Changes in Hepatitis E Virus Contamination during the Production of Liver Sausage from Naturally Contaminated Pig Liver and the Potential of Individual Production Parameters to Reduce Hepatitis E Virus Contamination in the Processing Chain
Abstract
1. Introduction
2. Materials and Methods
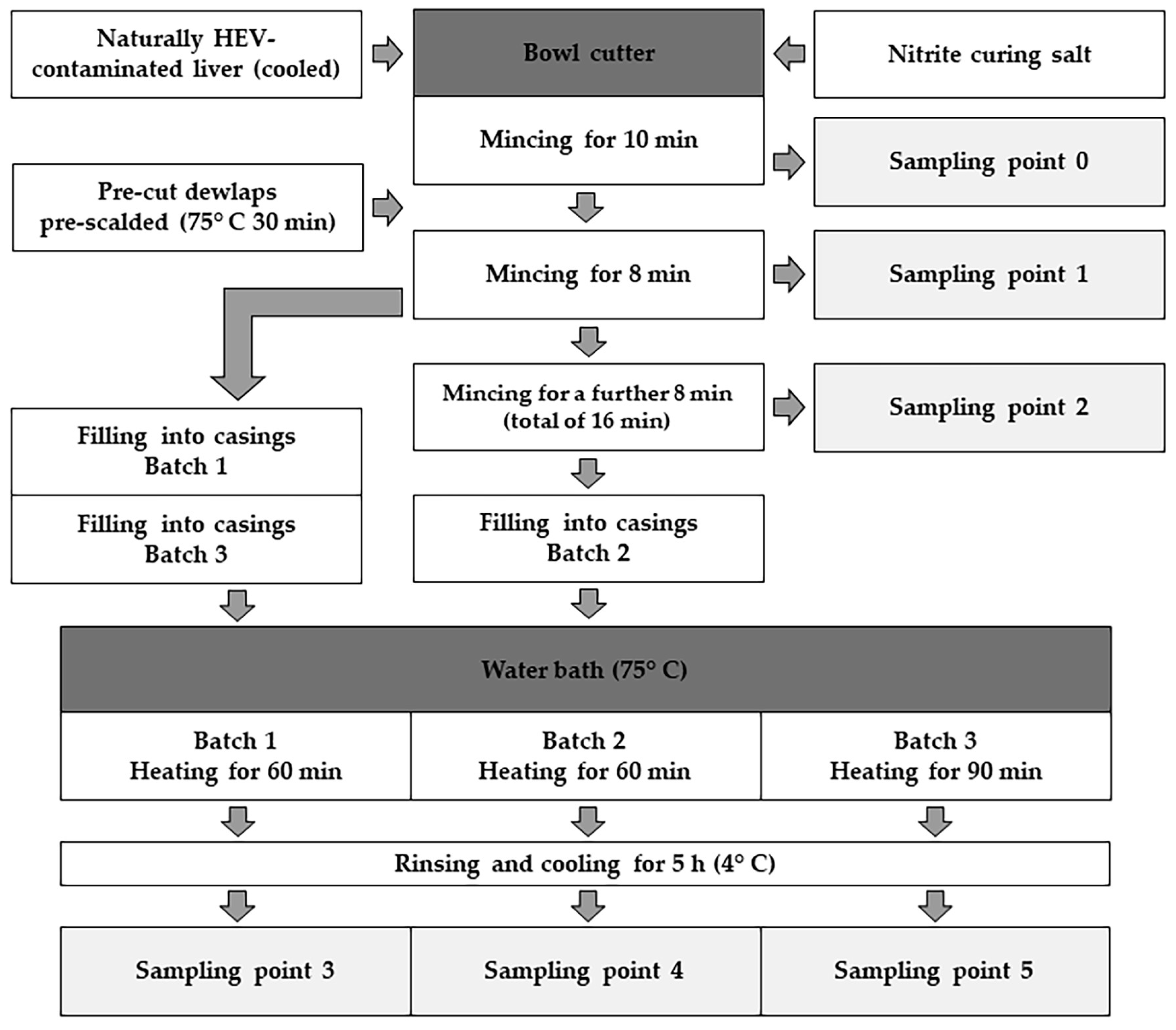
2.1. Production of Liver Sausage Based on an Industrial Recipe
2.2. Method for Extraction of HEV RNA
2.3. Spiking Experiment for the Quantification of HEV RNA in Liver Sausage under Field Conditions
2.4. Investigating the Impact of Modified Production Parameters on Viral Loads
2.5. Sampling Points during the Production Process
3. Results
3.1. Spiking Experiment for the Quantification of HEV RNA in Liver Sausage under Field Conditions
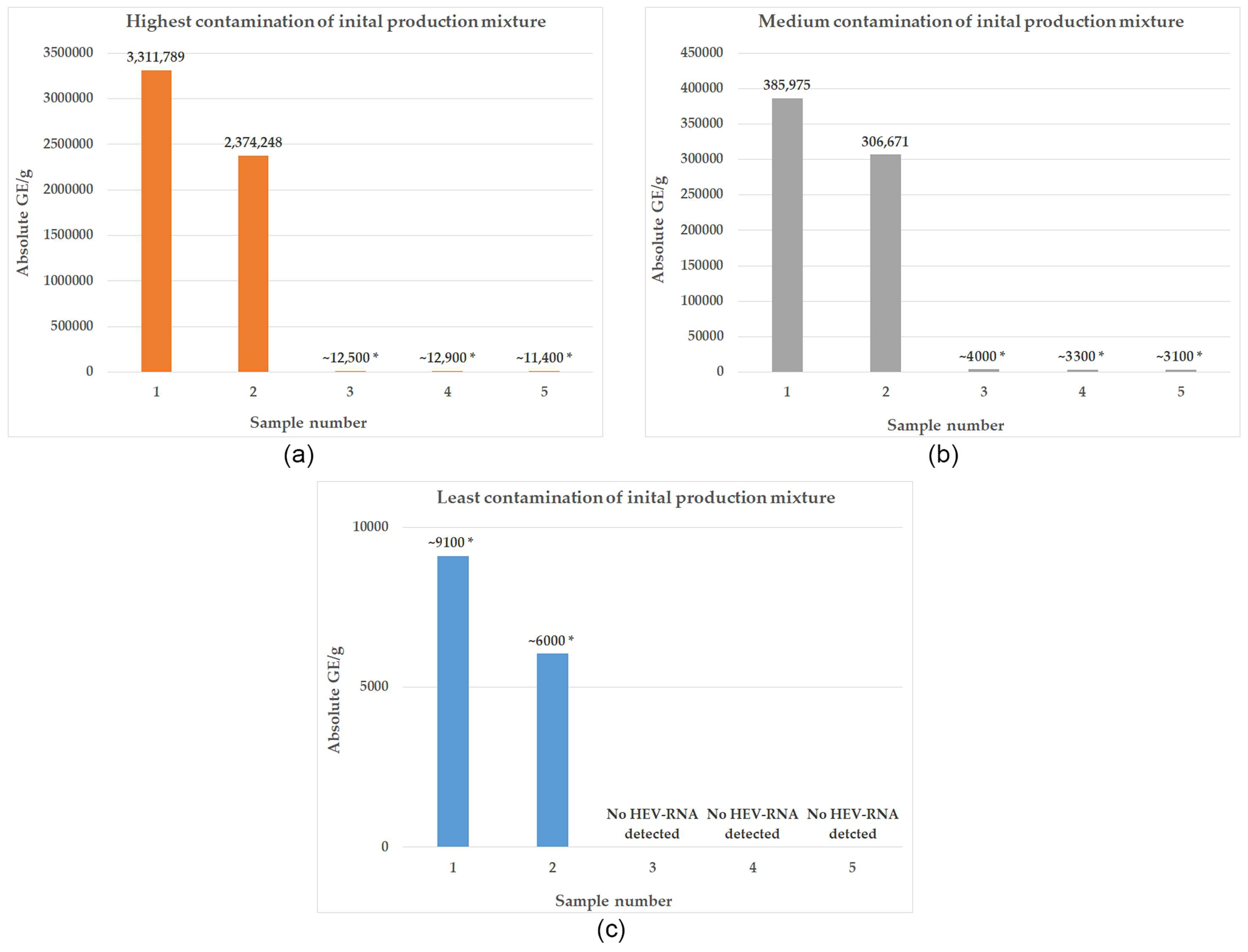
3.2. Development of HEV Contamination within the Production Process of Liver Sausage according to an Industrial Recipe
3.3. Potential of Individual Production Parameters to Reduce Contamination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.-S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis E. Lancet 2012, 379, 2477–2488. [Google Scholar] [CrossRef]
- Teshale, E.H.; Hu, D.J.; Holmberg, S.D. The two faces of hepatitis E virus. Clin. Infect. Dis. 2010, 51, 328–334. [Google Scholar] [CrossRef]
- Lewis, H.C.; Wichmann, O.; Duizer, E. Transmission routes and risk factors for autochthonous hepatitis E virus infection in Europe: A systematic review. Epidemiol. Infect. 2010, 138, 145–166. [Google Scholar] [CrossRef]
- Faber, M.; Willrich, N.; Schemmerer, M.; Rauh, C.; Kuhnert, R.; Stark, K.; Wenzel, J.J. Hepatitis E virus seroprevalence, seroincidence and seroreversion in the German adult population. J. Viral. Hepat. 2018, 25, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, J.M.; Gallian, P.; Dimeglio, C.; Saune, K.; Arnaud, C.; Pelletier, B.; Morel, P.; Legrand, D.; Tiberghien, P.; Izopet, J. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology 2016, 63, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Christou, L.; Kosmidou, M. Hepatitis E virus in the Western world--a pork-related zoonosis. Clin. Microbiol. Infect. 2013, 19, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Mauroy, A.; Pavio, N.; Purdy, M.A.; Rose, N.; Thiry, E.; de Oliveira-Filho, E.F. Hepatitis E Virus and Related Viruses in Animals. Transbound. Emerg. Dis. 2017, 64, 37–52. [Google Scholar] [CrossRef]
- Pavio, N.; Doceul, V.; Bagdassarian, E.; Johne, R. Recent knowledge on hepatitis E virus in Suidae reservoirs and transmission routes to human. Vet. Res. 2017, 48, 78. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.; Lunazzi, A.; Dorenlor, V.; Merbah, T.; Eono, F.; Eloit, M.; Madec, F.; Pavio, N. High prevalence of Hepatitis E virus in French domestic pigs. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 419–427. [Google Scholar] [CrossRef]
- Ponterio, E.; Di Bartolo, I.; Orrù, G.; Liciardi, M.; Ostanello, F.; Ruggeri, F.M. Detection of serum antibodies to hepatitis E virus in domestic pigs in Italy using a recombinant swine HEV capsid protein. BMC Vet. Res. 2014, 10, 133. [Google Scholar] [CrossRef]
- Boxman, I.L.A.; Verhoef, L.; Dop, P.Y.; Vennema, H.; Dirks, R.A.M.; Opsteegh, M. High prevalence of acute hepatitis E virus infection in pigs in Dutch slaughterhouses. Int. J. Food Microbiol. 2022, 379, 109830. [Google Scholar] [CrossRef]
- Baechlein, C.; Seehusen, F.; Nathues, H.; grosse Beilage, E.; Baumgärtner, W.; Grummer, B. Molecular detection of hepatitis E virus in German domestic pigs. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 25–31. [Google Scholar]
- Wenzel, J.J.; Preiss, J.; Schemmerer, M.; Huber, B.; Plentz, A.; Jilg, W. Detection of hepatitis E virus (HEV) from porcine livers in Southeastern Germany and high sequence homology to human HEV isolates. J. Clin. Virol. 2011, 52, 50–54. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Rutjes, S.A.; Reusken, C.B.; Stockhofe-Zurwieden, N.; Frankena, K.; de Jong, M.C.; de Roda Husman, A.M.; Poel, W.H. The course of hepatitis E virus infection in pigs after contact-infection and intravenous inoculation. BMC Vet. Res. 2009, 5, 7. [Google Scholar] [CrossRef]
- Ripellino, P.; Pianezzi, E.; Martinetti, G.; Zehnder, C.; Mathis, B.; Giannini, P.; Forrer, N.; Merlani, G.; Dalton, H.R.; Petrini, O.; et al. Control of Raw Pork Liver Sausage Production Can Reduce the Prevalence of HEV Infection. Pathogens 2021, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Chae, C. Localization of swine hepatitis E virus in liver and extrahepatic tissues from naturally infected pigs by in situ hybridization. J. Hepatol. 2003, 38, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Vergara, A. Hepatitis E Virus in the Food of Animal Origin: A Review. Foodborne Pathog. Dis. 2021, 18, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. Efsa J. 2017, 15, e04886. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; van der Poel, W.H. Survival and Elimination of Hepatitis E Virus: A Review. Food Environ. Virol. 2015, 7, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Günther, T.; Albert, T.; Johne, R. Effect of Sodium Chloride, Sodium Nitrite and Sodium Nitrate on the Infectivity of Hepatitis E Virus. Food Environ. Virol. 2020, 12, 350–354. [Google Scholar] [CrossRef]
- Wolff, A.; Günther, T.; Albert, T.; Schilling-Loeffler, K.; Gadicherla, A.K.; Johne, R. Stability of hepatitis E virus at different pH values. Int. J. Food Microbiol. 2020, 325, 108625. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Günther, T.; Johne, R. Stability of Hepatitis E Virus After Drying on Different Surfaces. Food Environ. Virol. 2022, 14, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Wolff, A.; Gadicherla, A.K.; Filter, M.; Schlüter, O. Stability of hepatitis E virus at high hydrostatic pressure processing. Int. J. Food Microbiol. 2021, 339, 109013. [Google Scholar] [CrossRef] [PubMed]
- Pexara, A.; Govaris, A. Foodborne Viruses an.....nd. Innovative Non-Thermal Food-Processing Technologies. Foods 2020, 9, 1520. [Google Scholar] [CrossRef] [PubMed]
- Schielke, A.; Filter, M.; Appel, B.; Johne, R. Thermal stability of hepatitis E virus assessed by a molecular biological approach. Virol. J. 2011, 8, 487. [Google Scholar] [CrossRef]
- Johne, R.; Trojnar, E.; Filter, M.; Hofmann, J. Thermal Stability of Hepatitis E Virus as Estimated by a Cell Culture Method. Appl. Environ. Microbiol. 2016, 82, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.J. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Int. J. Food Microbiol. 2008, 123, 32–37. [Google Scholar] [CrossRef]
- Hennechart-Collette, C.; Fraisse, A.; Guillier, L.; Perelle, S.; Martin-Latil, S. Evaluation of methods for elution of HEV particles in naturally contaminated sausage, figatellu and pig liver. Food Microbiol. 2019, 84, 103235. [Google Scholar] [CrossRef]
- Hinrichs, J.B.; Kreitlow, A.; Plötz, M.; Schotte, U.; Becher, P.; Gremmel, N.; Stephan, R.; Kemper, N.; Abdulmawjood, A. Development of a Sensitive and Specific Quantitative RT-qPCR Method for the Detection of Hepatitis E Virus Genotype 3 in Porcine Liver and Foodstuff. Foods 2024, 13, 467. [Google Scholar] [CrossRef]
- Koch, H.; Fuchs, M. Die Fabrikation Feiner Fleisch- und Wurstwaren, 24th ed.; Deutscher Fachverlag GmbH: Frankfurt am Main, Germany, 2016; pp. 379–380. [Google Scholar]
- Van der Poel, W.H.M.; Dalton, H.R.; Johne, R.; Pavio, N.; Bouwknegt, M.; Wu, T.; Cook, N.; Meng, X.J. Knowledge gaps and research priorities in the prevention and control of hepatitis E virus infection. Transbound. Emerg. Dis. 2018, 65 (Suppl. 1), 22–29. [Google Scholar] [CrossRef]
- Izopet, J.; Tremeaux, P.; Marion, O.; Migueres, M.; Capelli, N.; Chapuy-Regaud, S.; Mansuy, J.M.; Abravanel, F.; Kamar, N.; Lhomme, S. Hepatitis E virus infections in Europe. J. Clin. Virol. 2019, 120, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.A.; Bouwknegt, M.; van der Giessen, J.W.; de Roda Husman, A.M.; Reusken, C.B. Seroprevalence of hepatitis E virus in pigs from different farming systems in The Netherlands. J. Food Prot. 2014, 77, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Eiden, M.; Vina-Rodriguez, A.; Fast, C.; Dremsek, P.; Lange, E.; Ulrich, R.G.; Groschup, M.H. Natural and experimental hepatitis E virus genotype 3—Infection in European wild boar is transmissible to domestic pigs. Vet. Res. 2014, 45, 121. [Google Scholar] [CrossRef] [PubMed]
- Schotte, U.; Martin, A.; Brogden, S.; Schilling-Loeffler, K.; Schemmerer, M.; Anheyer-Behmenburg, H.E.; Szabo, K.; Müller-Graf, C.; Wenzel, J.J.; Kehrenberg, C.; et al. Phylogeny and spatiotemporal dynamics of hepatitis E virus infections in wild boar and deer from six areas of Germany during 2013-2017. Transbound. Emerg. Dis. 2022, 69, e1992–e2005. [Google Scholar] [CrossRef] [PubMed]
- Bouwknegt, M.; Lodder-Verschoor, F.; van der Poel, W.H.; Rutjes, S.A.; de Roda Husman, A.M. Hepatitis E virus RNA in commercial porcine livers in The Netherlands. J. Food Prot. 2007, 70, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Angeloni, G.; Ponterio, E.; Ostanello, F.; Ruggeri, F.M. Detection of hepatitis E virus in pork liver sausages. Int. J. Food Microbiol. 2015, 193, 29–33. [Google Scholar] [CrossRef]
- Montone, A.M.I.; De Sabato, L.; Suffredini, E.; Alise, M.; Zaccherini, A.; Volzone, P.; Di Maro, O.; Neola, B.; Capuano, F.; Di Bartolo, I. Occurrence of HEV-RNA in Italian Regional Pork and Wild Boar Food Products. Food Environ. Virol. 2019, 11, 420–426. [Google Scholar] [CrossRef]
- Pavio, N.; Merbah, T.; Thébault, A. Frequent hepatitis E virus contamination in food containing raw pork liver, France. Emerg. Infect. Dis. 2014, 20, 1925–1927. [Google Scholar] [CrossRef]
- Szabo, K.; Trojnar, E.; Anheyer-Behmenburg, H.; Binder, A.; Schotte, U.; Ellerbroek, L.; Klein, G.; Johne, R. Detection of hepatitis E virus RNA in raw sausages and liver sausages from retail in Germany using an optimized method. Int. J. Food Microbiol. 2015, 215, 149–156. [Google Scholar] [CrossRef]
- Moor, D.; Liniger, M.; Baumgartner, A.; Felleisen, R. Screening of Ready-to-Eat Meat Products for Hepatitis E Virus in Switzerland. Food Environ. Virol. 2018, 10, 263–271. [Google Scholar] [CrossRef]
- Boxman, I.L.A.; Jansen, C.C.C.; Hägele, G.; Zwartkruis-Nahuis, A.; Tijsma, A.S.L.; Vennema, H. Monitoring of pork liver and meat products on the Dutch market for the presence of HEV RNA. Int. J. Food Microbiol. 2019, 296, 58–64. [Google Scholar] [CrossRef]
- Barnaud, E.; Rogée, S.; Garry, P.; Rose, N.; Pavio, N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl. Environ. Microbiol. 2012, 78, 5153–5159. [Google Scholar] [CrossRef]
- Imagawa, T.; Sugiyama, R.; Shiota, T.; Li, T.C.; Yoshizaki, S.; Wakita, T.; Ishii, K. Evaluation of Heating Conditions for Inactivation of Hepatitis E Virus Genotypes 3 and 4. J. Food Prot. 2018, 81, 947–952. [Google Scholar] [CrossRef]
- Treagus, S.; Wright, C.; Baker-Austin, C.; Longdon, B.; Lowther, J. The Foodborne Transmission of Hepatitis E Virus to Humans. Food Environ. Virol. 2021, 13, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Rossen, L.; Nørskov, P.; Holmstrøm, K.; Rasmussen, O.F. Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int. J. Food Microbiol. 1992, 17, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; D’Agostino, M.; Johne, R. Potential Approaches to Assess the Infectivity of Hepatitis E Virus in Pork Products: A Review. Food Environ. Virol. 2017, 9, 243–255. [Google Scholar] [CrossRef]
- Cook, N.; D’Agostino, M.; Wood, A.; Scobie, L. Real-Time PCR-Based Methods for Detection of Hepatitis E Virus in Pork Products: A Critical Review. Microorganisms 2022, 10, 428. [Google Scholar] [CrossRef]
- Meester, M.; Tobias, T.J.; van den Broek, J.; Meulenbroek, C.B.; Bouwknegt, M.; van der Poel, W.H.M.; Stegeman, A. Farm biosecurity measures to prevent hepatitis E virus infection in finishing pigs on endemically infected pig farms. One Health 2023, 16, 100570. [Google Scholar] [CrossRef]
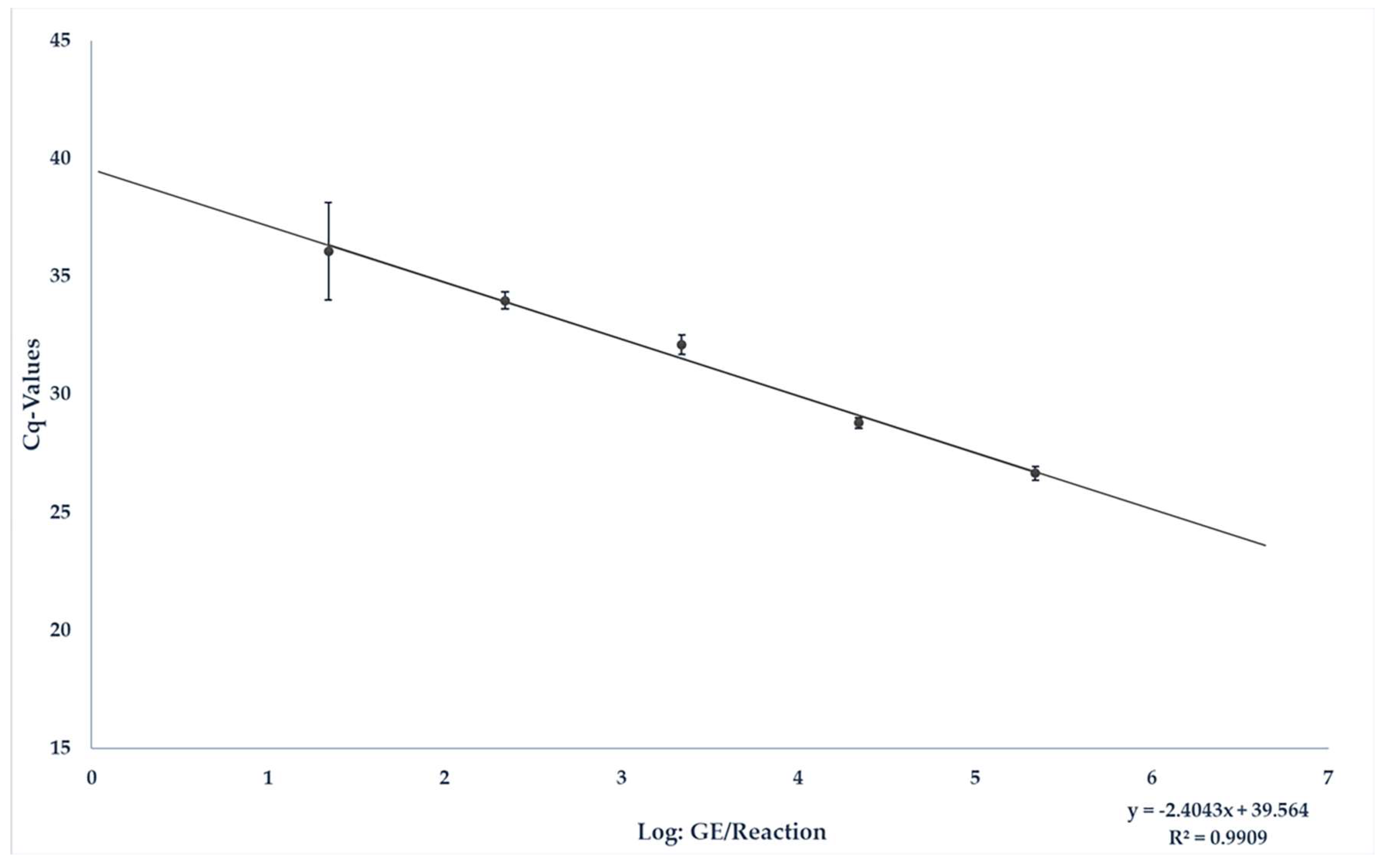
| GE/25 mg Sample | Positive/Tested | Average Cq-Value | Average Deviation |
|---|---|---|---|
| 1 × 107 | 9/9 | 25.86 | 1.29 |
| 1 × 106 | 9/9 | 27.62 | 1.86 |
| 1 × 105 | 9/9 | 31.61 | 0.9 |
| 1 × 104 | 7/9 | 33.93 | 0.8 |
| 1 × 103 | 7/9 | 36.1 | 4.47 |
| 1 × 102 | 2/9 | 36.88 | 2.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinrichs, J.B.; Kreitlow, A.; Siekmann, L.; Plötz, M.; Kemper, N.; Abdulmawjood, A. Changes in Hepatitis E Virus Contamination during the Production of Liver Sausage from Naturally Contaminated Pig Liver and the Potential of Individual Production Parameters to Reduce Hepatitis E Virus Contamination in the Processing Chain. Pathogens 2024, 13, 274. https://doi.org/10.3390/pathogens13040274
Hinrichs JB, Kreitlow A, Siekmann L, Plötz M, Kemper N, Abdulmawjood A. Changes in Hepatitis E Virus Contamination during the Production of Liver Sausage from Naturally Contaminated Pig Liver and the Potential of Individual Production Parameters to Reduce Hepatitis E Virus Contamination in the Processing Chain. Pathogens. 2024; 13(4):274. https://doi.org/10.3390/pathogens13040274
Chicago/Turabian StyleHinrichs, Jan Bernd, Antonia Kreitlow, Lisa Siekmann, Madeleine Plötz, Nicole Kemper, and Amir Abdulmawjood. 2024. "Changes in Hepatitis E Virus Contamination during the Production of Liver Sausage from Naturally Contaminated Pig Liver and the Potential of Individual Production Parameters to Reduce Hepatitis E Virus Contamination in the Processing Chain" Pathogens 13, no. 4: 274. https://doi.org/10.3390/pathogens13040274
APA StyleHinrichs, J. B., Kreitlow, A., Siekmann, L., Plötz, M., Kemper, N., & Abdulmawjood, A. (2024). Changes in Hepatitis E Virus Contamination during the Production of Liver Sausage from Naturally Contaminated Pig Liver and the Potential of Individual Production Parameters to Reduce Hepatitis E Virus Contamination in the Processing Chain. Pathogens, 13(4), 274. https://doi.org/10.3390/pathogens13040274






