Sarcocystis spp. of New and Old World Camelids: Ancient Origin, Present Challenges
Abstract
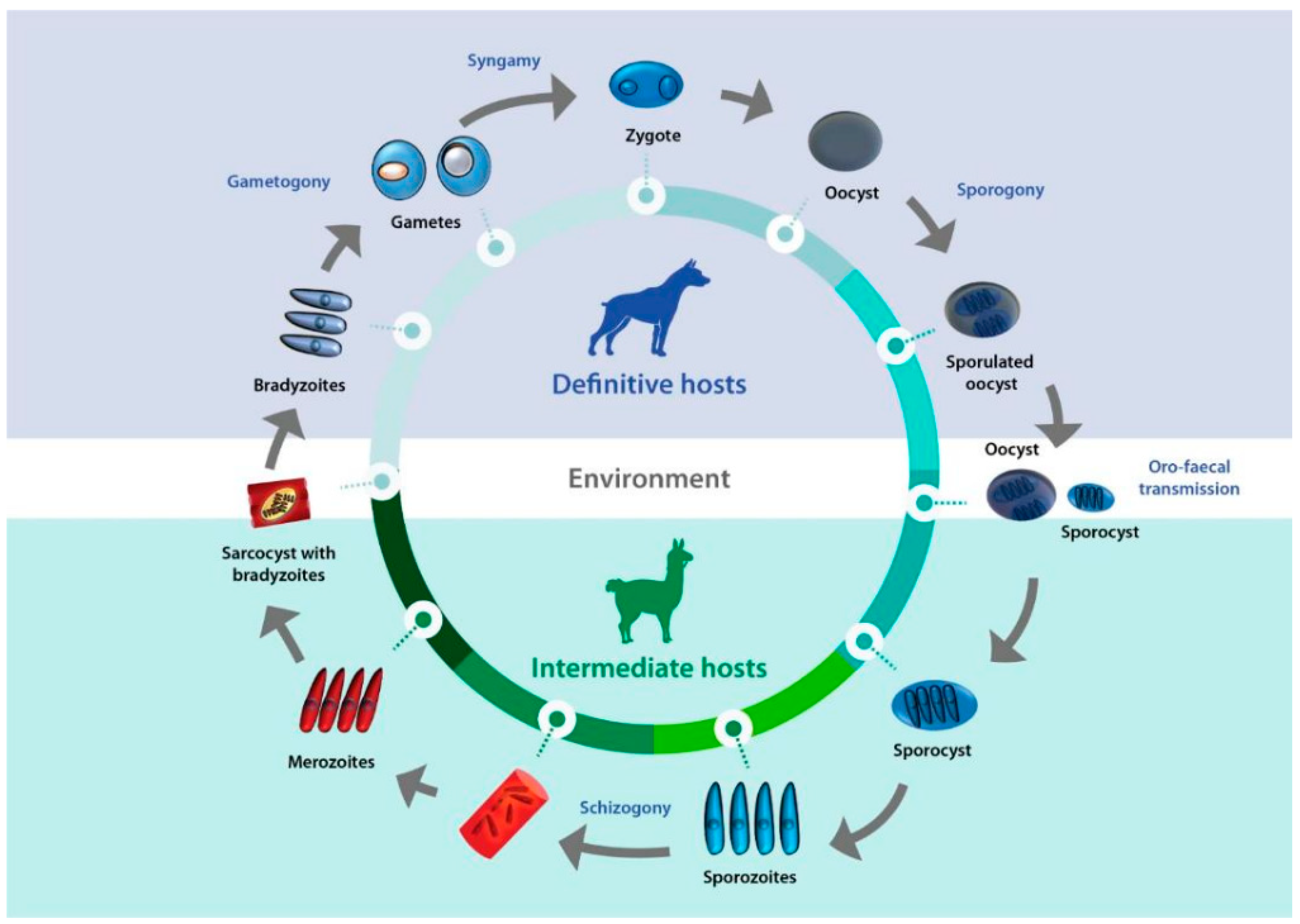
:1. Introduction
2. Sarcocystis Infecting OWCs and SACs
3. Definitive Hosts
4. Pathogenesis of Sarcocystosis in Camelids
5. Diagnosis
6. Epidemiology and Risk Factors
7. Parasite Biology and Host-Pathogen Interaction
8. Phylogeny of Camelids and Camelid-Infecting Sarcocystis spp.

9. Human Health Implications and Economic Losses Associated with Camelid Sarcocystosis
10. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Decker Franco, C.; Schnittger, L.; Florin-Christensen, M. Sarcocystis. In Parasitic Protozoa of Farm Animals and Pets; Springer International Publishing: Cham, Switzerland, 2018; pp. 103–124. ISBN 9783319701325. [Google Scholar]
- Fayer, R. Sarcocystis spp. in human infections. Clin. Microbiol. Rev. 2004, 17, 894–902. [Google Scholar] [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498710152. [Google Scholar]
- Dubey, J.P.; Rosenthal, B.M. Bovine Sarcocystosis: Sarcocystis species, diagnosis, prevalence, economic and public health considerations, and association of Sarcocystis species with eosinophilic myositis in cattle. Int. J. Parasitol. 2023, 53, 463–475. [Google Scholar] [CrossRef]
- Poulsen, C.S.; Stensvold, C.R. Current status of epidemiology and diagnosis of human sarcocystosis. J. Clin. Microbiol. 2014, 52, 3524–3530. [Google Scholar] [CrossRef]
- Fowler, M.E. Camelids are not ruminants. Zoo Wild Anim. Med. 2008, 375–385. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 13 December 2023).
- Midagari Camélidos Sudamericanos. Available online: https://www.midagri.gob.pe/portal/datero/40-sector-agrario/situacion-de-las-actividades-de-crianza-y-producci/298-camelidos-sudamericanos?start=1 (accessed on 13 December 2023).
- Zarrin, M.; Riveros, J.L.; Ahmadpour, A.; De Almeida, A.M.; Konuspayeva, G.; Vargas-Bello-Pérez, E.; Faye, B.; Hernández-Castellano, L.E. Camelids: New players in the international animal production context; Springer: Cham, Switzerland, 2020; Volume 52, pp. 903–913. [Google Scholar]
- Fowler, M.E. Husbandry and diseases of camelids. Rev. Sci. Tech. 1996, 15, 155–169. [Google Scholar] [CrossRef]
- Yacobaccio, H.D. The domestication of South American camelids: A Review. Anim. Front. 2021, 11, 43–51. [Google Scholar] [CrossRef]
- Mamani-Linares, L.W.; Gallo, C.B. Meat quality, proximate composition and muscle fatty acid profile of young llamas (Lama glama) supplemented with hay or concentrate during the dry season. Meat Sci. 2014, 96, 394–399. [Google Scholar] [CrossRef]
- Mamani-Linares, L.W.; Gallo, C.B. Meat quality attributes of the longissimus lumborum muscle of the Kh’ara genotype of llama (Lama glama) reared extensively in Northern Chile. Meat Sci. 2013, 94, 89–94. [Google Scholar] [CrossRef]
- Kadim, I.T.; Al-Amri, I.S.; Alkindi, A.Y.; Haq, Q.M.I. Nutritional values and health benefits of dromedary camel meat. Anim. Front. 2022, 12, 61–70. [Google Scholar] [CrossRef]
- Saeed, M.A.; Rashid, M.H.; Vaughan, J.; Jabbar, A. Sarcocystosis in South American camelids: The state of play revisited. Parasites Vectors 2018, 11, 146. [Google Scholar] [CrossRef]
- Saeed, M.A.; Vaughan, J.L.; Jabbar, A. An update on sarcocystosis in one-humped camels (Camelus dromedarius). Parasitology 2018, 145, 1367–1377. [Google Scholar] [CrossRef]
- Moré, G.; Regensburger, C.; Gos, M.L.; Pardini, L.; Verma, S.K.; Ctibor, J.; Serrano-Martínez, M.E.; Dubey, J.P.; Venturini, M.C. Sarcocystis masoni, n. sp. (Apicomplexa: Sarcocystidae), and redescription of Sarcocystis aucheniae from llama (Lama glama), guanaco (Lama guanicoe) and alpaca (Vicugna pacos). Parasitology 2016, 143, 617–626. [Google Scholar] [CrossRef]
- Giuliano, S.M.; Reategui Ordonez, J.; Barriga Marcopuda, X.; Florin-Christensen, M. Situación actual de la calidad de carne de camélidos sudamericanos (llama y alpaca) en Argentina y Perú y su relación con infestación con Sarcocystis aucheniae. In Proceedings of the XXIII Reunión Nacional de la Asociación Boliviana de Producción Animal—ABOPA, Oruro, Bolivia, 26–28 October 2023; Available online: https://www.cifumss.agro.bo/abopa/index.html (accessed on 22 February 2023).
- Dubey, J.P.; Hilali, M.; Van Wilpe, E.; Calero-Bernal, R.; Verma, S.K.; Abbas, I.E. A review of sarcocystosis in camels and redescription of Sarcocystis cameli and Sarcocystis ippeni sarcocysts from the one-humped camel (Camelus dromedarius). Parasitology 2015, 142, 1481–1492. [Google Scholar] [CrossRef]
- Dubey, J.P.; A’aji, N.N.; Mowery, J.D.; Verma, S.K.; Calero-Bernal, R. Identification of macroscopic sarcocysts of Sarcocystis cameli from one-humped camel (Camelus dromedarius) in Iraq. J. Parasitol. 2017, 103, 168–169. [Google Scholar] [CrossRef]
- Brumpt, E. Precis de Parasitologie, 2nd ed.; Masson et Cie: Paris, France, 1913. [Google Scholar]
- Quiroga, D.; Lombadero, O.; Zorrila, R. Sarcocystis tilpodi n.sp. en guanacos (Lama guanicoe) de la Repúplica Argentina. Gaceta Veterinaria 1969, 31, 67–70. [Google Scholar]
- Gorman, T.R.; Alcaíno, H.A.; Muñuz, H.; Cunazza, C. Sarcocystis sp. in guanaco (Lama guanicoe) and effect of temperature on its viability. Vet. Parasitol. 1984, 15, 95–101. [Google Scholar] [CrossRef]
- Leguía, G. Enfermedades Parasitarias y Atlas Parasitológico de Camélidos Sudamericanos; Primera, Ed.; De Mar: Lima, Perú, 1999. [Google Scholar]
- Leguía, G. The epidemiology and economic impact of llama parasites. Parasitol. Today 1991, 7, 54–56. [Google Scholar] [CrossRef]
- Carletti, T.; Martin, M.; Romero, S.; Morrison, D.A.; Marcoppido, G.; Florin-Christensen, M.; Schnittger, L. Molecular identification of Sarcocystis aucheniae as the macrocyst-forming parasite of llamas. Vet. Parasitol. 2013, 198, 396–400. [Google Scholar] [CrossRef]
- Metwally, D.M.; Al-Otaibi, T.T.; Al-Turaiki, I.M.; El-Khadragy, M.F.; Alajmi, R.A. Identification of Sarcocystis spp. in one-humped camels (Camelus dromedarius) from Riyadh and Dammam, Saudi Arabia, via histological and phylogenetic approaches. Animals 2020, 10, 1108. [Google Scholar] [CrossRef]
- Motamedi, G.R.; Dalimi, A.; Nouri, A.; Aghaeipour, K. Ultrastructural and molecular characterization of Sarcocystis isolated from camel (Camelus dromedarius) in Iran. Parasitol. Res. 2011, 108, 949–954. [Google Scholar] [CrossRef]
- Regensburger, C.; Gos, M.L.; Ctibor, J.; Moré, G. Morphological and molecular characteristics of Sarcocystis aucheniae isolated from meat of guanaco (Lama guanicoe). J. Food Qual. Hazards Cont. 2015, 2, 118–121. [Google Scholar]
- Schnieder, T.; Kaup, F.J.; Drommer, W.; Thiel, W.; Rommel, M. Fine structure and development of Sarcocystis aucheniae in llamas. Z. Parasitenkd. 1984, 70, 451–458. [Google Scholar] [CrossRef]
- Cornejo, R.; Chávez, A.; Leyva, V.; Falcón, N.; Panez, S.; Ticona, D. Relationship between the size of macrocysts of Sarcocystis aucheniae and its viability in Canis familiaris. Rev. Inv. Vet. Perú 2007, 18, 76–83. [Google Scholar]
- Zacarías, F.S.; Sam, R.T.; Ramos, D.D.; Lucas, O.A.; Lucas, J.L. Techniques for the isolation and purification of Sarcosytis aucheniae oocysts from small intestine of experimentally infected dogs. Rev. Inv. Vet. Perú 2013, 24, 396–403. [Google Scholar]
- Wu, Z.; Sun, J.; Hu, J.; Song, J.; Deng, S.; Zhu, N.; Yang, Y.; Tao, J. Morphological and molecular characterization, and demonstration of a definitive host for Sarcocystis masoni from an alpaca (Vicugna pacos) in China. Biology 2022, 11, 1016. [Google Scholar] [CrossRef]
- Hilali, M.; Mohamed, A. The dog (Canis familiaris) as the final host of Sarcocystis cameli (Mason 1910). Tropenmed. Parasitol. 1980, 31, 213–214. [Google Scholar] [PubMed]
- Hilali, M.; Fatani, A.; Al-Atiya, S. Isolation of tissue cysts of Toxoplasma, Isospora, Hammondia and Sarcocystis from Camel (Camelus dromedarius) meat in Saudi Arabia. Vet. Parasitol. 1995, 58, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Hilali, M.; Imam, E.S.; Hassan, A. The endogenous stages of Sarcocystis cameli (Mason, 1910). Vet. Parasitol. 1982, 11, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Hilali, M.; Nassar, A.M.; El-Ghaysh, A. Camel (Camelus dromedarius) and sheep (Ovis aries) meat as a source of dog infection with some coccidian parasites. Vet. Parasitol. 1992, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaffar, F.; Mehlhorn, H.; Bashtar, A.R.; Al-Rasheid, K.; Sakran, T.; El-Fayoumi, H. Life cycle of Sarcocystis camelicanis infecting the camel (Camelus dromedarius) and the dog (Canis familiaris), light and electron microscopic study. Parasitol. Res. 2009, 106, 189–195. [Google Scholar] [CrossRef]
- Ishag, M.Y.; Majid, A.; Magzoub, A. Isolation of a new Sarcocystis species from Sudanese camels (Camelus dromedarius). Int. J. Trop. Med. 2006, 1, 167–169. [Google Scholar]
- Godoy, R.; Vilca, M.; Gonzáles, A.; Leyva, V.; Sam, R. Saneamiento y detoxificación de carne de llama (Lama glama) infectada con Sarcocystis aucheniae mediante cocción, horneado, fritura y congelado. Rev. Inv. Vet. Perú 2007, 18, 51–56. [Google Scholar] [CrossRef]
- Miguel Vilca, L.; Julio Durán, O.; Daphne Ramos, D.; Juan Lucas, L. Saneamiento y eliminación de la toxicidad de carne de alpaca (Vicugna pacos) con sarcocistiosis mediante ahumado y curado. Rev. Inv. Vet. Perú 2013, 24, 537–543. [Google Scholar]
- Saito, M.; Taguchi, K.; Shibata, Y.; Kobayashi, T.; Shimura, K.; Itagaki, H. Toxicity and properties of the extract from Sarcocystis cruzi cysts. J. Vet. Med. Sci. 1995, 57, 1049–1051. [Google Scholar] [CrossRef]
- Irikura, D.; Saito, M.; Sugita-Konishi, Y.; Ohnishi, T.; Sugiyama, K.; Watanabe, M.; Yamazaki, A.; Izumiyama, S.; Sato, H.; Kimura, Y.; et al. Characterization of Sarcocystis fayeri ’s actin-depolymerizing factor as a toxin that causes diarrhea. Genes Cells 2017, 22, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Kamata, Y.; Saito, M.; Irikura, D.; Yahata, Y.; Ohnishi, T.; Bessho, T.; Inui, T.; Watanabe, M.; Sugita-Konishi, Y. A toxin isolated from Sarcocystis fayeri in raw horsemeat may be responsible for food poisoning. J. Food Prot. 2014, 77, 814–819. [Google Scholar] [CrossRef] [PubMed]
- La Perle, K.M.D.D.; Silveria, F.; Anderson, D.E.; Blomme, E.A.G.G. Dalmeny disease in an alpaca (Lama pacos): Sarcocystosis, eosinophilic myositis and abortion. J. Comp. Pathol. 1999, 121, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Gabor, M.; Gabor, L.J.; Srivastava, M.; Booth, M.; Reece, R. Chronic myositis in an Australian alpaca (Llama pacos) associated with Sarcocystis spp. J. Vet. Diagn. Investig. 2010, 22, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Chávez, A.; Leyva, V.; Panez, S.; Ticona, D.; García, W.; Pezo, D. Sarcocistiosis y la eficiencia productiva de la alpaca. Rev. Inv. Vet. Perú 2008, 19, 160–167. [Google Scholar] [CrossRef]
- Fatani, A.; El-Sebaie, A.; Hilali, M. Clinical and haematobiochemical changes in camels (Camelus dromedarius) experimentally inoculated with Sarcocystis cameli. J. Camel Pract. Res. 1996, 3, 11–15. [Google Scholar]
- Manal, Y.I.; El-Amin, E.; Osman, A. Camels experimentally infected with Sarcocystis. Sudan. J. Vet. Res. 2001, 17, 27–33. [Google Scholar]
- Valinezhad, A.; Oryan, A.; Ahmadi, N. Sarcocystis and its complications in camels (Camelus dromedarius) of Eastern provinces of Iran. Korean J. Parasitol. 2008, 46, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Metwally, D.M.; Al-Otaibi, T.T.; Semlali, A.; Alajmi, R.A. Sarcocystis camelicanis increases interleukin (IL)-6 expression in one-humped camels (Camelus dromedarius) from Riyadh and Al Qassim, Saudi Arabia. Biosci. Rep. 2021, 41, BSR20203140. [Google Scholar] [CrossRef] [PubMed]
- Rooney, A.L.; Limon, G.; Vides, H.; Cortez, A.; Guitian, J. Sarcocystis spp. in llamas (Lama glama) in southern Bolivia: A cross sectional study of the prevalence, risk factors and loss in income caused by carcass downgrades. Prev. Vet. Med. 2014, 116, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Gareh, A.; Soliman, M.; Saleh, A.A.; El-Gohary, F.A.; El-Sherbiny, H.M.M.; Mohamed, R.H.; Elmahallawy, E.K.; Kotb Elmahallawy, E. Epidemiological and histopathological investigation of Sarcocystis spp. in slaughtered dromedary camels (Camelus dromedarius) in Egypt. Vet. Sci. 2020, 7, 162. [Google Scholar] [CrossRef]
- Martin, M.; Decker Franco, C.; Romero, S.; Carletti, T.; Schnittger, L.; Florin-Christensen, M. Molecular detection of Sarcocystis aucheniae in the blood of llamas from Argentina. Rev. Argent. Microbiol. 2016, 48, 200–205. [Google Scholar] [CrossRef]
- Decker Franco, C.; Romero, S.; Ferrari, A.; Schnittger, L.; Florin-Christensen, M. Detection of Sarcocystis aucheniae in blood of llama using a duplex semi-nested PCR assay and its association with cyst infestation. Heliyon 2018, 4, e00928. [Google Scholar] [CrossRef]
- Moré, G.; Pardini, L.; Basso, W.; Marín, R.; Bacigalupe, D.; Auad, G.; Venturini, L.; Venturini, M.C. Seroprevalence of Neospora caninum, Toxoplasma gondii and Sarcocystis sp. in llamas (Lama glama) from Jujuy, Argentina. Vet. Parasitol. 2008, 155, 158–160. [Google Scholar] [CrossRef]
- Romero, S.; Carletti, T.; Decker Franco, C.; Moré, G.; Schnittger, L.; Florin-Christensen, M. Seropositivity to Sarcocystis infection of llamas correlates with breeding practices. Vet. Parasitol. Reg. Stud. Rep. 2017, 10, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Decker Franco, C.; Wieser, S.N.; Soria, M.; de Alba, P.; Florin-Christensen, M.; Schnittger, L. In silico identification of immunotherapeutic and diagnostic targets in the glycosylphosphatidylinositol metabolism of the coccidian Sarcocystis aucheniae. Transbound. Emerg. Dis. 2020, 67, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Wieser, S.N.; Decker-Franco, C.; de Alba, P.; Romero, S.; Ferrari, A.; Schnittger, L.; Florin-Christensen, M. Discovery of antigens and cellular mechanisms in the protozoan parasite Sarcocystis aucheniae using immunoproteomics. Parasitologia 2023, 3, 349–363. [Google Scholar] [CrossRef]
- Holmdahl, O.J.M.; Morrison, D.A.; Ellis, J.T.; Huong, L.T.T. Evolution of ruminant Sarcocystis (Sporozoa) parasites based on small subunit rDNA sequences. Mol. Phylogenet Evol. 1999, 11, 27–37. [Google Scholar] [CrossRef]
- Wieser, S.N.; Cafrune, M.W.; Romero, S.R.; Schnittger, L.; Florin-Christensen, M. Molecular identification of Sarcocystis aucheniae infesting Vicugna vicugna. Vet. Res. Commun. 2024, submitted.
- Beldomenico, P.M.; Uhart, M.M.; Beldomenico, P.M.; Uhart, M.; Bono, M.F.; Marull, C.; Baldi, R.; Peralta, J.L. Internal parasites of free-ranging guanacos from Patagonia. Vet. Parasitol. 2003, 118, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xin, S.; Zhu, N.; Yang, L.; Huang, W.; Hu, J.; Zhu, X.; Yang, Y. First report of Sarcocystis masoni in a captive alpaca (Vicugna pacos) from China. Front. Vet. Sci. 2021, 8, 759252. [Google Scholar] [CrossRef]
- Lucas, J.R.; Barrios-Arpi, M.; Rodríguez, J.; Balcázarnakamatsu, S.; Zarria, J.; Namiyama, G.; Taniwaki, N.; Gonzales-Viera, O. Ultrastructural description of Sarcocystis sp. in cardiac muscle of naturally infected alpacas (Vicugna pacos). Iran. J. Parasitol. 2019, 14, 174–179. [Google Scholar]
- Fernandez-F, F.; Gutiérrez-A, R.; Pacheco-S, V.; Chirinos-T, J.; Lombardo, D.M.; Olivera, L.V.M.; Bernabe-Ortiz, J.C.; López-Casaperalta, P. Determination of Sarcocystis lamacanis microcysts in the cardiac muscle of alpacas (Vicugna pacos) and their correlation with troponin CTnI. A study performed in the High Andean region of Southern Peru. Vet. Anim. Sci. 2022, 18, 100270. [Google Scholar] [CrossRef]
- Rodríguez, A.; Quispe-Solano, M.; Rodríguez, J.L.; Lucas, J.R. The occurrence of Sarcocystis spp. in the myocardium of alpacas (Vicugna pacos) with associated risk factors in the Peruvian Andes. Trop. Anim. Health Prod. 2023, 55, 66. [Google Scholar] [CrossRef]
- Latif, B.M.A.; Al-Delemi, J.K.; Mohammed, B.S.; Al-Bayati, S.M.; Al-Amiry, A.M. Prevalence of Sarcocystis spp. in meat-producing animals in Iraq. Vet. Parasitol. 1999, 84, 85–90. [Google Scholar] [CrossRef]
- Woldemeskel, M.; Gumi, B. Prevalence of sarcocysts in One-Humped camel (Camelus dromedarius) from Southern Ethiopia. J. Vet. Med. Ser. B 2001, 48, 223–226. [Google Scholar] [CrossRef]
- Fukuyo, M.; Battsetseg, G.; Byambaa, B. Prevalence of Sarcocystis infection in meat-producing animals in Mongolia. Southeast. Asian J. Trop. Med. Publ. Health 2002, 33, 490–495. [Google Scholar]
- Shekarforoush, S.S.; Shakerian, A.; Hasanpoor, M.M. Prevalence of Sarcocystis in slaughtered one-humped camels (Camelus dromedarius) in Iran. Trop. Anim. Health Prod. 2006, 38, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Hamidinejat, H.; Hekmatimoghaddam, S.; Jafari, H.; Sazmand, A.; Haddad Molayan, P.; Derakhshan, L.; Mirabdollahi, S. Prevalence and distribution patterns of Sarcocystis in camels (Camelus dromedarius) in Yazd Province, Iran. J. Parasit. Dis. 2013, 37, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.A.; Alzuraiq, A.A.; Mohammed, O.B. Prevalence and molecular detection of Sarcocystis spp. infection in the dromedary camel (Camelus dromedarius) in Riyadh City, Saudi Arabia. Biomed. Res. 2017, 28, 4962–4965. [Google Scholar]
- Al-Ani, F.K.; Amr, Z. Sarcocystis spp Prevalence in Camel Meat in Jordan. Dairy Vet. Sci. 2017, 4, 555643. [Google Scholar]
- Castro, E.; Sam, R.; López, T.; González, A.; Silva, M. Evaluación de la edad como factor de riesgo de seropositividad a Sarcocystis sp. en alpacas. Rev. Inv. Vet. Perú 2004, 15, 83–86. [Google Scholar] [CrossRef]
- Rochefort, B.S.; Root-Bernstein, M. History of canids in Chile and impacts on prey adaptations. Ecol. Evol. 2021, 11, 9892–9903. [Google Scholar] [CrossRef] [PubMed]
- Blazejewski, T.; Nursimulu, N.; Pszenny, V.; Dangoudoubiyam, S.; Namasivayam, S.; Chiasson, M.A.; Chessman, K.; Tonkin, M.; Swapna, L.S.; Hung, S.S.; et al. Systems-based analysis of the Sarcocystis neurona genome identifies pathways that contribute to a heteroxenous life cycle. mBio 2015, 6, e02445-14. [Google Scholar] [CrossRef]
- Rodríguez, A.E.; Couto, A.; Echaide, I.; Schnittger, L. Babesia bovis contains an abundant parasite-specific protein-free glycerophosphatidylinositol and the genes predicted for its assembly. Vet. Parasitol. 2010, 167, 227–235. [Google Scholar] [CrossRef]
- Delorenzi, M.; Sexton, A.; Shams-Eldin, H.; Schwarz, R.T.; Speed, T.; Schofield, L. Genes for glycosylphosphatidylinositol toxin biosynthesis in Plasmodium falciparum. Infect. Immun. 2002, 70, 4510–4522. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Florin-Christensen, M.; Flores, D.A.; Echaide, I.; Suarez, C.E.; Schnittger, L. The glycosylphosphatidylinositol-anchored protein repertoire of Babesia bovis and its significance for erythrocyte invasion. Ticks Tick. Borne Dis. 2014, 5, 343–348. [Google Scholar] [CrossRef]
- Deroost, K.; Pham, T.-T.; Opdenakker, G.; Van Den Steen, P.E. The immunological balance between host and parasite in Malaria. FEMS Microbiol. Rev. 2016, 46, 208–257. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.P.; Pinheiro, C.S.; Figueiredo, B.C.P.; Assis, N.R.G.; Morais, S.B.; Caliari, M.V.; Azevedo, V.; Castro-Borges, W.; Wilson, R.A.; Oliveira, S.C. Vaccination with enzymatically cleaved GPI-anchored proteins from Schistosoma mansoni induces protection against challenge infection. Clin. Dev. Immunol. 2012, 2012, 962538. [Google Scholar] [CrossRef] [PubMed]
- Hegazy-Hassan, W.; Zepeda-Escobar, J.A.; Ochoa-García, L.; Contreras-Ortíz, J.M.E.; Tenorio-Borroto, E.; Barbabosa-Pliego, A.; Aparicio-Burgos, J.E.; Oros-Pantoja, R.; Rivas-Santiago, B.; Díaz-Albiter, H.; et al. TcVac1 vaccine delivery by intradermal electroporation enhances vaccine induced immune protection against Trypanosoma cruzi infection in mice. Vaccine 2019, 37, 248–257. [Google Scholar] [CrossRef]
- Moubri, K.; Kleuskens, J.; Van de Crommert, J.; Scholtes, N.; Van Kasteren, T.; Delbecq, S.; Carcy, B.; Précigout, E.; Gorenflot, A.; Schetters, T. Discovery of a recombinant Babesia canis supernatant antigen that protects dogs against virulent challenge infection. Vet. Parasitol. 2018, 249, 21–29. [Google Scholar] [CrossRef]
- Florin-Christensen, M.; Sojka, D.; Ganzinelli, S.; Šnebergerová, P.; Suarez, C.E.; Schnittger, L. Degrade to survive: The intricate world of piroplasmid proteases. Trends Parasitol. 2023, 39, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guang, X.; Al-Fageeh, M.B.; Cao, J.; Pan, S.; Zhou, H.; Zhang, L.; Abutarboush, M.H.; Xing, Y.; Xie, Z.; et al. Camelid genomes reveal evolution and adaptation to desert environments. Nat. Commun. 2014, 5, 5188. [Google Scholar] [CrossRef]
- Díaz-Maroto, P.; Rey-Iglesia, A.; Cartajena, I.; Núñez, L.; Westbury, M.V.; Varas, V.; Moraga, M.; Campos, P.F.; Orozco-terWenge, P.; Marín, J.C.; et al. Ancient DNA reveals the lost domestication history of South American camelids in Northern Chile and across the Andes. eLife 2021, 10, e63390. [Google Scholar] [CrossRef]
- Wang, X.; Tedford, R.H.; Van Valkenburgh, B.; Wayne, R.K. Evolutionary History, moleecular sytematics and evolutionary ecology of Canidae. In The Biology of Conservation of World Canids; Oxford Press: Oxford, UK, 2004; pp. 39–54. [Google Scholar]
- Chavez, D.E.; Gronau, I.; Hains, T.; Dikow, R.B.; Frandsen, P.B.; Figueiró, H.V.; Garcez, F.S.; Tchaicka, L.; De Paula, R.C.; Rodrigues, F.H.G.; et al. Comparative genomics uncovers the evolutionary history, demography, and molecular adaptations of South American canids. Proc. Natl. Acad. Sci. USA 2022, 119, e2205986119. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Van Asch, B.; Zhang, A.B.; Oskarsson, M.C.R.; Klütsch, C.F.C.; Amorim, A.; Savolainen, P. Pre-Columbian origins of native American dog breeds, with only limited replacement by European dogs, confirmed by mtDNA analysis. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131142. [Google Scholar] [CrossRef] [PubMed]
- Salvá, B.K.; Zumalacárregui, J.M.; Figueira, A.C.; Osorio, M.T.; Mateo, J. Nutrient composition and technological quality of meat from alpacas reared in Peru. Meat Sci. 2009, 82, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Tejeda, L.; Peñarrieta, J.M.; Smith, M.A.; Bush, R.D.; Hopkins, D.L. Meat of South American camelids—Sensory quality and nutritional composition. Meat Sci. 2021, 171, 108285. [Google Scholar] [CrossRef] [PubMed]
- Carmanchahi, P.; Lichtenstein, G. Guanacos and People in Patagonia, 1st ed.; Carmanchahi, P., Lichtenstein, G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-031-06655-9. [Google Scholar]
- Soto Volkart, N.; Molina Uriarte, R. Evaluación Del Manejo de La Populación de Guanacos En El Área Agropecuaria de Magallanes; Informe Técnico; SAG Magallanes y Antártica Chilena: Punta Arnas, Chile, 2020.
- González, F.; Smulders, F.J.M.; Paulsen, P.; Skewes, O.; König, E. Anatomical investigations on meat cuts of guanacos (Lama guanicoe, Müller 1776) and chemical composition of selected muscles. Wien. Tierärtztliche Monatsschfift 2004, 91, 77–84. [Google Scholar]


| Intermediate Host | Species | Sarcocyst | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Shape, Size (Length × Width) | Cyst Wall | Location | |||||
| Type | Thickness (µm) | Villar Protrusions (vp) Size and Aspect | |||||
| OWC | S. cameli | Fusiform, microscopic (700 × 100 µm) and macroscopic (1.5–5.0 × 0.2–0.4 mm) | 9j | <2 | 3.0 × 0.5 µm finger-like vp | cardiac and skeletal muscle | [3,19,20,27,28] |
| S. ippeni | Globular, microscopic (100–120 × 50–100 µm) | 32 | 2.3–3.0 | 1.0 × 0.25–1.2 µm thorn-like vp | skeletal muscle | [3,19] | |
| SAC | S. aucheniae | Oval, macroscopic (0.5–2.0 × 0.2 cm) | 21 | 6–10 | 3–4.5 × 2.5–3.5 µm branched vp, cauliflower-like wall | skeletal muscle | [1,17,26,29,30] |
| S. masoni | Fusiform, microscopic (800 × 95 µm) | 9j | 2.5–3.5 | 2–2.8 × 0.5–0.7 µm conical to cylindrical vp | cardiac and skeletal muscle | 17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieser, S.N.; Giuliano, S.M.; Reategui Ordoñez, J.; Barriga Marcapura, X.; Olivera, L.V.M.; Chavez Fumagalli, M.A.; Schnittger, L.; Florin-Christensen, M. Sarcocystis spp. of New and Old World Camelids: Ancient Origin, Present Challenges. Pathogens 2024, 13, 196. https://doi.org/10.3390/pathogens13030196
Wieser SN, Giuliano SM, Reategui Ordoñez J, Barriga Marcapura X, Olivera LVM, Chavez Fumagalli MA, Schnittger L, Florin-Christensen M. Sarcocystis spp. of New and Old World Camelids: Ancient Origin, Present Challenges. Pathogens. 2024; 13(3):196. https://doi.org/10.3390/pathogens13030196
Chicago/Turabian StyleWieser, Sarah N., Susana M. Giuliano, Juan Reategui Ordoñez, Ximena Barriga Marcapura, Luis V. M. Olivera, Miguel Angel Chavez Fumagalli, Leonhard Schnittger, and Mónica Florin-Christensen. 2024. "Sarcocystis spp. of New and Old World Camelids: Ancient Origin, Present Challenges" Pathogens 13, no. 3: 196. https://doi.org/10.3390/pathogens13030196







