Identification and Characterization of Onchocerca volvulus Heat Shock Protein 70 (OvHSP70) as Novel Diagnostic Marker of Onchocerciasis in Human Urine
Abstract
1. Introduction
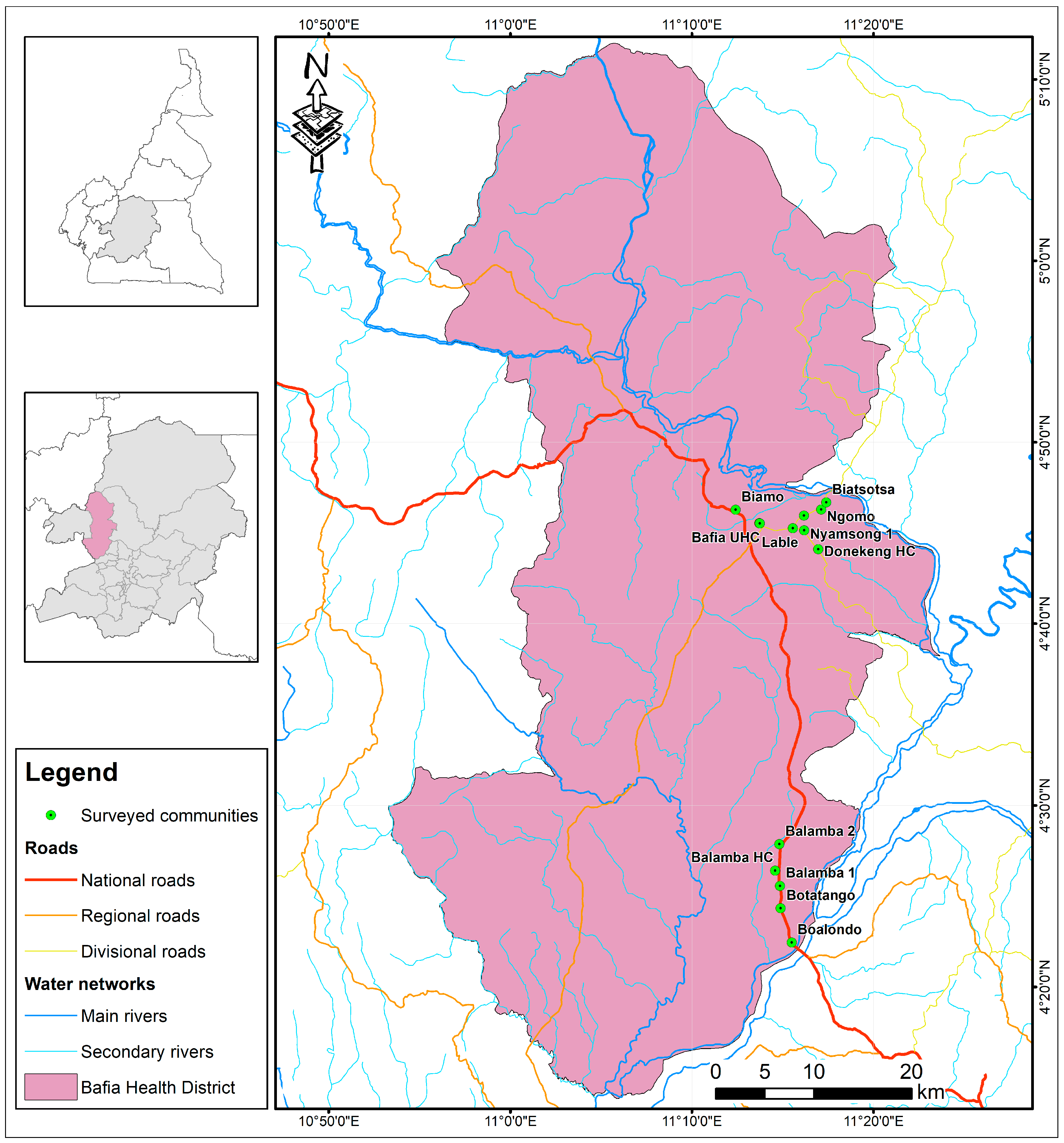
2. Materials and Methods
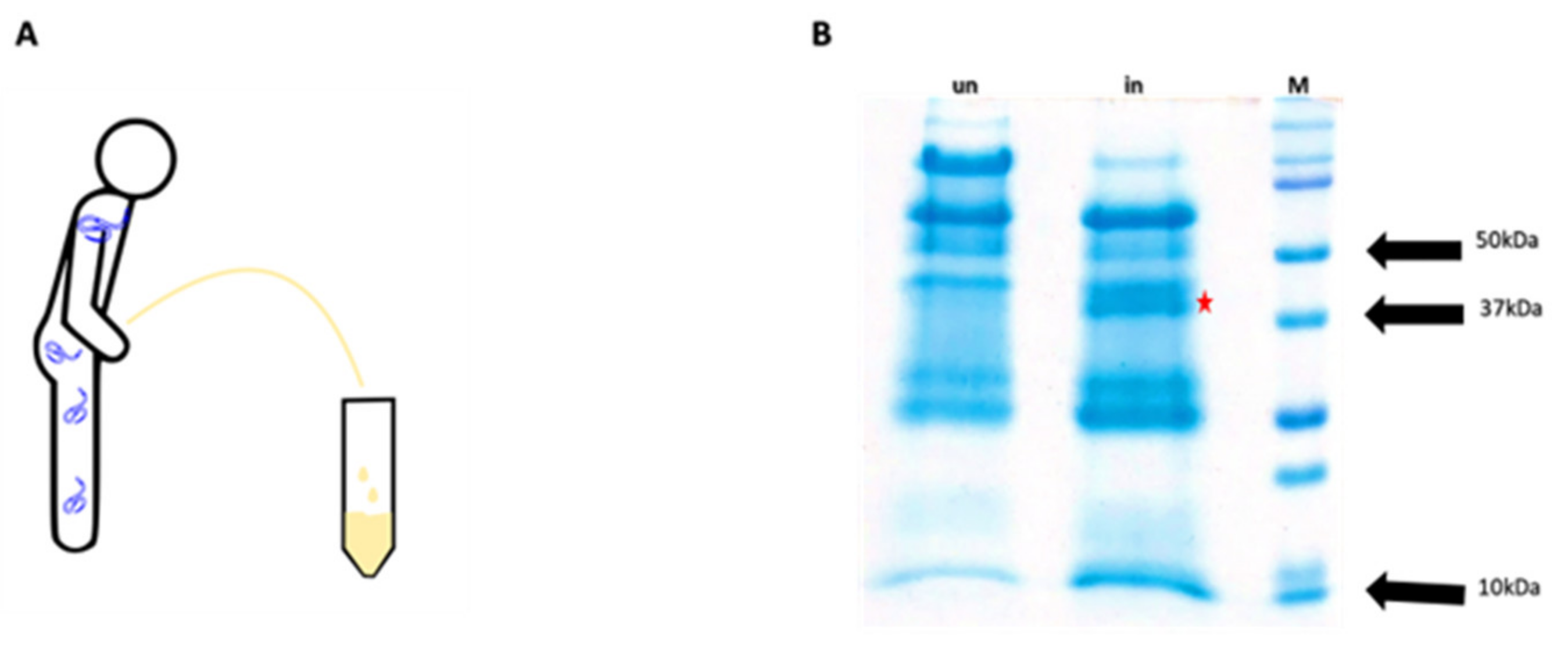
2.1. Extraction and Identification of OvHSP70 from Patient Urine
2.1.1. Urine Collection and Protein Precipitation
2.1.2. Acetone Precipitation of Urine Proteins
2.1.3. SDS PAGE
2.1.4. In-Gel Protein Digestion
2.1.5. LC/MS
2.1.6. Bioinformatics Analysis
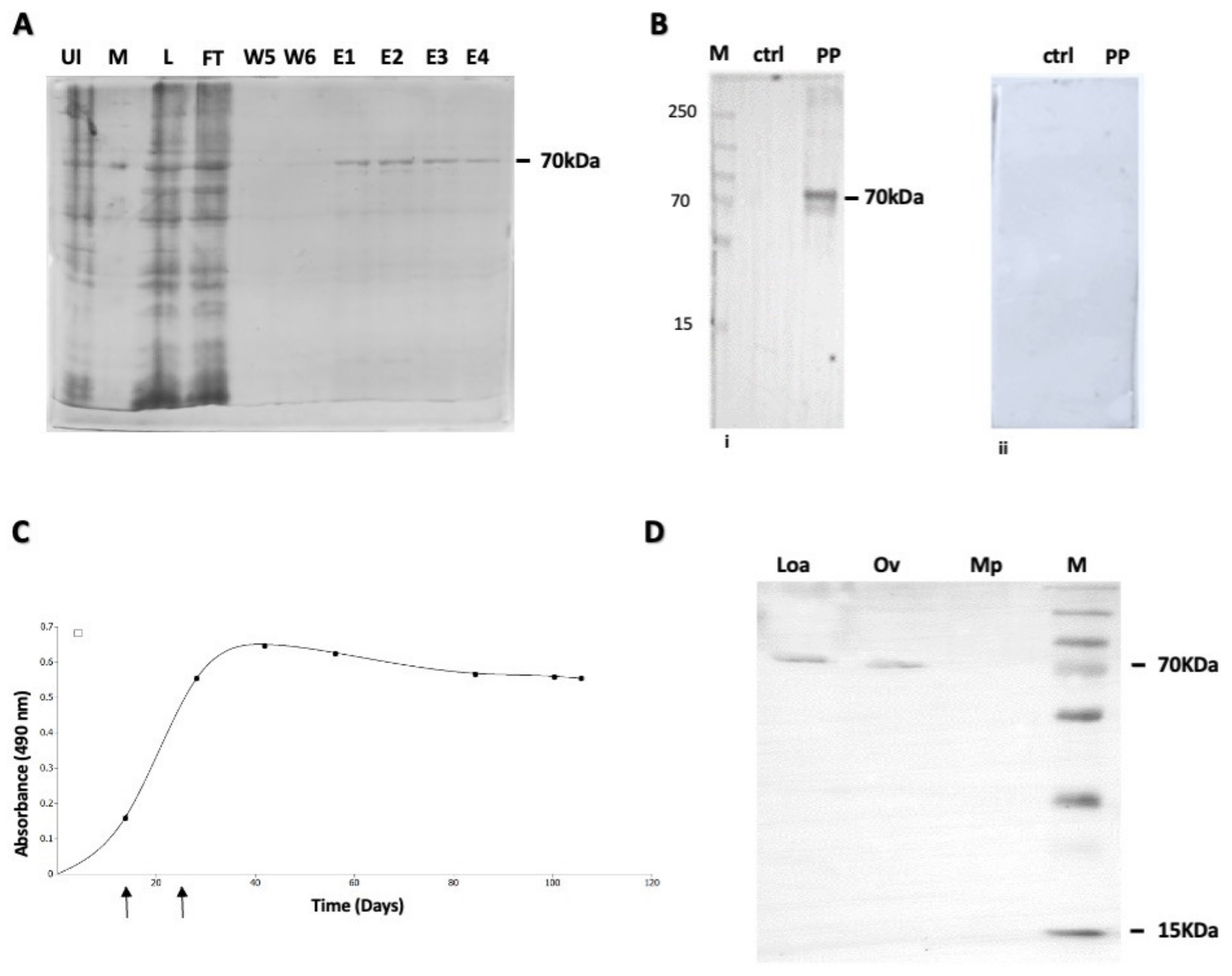
2.2. Cloning, Expression and Purification of Recombinant OvHSP70 Antigen
2.3. Rabbit Immunization and Production of Polyclonal Anti-OvHSP70 Antibodies
2.4. Western Blotting (WB)
2.5. Indirect OvHsp70 ELISA
2.6. Filter Retardation Assays (FRAs)
2.7. Statistical Analyses
3. Results
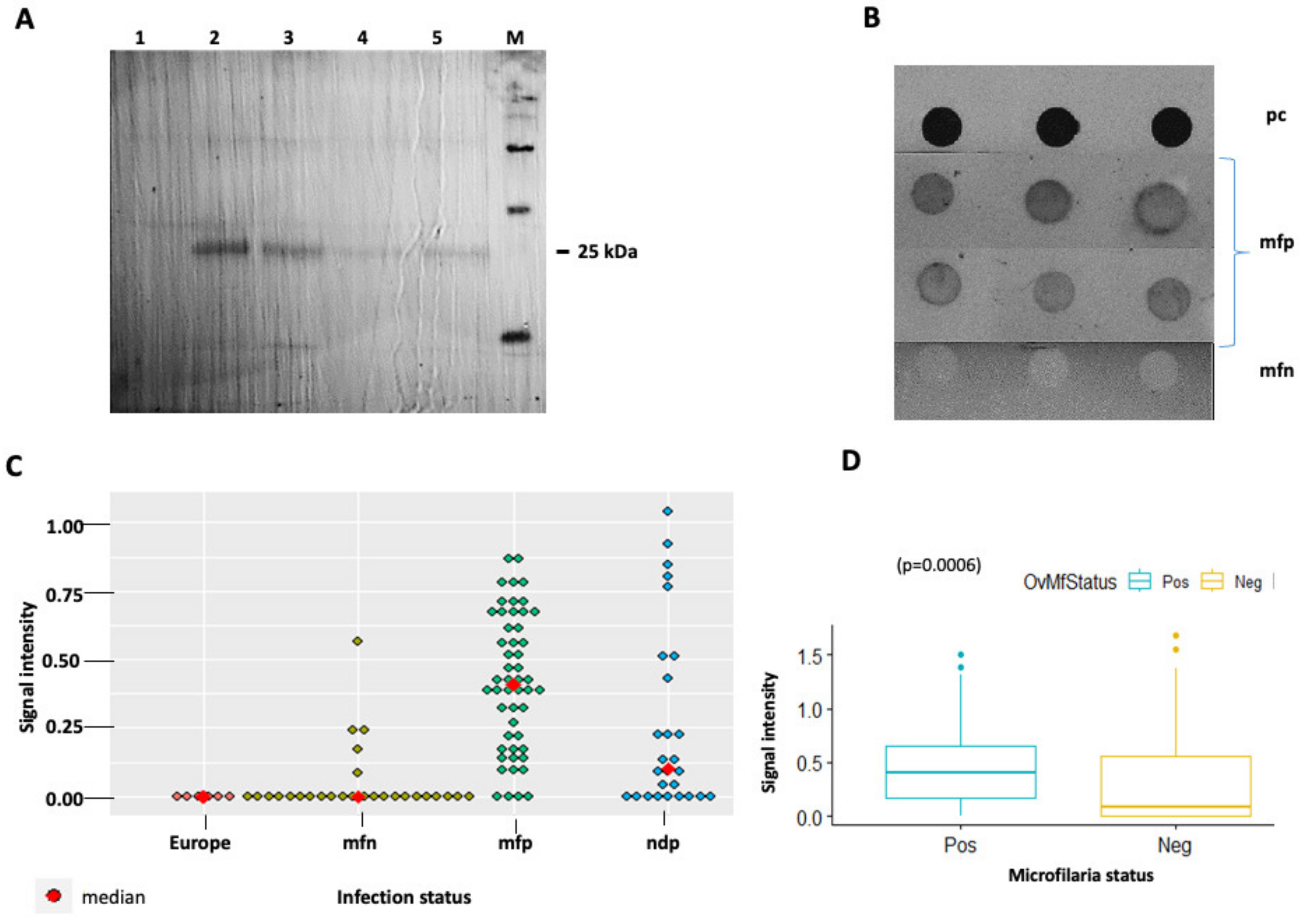
3.1. Identification of OvHSP70 in Urine of Infected Patients
3.2. Expression and Purification of OvHSP70; Generation of Polyclonal Anti-OvHSP70 Antibodies
3.3. Evaluation of Anti-OvHSP70 Specificity and Sensitivity Using Field Samples
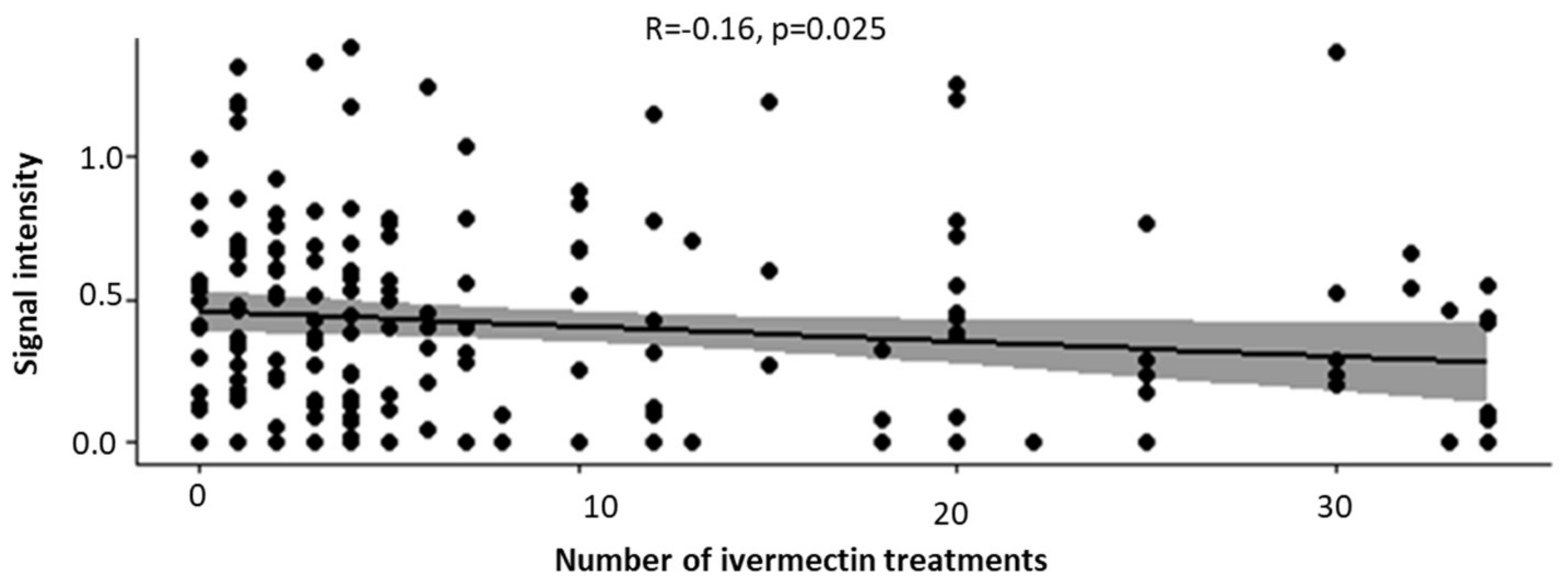
3.4. Increased Number of Ivermectin Treatments Reduces the Intensity of OvHSP70 Signals in Urine of Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Onchocerciasis Key Facts; World Health Organization: Geneva, Switzerland, 2019; pp. 1–5. [Google Scholar]
- CDC. Parasites—Onchocerciasis (Also Known as River Blindness) Epidemiology & Risk Factors; CDC: Atlanta, GA, USA, 2019; pp. 1–2.
- WHO. Onchocerciasis; WHO: Geneva, Switzerland, 2022; pp. 1–7. [Google Scholar]
- WHO. Elimination of human onchocerciasis: Progress report, 2018–2019—Élimination de l’onchocercose humaine: Rapport de situation, 2018–2019. Wkly. Epidemiol. Rec. = Relev. Épidémiol. Hebd. 2021, 94, 557–567. [Google Scholar]
- WHO. Weekly Epidemiological Record Relevé Épidémiologique Hebdomadaire; WHO: Geneva, Switzerland, 2022; Volume 2020, pp. 591–598. [Google Scholar]
- Yaméogo, L.; Levêque, C.; Hougard, J.-M. IRD Éditions Twenty-two Years. In Twenty-Two Years of Blackfly Control Control Pro-Gramme Onchocerciasis in the in West Africa; OpenEdition Books; pp. 101–107. Available online: https://books.openedition.org/irdeditions/28626?lang=en (accessed on 30 November 2023).
- Rebollo, M.P.; Zoure, H.; Ogoussan, K.; Sodahlon, Y.; Ottesen, E.A.; Cantey, P.T. Onchocerciasis: Shifting the target from con-trol to elimination requires a new first-step—Elimination mapping. Int. Health 2018, 10, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lobos, E.; Weiss, N.; Karam, M.; Taylort, H.R.; Otresen, E.A.; Nutman, T.B. Specific and early marker of infection. Science 1991, 16, 12–15. [Google Scholar]
- Golden, A.; Faulx, D.; Kalnoky, M.; Stevens, E.; Yokobe, L.; Peck, R.; Karabou, P.; Banla, M.; Rao, R.; Adade, K.; et al. Analysis of age-dependent trends in Ov16 IgG4 seroprevalence to onchocerciasis. Parasites Vectors 2016, 9, 338. [Google Scholar] [CrossRef]
- Richards, F.; Rizzo, N.; Espinoza, C.E.D.; Monroy, Z.M.; Valdez, C.G.C.; De Cabrera, R.M.; De Leon, O.; Zea-Flores, G.; Sau-erbrey, M.; Morales, A.L.; et al. One hundred years after its discovery in Guatemala by Rodolfo robles, Onchocerca volvu-lus transmission has been eliminated from the central endemic zone. Am. J. Trop. Med. Hyg. 2015, 93, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Golden, A.; Steel, C.; Yokobe, L.; Jackson, E.; Barney, R.; Kubofcik, J.; Peck, R.; Unnasch, T.R.; Nutman, T.B.; de los Santos, T.; et al. Extended Result Reading Window in Lateral Flow Tests Detecting Exposure to Onchocerca volvulus: A New Technology to Improve Epidemiological Surveillance Tools. PLoS ONE 2013, 8, e69231. [Google Scholar] [CrossRef]
- Vincent, J.A.; Lustigman, S.; Zhang, S.; Weil, G.J. A comparison of newer tests for the diagnosis of onchocerciasis. Ann. Trop. Med. Parasitol. 2000, 94, 253–258. [Google Scholar] [CrossRef]
- Esum, M.; Wanji, S.; Tendongfor, N.; Enyong, P. Co-endemicity of loiasis and onchocerciasis in the South West Province of Cameroon: Implications for mass treatment with ivermectin. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 673–676. [Google Scholar] [CrossRef]
- Wanji, S.; Chounna Ndongmo, W.P.; Fombad, F.F.; Kengne-Ouafo, J.A.; Njouendou, A.J.; Longang Tchounkeu, Y.F.; Koudou, B.; Bockarie, M.; Fobi, G.; Roungou, J.B.; et al. Impact of repeated annual community directed treatment with ivermectin on loiasis parasitological indicators in Cameroon: Implications for onchocerciasis and lymphatic filariasis elimination in areas co-endemic with Loa loa in Africa. PLoS Negl. Trop. Dis. 2018, 12, e0006750. [Google Scholar] [CrossRef]
- Osei-atweneboana, M.Y.; Awadzi, K.; Attah, S.K.; Boakye, D.A.; John, O.; Prichard, R.K. Phenotypic Evidence of Emerging Ivermectin Resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011, 5, e998. [Google Scholar] [CrossRef]
- Boatin, B.A.; Toé, L.; Alley, E.S.; Nagelkerke, N.J.D.; Borsboom, G.; Habbema, J.D.F. Detection of Onchocerca volvulus infection in low prevalence areas: A comparison of three diagnostic methods. Parasitology 2002, 125, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Ozoh, G.; Boussinesq, M.; Bissek, A.-C.; Kobangue, L.; Kombila, M.; Mbina, J.-R.; Enyong, P.; Noma, M.; Sékétéli, A.; Fobi, G. Evaluation of the diethylcarbamazine patch to evaluate onchocerciasis endemicity in Central Africa. Trop. Med. Int. Health 2007, 12, 123–129. [Google Scholar] [CrossRef]
- Zimmerman, A.P.; Ronald, H.G.; Edmundo, A.; Lynne, E.; Parag, P.; Joseph, K. Polymerase Chain Reaction-Based Diagnosis of Onchocerca volvulus Infection: Improved Detection of Patients with Onchocerciasis. J. Infect. Dis. 1994, 1994, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Ayong, L.S.; Tume, C.B.; Wembe, F.E.; Simo, G.; Asonganyi, T.; Lando, G.; Ngu, J.L. Development and evaluation of an anti-gen detection dipstick assay for the diagnosis of human onchocerciasis. Trop. Med. Int. Health 2005, 10, 228–233. [Google Scholar] [CrossRef]
- Bennuru, S.; Oduro-Boateng, G.; Osigwe, C.; Valle, P.D.; Golden, A.; Ogawa, G.M.; Cama, V.; Lustigman, S.; Nutman, T.B. In-tegrating multiple biomarkers to increase sensitivity for the detection of Oonchocerca volvulus infection. J. Infect. Dis. 2020, 221, 1805–1815. [Google Scholar] [CrossRef]
- Nana-Djeunga, H.C.; Bourguinat, C.; Pion, S.D.; Bopda, J.; Kengne-Ouafo, J.A.; Njiokou, F.; Prichard, R.K.; Wanji, S.; Kamgno, J.; Boussinesq, M. Reproductive Status of Onchocerca volvulus after Ivermectin Treatment in an Ivermectin-Naïve and a Fre-quently Treated Population from Cameroon. PLoS Negl. Trop. Dis. 2014, 8, e2824. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Namale Ssonko, V.; Oyet, W.; Lakwo, T.; Idro, R. Neurological manifestations in Onchocerca volvulus infec-tion: A review. Brain Res. Bull. 2019, 145, 39–44. [Google Scholar] [CrossRef]
- Mackenzie, C.D.; Behan-Braman, A.; Hauptman, J.; Geary, T. Assessing the Viability and Degeneration of the Medically Im-portant Filarial Nematodes. In Nematology—Concepts, Diagnosis and Control; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Desquesnes, M.; Sazmand, A.; Gonzatti, M.; Boulangé, A.; Bossard, G.; Thévenon, S.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S.; et al. Diagnosis of animal trypanosomoses: Proper use of current tools and future prospects. Parasites Vectors 2022, 15, 235. [Google Scholar] [CrossRef]
- Rosa, B.A.; Curtis, K.; Gilmore, P.E.; Martin, J.; Zhang, Q.; Sprung, R.; Weil, G.J.; Townsend, R.R.; Fischer, P.U.; Mitreva, M.; et al. Direct Proteomic Detection and Prioritization of 19 Onchocerciasis Biomarker Candidates in Humans Authors Direct Proteomic Detection and Prioritization of 19 Onchocerciasis Biomarker Candidates in Humans. Mol. Cell. Proteom. 2023, 22, 100454. [Google Scholar] [CrossRef]
- Selkirk, M.E.; Denham, D.A.; Partono, F.; Sutanto, I.; Maizels, R.M. Molecular Characterization of Antigens of Lymphatic Filarial Parasites. Parasitology 1986, 92, S15–S38. [Google Scholar] [CrossRef]
- Selkirk, M.E.; Denham, D.A.; Partono, F.; Maizels, R.M. Heat shock cognate 70 is a prominent immunogen in Brugian filariasis. J. Immunol. 1989, 143, 299–308. [Google Scholar] [CrossRef]
- Rothstein, N.; Rajan, T.V. Characterization of an hsp70 gene from the human filarial parasite, Brugia malayi (Nematoda). Mol. Biochem. Parasitol. 1991, 49, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, N.M.; Higashi, G.; Yates, J.; Rajan, T.V. Onchocerca volvulus heat shock protein 70 is a major immunogen in amicrofilaremic individuals from a filariasis-endemic area. Mol. Biochem. Parasitol. 1989, 33, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.W.; Braun, G.; Engelbrecht, F.; Salinas, G.; Sinha, K. Use of the Litomosoides sigmodontis-Mouse model in development of an Onchocerca vaccine. I-Molecular of O. volvulus antigens. Parasite 1994, 1, 29–30. [Google Scholar] [CrossRef]
- Collins, R.C.; Brandling-Bennett, A.D.; Holliman, R.B.; Campbell, C.C.; Darsie, R.F. Parasitological diagnosis of onchocerciasis: Comparisons of incubation media and incubation times for skin snips. Am. J. Trop. Med. Hyg. 1980, 29, 35–41. [Google Scholar] [CrossRef]
- Beretov, J.; Wasinger, V.C.; Schwartz, P.; Graham, P.H.; Li, Y. A Standardized and Reproducible Urine Preparation Protocol for Cancer Biomarkers Discovery. Biomark. Cancer 2014, 6, BIC.S17991. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the Head of Bacteriophage T4. Nature 1970, 227, 726–734. [Google Scholar] [CrossRef]
- Fairbanks, G.; Steck, T.L.; Wallach, D.F.H. Electrophoretic Analysis of the Major Polypeptides of the Human Erythrocyte Membrane. Biochemistry 1971, 10, 2606–2617. [Google Scholar] [CrossRef]
- Aathmanathan, V.S.; Jothi, N.; Prajapati, V.K.; Krishnan, M. Investigation of immunogenic properties of Hemolin from silkworm, Bombyx mori as carrier protein: An immunoinformatic approach. Sci. Rep. 2018, 8, 6957. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Nucleic Acids Research Nucleic Acids Research. Methods 1980, 12, 8235–8251. [Google Scholar]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Söderström, T.; Hanson, L.Å.; Öhman, L.; Stein, K.; Vann, W.F.; Schneerson, R. Studies on immunity against Escherichia coli K13 with monoclonal Anti-K13 and Anti-anti-K13. Infection 1985, 13, 309–312. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, no. bk. 1. In Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989; Available online: https://books.google.cm/books?id=8WViPwAACAAJ (accessed on 23 January 2024).
- Vigelsø, A.; Dybboe, R.; Hansen, C.N.; Dela, F.; Helge, J.W.; Grau, A.G. GAPDH and β-actin protein decreases with aging, making Stain-Free technology a superior loading control in Western blotting of human skeletal muscle. J. Appl. Physiol. 2015, 118, 386–394. [Google Scholar] [CrossRef]
- Šimundić, A.-M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 308890. [Google Scholar] [CrossRef] [PubMed]
- Unnasch, T.R.; Golden, A.; Cama, V.; Cantey, P.T. Diagnostics for onchocerciasis in the era of elimination. Int. Health 2018, 10, i20–i26. [Google Scholar] [CrossRef]
- Gbakima, A.A.; Nutman, T.B.; E Bradley, J.; A McReynolds, L.; Winget, M.D.; Hong, Y.; Scott, A.L. Immunoglobulin G subclass responses of children during infection with Onchocerca volvulus. Clin. Diagn. Lab. Immunol. 1996, 3, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Djune-Yemeli, L.; Domché, A.; Nana-Djeunga, H.C.; Donfo-Azafack, C.; Lenou-Nanga, C.G.; Masumbe-Netongo, P.; Kamgno, J. Relationship between skin snip and Ov16 ELISA: Two diagnostic tools for onchocerciasis in a focus in Cameroon after two decades of ivermectin-based preventive chemotherapy. PLoS Negl. Trop. Dis. 2022, 16, e0010380. [Google Scholar] [CrossRef]
- Richards, F.O.; Boatin, B.; Sauerbrey, M.; Sékétéli, A. Control of onchocerciasis today: Status and challenges. Trends Parasitol. 2001, 17, 558–563. [Google Scholar] [CrossRef]
- Remme, J.; Baker, R.H.; De Sole, G.; Dadzie, K.Y.; Walsh, J.F.; A Adams, M.; Alley, E.S.; Avissey, H.S. A community trial of ivermectin in the onchocerciasis focus of Asubende, Ghana. I. Effect on the microfilarial reservoir and the transmission of Onchocerca volvulus. Trop. Med. Parasitol. 1989, 40, 367–374. [Google Scholar] [PubMed]
- Diawara, L.; Traoré, M.O.; Badji, A.; Bissan, Y.; Doumbia, K.; Goita, S.F.; Konaté, L.; Mounkoro, K.; Sarr, M.D.; Seck, A.F.; et al. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: First evidence from studies in Mali and Senegal. PLoS Negl. Trop. Dis. 2009, 3, e497. [Google Scholar] [CrossRef] [PubMed]
- Lighaam, L.C.; Rispens, T. The Immunobiology of Immunoglobulin G4. Semin. Liver Dis. 2016, 36, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Pryce, J.; Unnasch, T.R.; Reimer, L.J. Evaluating the diagnostic test accuracy of molecular xenomonitoring methods for characterising the community burden of Onchocerciasis. PLoS Negl. Trop. Dis. 2021, 15, e0009812. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Langham, M.E.; de Stahl, E.M.; Figueroa, L.N.; Beltranena, F. Chemotherapy of onchocerciasis: A controlled clinical trial of topical diethylcarbamazine (DEC) in Guatemala. Tropenmed. Parasitol. 1980, 31, 357–364. [Google Scholar] [PubMed]
- Globisch, D.; Moreno, A.Y.; Hixon, M.S.; Nunes, A.A.K.; Denery, J.R.; Specht, S.; Hoerauf, A.; Janda, K.D. Onchocerca volvulus-neurotransmitter tyramine is a biomarker for river blindness. Proc. Natl. Acad. Sci. USA 2013, 110, 4218–4223. [Google Scholar] [CrossRef]
- Bennuru, S.; Cotton, J.A.; Ribeiro, J.M.C.; Grote, A.; Harsha, B.; Holroyd, N.; Mhashilkar, A.; Molina, D.M.; Randall, A.Z.; Shandling, A.D.; et al. Stage-specific transcriptome and proteome analyses of the filarial parasite Onchocerca volvulus and its Wolbachia endosymbiont. Mbio 2016, 7, e02028-16. [Google Scholar] [CrossRef]
- Patton, J.B.; Bennuru, S.; Eberhard, M.L.; Hess, J.A.; Torigian, A.; Lustigman, S.; Nutman, T.B.; Abraham, D. Development of Onchocerca volvulus in humanized NSG mice and detection of parasite biomarkers in urine and serum. PLoS Neglected Trop. Dis. 2018, 12, e0006977. [Google Scholar] [CrossRef]
- Wewer, V.; Peisker, H.; Gutbrod, K.; Al-Bahra, M.; Menche, D.; Amambo, N.G.; Fombad, F.F.; Njouendou, A.J.; Pfarr, K.; Wanji, S.; et al. Urine metabolites for the identification of Onchocerca volvulus infections in patients from Cameroon. Parasites Vectors 2021, 14, 397. [Google Scholar] [CrossRef] [PubMed]
- Shintouo, C.M.; Shey, R.A.; Nebangwa, D.N.; Esoh, K.K.; Nongley, N.F.; Nguve, J.E.; Giron, P.; Mutesa, L.; Vanhamme, L.; Souopgui, J.; et al. In silico design and validation of ovmane1, a chimeric antigen for human onchocerciasis diagnosis. Pathogens 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Yengo, B.N.; Shintouo, C.M.; Hotterbeekx, A.; Yaah, N.E.; Shey, R.A.; Quanico, J.; Baggerman, G.; Ayong, L.; Vanhamme, L.; Njemini, R.; et al. Immunoinformatics Design and Assessment of a Multiepitope Antigen (OvMCBL02) for Onchocerciasis Diagnosis and Monitoring. Diagnostics 2022, 12, 1440. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals. 2020. Available online: https://apps.who.int/iris/handle/10665/70809 (accessed on 23 January 2024).
- Bof, J.M.; Maketa, V.; Bakajika, D.K.; Ntumba, F.; Mpunga, D.; Murdoch, M.E.; Hopkins, A.; Noma, M.M.; Zouré, H.; Tekle, A.H.; et al. Onchocerciasis control in the Democratic Republic of Congo (DRC): Challenges in a post-war environment. Trop. Med. Int. Health 2015, 20, 48–62. [Google Scholar] [CrossRef]
- Kamga, G.R.; Dissak-Delon, F.N.; Nana-Djeunga, H.C.; Biholong, B.D.; Mbigha-Ghogomu, S.; Souopgui, J.; Zoure, H.G.M.; Boussinesq, M.; Kamgno, J.; Robert, A. Still mesoendemic onchocerciasis in two Cameroonian community-directed treatment with ivermectin projects despite more than 15 years of mass treatment. Parasites Vectors 2016, 9, 581. [Google Scholar] [CrossRef]
- Vlaminck, J.; Fischer, P.U.; Weil, G.J. Diagnostic Tools for Onchocerciasis Elimination Programs. Trends Parasitol. 2015, 31, 571–582. [Google Scholar] [CrossRef] [PubMed]
| MS Description | Protein 1 | Protein 2 |
|---|---|---|
| Checked | FALSE | FALSE |
| Protein FDR Confidence: Mascot | High | High |
| Master | Master Protein | Master Protein |
| Accession | A0A3P6S5U3 | A0A183HY13 |
| Description | Uncharacterized protein OS = Litomosoides sigmodontis OX = 42,156 GN = NLS_LOCUS128 PE = 3 SV = 1 | Uncharacterized protein OS = Onchocerca flexuosa OX = 387,005 GN = OFLC_LOCUS12375 PE = 3 SV = 1 |
| Exp. Q-value: Mascot | 0 | 0 |
| Coverage [%] | 11 | 12 |
| # Peptides | 6 | 8 |
| # PSMs | 6 | 8 |
| # Unique Peptides | 1 | 3 |
| # Aas | 680 | 1393 |
| MW [kDa] | 74.3 | 152.7 |
| calc. pI | 6.14 | 6.52 |
| Score Mascot: Mascot | 77 | 77 |
| # Peptides (by Search Engine): Mascot | 6 | 8 |
| # Protein Groups | 1 | 1 |
| Groups | Sensitivity (%) | Specificity (%) |
|---|---|---|
| mf+ versus mf- | 87% | 70% |
| mf+ versus European group | 87% | a100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambe, L.A.; Limunga, E.; Mbah, C.E.; Adela, N.; Eric, N.; Ngoe, M.; Sone, B.; Lochnit, G.; Tachu, J.B.; Wanji, S.; et al. Identification and Characterization of Onchocerca volvulus Heat Shock Protein 70 (OvHSP70) as Novel Diagnostic Marker of Onchocerciasis in Human Urine. Pathogens 2024, 13, 293. https://doi.org/10.3390/pathogens13040293
Ambe LA, Limunga E, Mbah CE, Adela N, Eric N, Ngoe M, Sone B, Lochnit G, Tachu JB, Wanji S, et al. Identification and Characterization of Onchocerca volvulus Heat Shock Protein 70 (OvHSP70) as Novel Diagnostic Marker of Onchocerciasis in Human Urine. Pathogens. 2024; 13(4):293. https://doi.org/10.3390/pathogens13040293
Chicago/Turabian StyleAmbe, Lum Abienwi, Elisabeth Limunga, Clarisse Engowei Mbah, Ngwewondo Adela, Ndumu Eric, Martha Ngoe, Bertrand Sone, Günter Lochnit, Julius Babila Tachu, Samuel Wanji, and et al. 2024. "Identification and Characterization of Onchocerca volvulus Heat Shock Protein 70 (OvHSP70) as Novel Diagnostic Marker of Onchocerciasis in Human Urine" Pathogens 13, no. 4: 293. https://doi.org/10.3390/pathogens13040293
APA StyleAmbe, L. A., Limunga, E., Mbah, C. E., Adela, N., Eric, N., Ngoe, M., Sone, B., Lochnit, G., Tachu, J. B., Wanji, S., Taubert, A., Hermosilla, C., & Kamena, F. (2024). Identification and Characterization of Onchocerca volvulus Heat Shock Protein 70 (OvHSP70) as Novel Diagnostic Marker of Onchocerciasis in Human Urine. Pathogens, 13(4), 293. https://doi.org/10.3390/pathogens13040293








