Prevalence of Toxoplasma gondii Antibodies and Risk Factors in Two Sympatric Invasive Carnivores (Procyon lotor and Nyctereutes procyonoides) from Zgorzelec County, Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
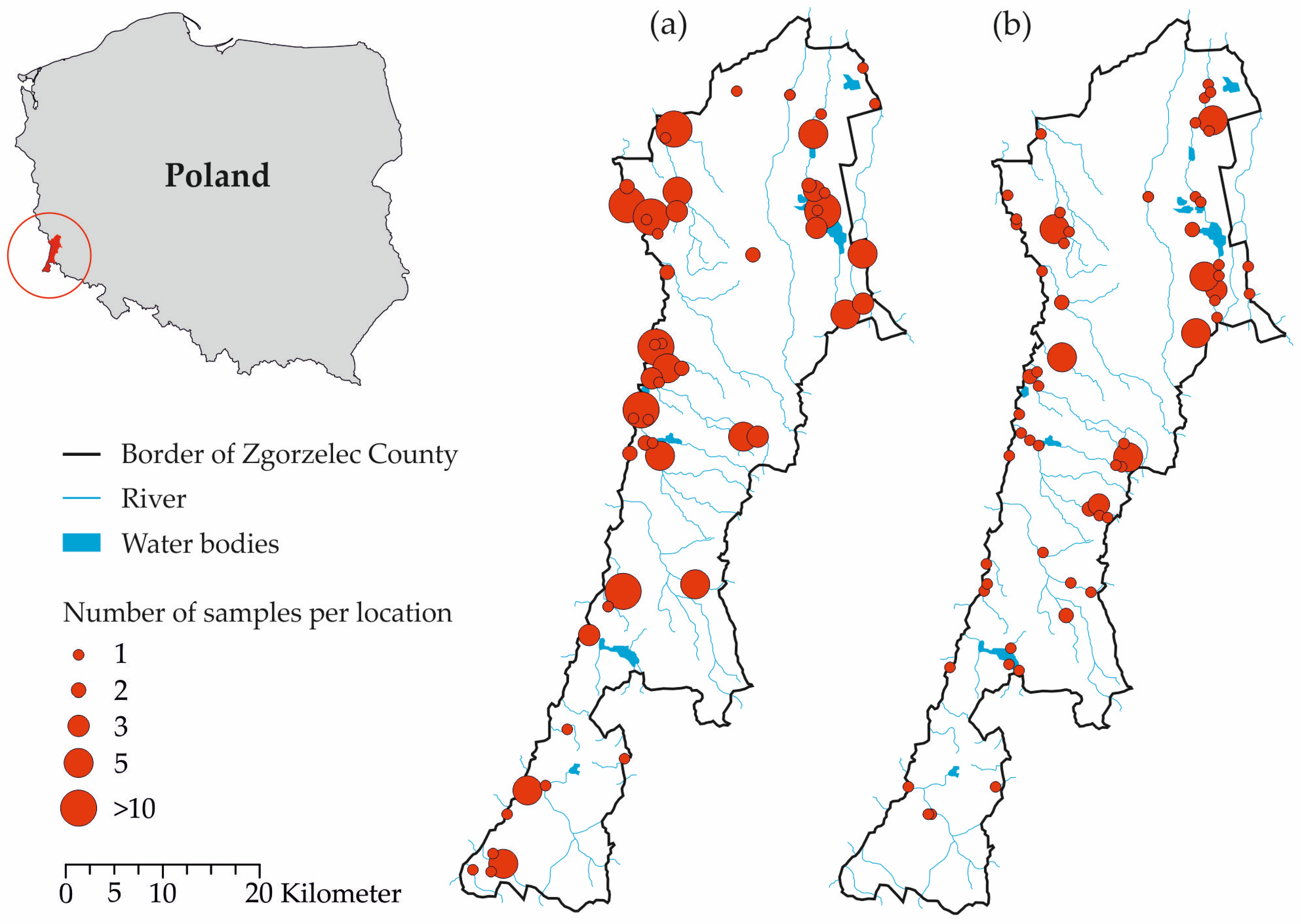
2.2. Sample Collection
2.3. Detection of T. gondii Antibodies
2.4. Statistical Analysis
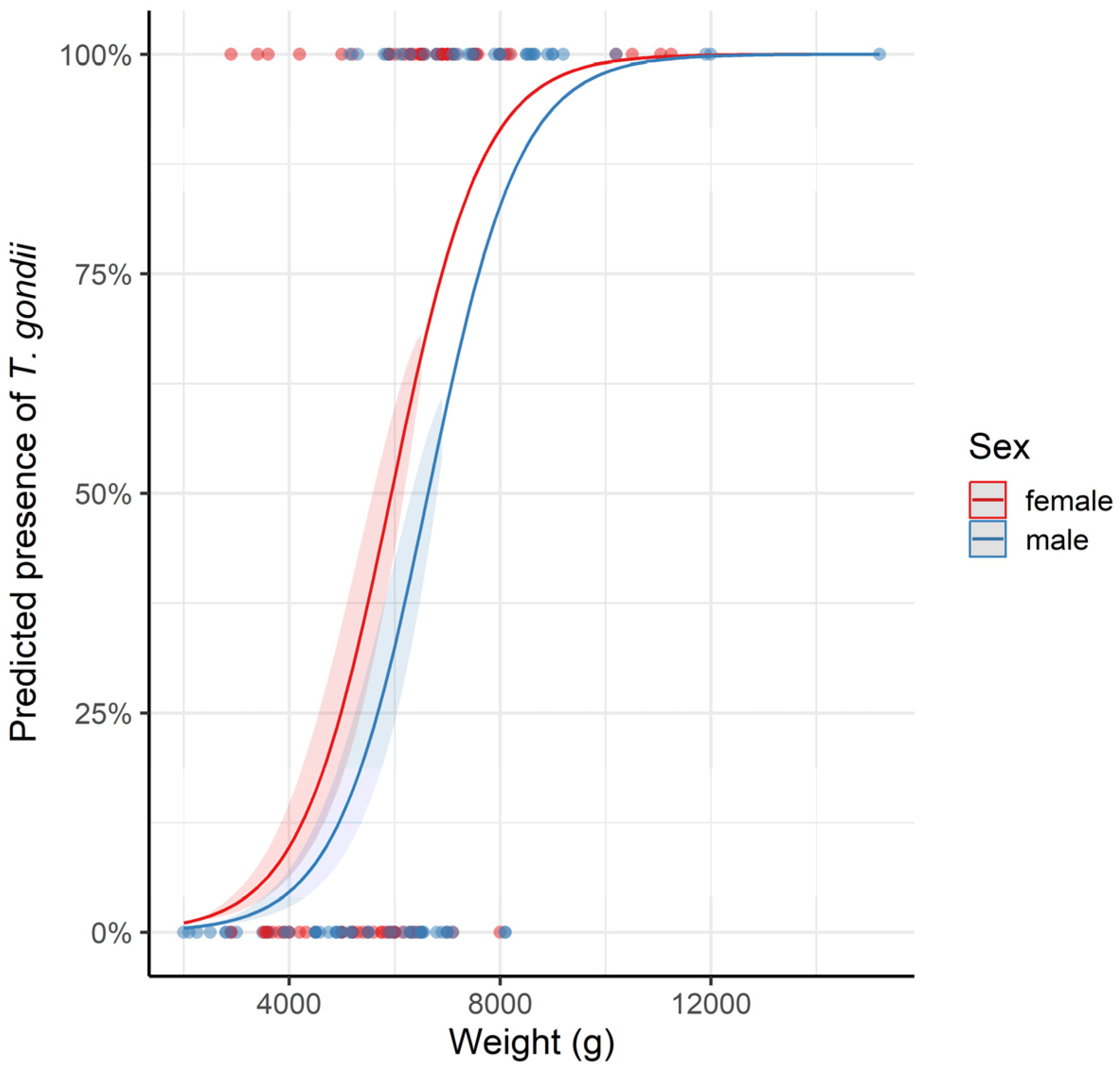
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P. Toxoplasmosis of Animals and Humans; CRC Press: London, UK; New York, NY, USA, 2021. [Google Scholar]
- Kaufmann, J.H. Raccoons and Allies. In Wild Mammals of North America: Biology, Management and Economics; Chapman, J.A., Feldhammer, G.A., Eds.; John Hopkins University Press: Baltimore, MD, USA, 1982; pp. 567–585. [Google Scholar]
- Salgado, I. Is the raccoon (Procyon lotor) out of control in Europe? Biodivers. Conserv. 2018, 27, 2243–2256. [Google Scholar] [CrossRef]
- Stope, M.B. The Raccoon (Procyon lotor) as a Neozoon in Europe. Animals 2023, 13, 273. [Google Scholar] [CrossRef]
- Fischer, M.L.; Sullivan, M.J.P.; Greiser, G.; Guerrero-Casado, J.; Heddergott, M.; Hohmann, U.; Keuling, O.; Lang, J.; Martin, I.; Michler, F.-U.; et al. Assessing and predicting the spread of non-native raccoons in Germany using hunting bag data and dispersal weighted models. Biol. Invasions 2016, 18, 57–71. [Google Scholar] [CrossRef]
- Fischer, M.L.; Salgado, I.; Beninde, J.; Klein, R.; Frantz, A.C.; Heddergott, M.; Cullingham, C.I.; Kyle, C.J.; Hochkirch, A. Multiple founder effects are followed by range expansion and admixture during the invasion process of the raccoon (Procyon lotor) in Europe. Divers. Distrib. 2017, 23, 409–420. [Google Scholar] [CrossRef]
- Frantz, A.C.; Schleimer, A.; Wittische, J.; Heddergott, M. Close spatial overlap between the genetic population boundaries of raccoons and the distribution of the raccoon roundworm in Germany. Hystrix It. J. Mamm. 2021, 32, 203–206. [Google Scholar] [CrossRef]
- Biedrzycka, A.; Zalewski, A.; Bartoszewicz, M.; Okarma, H.; Jędrzejewska, E. The genetic structure of raccoon introduced in Central Europe reflects multiple invasion pathways. Biol. Invasions 2014, 16, 1611–1625. [Google Scholar] [CrossRef]
- Dziech, A.; Wierzbicki, H.; Moska, M.; Zatoń-Dobrowolska, M. Invasive and Alien Mammal Species in Poland-A Review. Diversity 2023, 15, 138. [Google Scholar] [CrossRef]
- Nowak, E. Nyctereutes procyonoides (Gray, 1834)–Marderhund. In Handbuch der Säugetiere Europas; AULA: Wiesbaden, Germany, 1993; Volume 5. [Google Scholar]
- Kauhala, K.; Kowalczyk, R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: History of colonization, features behind its success, and threats to native fauna. Curr. Zool. 2011, 57, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.; Pielowski, Z. Die Verbreitung des Marderhundes in Polen im Zusammenhang mit seiner Einbürgerung und Ausbreitung in Europa. Acta Theriol. 1964, 9, 81–110. [Google Scholar] [CrossRef]
- Drygala, F.; Korablev, N.; Ansorge, H.; Fickel, J.; Isomursu, M.; Elmeros, M.; Kowalczyk, R.; Baltrunaite, L.; Balciauskas, L.; Saarma, U.; et al. Homogenous population genetic structure of the non-native raccoon dog (Nyctereutes procyonoides) in Europe as a result of rapid population expansion. PLoS ONE 2016, 11, e0153098. [Google Scholar] [CrossRef] [PubMed]
- Drygala, F.; Zoller, H. Spatial use and interaction of the invasive raccoon dog and the native red fox in Central Europe: Competition or coexistence? Eur. J. Wildl. Res. 2013, 59, 683–691. [Google Scholar] [CrossRef]
- Beltrán-Beck, B.; García, F.J.; Gortázar, C. Raccoons in Europe: Disease hazards due to the establishment of an invasive species. Eur. J. Wildl. Res. 2012, 58, 5–15. [Google Scholar] [CrossRef]
- Sutor, A.; Schwarz, S.; Conraths, F.J. The biological potential of the raccoon dog (Nyctereutes procyonoides, Gray 1834) as an invasive species in Europe—New risks for disease spread? Acta Theriol. 2014, 59, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Laurimaa, L.; Süld, K.; Davison, J.; Moks, E.; Valdmann, H.; Saarma, U. Alien species and their zoonotic parasites in native and introduced ranges: The raccoon dog example. Vet. Parasitol. 2016, 219, 24–33. [Google Scholar] [CrossRef]
- Stope, M. Wild raccoons in Germany as a reservoir for zoonotic agents. Eur. J. Wildl. Res. 2019, 65, 94. [Google Scholar] [CrossRef]
- Heddergott, M. Der Waschbärspulwurm (Baylisascaris procyonis) in Deutschland: Eine Übersicht. Beitr. Jagd Wildforsch. 2020, 45, 165–175. [Google Scholar]
- Heddergott, M.; Frantz, A.C.; Pohl, D.; Osten-Sacken, N.; Steinbach, P. Detection of Cryptosporidium spp. infection in wild raccoons (Procyon lotor) from Luxembourg using an ELISA Approach. Acta Parasit. 2020, 65, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Heddergott, M.; Steinbach, P.; Schwarz, S.; Anheyer-Behmenburg, H.; Sutor, A.; Schliephake, A.; Jeschke, D.; Striese, M.; Müller, F.; Meyer-Kayser, E.; et al. Geographic distribution of raccoon roundworm, Baylisascaris procyonis, Germany and Luxembourg. Emerg. Infect. Dis. 2020, 26, 821. [Google Scholar] [CrossRef]
- Kjær, L.J.; Jensen, L.M.; Chriél, M.; Bødker, R.; Petersen, H.H. The raccoon dog (Nyctereutes procyonoides) as a reservoir of zoonotic diseases in Denmark. Int. J. Parasitol. Parasites Wildl. 2021, 16, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Solarczyk, P.; Dabert, M.; Frantz, A.C.; Osten-Sacken, N.; Trzebny, A.; Wojtkowiak-Giera, A.; Heddergott, M. Zoonotic Giardia duodenalis sub-assemblage BIV in wild raccoons (Procyon lotor) from Germany and Luxembourg. Zoonoses Public Health 2021, 68, 538–543. [Google Scholar] [CrossRef]
- Myśliwy, I.; Perec-Matysiak, A.; Hildebrand, J. Invasive raccoon (Procyon lotor) and raccoon dog (Nyctereutes procyonoides) as potential reservoirs of tick-borne pathogens: Data review from native and introduced areas. Parasites Vectors 2022, 15, 126. [Google Scholar] [CrossRef]
- Kornacka-Stackonis, A. Toxoplasma gondii infection in wild omnivorous and carnivorous animals in Central Europe–A brief overview. Vet. Parasitol. 2022, 304, 109701. [Google Scholar] [CrossRef]
- Reinhardt, N.P.; Wassermann, M.; Härle, J.; Romig, T.; Kurzrock, L.; Arnold, J.; Großmann, E.; Mackenstedt, U.; Straubinger, R.K. Helminths in invasive raccoons (Procyon lotor) from Southwest Germany. Pathogens 2023, 12, 919. [Google Scholar] [CrossRef]
- Heddergott, M.; Lippert, S.; Schliephake, A.; Gaede, W.; Schleimer, A.; Frantz, A.C. Spread of the zoonotic nematode Baylisascaris procyonis into a Naive Raccoon Population. EcoHealth 2023, 20, 263–272. [Google Scholar] [CrossRef]
- Kornacka, A.; Cybulska, A.; Bień, J.; Goździk, K.; Moskwa, B. The usefulness of direct agglutination test, enzyme-linked immunosorbent assay and polymerase chain reaction for the detection of Toxoplasma gondii in wild animals. Vet. Parasitol. 2016, 228, 85–89. [Google Scholar] [CrossRef]
- Kornacka, A.; Cybulska, A.; Moskwa, B. Comparison of sensitivity of two primer sets for the detection of Toxoplasma gondii DNA in wildlife. Acta Parasitol. 2018, 63, 634–639. [Google Scholar] [CrossRef]
- Kornacka, A.; Cybulska, A.; Popiołek, M.; Kuśmierek, N.; Moskwa, B. Survey of Toxoplasma gondii and Neospora caninum in raccoons (Procyon lotor) from the Czech Republic, Germany and Poland. Vet. Parasitol. 2018, 262, 47–50. [Google Scholar] [CrossRef]
- Sroka, J.; Karamon, J.; Wójcik-Fatla, A.; Dutkiewicz, J.; Bilska-Zając, E.; Zając, V.; Piotrowska, W.; Cencek, T. Toxoplasma gondii infection in selected species of free-living animals in Poland. Ann. Agric. Environ. Med. 2019, 26, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Heddergott, M.; Müller, F.; Steinbach, P.; Jeschke, D.; Stubbe, M.; Frantz, A.C.; Stubbe, A.; Ansorge, H.; Osten-Sacken, N. First detection and low prevalence of Pearsonema spp. in wild raccoons (Procyon lotor) from Central Europe. Int. J. Parasitol. Parasites Wildl. 2022, 19, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Heddergott, M.; Frantz, A.C.; Stubbe, M.; Stubbe, A.; Ansorge, H.; Osten-Sacken, N. Seroprevalence and risk factors of Toxoplasma gondii infection in invasive raccoons (Procyon lotor) in Central Europe. Parasitol. Res. 2017, 116, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Heddergott, M.; Pohl, D.; Steinbach, P.; Salazar, L.C.; Müller, F.; Frantz, A.C. Determinants and effects of sinus worm Skrjabingylus nasicola (Nematoda: Metastrongyloidae) infestation in invasive American mink Neovison vison in Germany. Parasitol. Res. 2016, 115, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Hamir, A.N.; Shen, S.K.; Thulliez, P.; Rupprecht, C.E. Experimental Toxoplasma gondii infection in raccoons (Procyon lotor). J. Parasitol. 1993, 79, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Heddergott, M.; Müller, F. Hohe Prävalenz von Antikörpern gegen Toxoplasma gondii im Blutserum von Waschbären (Procyon lotor) aus der nordwestlichen hessischen Rhön, Deutschland. Beitr. Jagd Wildforsch. 2020, 45, 125–132. [Google Scholar]
- Brown, L.D.; Cat, T.T.; DasGupta, A. Interval estimation for a binomial proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: New York, NY, USA, 2013. [Google Scholar]
- Bartoń, K.M. Multi-Model Inference (1.46.0). 2022. Available online: https://CRAN.Rproject.org/package=MuMIn (accessed on 8 December 2023).
- Cade, B.S. Model averaging and muddled multimodel inferences. Ecology 2015, 96, 2370–2382. [Google Scholar] [CrossRef] [PubMed]
- Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science. R Package Version 2.8.10. 2021. Available online: https://CRAN.R-project.org/package=sjPlot (accessed on 1 December 2023).
- Burridge, M.J.; Bigler, W.J.; Forrester, D.J.; Hennemann, J.M. Serologic survey for Toxoplasma gondii in wild animals in Florida. J. Am. Vet. Med. Assoc. 1979, 175, 964–967. [Google Scholar]
- Smith, D.D.; Frenkel, J.K. Prevalence of antibodies to Toxoplasma gondii in wild mammals of Missouri and east central Kansas: Biologic and ecologic considerations of transmission. J. Wildl. Dis. 1995, 31, 15–21. [Google Scholar] [CrossRef]
- Mitchell, M.A.; Hungerford, L.L.; Nixon, C.; Esker, T.; Sullivan, J.; Koerkenmeier, R.; Dubey, J.P. Serological survey for selected infectious disease agents in racoons from Illinois. J. Wildl. Dis. 1999, 35, 347–355. [Google Scholar] [CrossRef]
- Hill, R.E., Jr.; Zimmerman, J.J.; Wills, R.W.; Patton, S.; Clark, W.R. Seroprevalence of antibodies against Toxoplasma gondii in free-ranging mammals in Iowa. J. Wildl. Dis. 1998, 34, 811–815. [Google Scholar] [CrossRef][Green Version]
- Hancock, K.; Thiele, L.A.; Zajac, A.M.; Elvinger, F.; Lindsay, D.S. Prevalence of antibodies to Toxoplasma gondii in raccoons (Procyon lotor) from an urban area of Northern Virginia. J. Parasitol. 2005, 91, 694–695. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.S.; Richardson, D.J.; Lindsay, D.S. Prevalence of agglutinating antibodies to Toxoplasma gondii in striped skunks (Mephitis mephitis), opossums (Didelphis virginiana), and raccoons (Procyon lotor) from Connecticut. J. Parasitol. 2006, 92, 664–665. [Google Scholar] [CrossRef]
- Hwang, Y.T.; Pitt, J.A.; Quirk, T.W.; Dubey, J.P. Seroprevalence of Toxoplasma gondii in mesocarnivores of the Canadian prairies. J. Parasitol. 2007, 93, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Gerhold, R.W.; Saraf, P.; Chapman, A.; Zou, X.; Hickling, G.; Stiver, W.H.; Houston, A.; Souza, M.; Su, C. Toxoplasma gondii seroprevalence and genotype diversity in select wildlife species from the southeastern United States. Parasites Vectors 2017, 10, 508. [Google Scholar] [CrossRef]
- Gey, A.B. Synopsis der Parasitenfauna des Waschbären (Procyon lotor) unter Berücksichtigung von Befunden aus Hessen. Ph.D. Thesis, Justus-Liebig Universität, Fachbereich Veterinärmedizin, Gießen, Germany, 1998. [Google Scholar]
- Sato, S.; Kabeya, H.; Makino, T.; Suzuki, K.; Asano, M.; Inoue, S.; Sentsui, H.; Nogami, S.; Maruyama, S. Seroprevalence of Toxoplasma gondii infection in feral raccoons (Procyon lotor) in Japan. J. Parasitol. 2011, 97, 956–957. [Google Scholar] [CrossRef]
- Engel, L.; Hamedy, A.; Kornacka-Stackonis, A.; Langner, T.; Birka, S.; Koethe, M. Toxoplasma gondii in raccoons (Procyon lotor) in Germany: A serosurvey based on meat juice. Parasitol. Res. 2022, 121, 3417–3425. [Google Scholar] [CrossRef]
- Gamble, H.R.; Dubey, J.P.; Lambillotte, D.N. Comparison of a commercial ELISA with the modified agglutination test for detection of Toxoplasma infection in the domestic pig. Vet. Parasitol. 2005, 128, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Verma, S.K.; Kwok, O.C.H.; Pedersen, K.; Rosenthal, B.M.; Su, C. White-tailed deer (Odocoileus virginianus) are a reservoir of a diversity of Toxoplasma gondii strains in the USA and pose a risk to consumers of undercooked venison. Parasitology 2020, 147, 775–781. [Google Scholar] [CrossRef]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Verma, S.K.; Kwok, O.C.H.; Pedersen, K.; Rosenthal, B.M.; Su, C. Genotyping of viable Toxoplasma gondii from the first national survey of feral swine revealed evidence for sylvatic transmission cycle, and presence of highly virulent parasite genotypes. Parasitology 2020, 147, 295–302. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H. Recent epidemiologic and clinical Toxoplasma gondii infections in wild canids and other carnivores: 2009–2020. Vet. Parasitol. 2021, 290, 109337. [Google Scholar] [CrossRef]
- Heddergott, M.; Osten-Sacken, N.; Steinbach, P.; Frantz, A.C. Seroprevalence of Toxoplasma gondii in free-living European mouflon (Ovis orientalis musimon) hunted in central Germany. Parasite 2018, 25, 21. [Google Scholar] [CrossRef]
- Heddergott, M.; Steinbach, P.; Pohl, D.; Frantz, A.C. First report on the sero-epidemiology of Toxoplasma gondii infection in German roe deer (Capreolus capreolus). Parasite 2018, 25, 52. [Google Scholar] [CrossRef]
- Almeria, S.; Cabezon, O.; Paniagua, J.; Cano-Terriza, D.; Jimenez-Ruiz, S.; Arenas-Montes, A.; Dubey, J.P.; Garcia-Bocanegra, I. Toxoplasma gondii in sympatric domestic and wild ungulates in the Mediterranean ecosystem. Parasitol. Res. 2018, 117, 665–671. [Google Scholar] [CrossRef]
- Dubey, J.P.; Sundar, N.; Nolden, C.A.; Samuel, M.D.; Velmurugan, G.V.; Bandini, L.A.; Kwok, O.C.H.; Bodenstein, B.; Su, C. Characterization of Toxoplasma gondii from raccoons (Procyon lotor), coyotes (Canis latrans), and striped skunks (Mephitis mephitis) in Wisconsin identified several atypical genotypes. J. Parasitol. 2007, 93, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Turčeková, L.; Hurníková, Z.; Spišák, F.; Miterpáková, M.; Chovancová, B. Toxoplasma gondii in protected wildlife in the Tatra National Park (TANAP), Slovakia. Ann. Agric. Environ. Med. 2014, 21, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.B.; Cong, W.; Hou, J.; Ma, J.G.; Zhang, X.X.; Zhu, X.Q.; Meng, Q.F.; Zhou, D.H. Seroprevalence and risk factors of Toxoplasma gondii infection in farmed raccoon dogs (Nyctereutes procyonoides) in China. Vector Borne Zoonotic Dis. 2017, 17, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, S.-Y.; Liu, Z.-L.; Zhang, X.-T.; Cui, D.-Y.; Li, J.-H.; Liu, Y.; Zhao, Q.; Ni, H.-B. Seroprevalence and risk factors of Toxoplasma gondii infection in domestic raccoon dogs in four provinces in northern China. Microb. Pathog. 2019, 128, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.-Y.; Chu, D.; Sun, H.-T.; Wang, D.; Xie, L.-H.; Xu, Y.; Li, J.-H.; Cui, D.-Y.; You, F.; Cai, Y.; et al. Prevalence and genotyping of Toxoplasma gondii infection in raccoon dogs (Nyctereutes procyonoides) in Northern China. Vector Borne Zoonotic Dis. 2019, 20, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Fredebaugh, S.L.; Mateus-Pinilla, N.E.; McAllister, M.; Warner, R.E.; Weng, H.-Y. Prevalence of antibody to Toxoplasma gondii in terrestrial wildlife in a natural area. J. Wildl. Dis. 2011, 47, 381–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beral, M.; Rossi, S.; Aubert, D.; Gasqui, P.; Terrier, M.E.; Klein, F.; Villena, I.; Abrial, D.; Gilot-Fromont, E.; Richomme, C.; et al. Environmental factors associated with the seroprevalence of Toxoplasma gondii in wild boars (Sus scrofa), France. EcoHealth 2012, 9, 303–309. [Google Scholar] [CrossRef]
- Barros, M.; Cabezon, O.; Dubey, J.P.; Almeria, S.; Ribas, M.P.; Escobar, L.E.; Ramos, B.; Medina-Vogel, G. Toxoplasma gondii infection in wild mustelids and cats across an urban-rural gradient. PLoS ONE 2018, 13, e0199085. [Google Scholar] [CrossRef]
- Matoba, Y.; Asano, M.; Masubuchi, H.; Asakawa, M. First Records of the Genera Eimeria and Isospora (Protozoa: Eimeriidae) obtained from feral raccoons (Procyon lotor) alien species in Japan and prevalence of serum antibodies to Toxoplasma gondii among the raccoons. Jpn. J. Zoo. Wildl. Med. 2002, 7, 87–90. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, E.; Takada, M.B.; Fujii, K.; Kobayashi, K.; Imai, K.; Kadohira, M. Association between seroprevalence of Toxoplasma gondii in raccoons and environmental factors of their habitats in Tokachi District, Hokkaido, Japan. J. Vet. Epidemol. 2015, 19, 108–113. [Google Scholar] [CrossRef]
- Niedziałkowska, M.; Jędrzejewski, W.; Mysłajek, R.W.; Nowak, S.; Jędrzejewska, B.; Schmidt, K. Environmental correlates of Eurasian lynx occurrence in Poland–Large scale census and GIS mapping. Biol. Conserv. 2006, 133, 63–69. [Google Scholar] [CrossRef]
- Gerngross, P.; Ambarli, H.; Angelici, F.M.; Anile, S.; Campbell, R.; Ferreras de Andres, P.; Gil-Sanchez, J.M.; Götz, M.; Jerosch, S.; Mengüllüoglu, D.; et al. Felis silvestris. The IUCN Red List of Threatened Species. 2022, p. e.T181049859A181050999. Available online: https://www.iucnredlist.org/species/181049859/181050999 (accessed on 1 December 2023).
- Engelmann, A.; Köhnemann, B.; Michler, F.-U.F. Nahrungsökologische Analyse von Exkrementen gefangener Waschbären (Procyon lotor L., 1758) aus dem Müritz-Nationalpark (Mecklenburg-Vorpommern) unter Berücksichtigung individueller Parameter. Beitr. Jagd Wildforsch. 2011, 36, 587–604. [Google Scholar]
- Michler, B.A. Koproskopische Untersuchungen zum Nahrungsspektrum des Waschbären Procyon lotos (Linné, 1758) im Müritz-Nationalpark (Mecklenburg-Vorpommern) Unter Besonderer Berücksichtigung des Artenschutzes und des Endoparasitenbefalls; Wildtierforschung in Mecklenburg-Vorpommern. Band 5; TINUS Press: Schwerin, Germany, 2020. [Google Scholar]
- Sutor, A.; Kauhala, K.; Ansorge, H. Diet of the raccoon dog Nyctereutes procyonoides—A canid with an opportunistic foraging strategy. Acta Theriol. 2010, 55, 165–176. [Google Scholar] [CrossRef]
- Gehrt, S.D.; Fritzell, E.K. Growth rates and intraspecific variation in body weights of raccoons (Procyon lotor) in Southern Texas. Am. Midl. Nat. 1999, 141, 19–27. [Google Scholar] [CrossRef]
- Jones, J.L.; Kruszon-Moran, D.; Wilson, M.; McQuillan, G.; Navin, T.; McAuley, J.B. Toxoplasma gondii infection in the United States: Seroprevalence and risk factors. Am. J. Epidemiol. 2001, 154, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Zimmerman, J.J.; Patton, S.; Beran, G.W.; Hill, H.T. The epidemiology of toxoplasmosis in Iowa swine farms with an emphasis on the roles of free-living mammals. Vet. Parasitol. 1992, 42, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Kauhala, K.; Helle, E.; Taskinen, K. Home range of the raccoon dog (Nyctereutes procyonoides) in southern Finland. J. Zool. 1993, 231, 95–106. [Google Scholar] [CrossRef]

| Procyon lotor | Nyctereutes procyonoides | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | No. Tested | No. Positive | Prevalence in % (95% CI) 1 | No. Tested | No. Positive | Prevalence in % (95% CI) |
| Gender | Male | 100 | 45 | 45.00 (35.63–54.76) | 49 | 14 | 28.57 (17.83–42.55) |
| Female | 97 | 51 | 52.58 (42.74–62.21) | 40 | 11 | 27.50 (16.06–43.03) | |
| Age | Juvenile | 81 | 27 | 33.33 (24.04–44.20) | 50 | 11 | 22.00 (12.67–35.48) |
| Adult | 116 | 69 | 59.48 (50.37–67.96) | 39 | 14 | 35.90 (22.76–51.66) | |
| Collection year | 2019 | 108 | 53 | 49.07 (39.85–58.37) | 38 | 10 | 26.32 (14.91–42.23) |
| 2020 | 89 | 43 | 48.31 (38.23–58.54) | 51 | 15 | 29.41 (18.70–43.12) | |
| Total | 197 | 96 | 48.73 (41.85–55.67) | 89 | 25 | 28.09 (19.81–38.26) | |
| Coefficients | Estimate | s.e. | z-Value | p-Value |
|---|---|---|---|---|
| (a) | ||||
| (Intercept) | −6.8193 | 1.1125 | −6.130 | <0.0001 |
| Weight | 0.0012 | 0.0002 | 6.425 | >0.0001 |
| Sex: Males | −0.8016 | 0.3685 | −2.175 | 0.0296 |
| (b) | ||||
| (Intercept) | −9.4400 | 2.3071 | −4.092 | −4.092 |
| Weight | 0.0016 | 0.0004 | 3.937 | 3.937 |
| Sex: Males | −1.3802 | 0.6614 | −2.087 | −2.087 |
| Age: Juvenile | 2.7118 | 0.9695 | 2.787 | 2.787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osten-Sacken, N.; Pikalo, J.; Steinbach, P.; Heddergott, M. Prevalence of Toxoplasma gondii Antibodies and Risk Factors in Two Sympatric Invasive Carnivores (Procyon lotor and Nyctereutes procyonoides) from Zgorzelec County, Poland. Pathogens 2024, 13, 210. https://doi.org/10.3390/pathogens13030210
Osten-Sacken N, Pikalo J, Steinbach P, Heddergott M. Prevalence of Toxoplasma gondii Antibodies and Risk Factors in Two Sympatric Invasive Carnivores (Procyon lotor and Nyctereutes procyonoides) from Zgorzelec County, Poland. Pathogens. 2024; 13(3):210. https://doi.org/10.3390/pathogens13030210
Chicago/Turabian StyleOsten-Sacken, Natalia, Jutta Pikalo, Peter Steinbach, and Mike Heddergott. 2024. "Prevalence of Toxoplasma gondii Antibodies and Risk Factors in Two Sympatric Invasive Carnivores (Procyon lotor and Nyctereutes procyonoides) from Zgorzelec County, Poland" Pathogens 13, no. 3: 210. https://doi.org/10.3390/pathogens13030210
APA StyleOsten-Sacken, N., Pikalo, J., Steinbach, P., & Heddergott, M. (2024). Prevalence of Toxoplasma gondii Antibodies and Risk Factors in Two Sympatric Invasive Carnivores (Procyon lotor and Nyctereutes procyonoides) from Zgorzelec County, Poland. Pathogens, 13(3), 210. https://doi.org/10.3390/pathogens13030210






