Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance
Abstract
1. Introduction
2. Increasing the Scientific Knowledge Base of IPV
3. Translating New Insights and Evidence into Programmatic Action
4. Expanding the IPV Supply Infrastructure for Global Demand
5. Developing the Next-Generation Polio Vaccine
- Poliovirus Virus-Like particles (PV VLPs)
- 2.
- S19 IPV
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- World Health Assembly. Global Eradication of Poliomyelitis by the Year 2000. Resolutions of the 41st World Health Assembly; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Lee, S.E.; Greene, S.A.; Burns, C.C.; Tallis, G.; Wassilak, S.G.F.; Bolu, O. Progress toward poliomyelitis eradication—Worldwide, January 2021–March 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 517–522. [Google Scholar] [CrossRef]
- Francis, T., Jr.; Korns, R.F.; Voight, R.B.; Boisen, M.; Hemphill, F.M.; Napier, J.A.; Tolchinsky, E. An evaluation of the 1954 poliomyelitis vaccine trials. Am. J. Public Health Nation’s Health 1955, 45 Pt 2, 1–63. [Google Scholar]
- Vidor, E. Poliovirus Vaccine—Inactivated. In Plotkin’s Vaccines, 8th ed.; Plotin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2018; Volume 48, pp. 841–865. [Google Scholar]
- van Wezel, A.L.; van Steenis, G.; van der Marel, P.; Osterhaus, A.D. Inactivated poliovirus vaccine: Current production methods and new developments. Clin. Infect. Dis. 1984, 6 (Suppl. S2), S335–S340. [Google Scholar] [CrossRef] [PubMed]
- Böttiger, M.; Larsson, B. Swedish inactivated polio vaccine: Laboratory standardization and clinical experience over a 30-year period. Biologicals 1992, 20, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.W.; Kew, O.M.; Cochi, S.L.; Aylward, R.B. Poliovirus Vaccine—Live. In Plotkin’s Vaccines, 8th ed.; Plotin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2018; Volume 49, pp. 866–917. [Google Scholar]
- Bahl, S.; Bhatnagar, P.; Sutter, R.W.; Roesel, S.; Zaffran, M. Global polio eradication—Way ahead. Indian J. Pediatr. 2018, 85, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative. 2005 Annual Report; World Health Organization: Geneva, Switzerland, 2006; WHO/Polio/06.02. [Google Scholar]
- Burns, C.C.; Diop, O.M.; Sutter, R.W.; Kew, O.M. Vaccine-derived polioviruses. J. Infect. Dis. 2014, 210 (Suppl. S1), S283–S293. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative. Polio Eradication & Endgame Strategic Plan 2013–2018; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Platt, L.R.; Estívariz, C.F.; Sutter, R.W. Vaccine-associated paralytic poliomyelitis: A review of the epidemiology and estimation of the global burden. J. Infect. Dis. 2014, 210, S380–S389. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative. Polio endgame & legacy—Implementation, best practices, and lessons learned. J. Infect. Dis. 2017, 216 (Suppl. S1), S1–S379. [Google Scholar]
- Thompson, K.M.; Kalkowski, D.A.; Badizagan, K. Looking back at prospective modeling of outbreak response strategies for managing global type 2 oral poliovirus vaccine (OPV2) cessation. Front. Public Health 2023, 11, 1098419. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the Strategic Advisory Group on Immunization October 2020—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2020, 48, 585–608. [Google Scholar]
- Macklin, G.R.; Mach, O. Fractional dose IPV in polio eradication. Lancet Infect. Dis. 2021, 21, 1061–1062. [Google Scholar] [CrossRef]
- Crothers, J.W.; Ross Colgate, E.; Cowan, K.J.; Dickson, D.M.; Walsh, M.; Carmolli, M.; Wright, P.F.; Norton, E.B.; Kirkpatrick, B.D. Intradermal fractional-dose inactivated polio vaccine (fIPV) adjuvanted with double mutant Enterotoxigenic Escherichia coli heat labile toxin (dmLT) is well-tolerated and augments a systemic immune response to all three poliovirus serotypes in a randomized placebo-controlled trial. Vaccine 2022, 40, 2705–2713. [Google Scholar] [CrossRef]
- Erdem, R.; De Coster, I.; Withanage, K.; Mercer, L.D.; Marchant, A.; Taton, M.; Cools, N.; Lion, E.; Cassels, F.; Higgins, D.; et al. Safety, tolerability, and immunogenicity of inactivated poliovirus vaccine with or without E. coli double mutant heat-labile toxin (dmLT) adjuvant in healthy adults; a phase 1 randomized study. Vaccine 2023, 41, 1657–1667. [Google Scholar] [CrossRef]
- Onorato, I.M.; Modlin, J.F.; McBean, A.M.; Thoms, M.L.; Losonsky, G.A.; Bernier, R.H. Mucosal immunity induced by enhance-potency inactivated and oral polio vaccines. J. Infect. Dis. 1991, 163, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ghendon, Y.U.; Sanakoyeva, I.I. Comparison of resistance of the intestinal tract to poliomyelitis vaccine (Sabin strains) in persons after naturally and experimentally acquired immunity. Acta Virol. 1961, 5, 265–273. [Google Scholar]
- Fine, P.E.; Carneiro, I.A. Transmissibility and persistence of oral polio vaccine viruses: Implications for the global poliomyelitis eradication initiative. Am. J. Epidemiol. 1999, 150, 1001–1021. [Google Scholar] [CrossRef] [PubMed]
- Brickley, E.B.; Connor, R.I.; Wieland-Alter, W.F.; Collett, M.S.; Hartford, M.; Van Der Avoort, H.; Boesch, A.W.; Weiner, J.A.; Ackerman, M.E.; McKinlay, M.A.; et al. Intestinal antibody responses to a live oral poliovirus vaccine challenge among adults previously immunized with inactivated polio vaccine in Sweden. BMJ Glob. Health 2019, 4, e001613. [Google Scholar] [CrossRef] [PubMed]
- Cuba IPV Study Collaborative Group. Randomized, placebo-controlled trial of inactivated poliovirus vaccine in Cuba. N. Engl. J. Med. 2007, 356, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Snider, C.J.; Zaman, K.; Wilkinson, A.L.; Binte Aziz, A.; Yunus, M.; Haque, W.; Jones, K.A.V.; Wei, L.; Estivariz, C.F.; Konopka-Anstadt, J.L.; et al. Poliovirus type 1 systemic humoral and intestinal mucosal immunity induced by monovalent oral poliovirus vaccine, fractional inactivated poliovirus vaccine, and bivalent oral poliovirus vaccine: A randomized controlled trial. Vaccine 2023, 41, 6083–6092. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.H.; Dang, D.A.; Nguyen, T.T.; Duong, T.H.; Tran, T.T.; Luong, N.T.; Jeyaseelan, V.; Lopez Cavestany, R.; Hendley, W.S.; Mainou, B.A.; et al. Inactivated poliovirus vaccine closing the type 2 immunity gap in Vietnam. J. Pediatr. Infect. Dis. Soc. 2022, 11, 413–416. [Google Scholar] [CrossRef]
- Marine, W.M.; Chin, T.D.; Gravelle, C.R. Limitation of fecal and pharyngeal poliovirus excretion in Salk-vaccinated children. A family study during a type 1 poliomyelitis epidemic. Am. J. Epidemiol. 1962, 76, 173–195. [Google Scholar] [CrossRef]
- Stickle, G. Observed and expected poliomyelitis in the United States, 1958–1961. Am. J. Public Health Nation’s Health 1964, 54, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Wahjuhono, G.; Revolusiana; Widhiastuti, D.; Sundoro, J.; Mardani, T.; Ratih, W.U.; Sutomo, R.; Safitri, I.; Sampurno, O.D.; Rana, B.; et al. Switch from oral to inactivated poliovirus vaccine in Yogyakarta Province, Indonesia: Summary of coverage, immunity, and environmental surveillance. J. Infect. Dis. 2014, 210 (Suppl. S1), S347–S352. [Google Scholar] [CrossRef]
- Snider, C.J.; Boualam, L.; Tallis, G.; Takashima, Y.; Abeyasinghe, R.; Lo, Y.R.; Grabovac, V.; Avagyan, T.; Aslam, S.K.; Eltayeb, A.O.; et al. Concurrent outbreaks of circulating vaccine-derived poliovirus types 1 and 2 affecting the Republic of the Philippines and Malaysia, 2019–2021. Vaccine 2023, 41 (Suppl. S1), A58–A69. [Google Scholar] [CrossRef]
- John, J.; Giri, S.; Karthikeyan, A.S.; Iturriza-Gomara, M.; Muliyil, J.; Abraham, A.; Grassly, N.C.; Kang, G. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: An open-label, randomised controlled trial. Lancet 2014, 384, 1505–1512. [Google Scholar] [CrossRef]
- Jafari, H.; Deshpande, J.M.; Sutter, R.W.; Bahl, S.; Verma, H.; Ahmad, M.; Kunwar, A.; Vishwakarma, R.; Agarwal, A.; Jain, S.; et al. Polio eradication. Efficacy of inactivated poliovirus vaccine in India. Science 2014, 345, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Hird, T.R.; Grassly, N.C. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012, 8, e1002599. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.P.; Molodecky, N.A.; Pons-Salort, M.; O’Reilly, K.M.; Grassly, N.C. Impact of inactivated poliovirus vaccine on mucosal immunity: Implications for the polio eradication endgame. Expert Rev. Vaccines 2015, 14, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Macklin, G.R.; Grassly, N.C.; Sutter, R.W.; Mach, O.; Bandyopadhyay, A.S.; Edmunds, W.J.; O’Reilly, K.M. Vaccine schedules and the effect on humoral and intestinal immunity against poliovirus: A systematic review and network meta-analysis. Lancet Infect. Dis. 2019, 19, 1121–1128. [Google Scholar] [CrossRef]
- Gamage, D.; Mach, O.; Ginige, S.; Weldon, W.C.; Oberste, M.S.; Jeyaseelan, V.; Sutter, R.W. Poliovirus Type 2 Seroprevalence Following Full- or Fractional-Dose Inactivated Poliovirus Vaccine in the Period After Sabin Type 2 Withdrawal in Sri Lanka. J. Infect. Dis. 2019, 219, 1887–1892. [Google Scholar] [CrossRef]
- Aziz, A.B.; Verma, H.; Jeyaseelan, V.; Yunus, M.; Nowrin, S.; Moore, D.D.; Mainou, B.A.; Mach, O.; Sutter, R.W.; Zaman, K. One Full or Two Fractional Doses of Inactivated Poliovirus Vaccine for Catch-up Vaccination in Older Infants: A Randomized Clinical Trial in Bangladesh. J. Infect. Dis. 2022, 226, 1319–1326. [Google Scholar] [CrossRef]
- Sharma, A.K.; Verma, H.; Estivariz, C.F.; Bajracharaya, L.; Rai, G.; Shah, G.; Sherchand, J.; Jones, K.A.V.; Mainou, B.A.; Chavan, S.; et al. Persistence of immunity following a single dose of inactivated poliovirus vaccine: A phase 4, open label, non-randomised clinical trial. Lancet Microbe 2023, 4, e923–e930. [Google Scholar] [CrossRef]
- Anand, A.; Molodecky, N.A.; Pallansch, M.A.; Sutter, R.W. Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule. Vaccine 2017, 35, 2993–2998. [Google Scholar] [CrossRef]
- Resik, S.; Tejeda, A.; Lago, P.M.; Diaz, M.; Carmenates, A.; Sarmiento, L.; Alemañi, N.; Galindo, B.; Burton, A.; Friede, M.; et al. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J. Infect. Dis. 2010, 201, 1344–1352. [Google Scholar] [CrossRef][Green Version]
- Resik, S.; Tejeda, A.; Sutter, R.W.; Diaz, M.; Sarmiento, L.; Alemañi, N.; Garcia, G.; Fonseca, M.; Hung, L.H.; Kahn, A.L.; et al. Priming after a fractional dose of inactivated poliovirus vaccine. N. Engl. J. Med. 2013, 368, 416–424. [Google Scholar] [CrossRef]
- Mohammed, A.J.; AlAwaidy, S.; Bawikar, S.; Kurup, P.J.; Elamir, E.; Shaban, M.M.; Sharif, S.M.; van der Avoort, H.G.; Pallansch, M.A.; Malankar, P.; et al. Fractional doses of inactivated poliovirus vaccine in Oman. N. Engl. J. Med. 2010, 362, 2351–2359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saleem, A.F.; Mach, O.; Yousafzai, M.T.; Khan, A.; Weldon, W.C.; Oberste, M.S.; Sutter, R.W.; Zaidi, A.K.M. Needle adapters for intradermal administration of fractional dose of inactivated poliovirus vaccine: Evaluation of immunogenicity and programmatic feasibility in Pakistan. Vaccine 2017, 35, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- Biya, O.; Manu, J.I.; Forbi, J.C.; Wa Nganda, G.; Ikwe, H.; Sule, A.; Edukugho, A.; Shehu, A.; Aliyu, N.; Barau, N.D.; et al. Notes from the Field: House-to-House Campaign Administration of Inactivated Poliovirus Vaccine—Sokoto State, Nigeria, November 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1290–1291. [Google Scholar] [CrossRef] [PubMed]
- Bullo, U.F.; Mehraj, J.; Raza, S.M.; Rasool, S.; Ansari, N.N.; Shaikh, A.A.; Phul, Z.A.; Memon, S.A.; Baloch, R.I.; Baloch, Z.A.; et al. An experience of mass administration of fractional dose inactivated polio vaccine through intradermal needle-free injectors in Karachi, Sindh, Pakistan. BMC Public Health 2021, 21, 44. [Google Scholar] [CrossRef]
- Pervaiz, A.; Mbaeyi, C.; Baig, M.A.; Burman, A.; Ahmed, J.A.; Akter, S.; Jatoi, F.A.; Mahamud, A.; Asghar, R.J.; Azam, N.; et al. Fractional-dose inactivated poliovirus vaccine campaign—Sindh Province, Pakistan, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1295–1299. [Google Scholar] [CrossRef]
- Resik, S.; Tejeda, A.; Mach, O.; Fonseca, M.; Diaz, M.; Alemany, N.; Garcia, G.; Hung, L.H.; Martinez, Y.; Sutter, R. Immune responses after fractional doses of inactivated poliovirus vaccine using newly developed intradermal jet injectors: A randomized controlled trial in Cuba. Vaccine 2015, 33, 307–313. [Google Scholar] [CrossRef]
- Cooper, L.V.; Erbeto, T.B.; Danzomo, A.A.; Abdullahi, H.W.; Boateng, K.; Adamu, U.S.; Shuaib, F.; Modjirom, N.; Gray, E.J.; Bandyopadhyay, A.S.; et al. Effectiveness of poliovirus vaccines against circulating vaccine-derived type 2 poliomyelitis in Nigeria between 2017 and 2022: A case control study. Lancet Infect. Dis. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Sáez-Llorens, X.; Clemens, R.; Leroux-Roels, G.; Jimeno, J.; Clemens, S.A.; Weldon, W.C.; Oberste, M.S.; Molina, N.; Bandyopadhyay, A.S. Immunogenicity and safety of a novel monovalent high-dose inactivated poliovirus type 2 vaccine in infants: A comparative, observer-blind, randomised, controlled trial. Lancet Infect. Dis. 2016, 16, 321–330. [Google Scholar] [CrossRef]
- Swartz, T.A.; Handsher, R.; Stoeckel, P.; Drucker, J.; Caudrelier, P.; Van Wezel, A.L.; Cohen, H.; Salk, D.; Salk, J. Immunologic memory induced at birth by immunization with inactivated polio vaccine in a reduced schedule. Eur. J. Epidemiol. 1989, 5, 143–145. [Google Scholar] [CrossRef]
- Samuel, B.U.; Cherian, T.; Sridharan, G.; Mukundan, P.; John, T.J. Immune response to intradermally injected inactivated poliovirus vaccine. Lancet 1991, 338, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, S.; Cherian, T.; Samuel, B.U.; Rajasingh, J.; Raghupathy, P.; John, T.J. Immune response of infants to fractional doses of intradermally administered inactivated poliovirus vaccine. Vaccine 1998, 16, 928–931. [Google Scholar] [CrossRef]
- Cadorna-Carlos, J.; Vidor, E.; Bonnet, M.-C. Randomized controlled study of fractional doses of inactivated poliovirus vaccine administered intradermally with a needle in the Philippines. Int. J. Infect. Dis. 2012, 16, e110–e116. [Google Scholar] [CrossRef]
- Soonawala, D.; Verdijk, P.; Wijmenga-Monsuur, A.J.; Boog, C.J.; Koedam, P.; Visser, L.G.; Rots, N.Y. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine 2013, 31, 3688–3694. [Google Scholar] [CrossRef] [PubMed]
- Bashorun, A.O.; Badjie Hydara, M.; Adigweme, I.; Umesi, A.; Danso, B.; Johnson, N.; Sambou, N.A.; Fofana, S.; Kanu, F.J.; Jeyaseelan, V.; et al. Intradermal administration of fractional doses of the inactivated poliovirus vaccine in a campaign: A pragmatic, open-label, non-inferiority trial in The Gambia. Lancet Glob. Health 2022, 10, e257–e268. [Google Scholar] [CrossRef]
- Trueba, G.; Jeyaseelan, V.; Lopez, L.; Mainou, B.A.; Zhang, Y.; Whittembury, A.; Olmedo Valarezo, A.J.; Baquero, G.; Romero de Aguinaga, R.; Zurita Salinas, L.J.; et al. Achieving high immunogenicity against poliovirus with fractional doses of inactivated poliovirus vaccine in Ecuador-results from a cross-sectional serological survey. Lancet Reg. Health Am. 2022, 11, 100235. [Google Scholar] [CrossRef]
- Ahmad, M.; Verma, H.; Deshpande, J.; Kunwar, A.; Bavdekar, A.; Mahantashetti, N.S.; Krishnamurthy, B.; Jain, M.; Mathew, M.A.; Pawar, S.D.; et al. Immunogenicity of fractional dose inactivated poliovirus vaccine in India. J. Pediatr. Infect. Dis. Soc. 2022, 11, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Snider, C.J.; Zaman, K.; Estivariz, C.F.; Yunus, M.; Weldon, W.C.; Wannemuehler, K.A.; Oberste, M.S.; Pallansch, M.A.; Wassilak, S.G.; Bari, T.I.A.; et al. Immunogenicity of full and fractional dose of inactivated poliovirus vaccine for use in routine immunisation and outbreak response: An open-label, randomised controlled trial. Lancet 2019, 393, 2624–2634. [Google Scholar] [CrossRef]
- Anand, A.; Zaman, K.; Estívariz, C.F.; Yunus, M.; Gary, H.E.; Weldon, W.C.; Bari, T.I.; Steven Oberste, M.; Wassilak, S.G.; Luby, S.P.; et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial. Vaccine 2015, 33, 6816–6822. [Google Scholar] [CrossRef]
- Louis-Auguste, J.; Kelly, P. Tropical enteropathies. Curr. Gastroenterol. Rep. 2017, 19, 29. [Google Scholar] [CrossRef]
- Patriarca, P.A.; Wright, P.F.; John, T.J. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: Review. Clin. Infect. Dis. 1991, 13, 926–939. [Google Scholar] [CrossRef]
- Posey, D.L.; Linkins, R.W.; Oliveria, M.J.; Monteiro, D.; Patriarca, P.A. The effect of diarrhea on oral poliovirus vaccine failure in Brazil. J. Infect. Dis. 1997, 175, S258–S263. [Google Scholar] [CrossRef]
- Hodges, P.; Tembo, M.; Kelly, P. Intestinal Biopsies for the Evaluation of Environmental Enteropathy and Environmental Enteric Dysfunction. J. Infect. Dis. 2021, 224, S856–S863. [Google Scholar] [CrossRef]
- Marie, C.; Ali, A.; Chandwe, K.; Petri, W.A., Jr.; Kelly, P. Pathophysiology of environmental enteric dysfunction and its impact on oral vaccine efficacy. Mucosal Immunol. 2018, 11, 1290–1298. [Google Scholar] [CrossRef]
- Mashunye, T.R.; Ndwandwe, D.E.; Dube, K.R.; Shey, M.; Shelton, M.; Wiysonge, C.S. Fractional dose compared with standard dose inactivated poliovirus vaccine in children: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- de Quadros, C.A.; Hersh, B.S.; Olivé, J.M.; Andrus, J.K.; da Silveira, C.M.; Carrasco, P.A. Eradication of wild poliovirus from the Americas: Acute flaccid paralysis surveillance, 1988–1995. J. Infect. Dis. 1997, 175, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Burr, R.K.; Sutter, R.W.; Murphy, T.V.; Advisory Committee on Immunization Practices. Poliomyelitis prevention in the United States. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2000, 49, 1–22. [Google Scholar] [PubMed]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2012—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2012, 87, 201–216. [Google Scholar]
- Global Vaccine Alliance to Deploy Six-in-One Vaccine to Lower-Income Countries, Establish Innovative Mechanisms to Protect against Future Epidemic Threats. Available online: https://www.gavi.org/news/media-room/global-vaccine-alliance-deploy-six-one-vaccine-lower-income-countries (accessed on 1 January 2024).
- Bahl, S.; Verma, H.; Bhatnagar, P.; Haldar, P.; Satapathy, A.; Kumar, K.N.; Horton, J.; Estivariz, C.F.; Anand, A.; Sutter, R. Fractional-dose inactivated poliovirus vaccine immunization campaign—Telangana State, India, June 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 859–863. [Google Scholar] [CrossRef]
- Daly, C.; Molodecky, N.A.; Sreevatsava, M.; Belayneh, A.D.; Chandio, S.A.; Partridge, J.; Shaikh, A.; Laghari, M.; Agbor, J.; Safdar, R.M.; et al. Needle-free injectors for mass administration of fractional dose inactivated poliovirus vaccine in Karachi, Pakistan: A survey of caregiver and vaccinator acceptability. Vaccine 2020, 38, 1893–1898. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, March 2023: Conclusions and recommendations. Wkly. Epidemiol. Rec. 2023, 98, 239–256. [Google Scholar]
- Estivariz, C.F.; Kovacs, S.D.; Mach, O. Review of use of inactivated poliovirus vaccine in campaigns to control type 2 circulating vaccine derived poliovirus (cVDPV) outbreaks. Vaccine 2023, 41, A113–A121. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.S.; Lopez Cavestany, R.; Blake, I.M.; Macklin, G.; Cooper, L.; Grassly, N.; Nery, A.L.M.D.S.; Mach, O. Use of inactivated poliovirus vaccine for poliovirus outbreak response. Lancet Infect. Dis. 2023. online ahead of print. [Google Scholar] [CrossRef]
- World Health Organization. Introduction of New Vaccines. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/implementation/introduction-of-new-vaccines (accessed on 1 January 2024).
- Okayasu, H.; Sutter, R.W.; Jafari, H.S.; Takane, M.; Aylward, R.B. Affordable inactivated poliovirus vaccine: Strategies and progress. J. Infect. Dis. 2014, 210, S459–S464. [Google Scholar] [CrossRef]
- World Health Organization. WHO Action Plan for Poliovirus Containment, 4th ed.; World Health Organization: Geneva, Switzerland, 2022; Available online: https://polioeradication.org/wp-content/uploads/2022/07/WHO-Global-Action-Plan-for-Poliovirus-Containment-GAPIV.pdf (accessed on 1 January 2024).
- Shimizu, H. Development and introduction of inactivated poliovirus vaccines derived from Sabin strains in Japan. Vaccine 2016, 34, 1975–1985. [Google Scholar] [CrossRef]
- Available online: https://www.thelancet.com/cms/10.1016/j.lanwpc.2021.100133/attachment/8c660f65-c236-4b2e-a8ba-1f6a42983adc/mmc4.pdf (accessed on 1 January 2024).
- World Health Assembly. Resolutions and Decisions. WHA61.1. Poliomyelitis: Mechanism for management of potential risks to eradication. In Proceedings of the Sixty-First World Health Assembly, Geneva, Switzerland, 19–24 May 2008; pp. 1–2. [Google Scholar]
- Sutter, R.W.; Okayasu, H.; Kieny, M.P. Next generation inactivated poliovirus vaccine: The future has arrived. Clin. Infect. Dis. 2017, 64, 1326–1327. [Google Scholar] [CrossRef]
- Okayasu, H.; Sein, C.; Hamidi, A.; Bakker, W.A.; Sutter, R.W. Development of inactivated poliovirus vaccine from Sabin strains: A progress report. Biologicals 2016, 44, 581–587. [Google Scholar] [CrossRef] [PubMed]
- United Nations Children’s Fund. Available online: https://www.unicef.org/supply/media/17981/file/IPV-vaccine-prices-17-07-2023.pdf (accessed on 1 January 2024).
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef]
- Fox, H.; Knowlson, S.; Minor, P.D.; Macadam, A.J. Genetically Thermo-Stabilised, Immunogenic Poliovirus Empty Capsids; a Strategy for Non-replicating Vaccines. PLoS Pathog. 2017, 13, e1006117. [Google Scholar] [CrossRef]
- Marsian, J.; Fox, H.; Bahar, M.W.; Kotecha, A.; Fry, E.E.; Stuart, D.I.; Macadam, A.J.; Rowlands, D.J.; Lomonossoff, G.P. Plant-made polio type 3 stabilized VLPs—A candidate synthetic polio vaccine. Nat. Commun. 2017, 8, 245. [Google Scholar] [CrossRef]
- Bahar, M.W.; Porta, C.; Fox, H.; Macadam, A.J.; Fry, E.E.; Stuart, D.I. Mammalian expression of virus-like particles as a proof of principle for next generation polio vaccines. npj Vaccines 2021, 6, 5. [Google Scholar] [CrossRef]
- Bahar, M.W.; Nasta, V.; Fox, H.; Sherry, L.; Grehan, K.; Porta, C.; Macadam, A.J.; Stonehouse, N.J.; Rowlands, D.J.; Fry, E.E.; et al. A conserved glutathione binding site in poliovirus is a target for antivirals and vaccine stabilisation. Commun. Biol. 2022, 5, 1293. [Google Scholar] [CrossRef]
- Adeyemi, O.O.; Nicol, C.; Stonehouse, N.J.; Rowlands, D.J. Increasing type 1 poliovirus capsid stability by thermal selection. J. Virol. 2017, 91, e01586-16. [Google Scholar] [CrossRef]
- Sherry, L.; Grehan, K.; Snowden, J.S.; Knight, M.L.; Adeyemi, O.O.; Rowlands, D.J.; Stonehouse, N.J. Comparative molecular biology approaches for the production of Poliovirus Virus-Like Particles using Pichia pastoris. mSphere 2020, 5, e00838-19. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Grehan, K.; Swanson, J.J.; Bahar, M.W.; Porta, C.; Fry, E.E.; Stuart, D.I.; Rowlands, D.J.; Stonehouse, N.J. Production and characterisation of stabilised PV-3 Virus-like Particles using Pichia pastoris. Viruses 2022, 14, 2159. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Swanson, J.J.; Grehan, K.; Xu, H.; Uchida, M.; Jones, I.M.; Stonehouse, N.J.; Rowlands, D.J. Protease-independent production of Poliovirus Virus-like Particles in Pichia pastoris: Implications for efficient vaccine development and Insights into capsid assembly. Microbiol. Spectr. 2023, 11, e0430022. [Google Scholar] [CrossRef]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, e00215-18. [Google Scholar] [CrossRef] [PubMed]
- Knowlson, S.; Burlison, J.; Giles, E.; Fox, H.; Macadam, A.J.; Minor, P.D. New strains intended for the production of Inactivated Polio Vaccine at low-containment after eradication. PLoS Pathog. 2015, 11, e1005316. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. CAG-TC3-20180630-EN.pdf. Available online: https://polioeradication.org/ (accessed on 1 January 2024).
- World Health Organization. Polio vaccines: WHO position paper—June 2022. Wkly. Epidemiol. Rec. 2022, 97, 277–300. [Google Scholar]
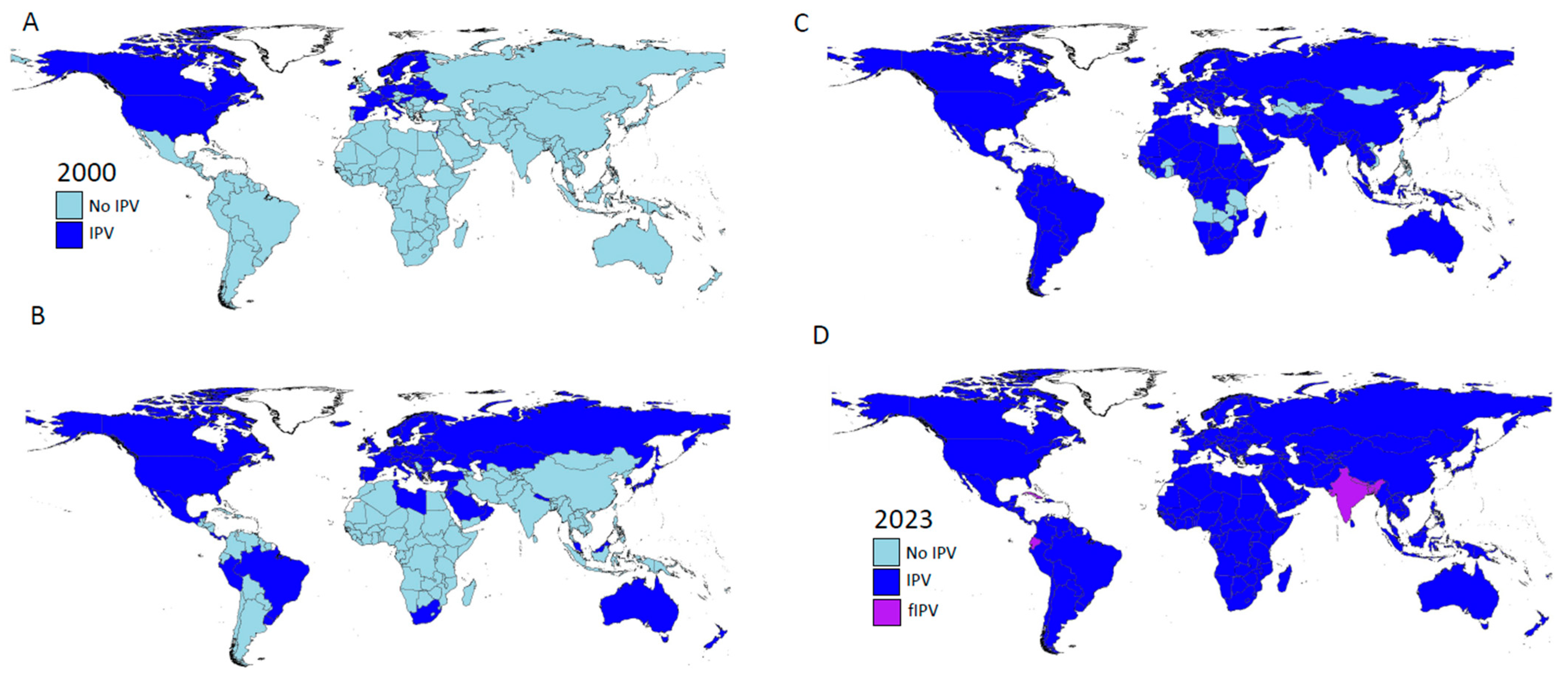

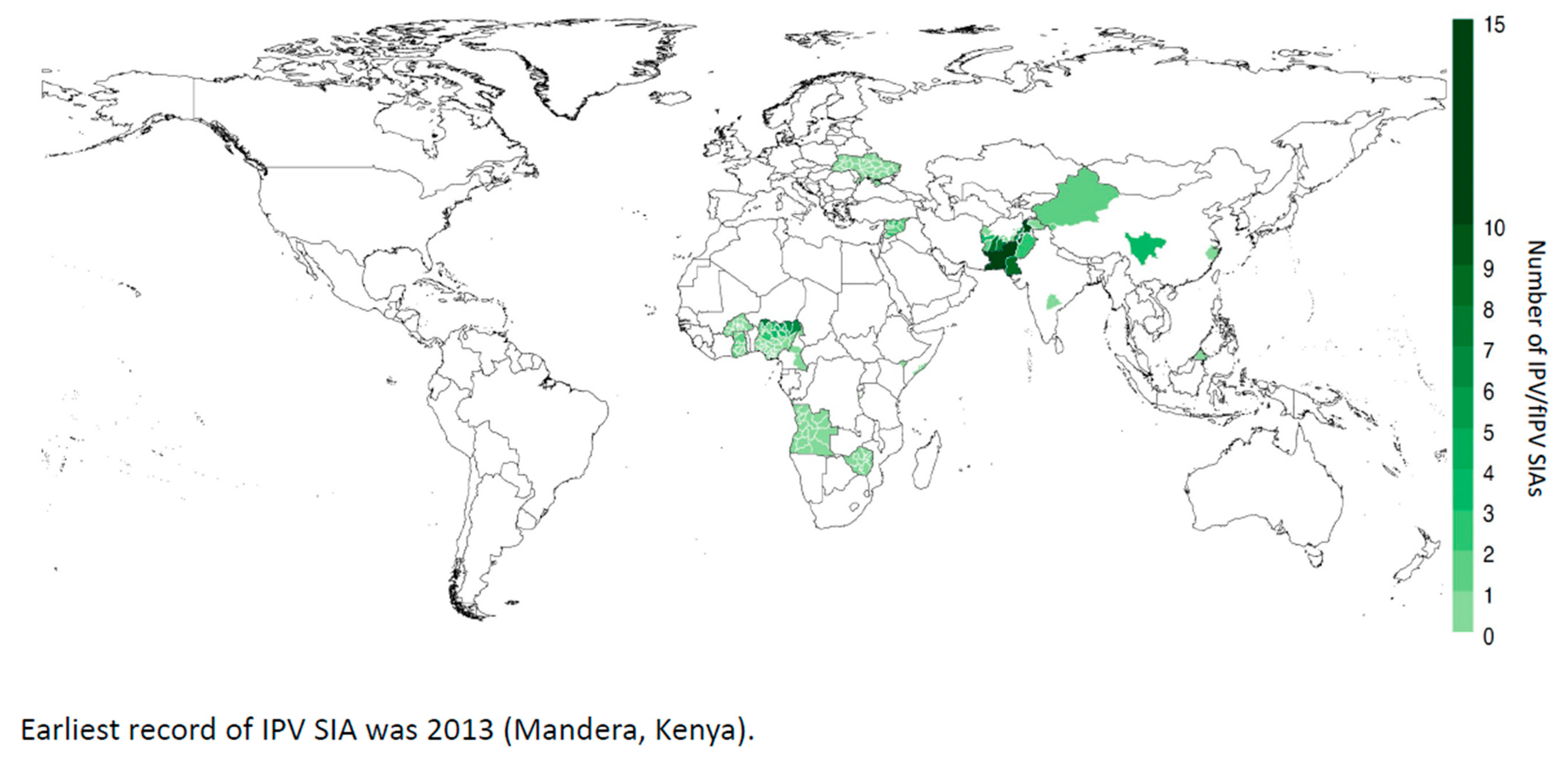
| IPV Effect | Advantages | Geographic Focus | References |
|---|---|---|---|
| Induces humoral immunity | Provides life-long immunity from paralytic poliomyelitis | Globally | Francis [3], Vidor [4] |
| Closes humoral immunity gaps in previously IPV- or OPV-vaccinated individuals | Prevents paralytic disease after poliovirus exposure | Globally | Vidor [4], Dang [25] |
| Boosts humoral antibody levels | Can reduce excretion of poliovirus after infection | Globally | Marine [26], Sutter [7] |
| Generates herd immunity | Less than 100% IPV coverage seem to induce herd immunity | Shown in countries with good hygiene and sanitation–size of effect unknown in high force-of-infection communities, but possibly in Indonesia and Malaysia | Stickle [27], Wahjuhono [28], Snider [29] |
| Induces pharyngeal mucosal immunity | Reduces oral-to-oral transmission of poliovirus | Mode of transmission more important in industrialized countries | Sutter [7], Onorato [19], Fine [21] |
| Induces a “modest” degree of mucosal intestinal immunity | Shorter excretion, decrease viral output in stool, and lower frequency → reduction of 95% in excreted poliovirus virions over course of infection | More important in countries with good hygiene and sanitation | Sutter [7], Ghendon [20], Fine [21], Brickley [22], Cuba IPV Collaborative Study Group [23] |
| Boosts intestinal mucosal immunity in OPV-vaccinated individuals | Reduces fecal-to-oral transmission of poliovirus–from 39 to 76% depending on age | More important in tropical developing countries | John [30], Jafari [31], Snider [24,29], Hird [32], Parker [33], Macklin [34], Gamage [35] |
| Later administration (age ≥3 mos) overcomes interference of maternally derived antibody | A single dose at 9 mos can lead to almost 100% seroconversion | Globally | Aziz [36], Sharma [37], Anand [38] |
| Induces priming immune responses in non-responders | A second dose can effectively boost humoral immunity rapidly <7 days | Globally | Resik [39,40], Mohammad [41] |
| Priming immune response duration | Demonstrated to persist at least 28 mos | Globally | Sharma [37] |
| Immunogenicity not affected by tropical enteropathy | IPV immunogenic in populations affected by tropical enteropathy (in contrast to OPV) | More important in tropical developing countries | Saleem [42] |
| IPV can be administered in house-to-house campaigns | Demonstrated in SIA in Northern Nigeria → may substantially increase coverage | More important in areas with suboptimal health infrastructure | Biya [43] |
| Fractional-dose IPV (fIPV) can reduce cost of polio immunization schedule or use in campaigns | Two doses of fIPV can induce nearly 100% immunity if given in appropriate schedule but only uses 40% of a full dose of IPV | More important for countries not currently supported by GAVI, such as India | Aziz [36], Sharma [37] |
| Administration of fIPV with intradermal jet injector Tropis® | Facilitates intradermal administration and results in dose saving | More important in house-to-house campaigns attempting to reach a high proportion of the target population | Bullo [44], Pervaiz [45], Resik [46] |
| Single-dose IPV vaccine effectiveness close to 90% in Nigeria | Should lead to re-evaluation of strategy to achieve and maintain population immunity in “consequential geographies” | More relevant to tropical areas with suboptimal health infrastructure | Cooper [47] |
| Seed Strains | Manufacturers | WHO Pre-Qualification Date | Presentation |
|---|---|---|---|
| Wild poliovirus | Sanofi S.A., Paris, France | 9DEC2005 19DEC2014 | 10-dose IPV DTaP-HBV-Hib-IPV |
| Bilthoven Biologicals B.V., Bilthoven, The Netherlands (a subsidiary of SII) | 6DEC2010 28NOV2014 | 1-dose IPV 5-dose IPV | |
| AJ Vaccines A/S, København, Denmark | 23DEC2010 21APR2020 | 1-dose IPV 5-dose IPV | |
| Serum Institute of India (SII), Pune, India (IPV from Bilthoven Biologicals B.V.) | 28OCT2016 11JUL2019 | 1 + 2 + 5-dose IPV 10-dose IPV | |
| Sanofi Healthcare India Private Ltd., India (with IPV from Sanofi S.A., France) | 01OCT2018 22APR2022 | 5-dose IPV 10-dose IPV | |
| Sabin strains | Sinovac Biotec Co., Ltd., Beijing, China § | 06JUN2022 | Single dose IPV |
| LG Chem Ltd., Seoul, Republic of Korea § | 01JUN2021 21DEC2020 | Single dose IPV 5-dose IPV | |
| Beijing Institute of Biological Product Co., Ltd., Beijing, China | 15FEB2022 | Single dose IPV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutter, R.W.; Eisenhawer, M.; Molodecky, N.A.; Verma, H.; Okayasu, H. Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance. Pathogens 2024, 13, 224. https://doi.org/10.3390/pathogens13030224
Sutter RW, Eisenhawer M, Molodecky NA, Verma H, Okayasu H. Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance. Pathogens. 2024; 13(3):224. https://doi.org/10.3390/pathogens13030224
Chicago/Turabian StyleSutter, Roland W., Martin Eisenhawer, Natalia A. Molodecky, Harish Verma, and Hiromasa Okayasu. 2024. "Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance" Pathogens 13, no. 3: 224. https://doi.org/10.3390/pathogens13030224
APA StyleSutter, R. W., Eisenhawer, M., Molodecky, N. A., Verma, H., & Okayasu, H. (2024). Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance. Pathogens, 13(3), 224. https://doi.org/10.3390/pathogens13030224




