Trichomonas vaginalis Legumain-2, TvLEGU-2, Is an Immunogenic Cysteine Peptidase Expressed during Trichomonal Infection
Abstract
:1. Introduction
2. Materials and Methods
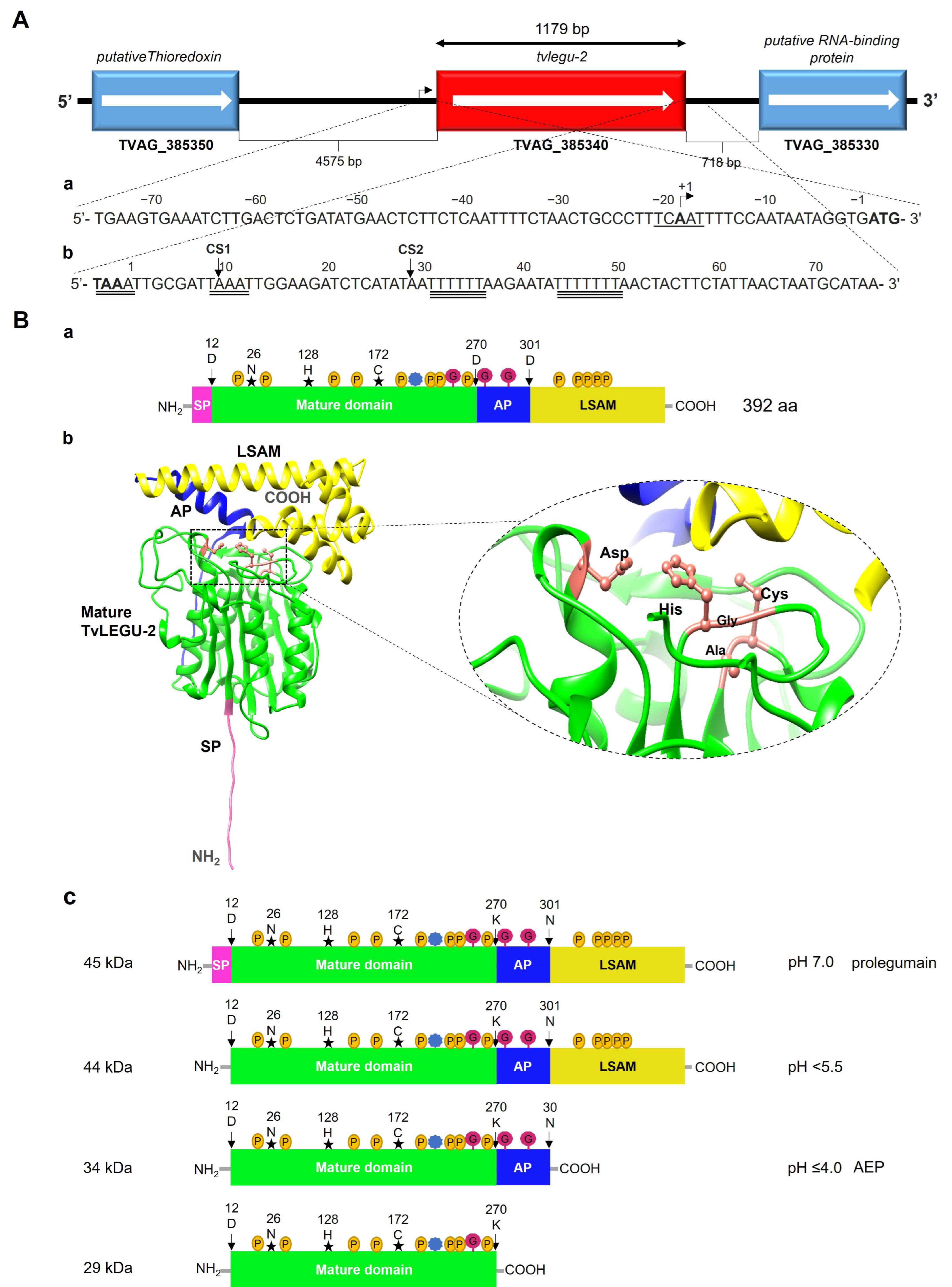
2.1. Bioinformatics Analysis of tvlegu-2 and TvLEGU-2
2.2. Trichomonas Vaginalis In Vitro Cultures
2.3. qPCR
2.4. Cloning and Expression of TvLEGU-2r
2.5. Production of α-TvLEGU-2 and α-CNCD188 Antibodies
2.6. Protein Extracts for 1-D and 2-D Gel Electrophoresis (1-DE and 2-DE)
2.7. Western Blot
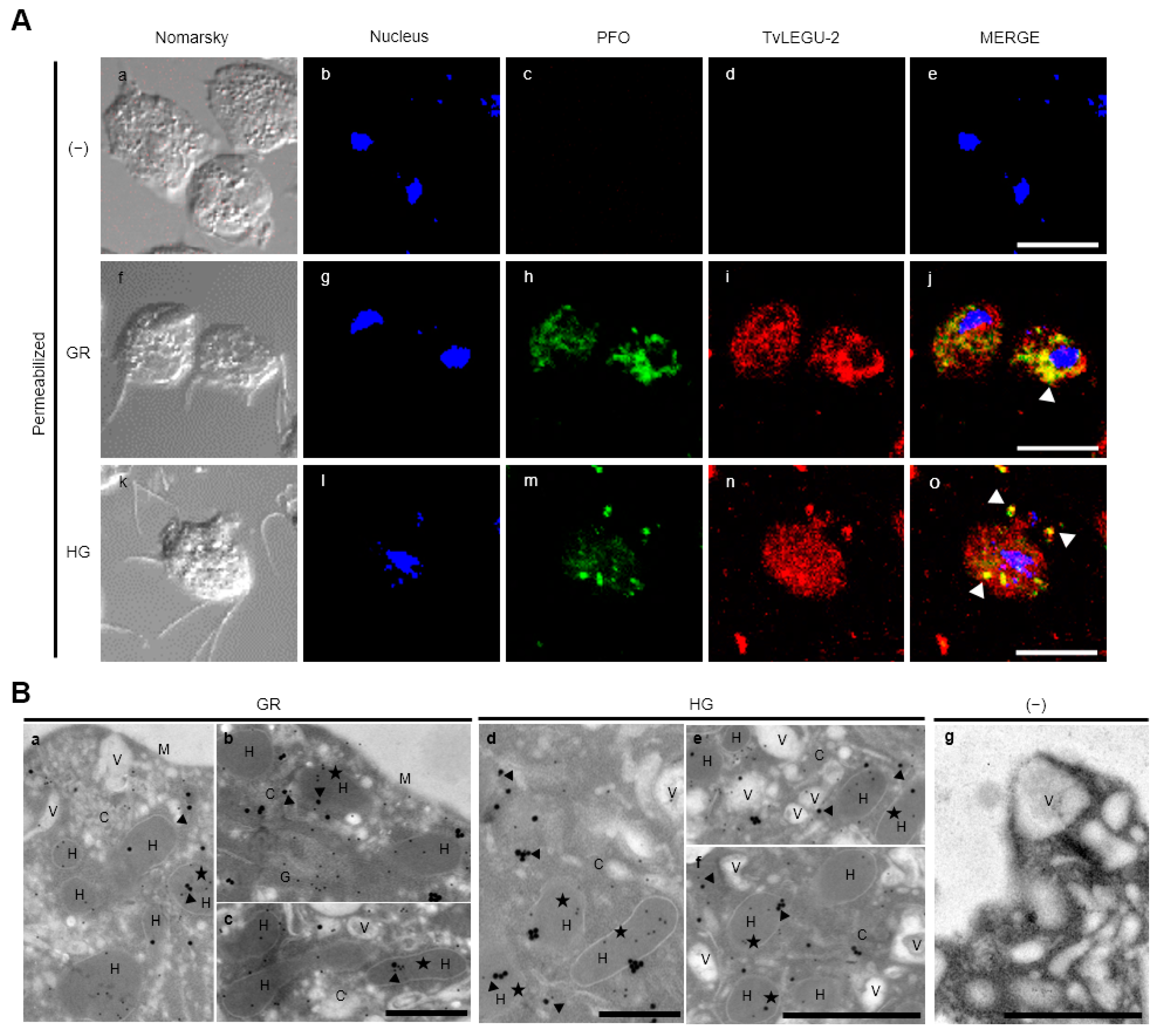
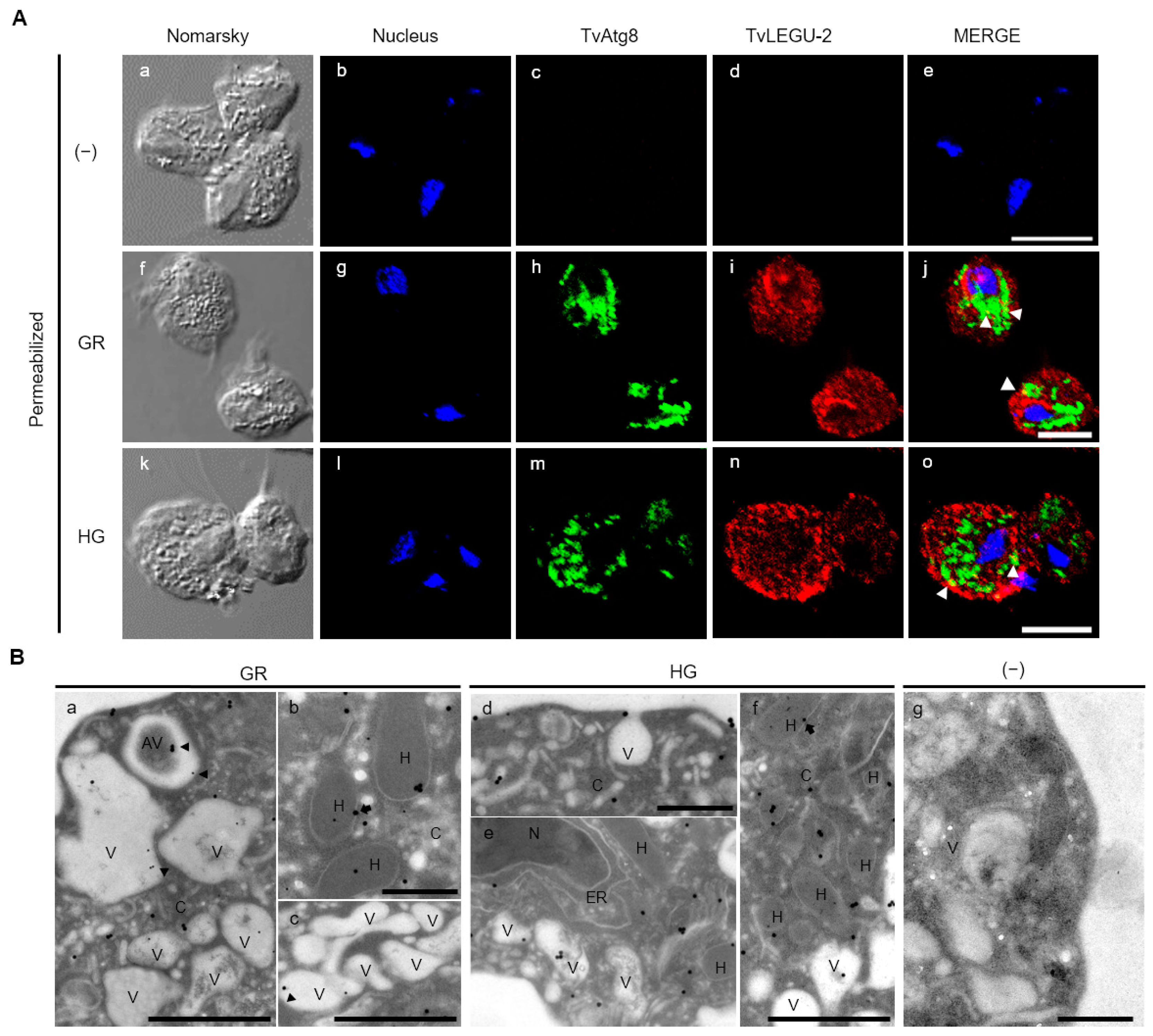
2.8. TvLEGU-2 Localization by Indirect Immunofluorescence Assays (IFA)
2.9. Transmission Electron Microscopy Immunolabeling
2.10. In Vitro Secretion Assay
2.11. Statistical Analysis
3. Results
3.1. In Silico Analysis of the tvlegu-2 Gene and Deduced Amino Acid Sequence of the TvLEGU-2 Protein
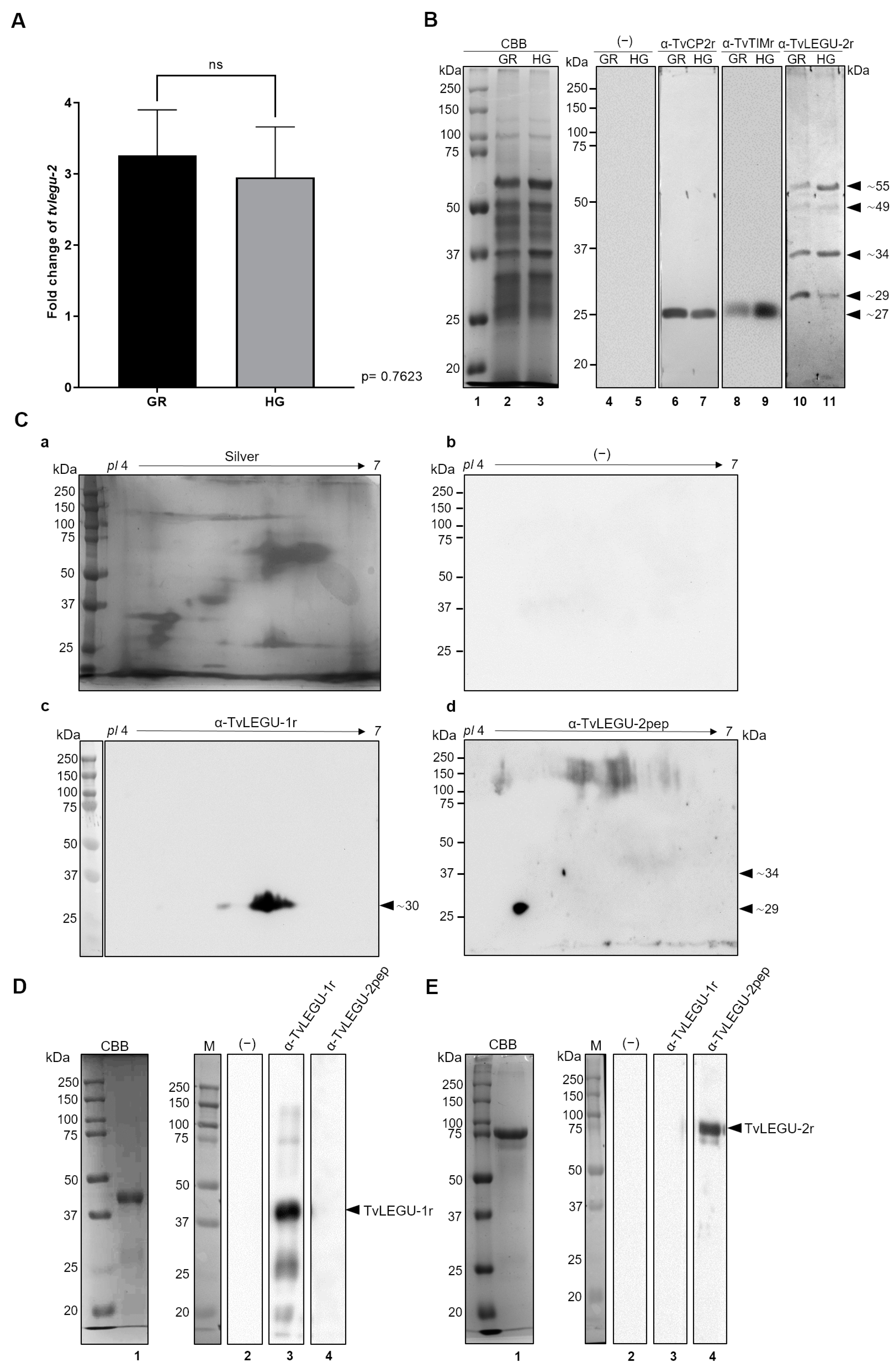
3.2. Glucose Conditions Have No Impact on tvlegu-2 Gene Expression
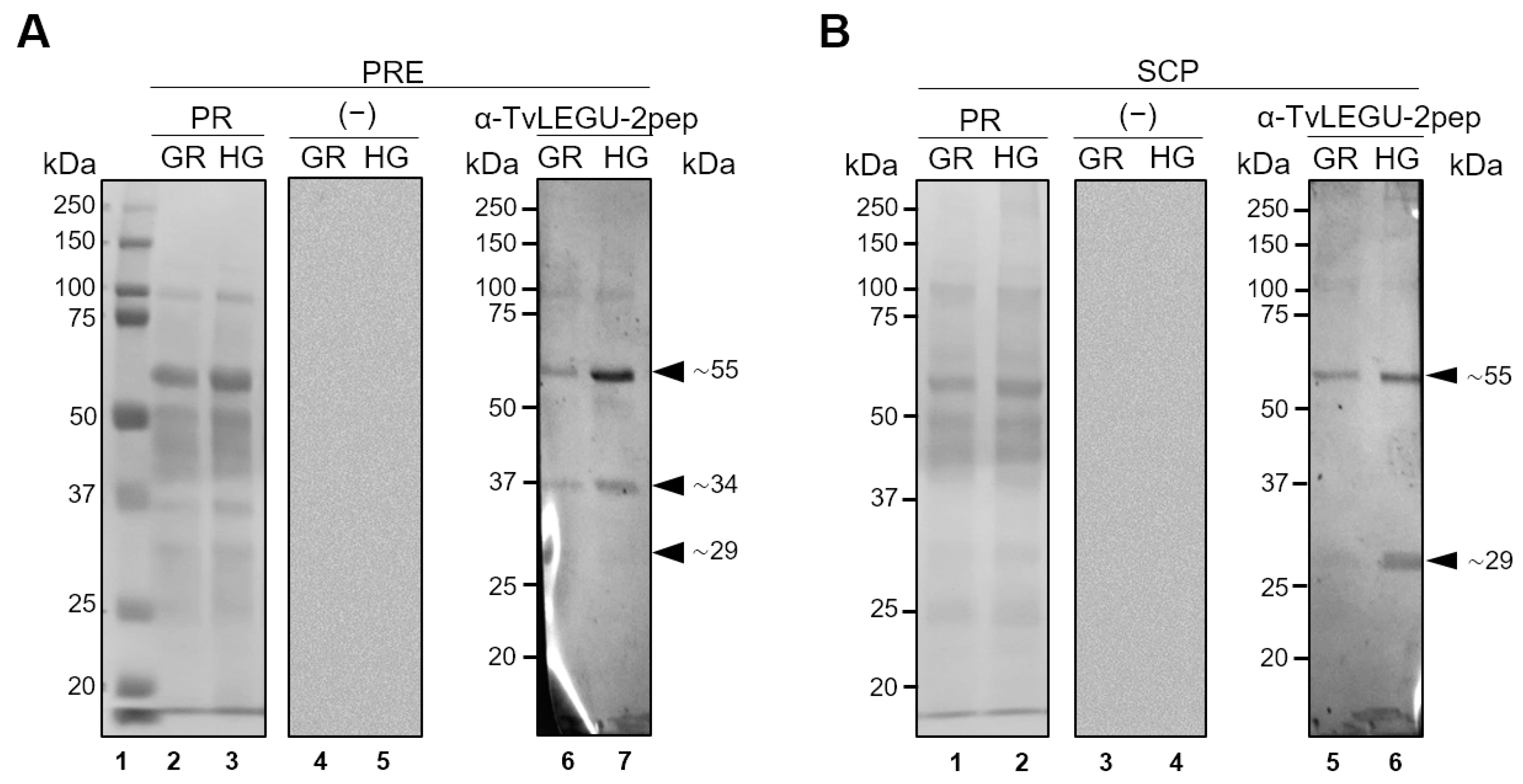
3.3. Glucose Influences TvLEGU-2 Processing
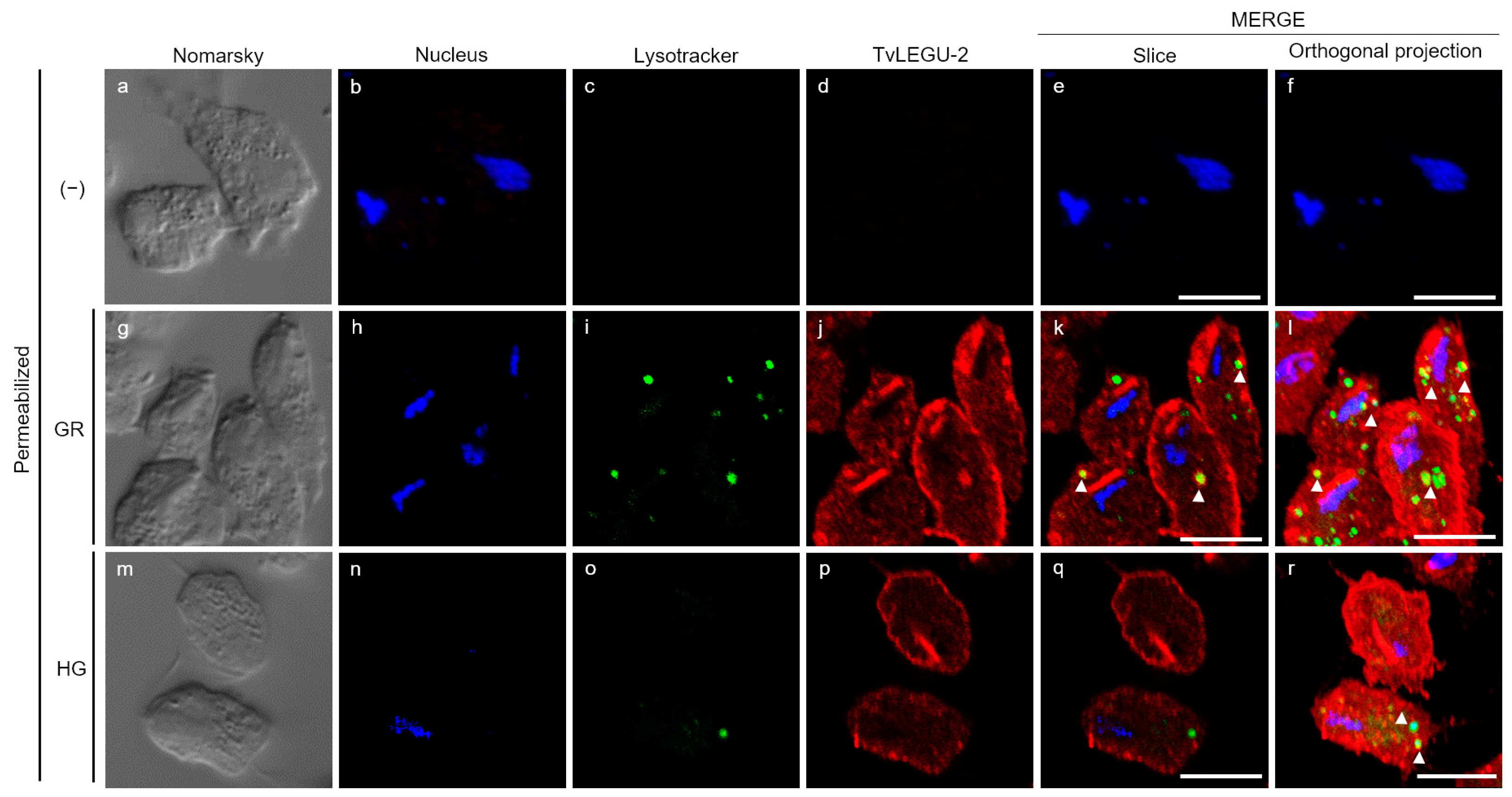
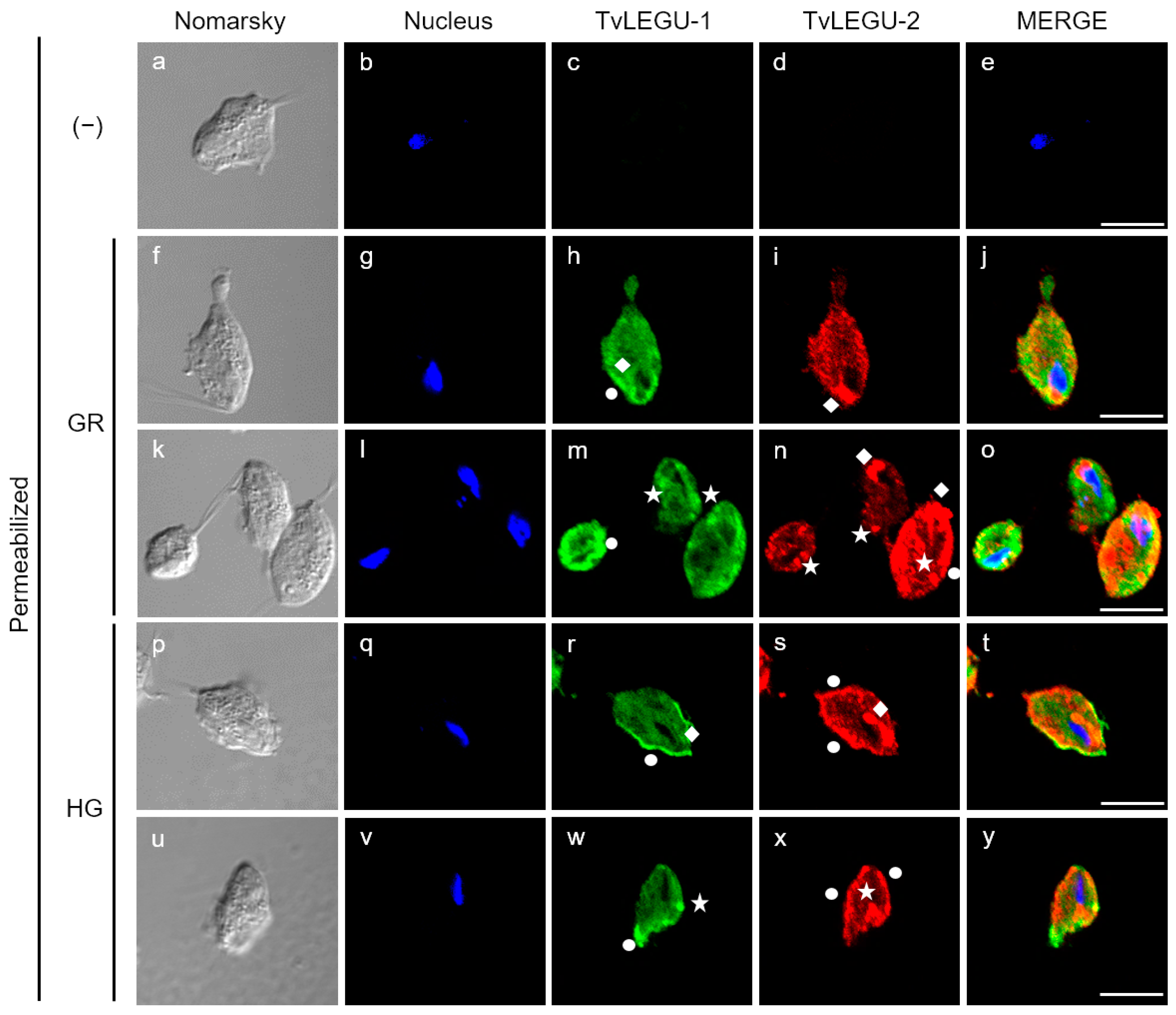
3.4. Subcellular Localization of TvLEGU-2 in T. vaginalis under Different Glucose Conditions
3.5. TvLEGU-2 Is Secreted under Both Glucose Conditions
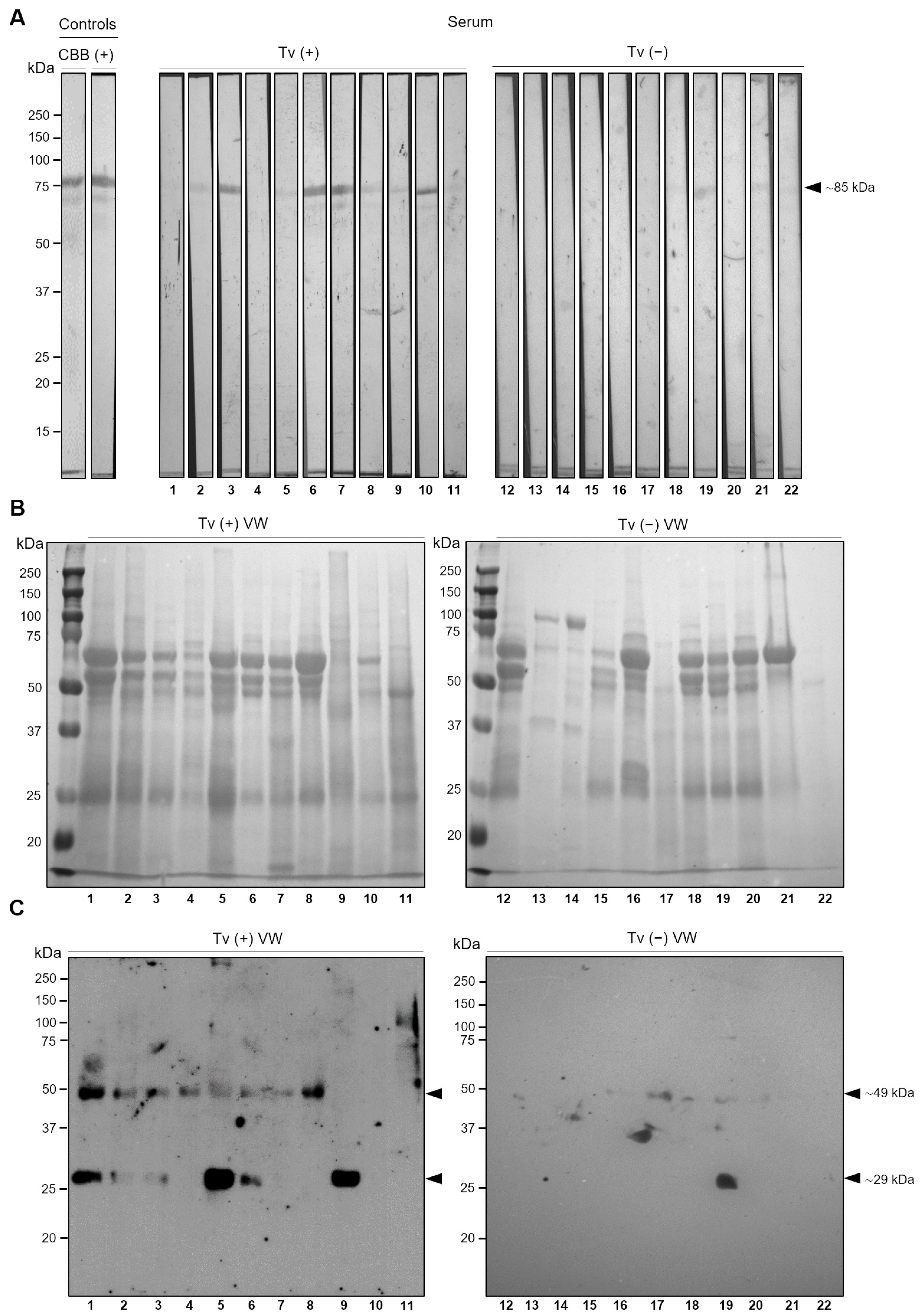
3.6. TvLEGU-2 Is an Immunogenic Peptidase Present during T. vaginalis Infection
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Taylor, M.M. Chlamydia, gonorrhea, trichomoniasis, and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548. [Google Scholar] [CrossRef] [PubMed]
- Swygard, H.; Sena, A.; Hobbs, M.; Cohen, M. Trichomoniasis: Clinical manifestations, diagnosis and management. Sex. Transm. Dis. 2004, 80, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Soper, D. Trichomoniasis: Under control or undercontrolled? Am. J. Obstet. Gynecol. 2004, 190, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Viikki, M. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 2000, 39, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Graham, S.; Yu, S.Z.; Marshall, J.; Zielezny, M.; Chen, Y.X.; Sun, M.; Tang, S.L.; Jiao, C.S.; Xu, J.L.; et al. Trichomonas vaginalis and cervical cancer: A prospective study in China. Ann. Epidemiol. 1995, 5, 325–332. [Google Scholar] [CrossRef]
- Stark, J.R.; Judson, G.; Alderete, J.F.; Mundodi, V.; Kucknoor, A.S.; Giovannucci, E.L.; Mucci, L.A. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians’ Health Study. J. Natl. Cancer Inst. 2009, 101, 1406–1411. [Google Scholar] [CrossRef]
- Carlton, J.M.; Hirt, R.P.; Silva, J.C.; Delcher, A.L.; Schatz, M.; Zhao, Q.; Wortman, J.R.; Bidwell, S.L.; Alsmark, U.C.; Besteiro, S.; et al. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 2007, 315, 207–212. [Google Scholar] [CrossRef]
- Neale, K.A.; Alderete, J.F. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect. Immun. 1990, 58, 157–162. [Google Scholar] [CrossRef]
- Ramón-Luing, L.A.; Rendón-Gandarilla, F.J.; Cárdenas-Guerra, R.E.; Rodríguez-Cabrera, N.A.; Ortega-López, J.; Avila-González, L.; Angel-Ortiz, C.; Herrera-Sánchez, C.N.; Mendoza-García, M.; Arroyo, R. Immunoproteomics of the active degradome to identify biomarkers for Trichomonas vaginalis. Proteomics 2010, 10, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Guerra, R.E.; Arroyo, R.; Rosa de Andrade, I.; Benchimol, M.; Ortega-López, J. The iron-induced cysteine proteinase TvCP4 plays a key role in Trichomonas vaginalis haemolysis. Microbes Infect. 2013, 15, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Angulo, E.E.; Rendón-Gandarilla, F.J.; Puente-Rivera, J.; Calla-Choque, J.S.; Cárdenas-Guerra, R.E.; Ortega-López, J.; Quintas-Granados, L.I.; Alvarez-Sánchez, M.E.; Arroyo, R. The effects of environmental factors on the virulence of Trichomonas vaginalis. Microbes Infect. 2012, 14, 1411–1427. [Google Scholar] [CrossRef] [PubMed]
- Alderete, J.F.; Newton, E.; Dennis, C.; Neale, K.A. Antibody in sera of patients infected with Trichomonas vaginalis is to trichomonad proteinases. Genitourin. Med. 1991, 67, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Bózner, P.; Gombosová, A.; Valent, M.; Demes, P.; Alderete, J.F. Proteinases of Trichomonas vaginalis: Antibody response in patients with urogenital trichomoniasis. Parasitology 1992, 105 Pt 3, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Hernández, H.M.; Marcet, R.; Sarracent, J. Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis. Parasite 2014, 21, 54. [Google Scholar] [CrossRef]
- Arroyo, R.; Cárdenas-Guerra, R.E.; Figueroa-Angulo, E.E.; Puente-Rivera, J.; Zamudio-Prieto, O.; Ortega-López, J. Trichomonas vaginalis Cysteine Proteinases: Iron Response in Gene Expression and Proteolytic Activity. BioMed Res. Int. 2015, 2015, 946787. [Google Scholar] [CrossRef]
- Alvarez-Sánchez, M.E.; Avila-González, L.; Becerril-García, C.; Fattel-Facenda, L.V.; Ortega-López, J.; Arroyo, R. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb. Pathog. 2000, 28, 193–202. [Google Scholar] [CrossRef]
- Ramón-Luing, L.A.; Rendón-Gandarilla, F.J.; Puente-Rivera, J.; Avila-González, L.; Arroyo, R. Identification and characterization of the immunogenic cytotoxic TvCP39 proteinase gene of Trichomonas vaginalis. Int. J. Biochem. Cell Biol. 2011, 43, 1500–1511. [Google Scholar] [CrossRef]
- Miranda-Ozuna JF, T.; Rivera-Rivas, L.A.; Cárdenas-Guerra, R.E.; Hernández-García, M.S.; Rodríguez-Cruz, S.; González-Robles, A.; Chavez-Munguía, B.; Arroyo, R. Glucose restriction increases Trichomonas vaginalis cellular damage towards HeLa cells and proteolytic activity of cysteine proteinases (CPs), such as TvCP2. Parasitology 2019, 146, 1156–1166. [Google Scholar] [CrossRef]
- Rivera-Rivas, L.A.; Lorenzo-Benito, S.; Sánchez-Rodríguez, D.B.; Miranda-Ozuna JF, T.; Euceda-Padilla, E.A.; Ortega-López, J.; Chávez-Munguía, B.; Lagunes-Guillén, A.; Velázquez-Valassi, B.; Jasso-Villazul, L.; et al. The effect of iron on Trichomonas vaginalis TvCP2: A cysteine proteinase found in vaginal secretions of trichomoniasis patients. Parasitology 2020, 147, 760–774. [Google Scholar] [CrossRef]
- Mendoza-López, M.R.; Becerril-Garcia, C.; Fattel-Facenda, L.V.; Avila-Gonzalez, L.; Ruíz-Tachiquín, M.E.; Ortega-Lopez, J.; Arroyo, R. CP30, a cysteine proteinase involved in Trichomonas vaginalis cytoadherence. Infect. Immun. 2000, 68, 4907–4912. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Gandarilla, F.J.; Ramón-Luing, L.A.; Ortega-López, J.; Rosa de Andrade, I.; Benchimol, M.; Arroyo, R. The TvLEGU-1, a legumain-like cysteine proteinase, plays a key role in Trichomonas vaginalis cytoadherence. BioMed Res. Int. 2013, 2013, 561979. [Google Scholar] [CrossRef] [PubMed]
- León-Félix, J.; Ortega-López, J.; Orozco-Solís, R.; Arroyo, R. Two novel asparaginyl endopeptidase-like cysteine proteinases from the protist Trichomonas vaginalis: Their evolutionary relationship within the clan CD cysteine proteinases. Gene 2004, 335, 25–35. [Google Scholar] [CrossRef]
- Riestra, A.M.; Gandhi, S.; Sweredoski, M.J.; Moradian, A.; Hess, S.; Urban, S.; Johnson, P.J. A Trichomonas vaginalis Rhomboid Protease and Its Substrate Modulate Parasite Attachment and Cytolysis of Host Cells. PLoS Pathogens 2015, 11. [Google Scholar] [CrossRef]
- Stáfková, J.; Rada, P.; Meloni, D.; Zárský, V.; Smutná, T.; Zimmann, N.; Harant, K.; Pompach, P.; Hrdý, I.; Tachezy, J. Dynamic secretome of Trichomonas vaginalis: Case study of-amylases. Mol. Cell. Proteom. 2018, 17, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Nievas, Y.R.; Coceres, V.M.; Midlej, V.; de Souza, W.; Benchimol, M.; Pereira-Neves, A.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J.; de Miguel, N. Membrane-shed vesicles from the parasite Trichomonas vaginalis: Characterization and their association with cell interaction. Cell. Mol. Life Sci. 2018, 75, 2211–2226. [Google Scholar] [CrossRef]
- Zimmann, N.; Rada, P.; Žárský, V.; Smutná, T.; Záhonová, K.; Dacks, J.; Harant, K.; Hrdý, I.; Tachezy, J. Proteomic Analysis of Trichomonas vaginalis Phagolysosome, Lysosomal Targeting, and Unconventional Secretion of Cysteine Peptidases. Mol. Cell. Proteom. MCP 2022, 21, 100174. [Google Scholar] [CrossRef]
- Dias-Lopes, G.; Wiśniewski, J.R.; de Souza, N.P.; Vidal, V.E.; Padrón, G.; Britto, C.; Cuervo, P.; de Jesus, J.B. In-Depth Quantitative Proteomic Analysis of Trophozoites and Pseudocysts of Trichomonas vaginalis. J. Proteome Res. 2018, 17, 3704–3718. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, J.B.; Cuervo, P.; Junqueira, M.; Britto, C.; ESilva-Filho, F.C.; Soares, M.J.; Cupolillo, E.; Fernandes, O.; Domont, G.B. A further proteomic study on the effect of iron in the human pathogen Trichomonas vaginalis. Proteomics 2007, 7, 1961–1972. [Google Scholar] [CrossRef]
- Miranda-Ozuna, J.F.; Hernández-García, M.S.; Brieba, L.G.; Benítez-Cardoza, C.G.; Ortega-López, J.; González-Robles, A.; Arroyo, R. The Glycolytic Enzyme Triosephosphate Isomerase of Trichomonas vaginalis Is a Surface-Associated Protein Induced by Glucose That Functions as a Laminin- and Fibronectin-Binding Protein. Infect. Immun. 2016, 84, 2878–2894. [Google Scholar] [CrossRef]
- dos Santos, O.; de Vargas Rigo, G.; Frasson, A.P.; Macedo, A.J.; Tasca, T. Optimal Reference Genes for Gene Expression Normalization in Trichomonas vaginalis. PLoS ONE 2015, 10, e0138331. [Google Scholar] [CrossRef]
- Goulas, T.; Cuppari, A.; Garcia-Castellanos, R.; Snipas, S.; Glockshuber, R.; Arolas, J.L.; Gomis-Rüth, F.X. The pCri System: A vector collection for recombinant protein expression and purification. PLoS ONE 2014, 9, e112643. [Google Scholar] [CrossRef]
- Meza-Cervantez, P.; González-Robles, A.; Cárdenas-Guerra, R.E.; Ortega-López, J.; Saavedra, E.; Pineda, E.; Arroyo, R. Pyruvate:ferredoxin oxidoreductase (PFO) is a surface-associated cell-binding protein in Trichomonas vaginalis and is involved in trichomonal adherence to host cells. Microbiology 2011, 157 Pt 12, 3469–3482. [Google Scholar] [CrossRef]
- Hernández-García, M.S.; Miranda-Ozuna, J.F.T.; Salazar-Villatoro, L.; Vázquez-Calzada, C.; Ávila-González, L.; González-Robles, A.; Ortega-López, J.; Arroyo, R. Biogenesis of Autophagosome in Trichomonas vaginalis during Macroautophagy Induced by Rapamycin-treatment and Iron or Glucose Starvation Conditions. J. Eukaryot. Microbiol. 2019, 66, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.R.; Johnson, P.J. Analysis of a ubiquitous promoter element in a primitive eukaryote: Early evolution of the initiator element. Mol. Cell. Biol. 1999, 19, 2380–2388. [Google Scholar] [CrossRef]
- Espinosa, N.; Hernández, R.; López-Griego, L.; López-Villaseñor, I. Separable putative polyadenylation and cleavage motifs in Trichomonas vaginalis mRNAs. Gene 2002, 289, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Dall, E.; Brandstetter, H. Structure and function of legumain in health and disease. Biochimie 2016, 122, 126–150. [Google Scholar] [CrossRef]
- McLuskey, K.; Mottram, J.C. Comparative structural analysis of the caspase family with other clan CD cysteine peptidases. Biochem. J. 2015, 466, 219–232. [Google Scholar] [CrossRef]
- Chen, J.M.; Rawlings, N.D.; Stevens, R.A.; Barrett, A.J. Identification of the active site of legumain links it to caspases, clostripain and gingipains in a new clan of cysteine endopeptidases. FEBS Lett. 1998, 441, 361–365. [Google Scholar] [CrossRef]
- Dall, E.; Brandstetter, H. Activation of legumain involves proteolytic and conformational events, resulting in a context-and substrate-dependent activity profile. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Singh, S.; Ganesh, S. The laforin-malin complex negatively regulates glycogen synthesis by modulating cellular glucose uptake via glucose transporters. Mol. Cell. Biol. 2012, 32, 652–663. [Google Scholar] [CrossRef]
- Semache, M.; Ghislain, J.; Zarrouki, B.; Tremblay, C.; Poitout, V. Pancreatic and duodenal homeobox-1 nuclear localization is regulated by glucose in dispersed rat islets but not in insulin-secreting cell lines. Islets 2014, 6, e982376. [Google Scholar] [CrossRef] [PubMed]
- Matsui, C.; Takatani-Nakase, T.; Maeda, S.; Nakase, I.; Takahashi, K. Potential Roles of GLUT12 for Glucose Sensing and Cellular Migration in MCF-7 Human Breast Cancer Cells under High Glucose Conditions. Anticancer Res. 2017, 37, 6715–6722. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.Y.; Chen, R.M.; Lin, H.C.; Cheng, W.H.; Lin, H.A.; Lin, W.N.; Huang, P.J.; Chiu, C.H.; Tang, P. Potential role of autophagy in proteolysis in Trichomonas vaginalis. J. Microbiol. Immunol. Infect. 2019, 52, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Mancilla-Olea, M.I.; Ortega-López, J.; Figueroa-Angulo, E.E.; Avila-González, L.; Cárdenas-Guerra, R.E.; Miranda-Ozuna JF, T.; González-Robles, A.; Hernández-García, M.S.; Sánchez-Ayala, L.; Arroyo, R. Trichomonas vaginalis cathepsin D-like aspartic proteinase (Tv-CatD) is positively regulated by glucose and degrades human hemoglobin. Int. J. Biochem. Cell Biol. 2018, 97, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fouts, A.C.; Kraus, S.J. Trichomonas vaginalis: Reevaluation of its clinical presentation and laboratory diagnosis. J. Infect. Dis. 1980, 141, 137–143. [Google Scholar] [CrossRef]
- Spence, M.R.; Hollander, D.H.; Smith, J.; McCaig, L.; Sewell, D.; Brockman, M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex. Transm. Dis. 1980, 7, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Šoba, B.; Skvarč, M.; Matičič, M. Trichomoniasis: A brief review of diagnostic methods and our experience with real-time PCR for detecting infection. Acta Dermatovenerol. Alp. Pannonica Adriat. 2015, 24, 7–10. [Google Scholar] [CrossRef]
- Mendling, W. Vaginal Microbiota. Adv. Exp. Med. Biol. 2016, 902, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Moheimani, F.; Kim, C.H.; Rahmanto, A.S.; van Reyk, D.M.; Davies, M.J. Inhibition of lysosomal function in macrophages incubated with elevated glucose concentrations: A potential contributory factor in diabetes-associated atherosclerosis. Atherosclerosis 2012, 223, 144–151. [Google Scholar] [CrossRef]
- Lamark, T.; Johansen, T. Mechanisms of Selective Autophagy. Annu. Rev. Cell Dev. Biol. 2021, 37, 143–169. [Google Scholar] [CrossRef]
- Coombs, G.H. Proteinases of Leishmania mexicana and other flagellate protozoa. Parasitology 1982, 84, 149–155. [Google Scholar] [CrossRef]
- Hernández-Gutierrez, R.; Ortega-López, J.; Arroyo, R. A 39-kDa cysteine proteinase CP39 from Trichomonas vaginalis, which is negatively affected by iron may be involved in trichomonal cytotoxicity. J. Eukaryot. Microbiol. 2003, 50, 696–698. [Google Scholar] [CrossRef]
- Hernández-Gutiérrez, R.; Avila-González, L.; Ortega-López, J.; Cruz-Talonia, F.; Gómez-Gutierrez, G.; Arroyo, R. Trichomonas vaginalis: Characterization of a 39-kDa cysteine proteinase found in patient vaginal secretions. Exp. Parasitol. 2004, 107, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U.; Costello, C.E.; Hayes, G.R.; Beach, D.H.; Gilbert, R.O.; Lucas, J.J.; Singh, B.N. Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J. Biol. Chem. 2005, 280, 23853–23860. [Google Scholar] [CrossRef]
- Kummer, S.; Hayes, G.R.; Gilbert, R.O.; Beach, D.H.; Lucas, J.J.; Singh, B.N. Induction of human host cell apoptosis by Trichomonas vaginalis cysteine proteases is modulated by parasite exposure to iron. Microb. Pathog. 2008, 44, 197–203. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, Y.; Xu, C.; Liu, Q.; Peng, B.; Kaufmann, G.F.; Chen, X.; Lan, B.; Wei, C.; Lu, D.; et al. Functional role of asparaginyl endopeptidase ubiquitination by TRAF6 in tumor invasion and metastasis. J. Natl. Cancer Inst. 2014, 106, dju012. [Google Scholar] [CrossRef] [PubMed]
- Karve, T.M.; Cheema, A.K. Small changes huge impact: The role of protein posttranslational modifications in cellular homeostasis and disease. J. Amino Acids 2011, 2011, 207691. [Google Scholar] [CrossRef]
- Lunde, N.N.; Haugen, M.H.; Bodin Larsen, K.B.; Damgaard, I.; Pettersen, S.J.; Kasem, R.; Rut, W.; Drag, M.; Poreba, M.; Johansen, H.T.; et al. Glycosylation is important for legumain localization and processing to active forms but not for cystatin E/M inhibitory functions. Biochimie 2017, 139, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Götz, B.; Klinkert, M.Q. Expression and partial characterization of a cathepsin B-like enzyme (Sm31) and a proposed ‘haemoglobinase’ (Sm32) from Schistosoma mansoni. Biochem. J. 1993, 290 Pt 3, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.; Hajdusek, O.; Dvorák, J.; Sajid, M.; Franta, Z.; Schneider, E.L.; Craik, C.S.; Vancová, M.; Buresová, V.; Bogyo, M.; et al. IrAE: An asparaginyl endopeptidase (legumain) in the gut of the hard tick Ixodes ricinus. Int. J. Parasitol. 2007, 37, 713–724. [Google Scholar] [CrossRef]
- Benchimol, M. Hydrogenosome autophagy: An ultrastructural and cytochemical study. Biol. Cell 1999, 91, 165–174. [Google Scholar] [CrossRef]
- de Jesus, J.B.; Cuervo, P.; Britto, C.; Sabóia-Vahia, L.; Suva-Filho FC, E.; Borges-Veloso, A.; Petrópolis, D.B.; Cupolillo, E.; Domont, G.B. Cysteine peptidase expression in Trichomonas vaginalis isolates D isplaying High- and low-virulence phenotypes. J. Proteome Res. 2009, 8, 1555–1564. [Google Scholar] [CrossRef]
- Lin, H.C.; Chu, L.J.; Huang, P.J.; Cheng, W.H.; Zheng, Y.H.; Huang, C.Y.; Hong, S.W.; Chen, L.C.; Lin, H.A.; Wang, J.Y.; et al. Proteomic signatures of metronidazole-resistant Trichomonas vaginalis reveal novel proteins associated with drug resistance. Parasites Vectors 2020, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Lunde, N.N.; Bosnjak, T.; Solberg, R.; Johansen, H.T. Mammalian legumain—A lysosomal cysteine protease with extracellular functions? Biochimie 2019, 166, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.M.; Kern, P.A. The role of glucose and glycosylation in the regulation of lipoprotein lipase synthesis and secretion in rat adipocytes. J. Biol. Chem. 1989, 264, 3177–3182. [Google Scholar] [CrossRef]
- Kahn, S.E.; Fujimoto, W.Y.; D’Alessio, D.A.; Ensinck, J.W.; Porte, D., Jr. Glucose stimulates and potentiates islet amyloid polypeptide secretion by the B-cell. Horm. Metab. Res. 1991, 23, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Hironaka, I.; Iwase, T.; Sugimoto, S.; Okuda, K.; Tajima, A.; Yanaga, K.; Mizunoe, Y. Glucose triggers ATP secretion from bacteria in a growth-phase-dependent manner. Appl. Environ. Microbiol. 2013, 79, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Lunde, N.N.; Holm, S.; Dahl, T.B.; Elyouncha, I.; Sporsheim, B.; Gregersen, I.; Abbas, A.; Skjelland, M.; Espevik, T.; Solberg, R.; et al. Increased levels of legumain in plasma and plaques from patients with carotid atherosclerosis. Atherosclerosis 2017, 257, 216–223. [Google Scholar] [CrossRef]
- Yamane, T.; Kato-Ose, I.; Sakamoto, T.; Nakano, Y. Secretion of Legumain Increases in Conditioned Medium from DJ-1-Knockout Cells and in Serum from DJ-1-Knockout Mice. Open Biochem. J. 2018, 12, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Johansen, H.T.; Nilsen, H.; Haugen, M.H.; Pettersen, S.J.; Mælandsmo, G.M.; Abrahamson, M.; Solberg, R. Intra- and extracellular regulation of activity and processing of legumain by cystatin E/M. Biochimie 2012, 94, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Laha, T.; Sripa, J.; Sripa, B.; Pearson, M.; Tribolet, L.; Kaewkes, S.; Sithithaworn, P.; Brindley, P.J.; Loukas, A. Asparaginyl endopeptidase from the carcinogenic liver fluke, Opisthorchis viverrini, and its potential for serodiagnosis. Int. J. Infect. Dis. 2008, 12, e49–e59. [Google Scholar] [CrossRef]
- Ju, J.W.; Joo, H.N.; Lee, M.R.; Cho, S.H.; Cheun, H.I.; Kim, J.Y.; Lee, Y.H.; Lee, K.J.; Sohn, W.M.; Kim, D.M.; et al. Identification of a serodiagnostic antigen, legumain, by immunoproteomic analysis of excretory-secretory products of Clonorchis sinensis adult worms. Proteomics 2009, 9, 3066–3078. [Google Scholar] [CrossRef]
- Chang, S.H.; Chen, K.Y.; Wang, L.C. Identification and characterization of an asparaginyl endopeptidase from Angiostrongylus cantonensis. Parasitol. Res. 2014, 113, 2143–2152. [Google Scholar] [CrossRef]
- Kang, J.M.; Lee, J.; Ju, H.L.; Ju, J.W.; Kim, J.H.; Pak, J.H.; Kim, T.S.; Hong, Y.; Sohn, W.M.; Na, B.K. Characterization of a gut-associated asparaginyl endopeptidase of Clonorchis sinensis. Exp. Parasitol. 2015, 153, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Holzhausen, J.; Haake, C.; Schicht, S.; Hinse, P.; Jordan, D.; Kremmer, E.; Strube, C. Biological function of Dictyocaulus viviparus asparaginyl peptidase legumain-1 and its suitability as a vaccine target. Parasitology 2018, 145, 378–392. [Google Scholar] [CrossRef] [PubMed]

| VW Microscopic Analysis b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample Number a | Patient ID | VEC d | WBC e | Bacteria | Yeast | Tv f | Tv Culture | Serum c |
| 1 | HGMM 718 | + | + | +++ | − | + | + | − |
| 2 | HGMM 766 | + | + | +++ | − | + | + | + |
| 3 | HGMM 826 | ++ | ++ | +++ | − | + | + | + |
| 4 | HGMM 827 | ++ | + | +++ | − | + | + | − |
| 5 | HGMM 842 | ++ | + | +++ | − | + | + | + |
| 6 | HGMM 949 | + | + | +++ | − | + | + | + |
| 7 | HGMM 990 | ++ | +++ | +++ | + | + | + | + |
| 8 | HGMM 999 | ++ | ++ | ++ | + | + | + | + |
| 9 | HGMM 1000 | ++ | ++ | ++ | − | + | + | + |
| 10 | HGMM 1005 | +++ | ++ | ++ | − | + | + | + |
| 11 | HGMM 1041 | + | ++ | ++ | − | + | + | − |
| 12 | HGMM 983 | + | + | ++ | − | − | − | − |
| 13 | HGMM 984 | ++ | ++ | ++ | − | − | − | − |
| 14 | HGMM 985 | ++ | ++ | ++ | − | − | − | − |
| 15 | HGMM 997 | ++ | +++ | +++ | + | − | − | − |
| 16 | HGMM 1012 | +++ | ++ | +++ | − | − | − | − |
| 17 | HGMM 1021 | +++ | ++ | +++ | + | − | − | − |
| 18 | HGMM 1025 | +++ | ++ | ++ | − | − | − | − |
| 19 | HGMM 1027 | ++ | ++ | ++ | − | − | − | + |
| 20 | HGMM 1031 | +++ | +++ | ++ | + | − | − | − |
| 21 | HGMM 1034 | + | + | + | + | − | − | + |
| 22 | HGMM 1035 | ++ | ++ | ++ | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Euceda-Padilla, E.A.; Mateo-Cruz, M.G.; Ávila-González, L.; Flores-Pucheta, C.I.; Ortega-López, J.; Talamás-Lara, D.; Velazquez-Valassi, B.; Jasso-Villazul, L.; Arroyo, R. Trichomonas vaginalis Legumain-2, TvLEGU-2, Is an Immunogenic Cysteine Peptidase Expressed during Trichomonal Infection. Pathogens 2024, 13, 119. https://doi.org/10.3390/pathogens13020119
Euceda-Padilla EA, Mateo-Cruz MG, Ávila-González L, Flores-Pucheta CI, Ortega-López J, Talamás-Lara D, Velazquez-Valassi B, Jasso-Villazul L, Arroyo R. Trichomonas vaginalis Legumain-2, TvLEGU-2, Is an Immunogenic Cysteine Peptidase Expressed during Trichomonal Infection. Pathogens. 2024; 13(2):119. https://doi.org/10.3390/pathogens13020119
Chicago/Turabian StyleEuceda-Padilla, Esly Alejandra, Miriam Guadalupe Mateo-Cruz, Leticia Ávila-González, Claudia Ivonne Flores-Pucheta, Jaime Ortega-López, Daniel Talamás-Lara, Beatriz Velazquez-Valassi, Lidia Jasso-Villazul, and Rossana Arroyo. 2024. "Trichomonas vaginalis Legumain-2, TvLEGU-2, Is an Immunogenic Cysteine Peptidase Expressed during Trichomonal Infection" Pathogens 13, no. 2: 119. https://doi.org/10.3390/pathogens13020119






