Involvement of MicroRNAs in the Hypersensitive Response of Capsicum Plants to the Capsicum Chlorosis Virus at Elevated Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Growth Conditions
2.2. RNA Isolation, Small RNA Library Construction. and Sequencing
2.3. Small RNA Sequencing Data Pre-Processing and microRNA Identification
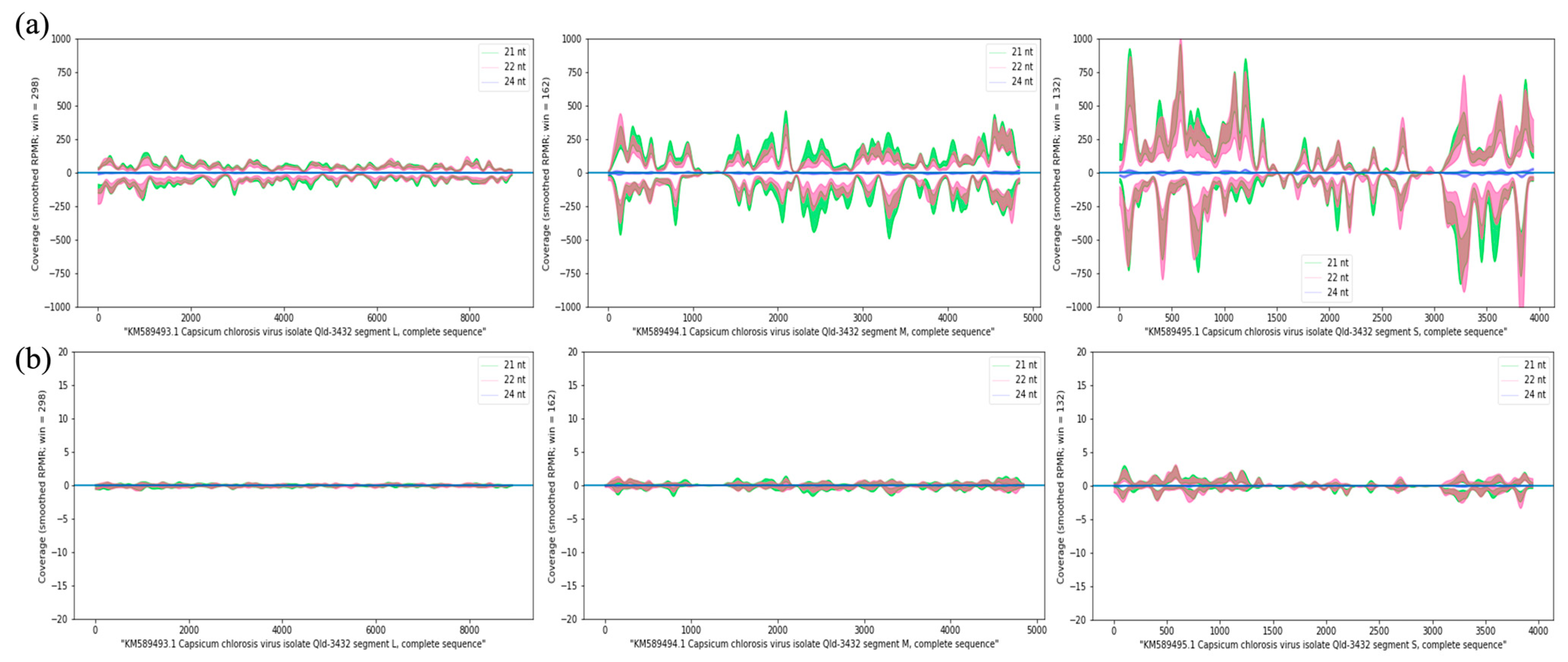
2.4. Analysis of Virus-Derived siRNAs
2.5. Differential Expression Analysis of miRNAs
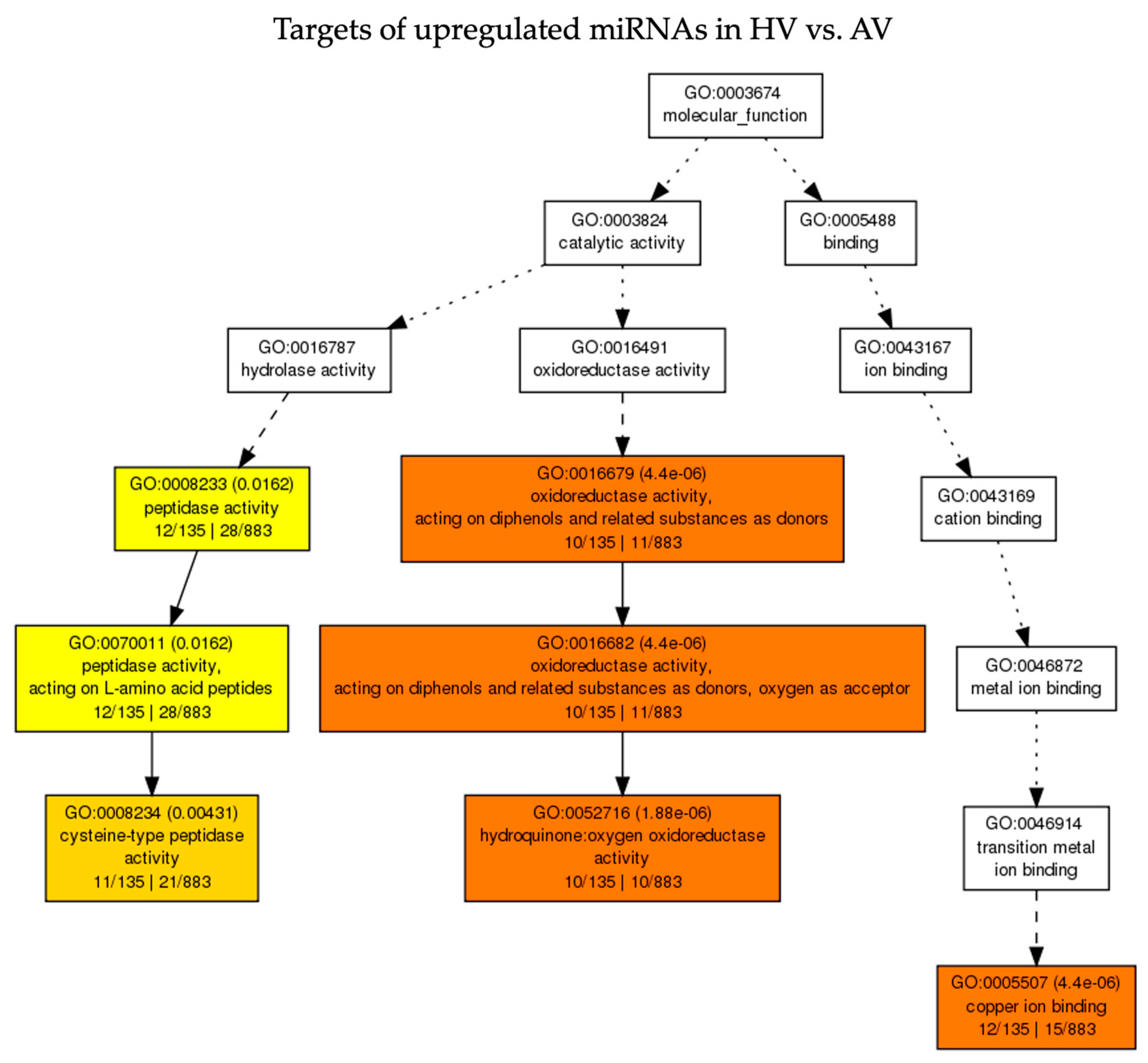
2.6. miRNA Target Prediction and Enrichment Analysis
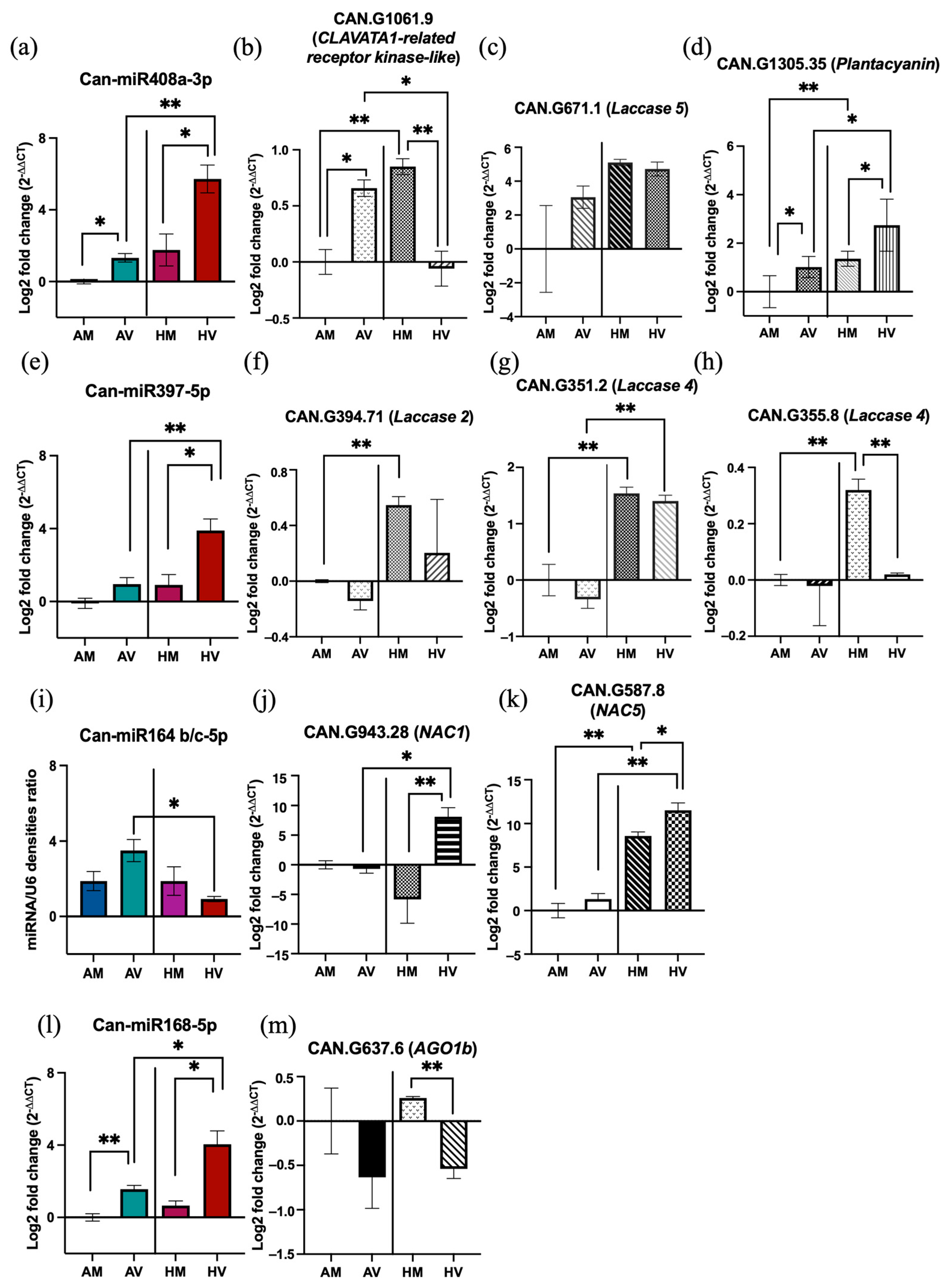
2.7. Evaluation of the Expression of miRNAs and Their Potential Target Genes
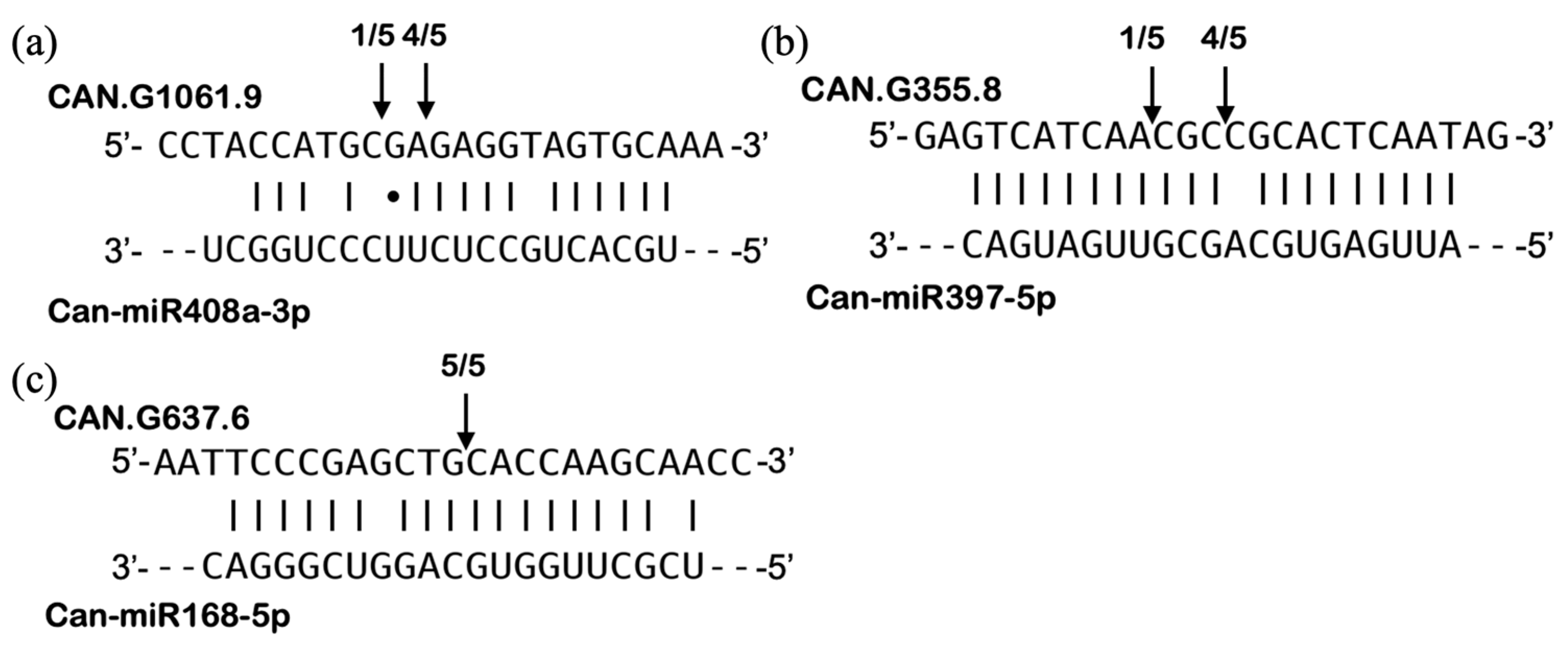
2.8. Cleavage Sites Validation of Target Genes of miRNAs
3. Results
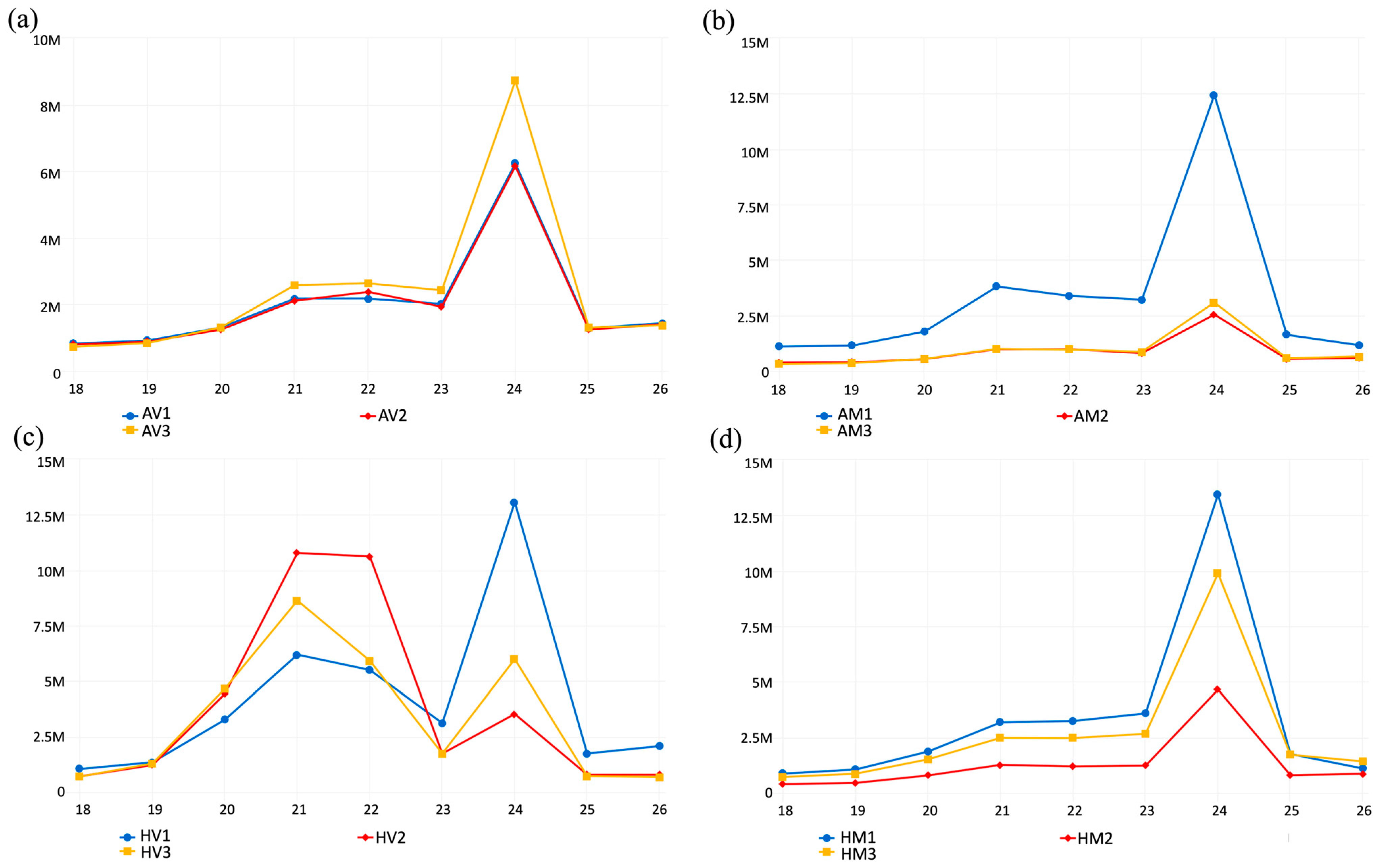
3.1. CaCV Symptom Development in Resistant Capsicum Plants at Elevated Temperatures
3.2. Small RNAs in CaCV-Infected and Virus-Free Capsicum Grown at Different Temperatures
3.3. Identification of Known and Novel miRNAs
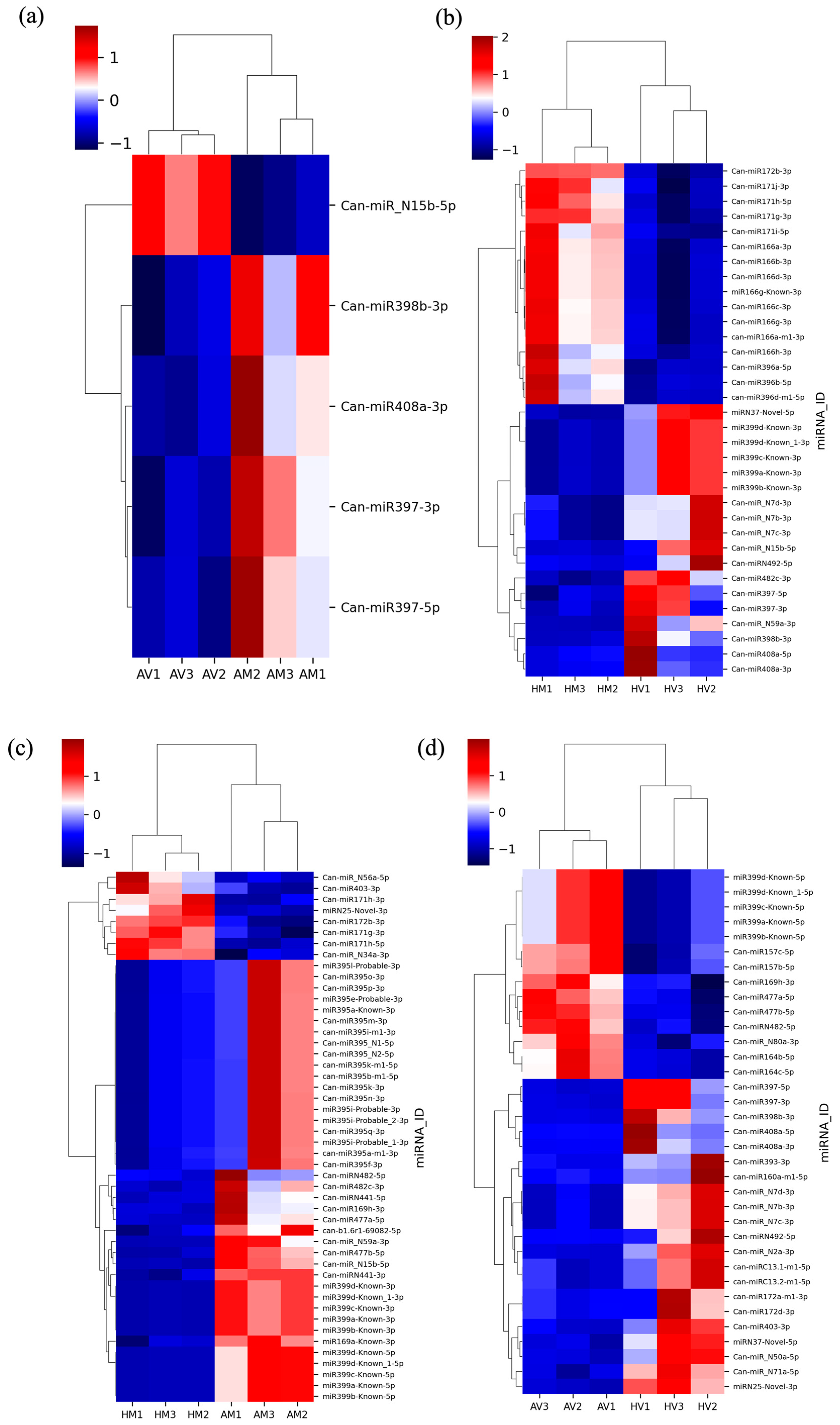
3.4. Differentially Expressed miRNAs
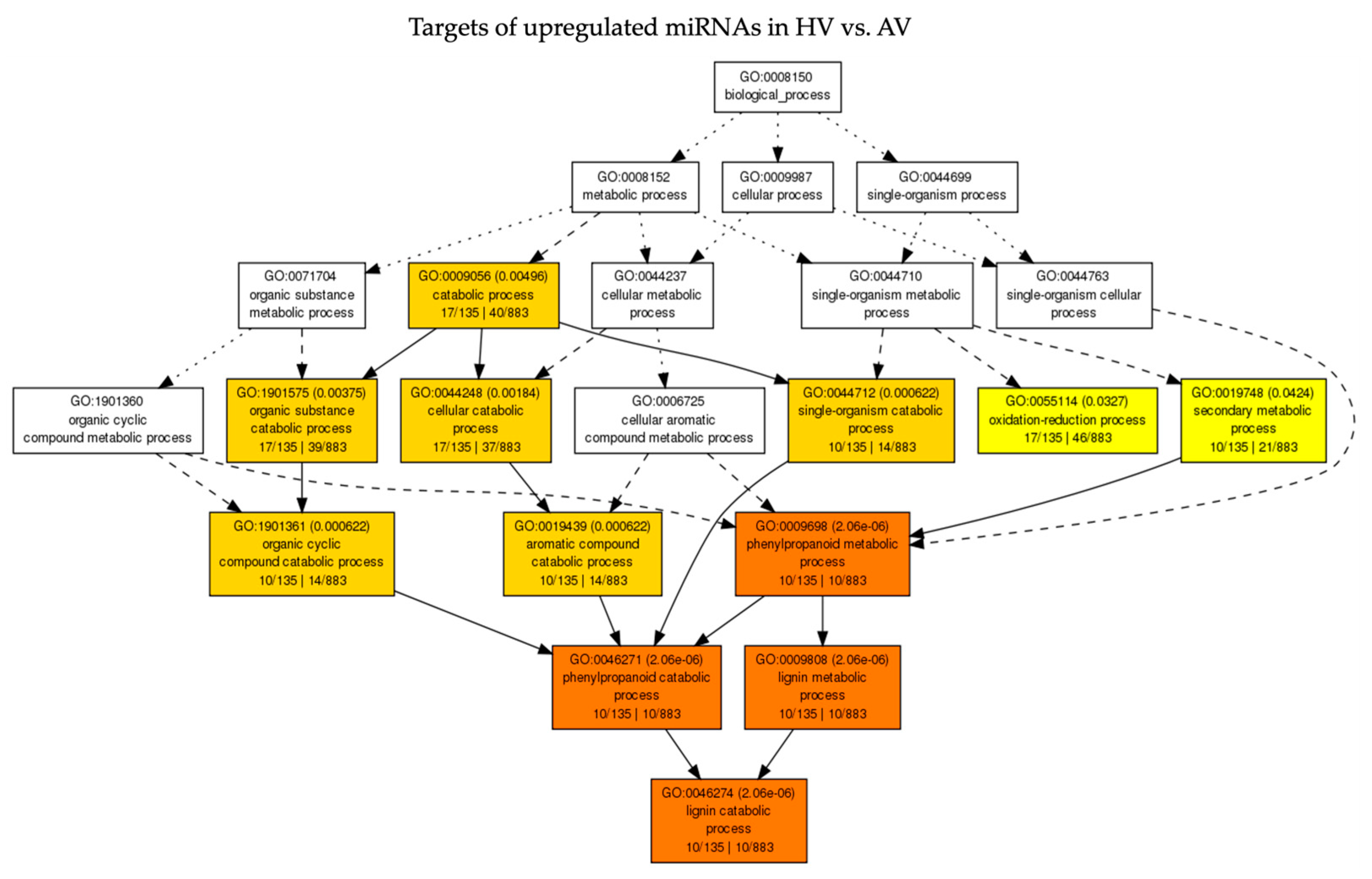
3.5. Functional Analysis of Predicted Target Genes of DE miRNAs in Response to CaCV under Ambient and High-Temperature Conditions
3.6. Validation of the Expression Patterns of Selected miRNAs and Their Target Genes
3.7. Identification of miRNA-Mediated Cleavage of Target Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripodi, P.; Kumar, S. The Capsicum Crop: An Introduction. In The Capsicum Genome; Ramchiary, N., Kole, C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–8. [Google Scholar]
- Zou, X.; Zhu, F. Origin, Evolution and Cultivation History of the Pepper. Acta Hortic. Sin. 2022, 49, 1371–1381. [Google Scholar] [CrossRef]
- Khaitov, B.; Umurzokov, M.; Cho, K.-M.; Lee, Y.-J.; Park, K. Importance and production of chilli pepper; heat tolerance and efficient nutrient use under climate change conditions. Korean J. Agric. Sci. 2019, 46, 769–779. [Google Scholar] [CrossRef]
- Penella, C.; Calatayud, A. Pepper Crop under Climate Change: Grafting as an Environmental Friendly Strategy. In Climate Resilient Agriculture: Strategies and Perspectives; IntechOpen: London, UK, 2018; pp. 128–155. [Google Scholar]
- Lu, J.; Guo, M.; Zhai, Y.; Gong, Z.; Lu, M. Differential Responses to the Combined Stress of Heat and Phytophthora capsici Infection Between Resistant and Susceptible Germplasms of Pepper (Capsicum annuum L.). J. Plant Growth Regul. 2017, 36, 161–173. [Google Scholar] [CrossRef]
- Parisi, M.; Alioto, D.; Tripodi, P. Overview of Biotic Stresses in Pepper (Capsicum spp.): Sources of Genetic Resistance, Molecular Breeding and Genomics. Int. J. Mol. Sci. 2020, 21, 2587. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Jain, R.K.; Krishnareddy, M.; Krishna Kumar, N.K.; Ravi, K.S.; Pappu, H.R. Emerging Problems of Tospoviruses (Bunyaviridae) and their Management in the Indian Subcontinent. Plant Dis. 2012, 96, 468–479. [Google Scholar] [CrossRef]
- Sharman, M.; Thomas, J.E.; Tree, D.; Persley, D.M. Natural host range and thrips transmission of capsicum chlorosis virus in Australia. Australas. Plant Pathol. 2020, 49, 45–51. [Google Scholar] [CrossRef]
- Persley, D.M.; Thomas, J.E.; Sharman, M. Tospoviruses—An Australian perspective. Australas. Plant Pathol. 2006, 35, 161–180. [Google Scholar] [CrossRef]
- Health, E.; Panel, o.P.; Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Gonthier, P.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Pest categorisation of Capsicum chlorosis virus. Eur. Food Saf. Auth. J. 2022, 20, e07337. [Google Scholar] [CrossRef]
- Chen, K.; Xu, Z.; Yan, L.; Wang, G. Characterization of a New Strain of Capsicum chlorosis virus from Peanut (Arachis hypogaea L.) in China. J. Phytopathol. 2007, 155, 178–181. [Google Scholar] [CrossRef]
- Krishnareddy, M.; Rani, R.U.; Kumar, K.S.A.; Reddy, K.M.; Pappu, H.R. Capsicum chlorosis virus (Genus Tospovirus) Infecting Chili Pepper (Capsicum annuum) in India. Plant. Dis. 2008, 92, 1469. [Google Scholar] [CrossRef]
- Widana Gamage, S.M.K.; McGrath, D.J.; Persley, D.M.; Dietzgen, R.G. Transcriptome Analysis of Capsicum Chlorosis Virus-Induced Hypersensitive Resistance Response in Bell Capsicum. PLoS ONE 2016, 11, e0159085. [Google Scholar] [CrossRef] [PubMed]
- McGarth, D. Capsicum Breeding for Tospovirus Resistance; Horticultural Australia Ltd.: Sydney, NSW, Australia, 2006. [Google Scholar]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.E.; Ullman, D.E.; German, T.L. Tospovirus-thrips interactions. Annu. Rev. Phytopathol. 2005, 43, 459–489. [Google Scholar] [CrossRef]
- Widana Gamage, S.; Persley, D.M.; Higgins, C.M.; Dietzgen, R.G. First complete genome sequence of a capsicum chlorosis tospovirus isolate from Australia with an unusually large S RNA intergenic region. Arch. Virol. 2015, 160, 869–872. [Google Scholar] [CrossRef]
- Honjo, M.N.; Emura, N.; Kawagoe, T.; Sugisaka, J.; Kamitani, M.; Nagano, A.J.; Kudoh, H. Seasonality of interactions between a plant virus and its host during persistent infection in a natural environment. Int. Soc. Microb. Ecol. J. 2020, 14, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive responses of potato (Solanum tuberosum L.) plants to heat stress and infection with potato virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Z.; Zhu, Y.; Hua, J. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol. Plant Microbe Interact. 2009, 22, 498–506. [Google Scholar] [CrossRef]
- Anfoka, G.; Moshe, A.; Fridman, L.; Amrani, L.; Rotem, O.; Kolot, M.; Zeidan, M.; Czosnek, H.; Gorovits, R. Tomato yellow leaf curl virus infection mitigates the heat stress response of plants grown at high temperatures. Sci. Rep. 2016, 6, 19715. [Google Scholar] [CrossRef]
- Canto, T.; Aranda, M.A.; Fereres, A. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Glob. Chang. Biol. 2009, 15, 1884–1894. [Google Scholar] [CrossRef]
- Velasquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Tsai, W.-A.; Brosnan, C.A.; Mitter, N.; Dietzgen, R.G. Perspectives on plant virus diseases in a climate change scenario of elevated temperatures. Stress Biol. 2022, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, B.C.; Calil, I.P.; Machado, J.P.B.; Santos, A.A.; Fontes, E.P.B. Immune Receptors and Co-receptors in Antiviral Innate Immunity in Plants. Front. Microbiol 2017, 7, 2139. [Google Scholar] [CrossRef]
- de Ronde, D.; Lohuis, D.; Kormelink, R. Identification and characterization of a new class of Tomato spotted wilt virus isolates that break Tsw-based resistance in a temperature-dependent manner. Plant Pathol. 2019, 68, 60–71. [Google Scholar] [CrossRef]
- Moury, B.; Selassie, K.G.; Marchoux, G.; Daubèze, A.-M.; Palloix, A. High temperature effects on hypersensitive resistance to Tomato Spotted Wilt Tospovirus (TSWV) in pepper (Capsicum chinense Jacq.). Eur. J. Plant Pathol. 1998, 104, 489–498. [Google Scholar] [CrossRef]
- Soler, S.; Diez, M.J.; Nuez, F. Effect of Temperature Regime and Growth Stage Interaction on Pattern of Virus Presence in TSWV-Resistant Accessions of Capsicum chinense. Plant Dis. 1998, 82, 1199–1204. [Google Scholar] [CrossRef]
- Szajko, K.; Yin, Z.; Marczewski, W. Accumulation of miRNA and mRNA Targets in Potato Leaves Displaying Temperature-Dependent Responses to Potato Virus Y. Potato Res. 2019, 62, 379–392. [Google Scholar] [CrossRef]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Liu, S.-R.; Zhou, J.-J.; Hu, C.-G.; Wei, C.-L.; Zhang, J.-Z. MicroRNA-Mediated Gene Silencing in Plant Defense and Viral Counter-Defense. Front. Microbiol. 2017, 8, 1801. [Google Scholar] [CrossRef]
- D’Ario, M.; Griffiths-Jones, S.; Kim, M. Small RNAs: Big Impact on Plant Development. Trends. Plant Sci. 2017, 22, 1056–1068. [Google Scholar] [CrossRef]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Zhang, J.; Wang, L.; Wu, J. Roles of Small RNAs in Virus-Plant Interactions. Viruses 2019, 11, 827. [Google Scholar] [CrossRef]
- Križnik, M.; Baebler, Š.; Gruden, K. Roles of small RNAs in the establishment of tolerant interaction between plants and viruses. Curr. Opin. Virol. 2020, 42, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Shivaprasad, P.V.; Chen, H.-M.; Patel, K.; Bond, D.M.; Santos, B.A.C.M.; Baulcombe, D.C. A MicroRNA Superfamily Regulates Nucleotide Binding Site–Leucine-Rich Repeats and Other mRNAs. Plant Cell 2012, 24, 859–874. [Google Scholar] [CrossRef]
- Li, F.; Pignatta, D.; Bendix, C.; Brunkard, J.O.; Cohn, M.M.; Tung, J.; Sun, H.; Kumar, P.; Baker, B. MicroRNA regulation of plant innate immune receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sahu, P.P.; Prasad, A.; Muthamilarasan, M.; Waseem, M.; Khan, Y.; Thakur, J.K.; Chakraborty, S.; Prasad, M. The Sw5a gene confers resistance to ToLCNDV and triggers an HR response after direct AC4 effector recognition. Proc. Natl. Acad. Sci. USA 2021, 118, e2101833118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ding, Z.; Wu, K.; Yang, L.; Li, Y.; Yang, Z.; Shi, S.; Liu, X.; Zhao, S.; Yang, Z.; et al. Suppression of Jasmonic Acid-Mediated Defense by Viral-Inducible MicroRNA319 Facilitates Virus Infection in Rice. Mol. Plant 2016, 9, 1302–1314. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Li, J.; Chen, J.P.; Zhang, H.M. Integrative Analysis of the microRNAome and Transcriptome Illuminates the Response of Susceptible Rice Plants to Rice Stripe Virus. PLoS ONE 2016, 11, e0146946. [Google Scholar] [CrossRef]
- Tao, H.; Jia, Z.; Gao, X.; Gui, M.; Li, Y.; Liu, Y. Analysis of the miRNA expression profile involved in the tomato spotted wilt orthotospovirus–pepper interaction. Virus. Res. 2022, 312, 198710. [Google Scholar] [CrossRef]
- Zhang, T.; Zhai, J.; Zhang, X.; Ling, L.; Li, M.; Xie, S.; Song, M.; Ma, C. Interactive Web-based Annotation of Plant MicroRNAs with iwa-miRNA. Genom. Proteom. Bioinform. 2022, 20, 557–567. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome. Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Kuang, Z.; Wang, Y.; Li, L.; Yang, X. miRDeep-P2: Accurate and fast analysis of the microRNA transcriptome in plants. Bioinformatics 2019, 35, 2521–2522. [Google Scholar] [CrossRef]
- Guo, Z.; Kuang, Z.; Wang, Y.; Zhao, Y.; Tao, Y.; Cheng, C.; Yang, J.; Lu, X.; Hao, C.; Wang, T.; et al. PmiREN: A comprehensive encyclopedia of plant miRNAs. Nucleic. Acids. Res. 2019, 48, D1114–D1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, J.; Feng, J.; Liu, B.; Feng, L.; Yu, X.; Li, G.; Zhai, J.; Meyers, B.C.; Xia, R. sRNAanno—A database repository of uniformly annotated small RNAs in plants. Hortic. Res. 2021, 8, 45. [Google Scholar] [CrossRef]
- Lunardon, A.; Johnson, N.R.; Hagerott, E.; Phifer, T.; Polydore, S.; Coruh, C.; Axtell, M.J. Integrated annotations and analyses of small RNA-producing loci from 47 diverse plants. Genome Res. 2020, 30, 497–513. [Google Scholar] [CrossRef]
- Meng, J.; Liu, D.; Sun, C.; Luan, Y. Prediction of plant pre-microRNAs and their microRNAs in genome-scale sequences using structure-sequence features and support vector machine. BMC Bioinform. 2014, 15, 423. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J.; Meyers, B.C. Revisiting Criteria for Plant MicroRNA Annotation in the Era of Big Data. Plant Cell 2018, 30, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic. Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Boden, M.; Mitter, N.; Carroll, B.J. SCRAM: A pipeline for fast index-free small RNA read alignment and visualization. Bioinformatics 2018, 34, 2670–2672. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bedre, R. Reneshbedre/Bioinfokit: Bioinformatics Data Analysis and Visualization Toolkit. Zenodo 2020. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic. Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Van Bel, M.; Diels, T.; Vancaester, E.; Kreft, L.; Botzki, A.; Van de Peer, Y.; Coppens, F.; Vandepoele, K. PLAZA 4.0: An integrative resource for functional, evolutionary and comparative plant genomics. Nucleic. Acids Res. 2017, 46, D1190–D1196. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic. Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zhang, W.; Li, H.; Shen, X.; Guo, Z. Separate enrichment analysis of pathways for up- and downregulated genes. J. R. Soc. Interface 2014, 11, 20130950. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The Control of the False Discovery Rate in Multiple Testing Under Dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Kang, K.; Zhang, X.; Liu, H.; Wang, Z.; Zhong, J.; Huang, Z.; Peng, X.; Zeng, Y.; Wang, Y.; Yang, Y.; et al. A novel real-time PCR assay of microRNAs using S-Poly(T), a specific oligo(dT) reverse transcription primer with excellent sensitivity and specificity. PLoS ONE 2012, 7, e48536. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, L.; Qiu, H.; Wu, Y.; Wang, Z.; Zai, Y.; Liu, L.; Qu, J.; Kang, K.; Gou, D. An improved method for detecting circulating microRNAs with S-Poly(T) Plus real-time PCR. Sci. Rep. 2015, 5, 15100. [Google Scholar] [CrossRef]
- Xie, S.; Zhu, Q.; Qu, W.; Xu, Z.; Liu, X.; Li, X.; Li, S.; Ma, W.; Miao, Y.; Zhang, L.; et al. sRNAPrimerDB: Comprehensive primer design and search web service for small non-coding RNAs. Bioinformatics 2019, 35, 1566–1572. [Google Scholar] [CrossRef]
- Tang, Z.; Yu, J.; Xie, J.; Lyu, J.; Feng, Z.; Dawuda, M.M.; Liao, W.; Wu, Y.; Hu, L. Physiological and Growth Response of Pepper (Capsicum annum L.) Seedlings to Supplementary Red/Blue Light Revealed through Transcriptomic Analysis. Agronomy 2019, 9, 139. [Google Scholar] [CrossRef]
- Tsai, W.A.; Shafiei-Peters, J.R.; Mitter, N.; Dietzgen, R.G. Effects of Elevated Temperature on the Susceptibility of Capsicum Plants to Capsicum Chlorosis Virus Infection. Pathogens 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mitter, N.; Dietzgen, R.G. Use of hairpin RNA constructs for engineering plant virus resistance. Methods Mol. Biol. 2012, 894, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Kasschau, K.D.; Xie, Z.; Allen, E.; Llave, C.; Chapman, E.J.; Krizan, K.A.; Carrington, J.C. P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA function. Dev. Cell 2003, 4, 205–217. [Google Scholar] [CrossRef]
- Song, C.; Wang, C.; Zhang, C.; Korir, N.K.; Yu, H.; Ma, Z.; Fang, J. Deep sequencing discovery of novel and conserved microRNAs in trifoliate orange (Citrus trifoliata). BioMed Cent. Genom. 2010, 11, 431. [Google Scholar] [CrossRef]
- Mallory, A.C.; Elmayan, T.; Vaucheret, H. MicroRNA maturation and action--the expanding roles of ARGONAUTEs. Curr. Opin. Plant Biol. 2008, 11, 560–566. [Google Scholar] [CrossRef]
- Varallyay, E.; Valoczi, A.; Agyi, A.; Burgyan, J.; Havelda, Z. Plant virus-mediated induction of miR168 is associated with repression of ARGONAUTE1 accumulation. Eur. Mol. Biol. Organ. J. 2010, 29, 3507–3519. [Google Scholar] [CrossRef]
- Varallyay, E.; Havelda, Z. Unrelated viral suppressors of RNA silencing mediate the control of ARGONAUTE1 level. Mol. Plant Pathol. 2013, 14, 567–575. [Google Scholar] [CrossRef]
- Fang, X.; Qi, Y. RNAi in Plants: An Argonaute-Centered View. Plant Cell 2016, 28, 272–285. [Google Scholar] [CrossRef]
- Fei, Y.; Mao, Y.; Shen, C.; Wang, R.; Zhang, H.; Huang, J. WPMIAS: Whole-degradome-based Plant MicroRNA-Target Interaction Analysis Server. Bioinformatics 2019, 36, 1937–1939. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, C.; Mei, J.; Chen, X.; Wu, Z.; Luo, X.; Cheng, J.; Tang, X.; Hu, K.; Li, S.C. Identification of MicroRNA Targets of Capsicum spp. Using MiRTrans-a Trans-Omics Approach. Front. Plant Sci. 2017, 8, 495. [Google Scholar] [CrossRef]
- Marques de Carvalho, L.; Benda, N.D.; Vaughan, M.M.; Cabrera, A.R.; Hung, K.; Cox, T.; Abdo, Z.; Allen, L.H.; Teal, P.E. Mi-1-Mediated Nematode Resistance in Tomatoes is Broken by Short-Term Heat Stress but Recovers Over Time. J. Nematol. 2015, 47, 133–140. [Google Scholar] [PubMed]
- Romero, A.M.; Kousik, C.S.; Ritchie, D.F. Temperature Sensitivity of the Hypersensitive Response of Bell Pepper to Xanthomonas axonopodis pv. vesicatoria. Phytopathology 2002, 92, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Whitham, S.; McCormick, S.; Baker, B. The N gene of tobacco confers resistance to tobacco mosaic virus in transgenic tomato. Proc. Natl. Acad. Sci. USA 1996, 93, 8776–8781. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.N.; Lee, J.H.; Kang, B.C.; Koh, S.W.; Joa, J.H.; Choi, K.S.; Ahn, J.J. HR-Mediated Defense Response is Overcome at High Temperatures in Capsicum Species. Plant Pathol. J. 2018, 34, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.G.; Park, J.H.; Lim, J.Y.; Kim, D.; Choi, Y.; Kim, S.; Reeves, G.; Yeom, S.I.; Lee, J.S.; Park, M.; et al. The hot pepper (Capsicum annuum) microRNA transcriptome reveals novel and conserved targets: A foundation for understanding MicroRNA functional roles in hot pepper. PLoS ONE 2013, 8, e64238. [Google Scholar] [CrossRef]
- Seo, E.; Kim, T.; Park, J.H.; Yeom, S.-I.; Kim, S.; Seo, M.-K.; Shin, C.; Choi, D. Genome-wide comparative analysis in Solanaceous species reveals evolution of microRNAs targeting defense genes in Capsicum spp. DNA Res. 2018, 25, 561–575. [Google Scholar] [CrossRef]
- Xu, X.-W.; Li, T.; Li, Y.; Li, Z.-X. Identification and Analysis of C. annuum microRNAs by High-Throughput Sequencing and Their Association with High Temperature and High Air Humidity Stress. Int. J. Bioautomat. 2015, 19, 459–472. [Google Scholar]
- Yang, S.; Zhang, Z.; Chen, W.; Li, X.; Zhou, S.; Liang, C.; Li, X.; Yang, B.; Zou, X.; Liu, F.; et al. Integration of mRNA and miRNA profiling reveals the heterosis of three hybrid combinations of Capsicum annuum varieties. GM Crops Food 2021, 12, 224–241. [Google Scholar] [CrossRef]
- Apostolova, E.; Hadjieva, N.; Ivanova, D.P.; Yahubyan, G.; Baev, V.; Gozmanova, M. MicroRNA expression dynamics reshape the cultivar-specific response of pepper (Capsicum annuum L.) to potato spindle tuber viroid (PSTVd) infection. Sci. Hortic. 2021, 278, 109845. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.J.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. Eur. Mol. Biol. Organ. J 2019, 38, e101948. [Google Scholar] [CrossRef] [PubMed]
- Kimmins, W.C.; Wuddah, D. Hypersensitive Resistance: Determination of Lignin in Leaves with a Localized Virus Infection. Phytopathology 1977, 67, 1012–1016. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.-X.; Abouelnasr, H.; Mohamed Hamed, A.; Kou, M.; Tang, W.; Yan, H.; Wang, X.; Wang, X.-X.; Zhang, Y.-G.; et al. Inhibition of miR397 by STTM technology to increase sweetpotato resistance to SPVD. J. Integr. Agric. 2022, 21, 2865–2875. [Google Scholar] [CrossRef]
- Ma, C.; Burd, S.; Lers, A. miR408 is involved in abiotic stress responses in Arabidopsis. Plant J. 2015, 84, 169–187. [Google Scholar] [CrossRef]
- Moxon, S.; Jing, R.; Szittya, G.; Schwach, F.; Rusholme Pilcher, R.L.; Moulton, V.; Dalmay, T. Deep sequencing of tomato short RNAs identifies microRNAs targeting genes involved in fruit ripening. Genome Res. 2008, 18, 1602–1609. [Google Scholar] [CrossRef]
- Mohorianu, I.; Schwach, F.; Jing, R.; Lopez-Gomollon, S.; Moxon, S.; Szittya, G.; Sorefan, K.; Moulton, V.; Dalmay, T. Profiling of short RNAs during fleshy fruit development reveals stage-specific sRNAome expression patterns. Plant J. 2011, 67, 232–246. [Google Scholar] [CrossRef]
- Linsen, S.E.; de Wit, E.; Janssens, G.; Heater, S.; Chapman, L.; Parkin, R.K.; Fritz, B.; Wyman, S.K.; de Bruijn, E.; Voest, E.E.; et al. Limitations and possibilities of small RNA digital gene expression profiling. Nat. Methods 2009, 6, 474–476. [Google Scholar] [CrossRef]
- Fuchs, R.T.; Sun, Z.; Zhuang, F.; Robb, G.B. Bias in ligation-based small RNA sequencing library construction is determined by adaptor and RNA structure. PLoS ONE 2015, 10, e0126049. [Google Scholar] [CrossRef] [PubMed]
- Dabney, J.; Meyer, M. Length and GC-biases during sequencing library amplification: A comparison of various polymerase-buffer systems with ancient and modern DNA sequencing libraries. BioTechniques 2012, 52, 87–94. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.E.; Pilon, M. MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J. Biol. Chem. 2008, 283, 15932–15945. [Google Scholar] [CrossRef]
- Trindade, I.; Capitão, C.; Dalmay, T.; Fevereiro, M.P.; Santos, D.M. miR398 and miR408 are up-regulated in response to water deficit in Medicago truncatula. Planta 2010, 231, 705–716. [Google Scholar] [CrossRef]
- Li, C.; Li, D.; Zhou, H.; Li, J.; Lu, S. Analysis of the laccase gene family and miR397-/miR408-mediated posttranscriptional regulation in Salvia miltiorrhiza. PeerJ 2019, 7, e7605. [Google Scholar] [CrossRef]
- Lu, S.; Li, Q.; Wei, H.; Chang, M.J.; Tunlaya-Anukit, S.; Kim, H.; Liu, J.; Song, J.; Sun, Y.H.; Yuan, L.; et al. Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc. Natl. Acad. Sci. USA 2013, 110, 10848–10853. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zhang, S.; Yu, Y.; Luo, Y.C.; Liu, Q.; Ju, C.; Zhang, Y.C.; Qu, L.H.; Lucas, W.J.; Wang, X.; et al. MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotechnol. J. 2014, 12, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Swetha, C.; Basu, D.; Pachamuthu, K.; Tirumalai, V.; Nair, A.; Prasad, M.; Shivaprasad, P.V. Major Domestication-Related Phenotypes in Indica Rice Are Due to Loss of miRNA-Mediated Laccase Silencing. Plant Cell 2018, 30, 2649–2662. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhou, J.; Gao, L.; Tang, Y. Plant miR397 and its functions. Funct. Plant Biol. 2021, 48, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tobimatsu, Y.; Zhou, R.; Pattathil, S.; Gallego-Giraldo, L.; Fu, C.; Jackson, L.A.; Hahn, M.G.; Kim, H.; Chen, F.; et al. Loss of function of cinnamyl alcohol dehydrogenase 1 leads to unconventional lignin and a temperature-sensitive growth defect in Medicago truncatula. Proc. Natl. Acad. Sci. USA 2013, 110, 13660–13665. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Rosas-Diaz, T.; Zhang, D.; Fan, P.; Wang, L.; Ding, X.; Jiang, Y.; Jimenez-Gongora, T.; Medina-Puche, L.; Zhao, X.; Feng, Z.; et al. A virus-targeted plant receptor-like kinase promotes cell-to-cell spread of RNAi. Proc. Natl. Acad. Sci. USA 2018, 115, 1388–1393. [Google Scholar] [CrossRef]
- Swida-Barteczka, A.; Szweykowska-Kulinska, Z. Micromanagement of Developmental and Stress-Induced Senescence: The Emerging Role of MicroRNAs. Genes 2019, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gomollon, S.; Mohorianu, I.; Szittya, G.; Moulton, V.; Dalmay, T. Diverse correlation patterns between microRNAs and their targets during tomato fruit development indicates different modes of microRNA actions. Planta 2012, 236, 1875–1887. [Google Scholar] [CrossRef]
- Kidner, C.A.; Martienssen, R.A. Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature 2004, 428, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, C.G.; Yoshimoto, N.; Maruyama-Nakashita, A.; Tsuchiya, Y.N.; Saito, K.; Takahashi, H.; Dalmay, T. Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J. 2009, 57, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llorca, M.; Munné-Bosch, S. Aging, stress, and senescence in plants: What can biological diversity teach us? Geroscience 2021, 43, 167–180. [Google Scholar] [CrossRef]
- Singh, A.; Permar, V.; Basavaraj, A.; Bhoopal, S.T.; Praveen, S. Effect of Temperature on Symptoms Expression and Viral RNA Accumulation in Groundnut Bud Necrosis Virus Infected Vigna unguiculata. Iran. J. Biotechnol. 2018, 16, 227–234. [Google Scholar] [CrossRef]
- Li, Z.; Peng, J.; Wen, X.; Guo, H. Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 2013, 25, 3311–3328. [Google Scholar] [CrossRef]
- Li, T.; Gonzalez, N.; Inzé, D.; Dubois, M. Emerging Connections between Small RNAs and Phytohormones. Trends. Plant Sci. 2020, 25, 912–929. [Google Scholar] [CrossRef]
- Podzimska-Sroka, D.; O’Shea, C.; Gregersen, P.L.; Skriver, K. NAC Transcription Factors in Senescence: From Molecular Structure to Function in Crops. Plants 2015, 4, 412–448. [Google Scholar] [CrossRef]
- Dalmadi, Á.; Miloro, F.; Bálint, J.; Várallyay, É.; Havelda, Z. Controlled RISC loading efficiency of miR168 defined by miRNA duplex structure adjusts ARGONAUTE1 homeostasis. Nucleic. Acids. Res. 2021, 49, 12912–12928. [Google Scholar] [CrossRef] [PubMed]


| miRNA ID | HV/AV_log2 (FC) | AV/AM_log2 (FC) | HV/HM_log2 (FC) | HM/AM_log2 (FC) |
|---|---|---|---|---|
| Can-miR408a-5p | 4.94651183 | 2.15675264 | ||
| Can-miR408a-3p | 4.52786751 | −1.431929 | 1.87576962 | |
| Can-miR398b-3p | 4.3647467 | −1.1693631 | 2.7875221 | |
| Can-miR397-5p | 3.41052681 | −1.3643359 | 1.80648604 | |
| Can-miR397-3p | 3.09698569 | −1.1034217 | 1.41436391 | |
| Can-miRN492-5p | 2.4800554 | 2.07163552 | ||
| Can-miR_N7b-3p | 2.22044295 | 1.95841442 | ||
| Can-miR_N7c-3p | 2.22044295 | 1.95841442 | ||
| Can-miR_N7d-3p | 2.16627692 | 1.84961374 | ||
| Can-miR393-3p | 2.00053338 | |||
| Can-miRN37-Novel-5p | 1.97325814 | 1.2736888 | ||
| Can-miR_N2a-3p | 1.66675404 | |||
| Can-miR172a-m1-3p | 1.62135413 | |||
| Can-miR172d-3p | 1.61286777 | |||
| Can-miR_N71a-5p | 1.60761055 | |||
| Can-miR_N50a-5p | 1.51467255 | |||
| Can-miR403-3p | 1.29105902 | 1.04987498 | ||
| Can-miRN25-Novel-3p | 1.27226491 | 1.27136116 | ||
| Can-miR160a-m1-5p | 1.25709852 | |||
| Can-miRC13.1-m1-5p | 1.19626839 | |||
| Can-miRC13.2-m1-5p | 1.19626839 | |||
| Can-miR157c-5p | −1.0923326 | |||
| Can-miR157b-5p | −1.1162378 | |||
| Can-miR169h-3p | −1.1385036 | −1.8141374 | ||
| Can-miR_N80a-3p | −1.4595388 | |||
| Can-miR477a-5p | −1.6760649 | −3.4314472 | ||
| Can-miR164b-5p | −1.7200209 | |||
| Can-miR164c-5p | −1.7417164 | |||
| Can-miR477b-5p | −1.7912909 | −2.9432463 | ||
| Can-miRN482-5p | −1.8617146 | −2.2237655 | ||
| Can-miR399a-Known-5p | −1.9889216 | −5.5813609 | ||
| Can-miR399b-Known-5p | −1.9889216 | −5.5813609 | ||
| Can-miR399c-Known-5p | −1.9889216 | −5.5813609 | ||
| Can-miR399d-Known_1-5p | −1.9889216 | −5.5813609 | ||
| Can-miR399d-Known-5p | −1.9889216 | −5.5813609 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-A.; Brosnan, C.A.; Mitter, N.; Dietzgen, R.G. Involvement of MicroRNAs in the Hypersensitive Response of Capsicum Plants to the Capsicum Chlorosis Virus at Elevated Temperatures. Pathogens 2024, 13, 745. https://doi.org/10.3390/pathogens13090745
Tsai W-A, Brosnan CA, Mitter N, Dietzgen RG. Involvement of MicroRNAs in the Hypersensitive Response of Capsicum Plants to the Capsicum Chlorosis Virus at Elevated Temperatures. Pathogens. 2024; 13(9):745. https://doi.org/10.3390/pathogens13090745
Chicago/Turabian StyleTsai, Wei-An, Christopher A. Brosnan, Neena Mitter, and Ralf G. Dietzgen. 2024. "Involvement of MicroRNAs in the Hypersensitive Response of Capsicum Plants to the Capsicum Chlorosis Virus at Elevated Temperatures" Pathogens 13, no. 9: 745. https://doi.org/10.3390/pathogens13090745







