002, 14, 1869–1883. [Google Scholar] [CrossRef]
- Kawabe, M.; Mizutani, K.; Yoshida, T.; Teraoka, T.; Yoneyama, K.; Yamaguchi, I.; Arie, T. Cloning of the pathogenicity-related gene FPD1 in Fusarium oxysporum f. sp. lycopersici. J. Gen. Plant Pathol. 2004, 70, 16–20. [Google Scholar] [CrossRef]
- Ruiz-Roldán, C.; Pareja-Jaime, Y.; González-Reyes, J.A.; Roncero, M.I. The Transcription Factor Con7-1 Is a Master Regulator of Morphogenesis and Virulence in Fusarium oxysporum. Mol. Plant Microbe Interact. 2015, 28, 55–68. [Google Scholar] [CrossRef]
- Denisov, Y.; Freeman, S.; Yarden, O. Inactivation of Snt2, a BAH/PHD-containing transcription factor, impairs pathogenicity and increases autophagosome abundance in Fusarium oxysporum. Mol. Plant Pathol. 2011, 12, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, W.; Xayamongkhon, H.; Haas, M.; Olivain, C.; van der Does, H.C.; Broz, K.; Rep, M.; Alabouvette, C.; Steinberg, C.; Kistler, H.C. EBR1 genomic expansion and its role in virulence of Fusarium species. Env. Microbiol. 2014, 16, 1982–2003. [Google Scholar] [CrossRef]
- Palos-Fernández, R.; Turrà, D.; Pietro, A.D. The Gal4-Type Transcription Factor Pro1 Integrates Inputs from Two Different MAPK Cascades to Regulate Development in the Fungal Pathogen Fusarium oxysporum. J. Fungi 2022, 8, 1242. [Google Scholar] [CrossRef]
- Ding, Z.; Lin, H.; Liu, L.; Lu, T.; Xu, Y.; Peng, J.; Ren, Y.; Xu, T.; Zhang, X. Transcription factor FoAce2 regulates virulence, vegetative growth, conidiation, and cell wall homeostasis in Fusarium oxysporum f. sp. cubense. Fungal Biol. 2024, 128, 1960–1967. [Google Scholar] [CrossRef]
- Su, J.; Wang, J.; Tang, J.; Yu, W.; Liu, J.; Dong, X.; Dong, J.; Chai, X.; Ji, P.; Zhang, L. Zinc finger transcription factor ZFP1 is associated with growth, conidiation, osmoregulation, and virulence in the Polygonatum kingianum pathogen Fusarium oxysporum. Sci. Rep. 2024, 14, 16061. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, Z.; Huang, L.; Liu, S.; Shen, Z.; Wang, Y.; Wang, H.; Zhang, H.; Li, D.; Song, F. CCR4-Not complex subunit Not2 plays critical roles in vegetative growth, conidiation and virulence in watermelon Fusarium wilt pathogen Fusarium oxysporum f. sp. niveum. Front. Microbiol. 2016, 7, 1449. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; An, B.; Luo, H.; He, C.; Wang, Q. The histone acetyltransferase FocGCN5 regulates growth, conidiation, and pathogenicity of the banana wilt disease causal agent Fusarium oxysporum f. sp. cubense tropical race 4. Res. Microbiol. 2022, 173, 103902. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.J.; Keller, N.P.; Yu, J.H.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Dominguez, Y.; Bok, J.W.; Berger, H.; Shwab, E.K.; Basheer, A.; Gallmetzer, A.; Scazzocchio, C.; Keller, N.; Strauss, J. Heterochromatic marks are associated with the repression of secondary metabolism clusters in Aspergillus nidulans. Mol. Microbiol. 2010, 76, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.W.; Noordermeer, D.; Kale, S.P.; Keller, N.P. Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol. Microbiol. 2006, 61, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- López-Berges, M.S.; Hera, C.; Sulyok, M.; Schäfer, K.; Capilla, J.; Guarro, J.; Di Pietro, A. The velvet complex governs mycotoxin production and virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Microbiol. 2013, 87, 49–65. [Google Scholar] [CrossRef]
- Li, P.; Pu, X.; Feng, B.; Yang, Q.; Shen, H.; Zhang, J.; Lin, B. FocVel1 influences asexual production, filamentous growth, biofilm formation, and virulence in Fusarium oxysporum f. sp. cucumerinum. Front. Plant Sci. 2015, 6, 312. [Google Scholar] [CrossRef]
- Lu, S.; Deng, H.; Lin, Y.; Huang, M.; You, H.; Zhang, Y.; Zhuang, W.; Lu, G.; Yun, Y. A Network of Sporogenesis-Responsive Genes Regulates the Growth, Asexual Sporogenesis, Pathogenesis and Fusaric Acid Production of Fusarium oxysporum f. sp. cubense. J. Fungi 2023, 10, 1. [Google Scholar] [CrossRef]
- Miller, M.A.; Olivas, W.M. Roles of Puf proteins in mRNA degradation and translation. Wiley Interdiscip. Rev. RNA 2011, 2, 471–492. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, X.; Wang, H.; Bi, Y.; Wang, J.; Yan, Y.; Li, D.; Song, F. Fusarium oxysporum f. sp. niveum Pumilio 1 Regulates Virulence on Watermelon through Interacting with the ARP2/3 Complex and Binding to an A-Rich Motif in the 3′ UTR of Diverse Transcripts. mBio 2023, 14, e0015723. [Google Scholar] [CrossRef]
- Lee, H.C.; Li, L.; Gu, W.; Xue, Z.; Crosthwaite, S.K.; Pertsemlidis, A.; Lewis, Z.A.; Freitag, M.; Selker, E.U.; Mello, C.C.; et al. Diverse pathways generate microRNA-like RNAs and Dicer-independent small interfering RNAs in fungi. Mol. Cell 2010, 38, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ling, J.; Liang, L.; Luo, Z.; Zhang, J.; Xie, B. Qip gene in Fusarium oxysporum is required for normal hyphae morphology and virulence. Mycology 2015, 6, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xie, L.; Wang, M.; Lin, Y.; Zhong, J.; Zhang, Y.; Zeng, J.; Kong, G.; Xi, P.; Li, H.; et al. FoQDE2-dependent milRNA promotes Fusarium oxysporum f. sp. cubense virulence by silencing a glycosyl hydrolase coding gene expression. PLoS Pathog. 2022, 18, e1010157. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Bi, Y.; He, C.; Situ, J.; Wang, M.; Kong, G.; Xi, P.; Jiang, Z.; Li, M. Unveiling microRNA-like small RNAs implicated in the initial infection of Fusarium oxysporum f. sp. cubense through small RNA sequencing. Mycology 2024, 1–16. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, H.; Zhao, G.; Yang, J.; Luo, Y.; Sun, S.; Wang, Z.; Li, S.; Jin, C. Genetical and O-glycoproteomic analyses reveal the roles of three protein O-mannosyltransferases in phytopathogen Fusarium oxysporum f. sp. cucumerinum. Fungal Genet. Biol. 2020, 134, 103285. [Google Scholar] [CrossRef]
- Xiong, X.; Gao, Y.; Wang, J.; Wang, H.; Lou, J.; Bi, Y.; Yan, Y.; Li, D.; Song, F. Palmitoyl Transferase FonPAT2-Catalyzed Palmitoylation of the FonAP-2 Complex Is Essential for Growth, Development, Stress Response, and Virulence in Fusarium oxysporum f. sp. Microbiol. Spectr. 2023, 11, e0386122. [Google Scholar] [CrossRef]
- Caffaro, C.E.; Hirschberg, C.B. Nucleotide sugar transporters of the Golgi apparatus: From basic science to diseases. Acc. Chem. Res. 2006, 39, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Hadley, B.; Maggioni, A.; Ashikov, A.; Day, C.J.; Haselhorst, T.; Tiralongo, J. Structure and function of nucleotide sugar transporters: Current progress. Comput. Struct. Biotechnol. J. 2014, 10, 23–32. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, X.; Wang, H.; Wang, J.; Bi, Y.; Yan, Y.; Cao, Z.; Li, D.; Song, F. Ero1-Pdi1 module-catalysed dimerization of a nucleotide sugar transporter, FonNst2, regulates virulence of Fusarium oxysporum on watermelon. Env. Microbiol. 2022, 24, 1200–1220. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Xiong, X.; Yan, Y.; Lou, J.; Noman, M.; Li, D.; Song, F. The Ser/Thr protein kinase FonKin4-poly(ADP-ribose) polymerase FonPARP1 phosphorylation cascade is required for the pathogenicity of watermelon fusarium wilt fungus. Front. Microbiol. 2024, 15, 1397688. [Google Scholar] [CrossRef]
- Li, B.; Gao, Y.; Mao, H.Y.; Borkovich, K.A.; Ouyang, S.Q. The SNARE protein FolVam7 mediates intracellular trafficking to regulate conidiogenesis and pathogenicity in Fusarium oxysporum f. sp. lycopersici. Env. Microbiol. 2019, 21, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhao, Q.; Yang, S.; Cai, Y.; Fang, W.; Abubakar, Y.S.; Lin, Y.; Yun, Y.; Zheng, W. Two distinct SNARE complexes mediate vesicle fusion with the plasma membrane to ensure effective development and pathogenesis of Fusarium oxysporum f. sp. cubense. Mol. Plant Pathol. 2024, 25, e13443. [Google Scholar] [CrossRef] [PubMed]
- Corral-Ramos, C.; Roca, M.G.; Di Pietro, A.; Roncero, M.I.; Ruiz-Roldán, C. Autophagy contributes to regulation of nuclear dynamics during vegetative growth and hyphal fusion in Fusarium oxysporum. Autophagy 2015, 11, 131–144. [Google Scholar] [CrossRef]
- Khalid, A.R.; Lv, X.; Naeem, M.; Mehmood, K.; Shaheen, H.; Dong, P.; Qiu, D.; Ren, M. Autophagy related gene (ATG3) is a key regulator for cell growth, development, and virulence of Fusarium oxysporum. Genes 2019, 10, 658. [Google Scholar] [CrossRef]
- Khalid, A.R.; Zhang, S.; Luo, X.; Mehmood, K.; Rahim, J.; Shaheen, H.; Dong, P.; Qiu, D.; Ren, M. Role of autophagy-related gene atg22 in developmental process and virulence of Fusarium oxysporum. Genes 2019, 10, 365. [Google Scholar] [CrossRef]
- Khalid, A.R.; Zhang, S.; Luo, X.; Shaheen, H.; Majeed, A.; Maqbool, M.; Zahid, N.; Rahim, J.; Ren, M.; Qiu, D. Functional analysis of autophagy-related gene ATG12 in potato dry rot fungus Fusarium oxysporum. Int. J. Mol. Sci. 2021, 22, 4932. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; He, D.; Gao, S.; Zhang, Y.; Wang, L. Hypothetical protein FoDbp40 influences the growth and virulence of Fusarium oxysporum by regulating the expression of isocitrate lyase. Front. Microbiol. 2022, 13, 1050637. [Google Scholar] [CrossRef]
- Huang, Z.; Lou, J.; Gao, Y.; Noman, M.; Li, D.; Song, F. FonTup1 functions in growth, conidiogenesis and pathogenicity of Fusarium oxysporum f. sp. niveum through modulating the expression of the tricarboxylic acid cycle genes. Microbiol. Res. 2023, 272, 127389. [Google Scholar] [CrossRef]
- Namiki, F.; Matsunaga, M.; Okuda, M.; Inoue, I.; Nishi, K.; Fujita, Y.; Tsuge, T. Mutation of an arginine biosynthesis gene causes reduced pathogenicity in Fusarium oxysporum f. sp. melonis. Mol. Plant Microbe Interact. 2001, 14, 580–584. [Google Scholar] [CrossRef]
- Corrales Escobosa, A.R.; Rangel Porras, R.A.; Meza Carmen, V.; Gonzalez Hernandez, G.A.; Torres Guzman, J.C.; Wrobel, K.; Roncero, M.I.; Gutierrez Corona, J.F. Fusarium oxysporum Adh1 has dual fermentative and oxidative functions and is involved in fungal virulence in tomato plants. Fungal Genet. Biol. 2011, 48, 886–895. [Google Scholar] [CrossRef]
- Corral-Ramos, C.; Roncero, M.I. Glycogen catabolism, but not its biosynthesis, affects virulence of Fusarium oxysporum on the plant host. Fungal Genet. Biol. 2015, 77, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, S.; Meacock, P.A. Thiamin metabolism and thiamin diphosphate-dependent enzymes in the yeast Saccharomyces cerevisiae: Genetic regulation. Biochim. Biophys. Acta 1998, 1385, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.H.; Marek, E.T.; Schardl, C.L.; Richey, M.G.; Chang, S.Y.; Smith, D.A. sti35, a stress-responsive gene in Fusarium spp. J. Bacteriol. 1990, 172, 4522–4528. [Google Scholar] [CrossRef] [PubMed]
- Maundrell, K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem. 1990, 265, 10857–10864. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roldán, C.; Puerto-Galán, L.; Roa, J.; Castro, A.; Di Pietro, A.; Roncero, M.I.; Hera, C. The Fusarium oxysporum sti35 gene functions in thiamine biosynthesis and oxidative stress response. Fungal Genet. Biol. 2008, 45, 6–16. [Google Scholar] [CrossRef]
- Wei, C.; Wen, C.; Zhang, Y.; Du, H.; Zhong, R.; Guan, Z.; Wang, M.; Qin, Y.; Wang, F.; Song, L.; et al. The FomYjeF Protein Influences the Sporulation and Virulence of Fusarium oxysporum f. sp. momordicae. Int. J. Mol. Sci. 2023, 24, 7260. [Google Scholar] [CrossRef]
- Gomez-Gil, L.; Camara Almiron, J.; Rodriguez Carrillo, P.L.; Olivares Medina, C.N.; Bravo Ruiz, G.; Romo Rodriguez, P.; Corrales Escobosa, A.R.; Gutierrez Corona, F.; Roncero, M.I. Nitrate assimilation pathway (NAP): Role of structural (nit) and transporter (ntr1) genes in Fusarium oxysporum f. sp. lycopersici growth and pathogenicity. Curr. Genet. 2018, 64, 493–507. [Google Scholar] [CrossRef]
- Caracuel, Z.; Martínez-Rocha, A.L.; Di Pietro, A.; Madrid, M.P.; Roncero, M.I. Fusarium oxysporum gas1 encodes a putative beta-1,3-glucanosyltransferase required for virulence on tomato plants. Mol. Plant Microbe Interact. 2005, 18, 1140–1147. [Google Scholar] [CrossRef]
- López-Fernández, L.; Ruiz-Roldán, C.; Pareja-Jaime, Y.; Prieto, A.; Khraiwesh, H.; Roncero, M.I. The Fusarium oxysporum gnt2, encoding a putative N-acetylglucosamine transferase, is involved in cell wall architecture and virulence. PLoS ONE 2013, 8, e84690. [Google Scholar] [CrossRef]
- Madrid, M.P.; Di Pietro, A.; Roncero, M.I. Class V chitin synthase determines pathogenesis in the vascular wilt fungus Fusarium oxysporum and mediates resistance to plant defence compounds. Mol. Microbiol. 2003, 47, 257–266. [Google Scholar] [CrossRef]
- Martín-Udíroz, M.; Madrid, M.P.; Roncero, M.I. Role of chitin synthase genes in Fusarium oxysporum. Microbiology 2004, 150, 3175–3187. [Google Scholar] [CrossRef] [PubMed]
- Martín-Urdíroz, M.; Roncero, M.I.; González-Reyes, J.A.; Ruiz-Roldán, C. ChsVb, a class VII chitin synthase involved in septation, is critical for pathogenicity in Fusarium oxysporum. Eukaryot. Cell 2008, 7, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Xie, X.L.; Lin, X.F.; Shi, J.X.; Ding, Z.J.; Ling, J.F.; Xi, P.G.; Zhou, J.N.; Leng, Y.; Zhong, S.; et al. Functional characterization of the gene FoOCH1 encoding a putative α-1,6-mannosyltransferase in Fusarium oxysporum f. sp. cubense. Fungal Genet. Biol. 2014, 65, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sheng, B.; Zhu, D.; Chen, J.; Cai, H.; Zhang, S.; Guo, C. Role of FoERG3 in Ergosterol Biosynthesis by Fusarium oxysporum and the Associated Regulation by Bacillus subtilis HSY21. Plant Dis. 2023, 107, 1565–1575. [Google Scholar] [CrossRef]
- Usman, S.; Ge, X.; Xu, Y.; Qin, Q.; Xie, J.; Wang, B.; Jin, C.; Fang, W. Loss of Phosphomannose Isomerase Impairs Growth, Perturbs Cell Wall Integrity, and Reduces Virulence of Fusarium oxysporum f. sp. cubense on Banana Plants. J. Fungi 2023, 9, 478. [Google Scholar] [CrossRef]
- Wei-na, G.A.O.; Zhan-ming, H.O.U. Research on the function of FolGLS2 gene in Fusarium oxysporum f. sp. lini. Chin. J. Oil Crop Sci. 2024, 46. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, Y.; Ebel, F.; Jin, C. Galactofuranose (Galf)-containing sugar chain contributes to the hyphal growth, conidiation and virulence of F. oxysporum f. sp. cucumerinum. PLoS ONE 2021, 16, e0250064. [Google Scholar] [CrossRef]
- Zhang, N.; Lv, F.; Qiu, F.; Han, D.; Xu, Y.; Liang, W. Pathogenic fungi neutralize plant-derived ROS via Srpk1 deacetylation. EMBO J. 2023, 42, e112634. [Google Scholar] [CrossRef]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Cañero, D.C.; Roncero, M.I. Functional analyses of laccase genes from Fusarium oxysporum. Phytopathology 2008, 98, 509–518. [Google Scholar] [CrossRef]
- Cañero, D.C.; Roncero, M.I.G. Influence of the chloride channel of Fusarium oxysporum on extracellular laccase activity and virulence on tomato plants. Microbiology 2008, 154, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Roberts, D.P.; Dery, P.D.; Meyer, S.L.F.; Lohrke, S.; Lumsden, R.D.; Hebbar, K.P. Broad spectrum anti-biotic activity and disease suppression by the potential biocontrol agent Burkholderia ambifaria BC-F. Crop Prot. 2002, 21, 129–135. [Google Scholar] [CrossRef]
- Nelson, P.E.; Dignani, M.C.; Anaissie, E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Pareek, M.; Almog, Y.; Bari, V.K.; Hazkani-Covo, E.; Onn, I.; Covo, S. Alternative functional rad21 paralogs in Fusarium oxysporum. Front. Microbiol. 2019, 10, 1370. [Google Scholar] [CrossRef]
- Ohara, T.; Inoue, I.; Namiki, F.; Kunoh, H.; Tsuge, T. REN1 is required for development of microconidia and macroconidia, but not of chlamydospores, in the plant pathogenic fungus Fusarium oxysporum. Genetics 2004, 166, 113–124. [Google Scholar] [CrossRef]
- Qian, H.; Song, L.; Wang, L.; Yang, Q.; Wu, R.; Du, J.; Zheng, B.; Liang, W. FolIws1-driven nuclear translocation of deacetylated FolTFIIS ensures conidiation of Fusarium oxysporum. Cell Rep. 2024, 43, 114588. [Google Scholar] [CrossRef]
- Tao, L.; Yu, J.H. AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiology 2011, 157, 313–326. [Google Scholar] [CrossRef]
- Etxebeste, O.; Garzia, A.; Espeso, E.A.; Ugalde, U. Aspergillus nidulans asexual development: Making the most of cellular modules. Trends Microbiol. 2010, 18, 569–576. [Google Scholar] [CrossRef]
- Azizullah; Noman, M.; Gao, Y.; Wang, H.; Xiong, X.; Wang, J.; Li, D.; Song, F. The SUMOylation Pathway Components Are Required for Vegetative Growth, Asexual Development, Cytotoxic Responses, and Programmed Cell Death Events in. J. Fungi 2023, 9, 94. [Google Scholar] [CrossRef]
- Moura, R.D.; de Castro, L.A.M.; Culik, M.P.; Fernandes, A.A.R.; Fernandes, P.M.B.; Ventura, J.A. Culture medium for improved production of conidia for identification and systematic studies of Fusarium pathogens. J. Microbiol. Methods 2020, 173, 105915. [Google Scholar] [CrossRef]
- Iida, Y.; Kurata, T.; Harimoto, Y.; Tsuge, T. Nitrite reductase gene upregulated during conidiation is involved in macroconidium formation in Fusarium oxysporum. Phytopathology 2008, 98, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yan, L.; Liang, W.; Yang, Q. Paralogous Cyp51s mediate the differential sensitivity of Fusarium oxysporum to sterol demethylation inhibitors. Pest. Manag. Sci. 2019, 75, 396–404. [Google Scholar] [CrossRef]
- Zhang, N.; Song, L.; Xu, Y.; Pei, X.; Luisi, B.F.; Liang, W. The decrotonylase FoSir5 facilitates mitochondrial metabolic state switching in conidial germination of Fusarium oxysporum. eLife 2021, 10. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2019, 10, 2993. [Google Scholar] [CrossRef]
- Henry, C.; Fontaine, T.; Heddergott, C.; Robinet, P.; Aimanianda, V.; Beau, R.; Beauvais, A.; Mouyna, I.; Prevost, M.C.; Fekkar, A.; et al. Biosynthesis of cell wall mannan in the conidium and the mycelium of Aspergillus fumigatus. Cell Microbiol. 2016, 18, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Rajam, M.V. RNAi-mediated down-regulation of fasciclin-like proteins (FoFLPs) in Fusarium oxysporum f. sp. lycopersici results in reduced pathogenicity and virulence. Microbiol. Res. 2022, 260, 127033. [Google Scholar] [CrossRef] [PubMed]
- Rispail, N.; Di Pietro, A. Fusarium oxysporum Ste12 controls invasive growth and virulence downstream of the Fmk1 MAPK cascade. Mol. Plant-Microbe Interact. 2009, 22, 830–839. [Google Scholar] [CrossRef]
- Asunción García-Sánchez, M.; Martín-Rodrigues, N.; Ramos, B.; de Vega-Bartol, J.J.; Perlin, M.H.; Díaz-Mínguez, J.M. fost12, the Fusarium oxysporum homolog of the transcription factor Ste12, is upregulated during plant infection and required for virulence. Fungal Genet. Biol. 2010, 47, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Wang, T.; Zhou, S.; Liang, X.; Xie, C.; Kang, Z.; Chen, D.; Zheng, L. The Small Secreted Protein FoSsp1 Elicits Plant Defenses and Negatively Regulates Pathogenesis in Fusarium oxysporum f. sp. cubense (Foc4). Front. Plant Sci. 2022, 13, 873451. [Google Scholar] [CrossRef]
- Nordzieke, D.E.; Fernandes, T.R.; El Ghalid, M.; Turrà, D.; Di Pietro, A. NADPH oxidase regulates chemotropic growth of the fungal pathogen Fusarium oxysporum towards the host plant. New Phytol. 2019, 224, 1600–1612. [Google Scholar] [CrossRef]
- Dong, X.; Ling, N.; Wang, M.; Shen, Q.; Guo, S. Fusaric acid is a crucial factor in the disturbance of leaf water imbalance in Fusarium-infected banana plants. Plant Physiol. Biochem. 2012, 60, 171–179. [Google Scholar] [CrossRef]
- López-Díaz, C.; Rahjoo, V.; Sulyok, M.; Ghionna, V.; Martín-Vicente, A.; Capilla, J.; Di Pietro, A.; López-Berges, M.S. Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Plant Pathol. 2018, 19, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Lee, S.H.; Kim, L.H.; Ryu, J.G.; Lee, S.; Seo, Y.; Kim, Y.H.; Busman, M.; Yun, S.H.; Proctor, R.H.; et al. Identification of a 12-gene Fusaric Acid Biosynthetic Gene Cluster in Fusarium Species Through Comparative and Functional Genomics. Mol. Plant Microbe Interact. 2015, 28, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Zhang, Y.; Liu, N.; Viljoen, A.; Mostert, D.; Zuo, C.; Hu, C.; Bi, F.; Gao, H.; et al. Fusaric acid instigates the invasion of banana by Fusarium oxysporum f. sp. cubense TR4. New Phytol. 2020, 225, 913–929. [Google Scholar] [CrossRef]
- Crutcher, F.K.; Liu, J.; Puckhaber, L.S.; Stipanovic, R.D.; Bell, A.A.; Nichols, R.L. FUBT, a putative MFS transporter, promotes secretion of fusaric acid in the cotton pathogen Fusarium oxysporum f. sp. vasinfectum. Microbiology 2015, 161, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J. Nitric oxide: An effective weapon of the plant or the pathogen? Mol. Plant Pathol. 2014, 15, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Mostert, D.; Yu, H.; Zhuo, M.; Li, G.; Zuo, C.; Haridas, S.; Webster, K.; Li, M.; et al. Virulence of banana wilt-causing fungal pathogen Fusarium oxysporum tropical race 4 is mediated by nitric oxide biosynthesis and accessory genes. Nat. Microbiol. 2024. [Google Scholar] [CrossRef]
- Bravo Ruiz, G.; Di Pietro, A.; Roncero, M.I. Combined action of the major secreted exo- and endopolygalacturonases is required for full virulence of Fusarium oxysporum. Mol. Plant Pathol. 2016, 17, 339–353. [Google Scholar] [CrossRef]
- García-Maceira, F.I.; Di Pietro, A.; Roncero, M.I. Cloning and disruption of pgx4 encoding an in planta expressed exopolygalacturonase from Fusarium oxysporum. Mol. Plant Microbe Interact. 2000, 13, 359–365. [Google Scholar] [CrossRef]
- García-Maceira, F.I.; Di Pietro, A.; Huertas-González, M.D.; Ruiz-Roldán, M.C.; Roncero, M.I. Molecular characterization of an endopolygalacturonase from Fusarium oxysporum expressed during early stages of infection. Appl. Env. Microbiol. 2001, 67, 2191–2196. [Google Scholar] [CrossRef]
- Gómez-Gómez, E.; Isabel, M.; Roncero, G.; Di Pietro, A.; Hera, C. Molecular characterization of a novel endo-beta-1,4-xylanase gene from the vascular wilt fungus Fusarium oxysporum. Curr. Genet. 2001, 40, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Huertas-González, M.D.; Gutierrez-Corona, J.F.; Martínez-Cadena, G.; Méglecz, E.; Roncero, M.I. Molecular characterization of a subtilase from the vascular wilt fungus Fusarium oxysporum. Mol. Plant Microbe Interact. 2001, 14, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Ruiz, G.; Ruiz-Roldán, C.; Roncero, M.I. Lipolytic system of the tomato pathogen Fusarium oxysporum f. sp. lycopersici. Mol. Plant Microbe Interact. 2013, 26, 1054–1067. [Google Scholar] [CrossRef]
- Gómez-Gómez, E.; Ruíz-Roldán, M.C.; Di Pietro, A.; Roncero, M.I.; Hera, C. Role in pathogenesis of two endo-beta-1,4-xylanase genes from the vascular wilt fungus Fusarium oxysporum. Fungal Genet. Biol. 2002, 35, 213–222. [Google Scholar] [CrossRef]
- Dong, Z.; Luo, M.; Wang, Z. An Exo-polygalacturonase Pgc4 regulates aerial hyphal growth and virulence in Fusarium oxysporum f. sp. cubense race 4. Int. J. Mol. Sci. 2020, 21, 5886. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Fu, Z.; Shi, W.; Ninkuu, V.; Li, G.; Yang, X.; Zeng, H. FoEG1, a secreted glycoside hydrolase family 12 protein from Fusarium oxysporum, triggers cell death and modulates plant immunity. Mol. Plant Pathol. 2021, 22, 522–538. [Google Scholar] [CrossRef]
- Michielse, C.B.; Reijnen, L.; Olivain, C.; Alabouvette, C.; Rep, M. Degradation of aromatic compounds through the β-ketoadipate pathway is required for pathogenicity of the tomato wilt pathogen Fusarium oxysporum f. sp. lycopersici. Mol. Plant Pathol. 2012, 13, 1089–1100. [Google Scholar] [CrossRef]
- Houterman, P.M.; Speijer, D.; Dekker, H.L.; DE Koster, C.G.; Cornelissen, B.J.; Rep, M. The mixed xylem sap proteome of Fusarium oxysporum-infected tomato plants. Mol. Plant Pathol. 2007, 8, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Lievens, B.; Houterman, P.M.; Rep, M. Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales. FEMS Microbiol. Lett. 2009, 300, 201–215. [Google Scholar] [CrossRef]
- Rep, M.; van der Does, H.C.; Meijer, M.; van Wijk, R.; Houterman, P.M.; Dekker, H.L.; de Koster, C.G.; Cornelissen, B.J. A small, cysteine-rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I-3-mediated resistance in tomato. Mol. Microbiol. 2004, 53, 1373–1383. [Google Scholar] [CrossRef]
- Jangir, P.; Mehra, N.; Sharma, K.; Singh, N.; Rani, M.; Kapoor, R. Secreted in Xylem Genes: Drivers of Host Adaptation in Fusarium oxysporum. Front. Plant Sci. 2021, 12, 628611. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Wang, G.; Xiao, J.; Ling, J.; Yang, Y.; Xie, B. A SIX1 Homolog in Fusarium oxysporum f. sp. conglutinans Is Required for Full Virulence on Cabbage. PLoS ONE 2016, 11, e0152273. [Google Scholar] [CrossRef]
- Rep, M.; Meijer, M.; Houterman, P.M.; van der Does, H.C.; Cornelissen, B.J. Fusarium oxysporum evades I-3-mediated resistance without altering the matching avirulence gene. Mol. Plant Microbe Interact. 2005, 18, 15–23. [Google Scholar] [CrossRef]
- Widinugraheni, S.; Niño-Sánchez, J.; van der Does, H.C.; van Dam, P.; García-Bastidas, F.A.; Subandiyah, S.; Meijer, H.J.G.; Kistler, H.C.; Kema, G.H.J.; Rep, M. A SIX1 homolog in Fusarium oxysporum f. sp. cubense tropical race 4 contributes to virulence towards Cavendish banana. PLoS ONE 2018, 13, e0205896. [Google Scholar] [CrossRef] [PubMed]
- Houterman, P.M.; Ma, L.; van Ooijen, G.; de Vroomen, M.J.; Cornelissen, B.J.; Takken, F.L.; Rep, M. The effector protein Avr2 of the xylem-colonizing fungus Fusarium oxysporum activates the tomato resistance protein I-2 intracellularly. Plant J. 2009, 58, 970–978. [Google Scholar] [CrossRef]
- Kashiwa, T.; Inami, K.; Fujinaga, M.; Ogiso, H.; Yoshida, T.; Teraoka, T.; Arie, T. An avirulence gene homologue in the tomato wilt fungus Fusarium oxysporum f. sp. lycopersici race 1 functions as a virulence gene in the cabbage yellows fungus F. oxysporum f. sp. conglutinans. J. Gen. Plant Pathol. 2013, 71, 412–421. [Google Scholar] [CrossRef]
- Thatcher, L.F.; Gardiner, D.M.; Kazan, K.; Manners, J.M. A highly conserved effector in Fusarium oxysporum is required for full virulence on Arabidopsis. Mol. Plant Microbe Interact. 2012, 25, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Houterman, P.M.; Gawehns, F.; Cao, L.; Sillo, F.; Richter, H.; Clavijo-Ortiz, M.J.; Schmidt, S.M.; Boeren, S.; Vervoort, J.; et al. The AVR2-SIX5 gene pair is required to activate I-2-mediated immunity in tomato. New Phytol. 2015, 208, 507–518. [Google Scholar] [CrossRef]
- Gawehns, F.; Houterman, P.M.; Ichou, F.A.; Michielse, C.B.; Hijdra, M.; Cornelissen, B.J.; Rep, M.; Takken, F.L. The Fusarium oxysporum effector Six6 contributes to virulence and suppresses I-2-mediated cell death. Mol. Plant Microbe Interact. 2014, 27, 336–348. [Google Scholar] [CrossRef]
- Niu, X.; Zhao, X.; Ling, K.S.; Levi, A.; Sun, Y.; Fan, M. The FonSIX6 gene acts as an avirulence effector in the Fusarium oxysporum f. sp. niveum—Watermelon pathosystem. Sci. Rep. 2016, 6, 28146. [Google Scholar] [CrossRef]
- van Dam, P.; Fokkens, L.; Ayukawa, Y.; van der Gragt, M.; Ter Horst, A.; Brankovics, B.; Houterman, P.M.; Arie, T.; Rep, M. A mobile pathogenicity chromosome in Fusarium oxysporum for infection of multiple cucurbit species. Sci. Rep. 2017, 7, 9042. [Google Scholar] [CrossRef]
- An, B.; Hou, X.; Guo, Y.; Zhao, S.; Luo, H.; He, C.; Wang, Q. The effector SIX8 is required for virulence of Fusarium oxysporum f. sp. cubense tropical race 4 to Cavendish banana. Fungal Biol. 2019, 123, 423–430. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Asai, S.; Gan, P.; Tsushima, A.; Ichihashi, Y.; Shibata, A.; Komatsu, K.; Houterman, P.M.; Rep, M.; Shirasu, K.; et al. A pair of effectors encoded on a conditionally dispensable chromosome of Fusarium oxysporum suppress host-specific immunity. Commun. Biol. 2021, 4, 707. [Google Scholar] [CrossRef]
- Jashni, M.K.; Dols, I.H.; Iida, Y.; Boeren, S.; Beenen, H.G.; Mehrabi, R.; Collemare, J.; de Wit, P.J. Synergistic Action of a Metalloprotease and a Serine Protease from Fusarium oxysporum f. sp. lycopersici Cleaves Chitin-Binding Tomato Chitinases, Reduces Their Antifungal Activity, and Enhances Fungal Virulence. Mol. Plant Microbe Interact. 2015, 28, 996–1008. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Wu, B.; Xie, J.; Viljoen, A.; Wang, W.; Mostert, D.; Xie, Y.; Fu, G.; Xiang, D.; et al. The M35 Metalloprotease Effector FocM35_1 Is Required for Full Virulence of. Pathogens 2021, 10, 670. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Song, H.; Luo, M.; Dong, Z. α-Pheromone Precursor Protein Foc4-PP1 Is Essential for the Full Virulence of Fusarium oxysporum f. sp. cubense Tropical Race 4. J. Fungi. 2023, 9, 365. [Google Scholar] [CrossRef]
- Yan, T.; Zhou, X.; Li, J.; Li, G.; Zhao, Y.; Wang, H.; Li, H.; Nie, Y.; Li, Y. FoCupin1, a Cupin_1 domain-containing protein, is necessary for the virulence of Fusarium oxysporum f. sp. cubense tropical race 4. Front. Microbiol. 2022, 13, 1001540. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Zhao, Y.; Liang, X.; Liu, S.; Zhang, Y.; Kang, Z.; Chen, D.; Zheng, L. Systemic screening of Fusarium oxysporum candidate effectors reveals FoSSP17 that suppresses plant immunity and contributes to virulence. Phytopathol. Res. 2023, 5. [Google Scholar] [CrossRef]
- Pareja-Jaime, Y.; Roncero, M.I.; Ruiz-Roldán, M.C. Tomatinase from Fusarium oxysporum f. sp. lycopersici is required for full virulence on tomato plants. Mol. Plant Microbe Interact. 2008, 21, 728–736. [Google Scholar] [CrossRef]
- Yang, Y.; An, B.; Guo, Y.; Luo, H.; He, C.; Wang, Q. A Novel Effector, FSE1, Regulates the Pathogenicity of Fusarium oxysporum f. sp. cubense Tropical Race 4 to Banana by Targeting the MYB Transcription Factor MaEFM-Like. J. Fungi 2023, 9, 472. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Wang, C.; Liu, S.; Yu, G.; Gao, M.; Qian, H.; Liu, M.; Luisi, B.F.; Gabriel, D.W.; et al. Acetylation of a fungal effector that translocates host PR1 facilitates virulence. eLife 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.M.; Mao, H.Y.; Li, S.J.; Feng, T.; Zhang, Z.Y.; Cheng, L.; Luo, S.J.; Borkovich, K.A.; Ouyang, S.Q. Fol-milR1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses. New Phytol. 2021, 232, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Li, S.; Jiang, N.; Sun, Q.; Xuan, Y.H.; Xia, Z. Comparative transcriptome analysis reveals that ATP synthases regulate Fusarium oxysporum virulence by modulating sugar transporter gene expressions in tobacco. Front. Plant Sci. 2022, 13, 978951. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Wang, L.; Wang, B.; Liang, W. The secreted ribonuclease T2 protein FoRnt2 contributes to Fusarium oxysporum virulence. Mol. Plant Pathol. 2022, 23, 1346–1360. [Google Scholar] [CrossRef]
- Chang, W.; Li, H.; Chen, H.; Qiao, F.; Zeng, H. Identification of mimp-associated effector genes in Fusarium oxysporum f. sp. cubense race 1 and race 4 and virulence confirmation of a candidate effector gene. Microbiol. Res. 2020, 232, 126375. [Google Scholar] [CrossRef]
- Guo, L.; Wang, J.; Liang, C.; Yang, L.; Zhou, Y.; Liu, L.; Huang, J. Fosp9, a Novel Secreted Protein, Is Essential for the Full Virulence of Fusarium oxysporum f. sp. Appl. Env. Microbiol. 2022, 88, e0060421. [Google Scholar] [CrossRef]
- Sasaki, K.; Ito, Y.; Hamada, Y.; Dowaki, A.; Jogaiah, S.; Ito, S.I. FoMC69 Gene in Fusarium oxysporum f. sp. radicis-lycopersici Is Essential for Pathogenicity by Involving Normal Function of Chlamydospores. Pathogens 2022, 11, 1433. [Google Scholar] [CrossRef]
- Sakane, K.; Akiyama, M.; Ando, A.; Shigyo, M.; Ito, S.I.; Sasaki, K. Identification of a novel effector gene and its functional tradeoff in Fusarium oxysporum f. sp. cepae that infects Welsh onion. Physiol. Mol. Plant Pathol. 2023, 123, 101939. [Google Scholar] [CrossRef]
- Liu, S.; Wu, B.; Yang, J.; Bi, F.; Dong, T.; Yang, Q.; Hu, C.; Xiang, D.; Chen, H.; Huang, H.; et al. A cerato-platanin family protein FocCP1 is essential for the penetration and virulence of Fusarium oxysporum f. sp. cubense tropical race 4. Int. J. Mol. Sci. 2019, 20, 3785. [Google Scholar] [CrossRef]
- Araiza-Cervantes, C.A.; Valle-Maldonado, M.I.; Patiño-Medina, J.A.; Alejandre-Castañeda, V.; Guzmán-Pérez, J.B.; Ramírez-Díaz, M.I.; Macias-Sánchez, K.L.; López-Berges, M.S.; Meza-Carmen, V. Transcript pattern analysis of Arf-family genes in the phytopathogen Fusarium oxysporum f. sp. lycopersici reveals the role of Arl3 in the virulence. Antonie Van. Leeuwenhoek 2021, 114, 1619–1632. [Google Scholar] [CrossRef]
- Calero-Nieto, F.; Di Pietro, A.; Roncero, M.I.; Hera, C. Role of the transcriptional activator xlnR of Fusarium oxysporum in regulation of xylanase genes and virulence. Mol. Plant Microbe Interact. 2007, 20, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Arjona, F.M.; Vitale, S.; Voxeur, A.; Dora, S.; Müller, S.; Sancho-Andrés, G.; Montesinos, J.C.; Di Pietro, A.; Sánchez-Rodríguez, C. Impairment of the cellulose degradation machinery enhances Fusarium oxysporum virulence but limits its reproductive fitness. Sci. Adv. 2022, 8, eabl9734. [Google Scholar] [CrossRef]
- Caracuel, Z.; Roncero, M.I.; Espeso, E.A.; González-Verdejo, C.I.; García-Maceira, F.I.; Di Pietro, A. The pH signalling transcription factor PacC controls virulence in the plant pathogen Fusarium oxysporum. Mol. Microbiol. 2003, 48, 765–779. [Google Scholar] [CrossRef]
- Jonkers, W.; Rep, M. Mutation of CRE1 in Fusarium oxysporum reverts the pathogenicity defects of the FRP1 deletion mutant. Mol. Microbiol. 2009, 74, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Zhou, X.; Yang, S.; Wen, Y.; You, H.; Zheng, Y.; Norvienyeku, J.; Shim, W.B.; Wang, Z. Fusarium oxysporum f. sp. lycopersici C2H2 transcription factor FolCzf1 is required for conidiation, fusaric acid production, and early host infection. Curr. Genet. 2019, 65, 773–783. [Google Scholar] [CrossRef]
- Ding, Z.-j.; Qi, Y.-x.; Zeng, F.-y.; Peng, J.; Xie, Y.-x.; Zhang, X. A transcription factor FoSwi6 regulates physiology traits and virulence in Fusarium oxysporum f. sp. cubense. Acta Phytopathol. Sin. 2018, 48, 601–610. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, T.; Zhu, W.; Li, L.; Fu, Q. A MADS-box transcription factor FoRlm1 regulates aerial hyphal growth, oxidative stress, cell wall biosynthesis and virulence in Fusarium oxysporum f. sp. cubense. Fungal Biol. 2020, 124, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; van Wijk, R.; Reijnen, L.; Manders, E.M.; Boas, S.; Olivain, C.; Alabouvette, C.; Rep, M. The nuclear protein Sge1 of Fusarium oxysporum is required for parasitic growth. PLoS Pathog. 2009, 5, e1000637. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Casado-Del Castillo, V.; Tello, V.; De Vega-Bartol, J.J.; Ramos, B.; Sukno, S.A.; Díaz Mínguez, J.M. The FTF gene family regulates virulence and expression of SIX effectors in Fusarium oxysporum. Mol. Plant Pathol. 2016, 17, 1124–1139. [Google Scholar] [CrossRef]
- Casado-Del Castillo, V.; Benito, E.P.; Díaz-Mínguez, J.M. The Role of the Fusarium oxysporum FTF2 Transcription Factor in Host Colonization and Virulence in Common Bean Plants (Phaseolus vulgaris L.). Pathogens 2023, 12, 380. [Google Scholar] [CrossRef]
- Imazaki, I.; Kurahashi, M.; Iida, Y.; Tsuge, T. Fow2, a Zn(II)2Cys6-type transcription regulator, controls plant infection of the vascular wilt fungus Fusarium oxysporum. Mol. Microbiol. 2007, 63, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; van Wijk, R.; Reijnen, L.; Cornelissen, B.J.; Rep, M. Insight into the molecular requirements for pathogenicity of Fusarium oxysporum f. sp. lycopersici through large-scale insertional mutagenesis. Genome Biol. 2009, 10, R4. [Google Scholar] [CrossRef]
- Ruiz-Roldán, M.C.; Garre, V.; Guarro, J.; Mariné, M.; Roncero, M.I. Role of the white collar 1 photoreceptor in carotenogenesis, UV resistance, hydrophobicity, and virulence of Fusarium oxysporum. Eukaryot. Cell 2008, 7, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Divon, H.H.; Ziv, C.; Davydov, O.; Yarden, O.; Fluhr, R. The global nitrogen regulator, FNR1, regulates fungal nutrition-genes and fitness during Fusarium oxysporum pathogenesis. Mol. Plant Pathol. 2006, 7, 485–497. [Google Scholar] [CrossRef]
- López-Berges, M.S.; Capilla, J.; Turrà, D.; Schafferer, L.; Matthijs, S.; Jöchl, C.; Cornelis, P.; Guarro, J.; Haas, H.; Di Pietro, A. HapX-mediated iron homeostasis is essential for rhizosphere competence and virulence of the soilborne pathogen Fusarium oxysporum. Plant Cell 2012, 24, 3805–3822. [Google Scholar] [CrossRef]
- López-Berges, M.S. ZafA-mediated regulation of zinc homeostasis is required for virulence in the plant pathogen Fusarium oxysporum. Mol. Plant Pathol. 2020, 21, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Guo, L.; Yang, L.; Huang, J. Foatf1, a bZIP transcription factor of Fusarium oxysporum f. sp. cubense, is involved in pathogenesis by regulating the oxidative stress responses of Cavendish banana (Musa spp.) . Physiol. Mol. Plant Pathol. 2013, 84, 76–85. [Google Scholar] [CrossRef]
- Qi, X.; Liu, L.; Wang, J. Stress response regulator FoSkn7 participates in the pathogenicity of Fusarium oxysporum f. sp. cubense race 4 by conferring resistance to exogenous oxidative stress. J. Gen. Plant Pathol. 2019, 85, 382–394. [Google Scholar] [CrossRef]
- Bailey, B.A.; Apel-Birkhold, P.C.; Luster, D.G. Expression of NEP1 by Fusarium oxysporum f. sp. erythroxyli After Gene Replacement and Overexpression Using Polyethylene Glycol-Mediated Transformation. Phytopathology 2002, 92, 833–841. [Google Scholar] [CrossRef]
- Ohara, T.; Tsuge, T. FoSTUA, encoding a basic helix-loop-helix protein, differentially regulates development of three kinds of asexual spores, macroconidia, microconidia, and chlamydospores, in the fungal plant pathogen Fusarium oxysporum. Eukaryot. Cell 2004, 3, 1412–1422. [Google Scholar] [CrossRef]
- Martínez-Rocha, A.L.; Roncero, M.I.; López-Ramirez, A.; Mariné, M.; Guarro, J.; Martínez-Cadena, G.; Di Pietro, A. Rho1 has distinct functions in morphogenesis, cell wall biosynthesis and virulence of Fusarium oxysporum. Cell Microbiol. 2008, 10, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Medina, M.A.; Macías-Sánchez, K.L. GTPase Rho1 regulates the expression of xyl3 and laccase genes in Fusarium oxysporum. Biotechnol. Lett. 2015, 37, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Gurdaswani, V.; Ghag, S.B.; Ganapathi, T.R. FocSge1 in Fusarium oxysporum f. sp. cubense race 1 is essential for full virulence. BMC Microbiol. 2020, 20, 255. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; An, B.; Wang, Q.; Guo, Y.; Luo, H.; He, C. SGE1 is involved in conidiation and pathogenicity of Fusarium oxysporum f. sp. cubense. Can. J. Microbiol. 2018, 64, 349–357. [Google Scholar] [CrossRef]
- He, D.; Feng, Z.; Gao, S.; Wei, Y.; Han, S.; Wang, L. Contribution of NADPH-cytochrome P450 Reductase to Azole Resistance in Fusarium oxysporum. Front. Microbiol. 2021, 12, 709942. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, E.; Li, J.; Weerasinghe, T.; Li, X. The Ubiquitous Wilt-Inducing Pathogen Fusarium oxysporum—A Review of Genes Studied with Mutant Analysis. Pathogens 2024, 13, 823. https://doi.org/10.3390/pathogens13100823
Jackson E, Li J, Weerasinghe T, Li X. The Ubiquitous Wilt-Inducing Pathogen Fusarium oxysporum—A Review of Genes Studied with Mutant Analysis. Pathogens. 2024; 13(10):823. https://doi.org/10.3390/pathogens13100823
Chicago/Turabian StyleJackson, Edan, Josh Li, Thilini Weerasinghe, and Xin Li. 2024. "The Ubiquitous Wilt-Inducing Pathogen Fusarium oxysporum—A Review of Genes Studied with Mutant Analysis" Pathogens 13, no. 10: 823. https://doi.org/10.3390/pathogens13100823





