Abstract
Continued investment in the development and application of mathematical models of poliovirus transmission, economics, and risks leads to their use in support of polio endgame strategy development and risk management policies. This study complements an earlier review covering the period 2000–2019 and discusses the evolution of studies published since 2020 by modeling groups supported by the Global Polio Eradication Initiative (GPEI) partners and others. We systematically review modeling papers published in English in peer-reviewed journals from 2020–2024.25 that focus on poliovirus transmission and health economic analyses. In spite of the long-anticipated end of poliovirus transmission and the GPEI sunset, which would lead to the end of its support for modeling, we find that the number of modeling groups supported by GPEI partners doubled and the rate of their publications increased. Modeling continued to play a role in supporting GPEI and national/regional policies, but changes in polio eradication governance, decentralized management and decision-making, and increased heterogeneity in modeling approaches and findings decreased the overall impact of modeling results. Meanwhile, the failure of the 2016 globally coordinated cessation of type 2 oral poliovirus vaccine use for preventive immunization and the introduction of new poliovirus vaccines and formulation, increased the complexity and uncertainty of poliovirus transmission and economic models and policy recommendations during this time.
1. Introduction
The over 100 publications related to polio eradication modeling since 2020 [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102] provide an indication of ongoing challenges in the quest to achieve the 1988 World Health Assembly (WHA) resolution to eradicate paralytic poliomyelitis (“polio”) [103]. The WHA target of achieving polio eradication by the year 2000 led a small group of core partners to establish the Global Polio Eradication Initiative (GPEI) to provide, mobilize, and coordinate the necessary planning, financial, and technical resources [104]. Although early action plans expected the achievement of polio eradication as a result of national efforts to strengthen their routine polio immunization programs and global improvements in surveillance, the strategies for meeting the year 2000 target also included immunization campaigns [105]. Over time, operational challenges and the complexity of polio eradication became increasingly apparent, with successive target deadlines for various objectives repeatedly missed [104]. Shortly before 2000, policymakers began to recognize the potential role of using insights from dynamic poliovirus transmission (e.g., [106,107,108,109]) and static health economics (e.g., [110]) modeling to support decision-making for the polio eradication endgame. These early studies helped to motivate the GPEI core partners to formally engage independent modeling groups to provide policy and decision insights, as discussed in a prior systematic review of poliovirus transmission and health economic modeling studies published in English from 2000 to 2019 [1].
Recognizing the importance of facilitating access for modelers to national, regional, and global data, the GPEI core partners developed a data sharing agreement to support polio modeling groups [1]. The prior review of 2000–2019 included documentation of all polio-related publications by the three independent modeling groups supported by GPEI core partners under the data sharing agreement as of 2019 [1]. Since 2019, however, the polio modeling landscape has evolved and expanded. While two groups, Kid Risk, Inc. (KRI, Orlando, FL, USA) and Imperial College (IC, London, UK), continued to perform independent modeling with support from GPEI partners, in 2020, the third modeling group included in the prior review [1], the Institute for Disease Modeling (IDM, Seattle, WA, USA), became part of the Bill and Melinda Gates Foundation (BMGF). With this transition, IDM modeling went fully under the operational control of an influential GPEI core partner that presumably could rely on internal modeling results for decision-making without external peer review. In addition, with personnel moves and the expansion of the GPEI data sharing agreement to allow each core partner to support more than one independent modeling group, several other groups also became GPEI-partner-supported polio modeling groups. These include the London School of Hygiene and Tropical Medicine (LSHTM), the South African Centre for Epidemiological Modelling and Analysis (SACEMA), and the Georgia Institute of Technology (GIT). In addition to studies by GPEI-supported polio modeling groups (henceforth “modeling groups”), the literature continues to include some poliovirus transmission and economic modeling studies published by others.
The complexity of modeling poliovirus transmission remains a challenge due to the presence of three distinct poliovirus serotypes (i.e., types 1, 2, and 3) and numerous strains of live polioviruses (LPVs), including wild polioviruses (WPVs), live attenuated oral poliovirus vaccines (OPVs), and OPV-related strains. OPV-related strains include vaccine-derived polioviruses (VDPVs) that can result in outbreaks of circulating VDPVs (cVDPVs, also referred to as “variant” polioviruses), if OPV-related viruses transmit in populations with low immunization coverage. The GPEI reports cases of WPVs and cVDPVs weekly, but not other strains. However, other OPV-related strains, including immunodeficiency-associated VDPVs (iVDPVs), which occur rarely in individuals with primary immunodeficiencies, represent an important concern for eradication because immunodeficient patients can become prolonged or chronic poliovirus excreters and potentially act as a local source for re-introduction in an otherwise polio-free population. OPV-related risks also include rare cases of vaccine-associated paralytic polio (VAPP) that can occur in OPV recipients or their close contacts.
Additional challenges arise from the complex historical use of inactivated poliovirus vaccines (IPV) and OPV, including different formulations of OPV. Historically, OPVs used Sabin OPV strains, for example, trivalent OPV (tOPV, containing types 1, 2, and 3), monovalent OPV (mOPV, containing type 1, 2, or 3, and identified as mOPV1, mOPV2, or mOPV3), or bivalent OPV (bOPV, containing types 1 and 3). More recently, however, the development of a novel type 2 OPV (nOPV2) strain led to its use instead of or in addition to mOPV2 in outbreak response immunization campaigns, with novel OPV types 1 and 3 rapidly being developed. In addition, while all IPV formulations remain trivalent, licensed vaccines use either wild seed strains (Salk IPV) or Sabin OPV seed strains (Sabin IPV), and delivery of IPV can occur standalone in full or fractional dosing or in combination with other antigens. All national immunization programs deliver poliovirus vaccines in their routine immunizations (RI) according to age-based national schedules. Some countries also deliver poliovirus vaccines in supplemental immunization activities (SIAs), which target individuals within a specific age range over a short period of time, either as planned preventive SIAs (pSIAs) or reactive outbreak response SIAs (oSIAs). Poliovirus modeling studies must ideally incorporate all relevant biological, epidemiological, risk, and economic data on different poliovirus types and strains to provide value to policymakers.
Since the body of literature has expanded significantly since the prior review [1], we sought to document the publications of the different modeling groups and to systematically review poliovirus transmission and economic modeling results published in English for 2020–2024.25. The prior review noted the absence of a single repository for global polio policy decisions [1], and this remains an ongoing challenge. For this update, we recognized the opportunity to review the GPEI annual reports, conclusions and recommendations of the World Health Organization Strategic Advisory Group of Experts on Immunization (SAGE) meetings, and reports of technical advisory groups to assess the impact of modeling in supporting polio policies and decisions. This review seeks to provide insights into the role and impact of modeling on national, regional, and global policies with respect to the ongoing polio endgame.
2. Materials and Methods
For each of the 102 studies that met our inclusion criteria [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102] (Figure 1), we used the same categories and hierarchy to classify the type of modeling study as in the prior review [1]: (i) integrated modeling (i.e., including both dynamic transmission and economic modeling), (ii) dynamic transmission models, subcategorized as differential-equation-based (DEB), stochastic compartmental (SC), individual-based (IB), and/or discrete event simulation (DES), (iii) economic analyses, or (iv) other. The “other” category (i.e., polio publications not reporting poliovirus transmission or health economic modeling) applies only to the modeling groups, which we categorized as: statistical analyses (further classified as risk assessment, vaccine effectiveness, or mucosal immunity studies), reviews, discussions of policy options, or perspectives/commentaries as before [1].
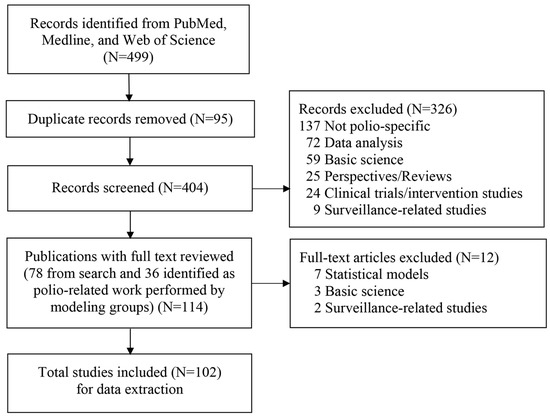
Figure 1.
Literature search process.
In this review, we provide a synopsis of all poliovirus-related publications by the six modeling groups as of early 2024 and all poliovirus transmission and economic modeling studies identified in our search and published between 2020–2024.25. We also summarize trends in the numbers and types of polio-related papers published by the modeling groups and other poliovirus transmission and economic modeling papers, for which we update the summary statistics from the prior review [1]. To appropriately discuss trends, we updated the attribution of studies published 2000–2019 [1] for 2 studies led by an LSHTM researcher (Dr. Kathleen O’Reilly) with IC coauthors [111,112] from IC to LSHTM. Assuming that the GPEI data sharing agreement with LSHTM could cover other polio researchers at LSHTM, in our analysis of trends, we changed the attribution of a 2001 transmission modeling study by Dr. Paul Fine [113] from the “other” transmission modeling group in prior review [1] to LSHTM. We also added other LSHTM polio-related publications between 2000 and 2019 (i.e., statistical analyses, vaccine effectiveness and immunology studies, reviews, and perspectives [114,115,116,117,118,119,120,121,122,123,124,125,126,127]), although we note the continued exclusion of poliovirus transmission modeling papers published by these LSHTM researchers prior to 2000 [106,107]. The shift of IDM into BMGF created challenges for characterizing the contributions of IDM and BMGF modelers. We searched for and included polio publications by IDM named- staff identified in the prior review still at IDM/BMGF (i.e., Drs. Guillaume Chabot-Couture, Michael Famulare, Steve Kroiss, Hil Lyons, and Kevin McCarthy) [1]. Similar to the prior review [1], which mentioned but did not include a 2017 BMGF-led statistical analysis that characterized population immunity in the Democratic Republic of the Congo [128], this updated review excludes results from serological surveys [129,130] and a statistical approach for analyzing and presenting polio surveillance data to supplement standard performance indicators [131].
In addition to characterizing trends in the numbers and types of publications, we identified papers that covered the same themes identified in the prior review [1]. We also added themes that emerged between 2020 and 2024, including nOPV, COVID-19/pandemic modeling, secondary effects of OPV, GPEI transition and integration, and containment risks. For the subset of studies that included poliovirus transmission modeling, we identified the specific population modeled.
As noted in the prior review [1], no depository of polio policy or GPEI decisions exists, which undermines efforts to systematically review and document the decision-support provided by modeling. However, while national, regional, and global health leaders and the GPEI leaders make policy decisions that SAGE does not review, for this analysis, we reviewed the published conclusions and recommendations from SAGE biannual meetings from inception in 1999 through April 2024 [132]. We found that SAGE discussions occasionally referred to input from technical advisory groups, which led us to review all available reports from the Advisory Committee on Polio Eradication (ACPE), which met nine times during 2004–2009, and the SAGE polio working group (SPWG), which met 27 times since its creation in 2008 through April 2024. From SPWG meeting notes for the record, we extracted the model group affiliations for SPWG meeting attendance. SPWG membership included Dr. Kimberly Thompson from KRI (term, meetings 1–14, 2008–2017), Dr. Nicholas Grassly from IC (meetings 1–19, 2008–2020), Dr. Guillaume Chabot-Couture from IDM/BMGF (meetings 15–27, 2017–present), and Dr. Kathleen O’Reilly from LSHTM (meetings 20–27, 2020–present). We identified participation in SPWG meetings by representatives of the groups noting participation as a SPWG member (M) or guest (G), and we noted whether a representative of the modeling group presented its work during the meeting or if some other speaker presented work from the modeling group (e.g., another modeling group or a GPEI partner). Finally, we also reviewed the 1999–2022 GPEI Annual Reports [133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156]. From all of the available reports, we searched the references to the modeling groups, including citations of their studies, and we extracted the context and nature of the modeling mentioned.
With changes in GPEI leadership (Dr. Bruce Aylward (before 2000–2014) [157], Dr. Hamid Jafari (2015) [158], Mr. Michel Zaffran (2016–2020) [159] and Mr. Adian O’Leary (2021–present) [159]), we observed that GPEI changed its governance structure, and we separately reported how GPEI strategies and timelines evolved over time [104]. These changes included the creation of the GPEI Strategy Committee in December 2014 [157]. From experience, we know of modeling presentations made to the GPEI Strategy Committee (e.g., KRI, IC, and IDM/BMGF all presented modeling related to responding to the global situation with type 2 outbreaks in April and May 2022), but in the absence of published meeting records or minutes, we could not report on the role of modeling input in GPEI Strategy Committee decisions.
3. Results
This section begins with a brief overview of each of the 102 studies identified by the search process summarized in Figure 1 [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102]. As before [1], we organize these by modeling group (i.e., KRI, IC, LSHTM, IDM/BMGF, SACEMA, GIT), and others. We then provide summary statistics of the included publications and characterize overall trends and themes, considering these in the context of the prior review [1]. Finally, we report the apparent impact of modeling in GPEI Annual Reports and SAGE conclusions and recommendations.
3.1. Studies That Met Inclusion Criteria Published 2020–2024.25
3.1.1. KRI
In 2020, KRI published reviews on several topics, including the prior review of modeling studies [1], potential use of vaccine patches for immunization, including IPV [2], and the performance of the United States Vaccine Injury Compensation Program (VICP): 1988–2019 [3], which highlighted VAPP risks as a key motivation for VICP establishment. KRI also published a commentary related to incentives for developing vaccine patches [4] and an economic analysis related to developing better defaults for valuing health outcomes based on health opportunity costs [5].
Prior to and during the COVID-19 pandemic, KRI published several studies related to updating the inputs of its global integrated model, including 2019 updates for iVDPV risks [160] (included in the prior review [1]). Following the release of the 2019–2023 GPEI strategic plan [161] and post-cessation strategy [162], KRI updated its integrated model inputs to use retrospective immunization and epidemiology data through the end of 2018 and prospective inputs consistent with available information about recent programmatic performance and updated plans. The third special issue of the journal Risk Analysis on Global Poliovirus Risk Management and Modeling, published in February 2021 [6], included many of these studies, several of which appeared online in 2020. In January 2020 (prior to the COVID-19 pandemic), the KRI update of its integrated model reported the GPEI was off-track with respect to achieving its polio eradication goals [161] and attributed this primarily to the low quality of OPV SIAs [7]. For this integrated model update [7], KRI reflected on key differences between the optimistic and ideal risk management assumptions it used prior to 2016 [163,164] and actual performance and plans after 2016 [8]. Due to its expectation of ongoing transmission of WPV1 in Pakistan and Afghanistan [7], KRI built on its prior Pakistan and Afghanistan modeling [165,166,167,168] to update its DEB model for these countries (as a single epidemiological reservoir) and identified pSIA strategies that could end WPV1 transmission within the timeline of the 2019–2023 GPEI strategic plan [9]. KRI also developed a DEB model of poliovirus transmission for northeast Nigeria to characterize the transmission of WPV1 that occurred in Borno and Yobe in 2016 [10]. Using this DEB as the basis for an SC model, KRI characterized the confidence of no circulation or undetected WPV1 transmission as a function of time for Borno and Yobe [11], which the Africa Regional Commission for the Certification of Poliomyelitis Eradication used to support its regional certification in 2020 [169]. KRI also updated its characterization of post-OPV cessation risks related to the misuse or inadvertent use of OPV after homotypic cessation, and updated its probability estimates for the need to restart OPV2 production and use in preventive immunization [12]. To support integrated analyses, KRI published updated cost estimates for poliovirus immunization and valuations of polio health outcomes [13], including consideration of health opportunity costs [5]. With global certification of WPV3 eradication in 2019 [170] and reductions of bOPV pSIAs, in late 2020, KRI published a DEB modeling study of the expected reductions of VAPP and cVDPV cases and vaccine dose requirements for a policy of globally coordinated cessation of type 3 OPV (OPV3) before type 1 OPV (OPV1) compared to ongoing bOPV use [14].
Following the May 2020 GPEI publication of an addendum to its 2019–2023 strategic plan that emphasized the use of nOPV2 for oSIAs [171], KRI applied its global model to explore the impacts of using nOPV2 compared to Sabin mOPV2 for oSIAs and reported that even with ideal characteristics, nOPV2 would not likely end all type 2 outbreaks and transmissions [15], which it also discussed in a commentary [16]. Considering pre-COVID-19 conditions, KRI updated its 2010 integrated analysis of the health and economic benefits of the GPEI [172] based on the status of the GPEI and its plans as of 2019 [17], which included consideration of the possibility of ending WPV1 transmission in Pakistan and Afghanistan [9]. KRI also applied its integrated model to consider the health and economic impacts of IPV or tOPV in RI with or without OPV use for oSIAs for different control and eradication scenarios for 2019–2029 [18].
After the emergence of COVID-19, a high-profile suggestion to use OPV to prevent COVID-19 in the United States [173,174] motivated KRI to perform a health economic analysis that estimated VAPP risks and costs with no anticipation of expected benefits for reducing COVID-19 [19] (and associated letter [175]). KRI explored the impact of disruptions caused by the COVID-19 pandemic on global polio eradication in a 2021 study [20], which included updating its assumptions related to long-range exportations. In 2021, KRI published two studies related to the hypothetical emergence of a novel poliovirus in 2020 to simulate a pandemic with poliovirus (instead of COVID-19) and demonstrated the consequences of policy decisions for control or eradication using nonpharmaceutical interventions [21] and vaccines [22]. KRI also updated its characterization of poliovirus transmission in Pakistan and Afghanistan post-COVID-19 to explore the impacts of different vaccine options for oSIAs (e.g., mOPV2, tOPV) in the context of the cocirculation of WPV1 and cVDPV2 [23]. A separate KRI study estimated the expected costs of poliovirus surveillance and immunization campaign quality monitoring for Pakistan and Afghanistan for 2019–2023 [24].
Outbreak response emerged as a theme for several KRI publications during this period. In 2021, KRI characterized the trade-offs of different OPV2 vaccines (i.e., mOPV2 or nOPV2) for oSIAs and the consequences of delaying oSIAs to wait for nOPV2 instead of using available mOPV2 [25]. This analysis emphasized the importance of responding quickly and well with available vaccine supplies (i.e., mOPV2) and anticipated that delays in waiting for nOPV2 would likely lead to over a thousand paralytic cases and greater global cVDPV2 transmission [25]. Despite this analysis, countries in the WHO African region chose to delay oSIAs to wait for nOPV2, which increased the number and spread of reported cVDPV2 cases and motivated KRI to ask, “What kind of world do we want?” in a commentary [26]. A separate commentary raised questions about the effectiveness of a nOPV2 for oSIAs and highlighted the increasingly complicated polio endgame [27].
Following up on discussions of secondary vaccine effects of OPV raised prior to the development of COVID-19 vaccines [19], KRI published a systematic review of secondary health effects of vaccines and policy insights for health economic analyses [28] and separately discussed the challenges of assessing the benefits and costs of non-polio and shared activities for polio and non-polio interventions in polio health economic analyses [29].
In 2022, following the publication of a new GPEI strategic plan for 2022–2026 [176], KRI published 2 analyses related to questions asked by GPEI partners during the development of the plan about the overall cases of polio prevented by poliovirus vaccines and GPEI [30] and the health and economic consequences of a shift to control in 2022 [31] (as an update to a 2007 KRI analysis of control vs. eradication [177]). Although KRI performed these studies in response to specific questions raised by GPEI partners, GPEI did not subsequently refer to the results of these studies.
With new milestones [104] established in the new plan [176], KRI again updated its DEB model for Pakistan and Afghanistan [32]. Assuming the application of a pSIA strategy that KRI identified as successful for ending WPV1 transmission in its model [32], KRI used this analysis as the basis for an SC model to characterize the confidence of no undetected WPV1 transmission as a function of time [33]. The Global Commission for Certification of Poliomyelitis Eradication (GCC) used these results [32,33] to support a potential shortening of the time required to certify the end of indigenous WPV1 transmission after the last reported case or positive environmental sample to less than 3 years, depending on the quality of the surveillance and immunization information [178].
In 2022, KRI removed its assumptions related to OPV restart as a policy option in its global integrated model for consistency with observed experience. This change implied the potential use of OPV vaccines available for oSIAs for the full model time horizon of prospective models, and ended KRI characterization of the probability of OPV restart as a metric to characterize OPV cessation failure [34]. One application of the KRI global model published in late 2022 showed the trade-offs of different OPV2 vaccine options and oSIA characteristics (i.e., scope, timeliness, quality) [34], which identified aggressive options that could potentially lead to a high probability of cVDPV2 transmission dying out by the end of the model time horizon and characterized the low chances of success for the status quo GPEI strategy and policies. A separate study applied a DEB model of a hypothetical population with cocirculation of types 1 and 2 and demonstrated the advantages of coadministration of both types (e.g., tOPV or bOPV and nOPV2) compared to sequential or alternating strategies [35]. KRI also performed a look-back analysis to learn from its prior prospective modeling of oSIAs for managing OPV2 cessation [36].
In 2023, KRI explored the complexity of modeling the increase in vaccine options (i.e., nOPVs, different IPV formulations) and the dynamics of starting and/or stopping OPV in national immunization programs after OPV cessation [37]. KRI commented on polio eradication hurdles [38] and the need for updating expectations for nOPV2 [39]. KRI also reviewed the experience with the OPV2 stockpiles and discussed the challenges of forecasting vaccine demand given uncertainty about prospective national, regional, and global policies [40]. With ongoing discussions about potential global coordination of bOPV cessation, KRI also applied its global integrated model to characterize the expected outcomes of coordinated global cessation of bOPV use in 2027 without bOPV pSIAs [41], as suggested by the 2022–2026 strategic plan [176]. In addition to exploring the expected annual paralytic cases by type over time, KRI also explored the worst-case scenarios [42]. In the context of low expectations of successful bOPV cessation, a related health economic analysis anticipated a relatively low value of antiviral drugs [43].
Finally, in 2023–2024, KRI published a DEB model of the cVDPV2 transmission and IPV oSIA for the imported cVDPV2 outbreak that occurred in 2022 in New York State [44]. KRI used this as the basis for an SC model analysis that explored confidence about no circulation as a function of time since the last positive surveillance signal [45], which helped to support the New York State and national leaders feel more confident about the end of the outbreak. KRI also explored the trade-offs of different poliovirus vaccine options for outbreak response in IPV-using countries, such as the US 2022 cVDPV2 outbreak, and considered a hypothetical cVDPV1 outbreak to show differences between poliovirus types [46], which supported the 2023 US decisions to offer IPV to adults [179] and deliberations by the Advisory Committee on Immunization Practices (ACIP) about the potential use of OPV in the US for oSIAs at its February 2024 meeting. This study also included a systematic review of modeling studies that included IPV use in outbreak response [46].
3.1.2. IC
The studies identified for IC for 2020–2024.25 showed its continued focus on statistical analyses of existing surveillance data and data collected as part of prospective trials or challenge studies and included a new focus area related to the development of a poliovirus direct detection method. With respect to transmission modeling, IC applied an SC model to characterize the spread of serotype-2 vaccine-derived-poliovirus outbreaks in Pakistan and Afghanistan to inform outbreak control strategies in the context of the COVID-19 pandemic [47], which built on a statistical analysis that quantified movement patterns and vaccination status in high-risk mobile populations [48].
Statistical analyses published by IC included an analysis of immune predictors of OPV immunogenicity among infants in South India [49]. One IC study used poliovirus genetic sequences from GenBank and demonstrated the application of phylogenetic and phylogeospatial modeling to infer geospatial transmission patterns of poliovirus transmission during the 2010 Tajikistan outbreak [50]. Another analysis characterized the variability in the sensitivity of environmental surveillance sites in Nigeria to detect poliovirus and other enteroviruses [51]. Two IC studies focused on risk factors for cVDPV2 transmission in Africa after 2016 [52,53]. The first of these highlighted the need for larger and faster OPV2 oSIAs to stop cVDPV2 transmission [52], and the second identified risk factors for VDPV2 emergence and suggested priorities for nOPV2 use [53]. IC reported the results of several vaccine effectiveness studies, including a phase 4 clinical trial on the effect of maternal immunization with multivalent vaccines containing IPV in infants [54] and the effectiveness of nOPV2 and mOPV2 against cVDPV2s in Nigeria between 2017 and 2022 [55]. IC commented on the first Africa-based nOPV2 clinical trial [56] and contributed to perspectives related to the impacts of one billion doses and WHO prequalification of nOPV2 [57] and the use of nOPV2 for oSIAs [58].
IC invested substantial effort in the research, development, and implementation of direct detection with nanopore sequencing (DDNS) for poliovirus surveillance [59,60,61,62]. This included publications that characterized rapid and sensitive direct detection and identification of poliovirus from stool and environmental surveillance samples using nanopore sequencing [59] and an analysis of the time taken to detect and respond to polio outbreaks in Africa considering the potential impact of direct molecular detection and nanopore sequencing [60]. IC also published a comparison of eleven RNA extraction methods for direct molecular detection of polioviruses from stool [61] and a prospective validation study of DDNS that reported preliminary cost estimates [62]. IC also included poliovirus surveillance in a consortium effort that it led with the goal of defining a research agenda for broad environmental wastewater surveillance of pathogens [63].
Following the detection of imported cVDPV2 transmission in the UK, IC published a commentary about the detection as a wake-up call [64] and a statistical analysis of the sustained detection by enhanced environmental surveillance in London sewage between February and July 2022 [65]. IC contributed substantially (i.e., three of eight coauthors) to a review of the use of IPV for oSIAs [66] prepared at the request of the SPWG for discussion at its 24th meeting (August 2022) and the October 2022 SAGE meeting.
3.1.3. LSHTM
Our review identified one SC modeling study that explored the effect of population partitioning on the probability of silent poliovirus transmission, which included a coauthor affiliated with both LSHTM and SACEMA [67]. The lead author of this study [67] published several poliovirus transmission modeling studies counted as “other transmission studies” in the prior review [1], in collaboration with a polio modeler from the University of Michigan (Dr. James Koopman). This study [67] represented the only publication that met the review inclusion criteria for SACEMA, although for this review, we attributed it to LSHTM.
As discussed earlier, several IC studies in the prior review included a researcher who moved to LSHTM in 2018 [1]. Not surprisingly, given prior collaboration, several LSHTM-led studies published in 2020–2024.25 included coauthors from IC [68,69,70]. One study included a coauthor from IDM/BMGF [71]. LSHTM reported statistical epidemiological analyses that characterized changes in the epidemiology of poliovirus serotype 2 following OPV2 cessation [68], which followed discussions held during a January 2020 meeting that included all of the groups. Other LSHTM studies explored the optimization of environmental surveillance to detect poliovirus importations into England and Wales [69] and characterized the epidemiology of cVDPV2 global outbreaks between 2016 and 2020 [70]. Another statistical analysis characterized the impact of surveillance and other factors on the detection of emergent and circulating vaccine-derived polioviruses [71]. We identified several LSHTM reviews related to the polio endgame [72], the accelerated roll-out of the nOPV2 vaccine [73], and challenges with achieving polio eradication given population immunity as of early 2024 [74]. LSHTM coauthors also commented on the challenges of informative wastewater sampling for SARS-CoV-2, considering lessons learned from polio eradication [75]. LSHTM also published a vaccine effectiveness study of an mOPV2 challenge dose given to IPV-vaccinated children [76], a mucosal immunity study for nOPV2 in healthy adults [77], and a review of polio mucosal immunity studies [78].
3.1.4. IDM/BMGF
IDM/BMGF published three poliovirus transmission modeling related studies between 2020 and 2024.25 [79,80,81]. Using an IB model, the first study incorporated the results from a mOPV2 clinical trial performed in Bangladesh, which suggested that household and community structure played an important role in limiting transmission [79]. In 2023, the second study described the development of a DEB model that endogenously included OPV2 reversion for the same population [80]. The third study applied a DEB model to revisit the role of time-varying viral shedding in modeling environmental surveillance using the 2013 poliovirus outbreak in Israel [81].
IDM/BMGF also published multiple statistical analyses. One study modeled the genetic sequences of type 2 polioviruses and identified positive selection and tight transmission bottlenecks that substantially influenced the early evolution of OPV2 [82]. Another study of disease surveillance investments and administrative data for Pakistan showed the limited value of the available information for improving surveillance quality [83]. Studies in 2023 developed a real-time prediction model of cVDPV2 outbreaks to aid oSIA decisions [84] and analyzed changes in population immunity for all three types of polioviruses since the 2016 shift from tOPV to bOPV for selected countries and regions [85]. The final IDM/BMGF publication applied a time series statistical model to estimate the vaccine effectiveness of nOPV2 in oSIAs in Nigeria, which also reported similar vaccine effectiveness of mOPV2, but substantially lower effectiveness for IPV than for either OPV2 [86].
3.1.5. GIT
GIT published its first two poliovirus transmission modeling studies in 2024, including a peer-reviewed conference publication [87] and a related journal article [88]. Both studies described the application of a DEB model it developed to characterize cVDPV2 transmission and the impacts of interventions that aim to control outbreaks in Nigeria, and they identified the need for more aggressive oSIAs (i.e., more rounds and broader coverage, particularly in under-vaccinated communities) [87,88].
3.1.6. Economic Analyses Published by Other Groups
The search identified two other studies published in 2020 that presented game theoretic applications to polio, which we included as other economic analyses [89,90]. The first integrated a DEB model with two age classes (i.e., [180] identified in the prior review [1]) into the game theoretic model, which led us to characterize it as an integrated model [89]. The study concluded that achieving polio eradication would require a mandatory vaccination policy [89]. The second study used survey data related to perceptions of the population benefits of OPV and statistical model inputs informed by poliovirus transmission characteristics in a model that suggested that prosocial behavior to receive bOPV in Israel contributed some to the oSIA vaccine uptake after the 2013 WPV1 outbreak [90].
The search found three other polio-related economic modeling studies. These include cost-effectiveness analyses of three poliovirus immunization schedules in Shanghai, China [91] and various immunization schedules with Sabin IPV in Hangzhou, China [92]. The final study assumed OPV could provide protection from COVID-19 due to secondary effects and suggested the need to prioritize clinical trials and other studies that could resolve the uncertainty about the effects of OPV on COVID and other non-poliovirus diseases [93].
3.1.7. Poliovirus Transmission Modeling Studies Published by Other Groups
Our review identified nine other studies that presented DEB models of poliovirus transmission for theoretical populations using different numerical simulation methods [94,95,96,97,98,99,100,101,102].
3.2. Trends in Characteristics of Polio Modeling Studies
Table 1 provides a high-level comparison between the numbers and types of studies included in this review compared to those in the prior review for 2000–2019 [1] (with the updates discussed above). Notably, the 102 studies identified for the 4.25 years covered in this review imply an increase in the annual rate of polio modeling papers published over time of approximately 2, 5, 9, 22, and 24 papers/year, respectively, for 2000–2004, 2005–2009, 2010–2014, 2015–2019, and 2020–2024.25. Among the modeling groups, KRI published the largest number of studies for 2020–2024.25 (46 publications), followed by IC (20 publications), and LSHTM (12 publications).

Table 1.
Characteristics of included studies with comparison to results reported for 2000–2019 [1].
Table 2 summarizes the themes explored in the included studies by all groups with publications discussed in the prior section. We identified publications by multiple groups for several key themes from the prior review [1], as well as several new themes. Given the global situation with ongoing poliovirus transmission, particularly for cVDPV2s, we identified publications by all the groups in Table 2 on: outbreak response speed (and quality), population immunity, and novel OPV, particularly nOPV2. All groups discussed changes in population immunity in multiple publications, albeit with different definitions. As in the prior review [1], outbreak response speed and quality represented a major theme, with all groups demonstrating the need for improvements in oSIAs. With the use of nOPV2 in oSIAs starting in 2021, our review identified publications by all groups related to nOPV2, many of which included analyses related to nOPV2 use in oSIAs. With respect to OPV cessation dynamics, several groups explored the evolution of OPV, which included some prospective modeling related to potential bOPV cessation.

Table 2.
Summary of themes explored and specific populations in poliovirus transmission model studies by group.
Also represented in Table 2 as related to OPV cessation dynamics, we noted multiple studies by different groups on the failure of the 2016 globally coordinated cessation of OPV2 use for preventive immunization, which included retrospective statistical analyses of epidemiological data and poliovirus transmission modeling. With substantial GPEI investments in the expansion of environmental surveillance, Table 2 also shows numerous studies by different groups related to surveillance. Notably, given multiple publications by IC related to its development of DDNS, we broadened the category from environmental surveillance to surveillance.
Most of the modeling groups also published on the topic of IPV use. These publications included IPV use for oSIAs, with some studies motivated by actual experiences (e.g., the 2022 cVDPV2 outbreak in the United States). In addition, multiple groups published studies related to the role of IPV use after OPV cessation, but we did not include the studies that simply added IPV to RI consistent with the prerequisites for OPV2 cessation in Table 2. Not surprisingly, most modeling groups published studies motivated by the global experience with the COVID-19 pandemic, including many studies related to disruptions in national and GPEI immunization activities. Two groups published studies related to undetected circulation in different geographies. Only KRI published studies related to the topics of expanded age group SIAs, silent transmission on an IPV background and/or delayed detection of transmission due to IPV use, vaccine stockpile, iVDPVs, containment, secondary effects of OPV, and GPEI transition and integration.
The bottom portion of Table 2 shows the geographic scope covered by 45 studies that included poliovirus transmission modeling [7,9,10,11,12,14,15,17,18,19,20,21,22,23,25,30,31,32,33,34,35,36,41,42,43,44,45,46,47,67,79,80,81,87,88,89,94,95,96,97,98,99,100,101,102]. Not surprisingly, two groups published studies on Pakistan, Afghanistan and Nigeria, which continue to represent critical geographies for polio eradication. Populations modeled by only one group include Bangladesh, Israel, and the United States, and only KRI performed global analyses.
3.3. Modeling in GPEI Annual Reports or SAGE Conclusions and Recommendations
Table 3 provides excerpts of references to modeling and/or modeling groups and the sources for results reported that we identified in the GPEI Annual Reports. These discussions of modeling activities and/or references to studies and results published by the modeling groups provide some indication that GPEI broadly relied on modeling inputs. The rightmost two columns show the modeling group(s) referred to by name or by the journal of their publication, and the references to studies from which the GPEI Annual Reports included mention of specific results. Between 2005 and 2018, the GPEI Annual Reports referred to results from studies published by 4 modeling groups: LSHTM [121], KRI [172,177,181,182,183], IC [184,185,186,187], and IDM [188], and we noted multiple references to the results from older studies over time. References to the results from modeling studies occurred in 4 of 5 years for the 2005–2009, 2010–2014, and 2015–2019 time periods. In the 2020–2022 GPEI Annual Reports, only the 2022 report even alluded to the results from modeling studies by including a link to a 2022 investment case [189] that presented the results of an unpublished update of a prior study [188] and mentioned the outdated results of a 2007 publication [177]. Notably, the investment case did not mention a published 2021 updated economic analysis of the GPEI [17]. Two other relevant studies published shortly after publication of the investment case included quantification of the cases of polio prevented by polio vaccines and GPEI [30] and an update of the consequences of shifting to control in 2022 [31] (as an update for an outdated study of control vs. eradication [177]).

Table 3.
Discussions of work related to polio modeling and groups in GPEI Annual Reports [133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156].
Table 4 provides excerpts of discussions of polio modeling (and economic analyses) we identified in SAGE meeting conclusions and recommendations published in the Weekly Epidemiological Record [132]. Although every regular SAGE meeting agenda included polio eradication (annually 2000–2002 and biannually 2003–2024), Table 4 only includes meetings for which we identified discussions of polio modeling results. The third column provides references to publications specifically discussed as studies or reviews, as SAGE noted in the conclusions and recommendations. The fourth column provides references to publications we could identify in our review of available presentations to SAGE. Although we identified multiple references to modeling and/or the modeling groups, we identified only five explicit references to specific studies or reviews published by the modeling groups (i.e., KRI [172,177], IC [66,184,185]) and one acknowledgment of IC unpublished work supporting WHO. However, our review of the available presentations made to SAGE identified the inclusion of results from the modeling groups in most of its meetings. We recognized that some of the SAGE meetings included discussions of topics for which relevant modeling available at the time did not get presented (e.g., the role of older age groups in transmission, the economics of using IPV in outbreak response, trade-offs between vaccine choices for outbreak response, including in areas with cocirculation of poliovirus types 1 and 2). We identified some unpublished and not peer-reviewed (to date) modeling results presented (see notes at the bottom of Table 4), most notably predictions about the number of cVDPV2 outbreaks to expect after OPV2 cessation that did not imply any dependence on the implementation of oSIAs. We also observed multiple references to both ACPE and the SPWG. This motivated us to also review the available notes for the record from the face-to-face meetings of these groups. We know from experience that modeling groups attended and presented at some ACPE meetings, but we did not find agendas for all ACPE meetings, and we did not find specific references to the modeling publications presented in the ACPE meeting reports.

Table 4.
Polio modeling discussions identified in SAGE meeting conclusions and recommendations.
Table 5 shows the representation of the modeling groups in the 27 face-to-face SPWG meetings and the dates of the meetings. The third through sixth columns show the roles for four of the modeling groups (i.e., KRI, IC, LSHTM, and IDM/BMGF) by indicating the meetings for which representatives from the group participated as a member (M), an invited external participant (P), or a rapporteur (R). The SPWG generally holds closed meetings, with participation limited to committee members and invited participants, although some representatives of some GPEI core partners also attended SPWG meetings. Representatives of the modeling groups, attended different numbers of total SPWG meetings to date: KRI (14), IC (22), LSHTM (12), and IDM/BMGF (24). As shown in Table 5, which uses an asterisk (*) to indicate a presentation, members frequently presented work from their groups and nearly all invited external participants presented work from their group while at the meeting. The seventh column shows the topic presented by modeling groups (and indicates the group). We identified some presentations that included work from modeling groups not attending the meeting. We included the topic of the presentation and added a note to indicate these presentations. This led to the inclusion of the topics of some presentations in the seventh column with no group indicated, as well as some notes showing presentations given by a group representative that included work from one or more other modeling groups in addition to their own work. As shown in the rightmost column in Table 5, between 2008 and 2014 the SPWG reports included references to specific studies by different modeling groups. However, since 2015, while some SPWG notes for the record mentioned presentations by modeling groups, they did not include references to specific studies (e.g., the IC review of IPV use for oSIAs [66]). In the absence of reviewing each presentation, we could not identify all of the modeling results presented to the SPWG.

Table 5.
Representation of modeling at face-to-face SAGE polio workgroup meetings.
Overall, it appears that poliovirus transmission and economic modeling results played a much larger role in GPEI and SAGE discussions in earlier time periods than during 2020–2024.25, although we see a strong representation of consideration of the results of the modeling groups for 2000–2024.
4. Discussion
Publications by the modeling groups since 2020 have continued to show the application of different approaches and active communication of model results to policymakers. However, we observed fewer references to modeling results in GPEI Annual Reports since 2020 than during the prior 15 years, and a decrease in the impact of the results of poliovirus transmission and economic modeling on decisions. Notably, in some cases, the reduced impact of modeling occurred despite the agreement of the multiple modeling groups. For example, although multiple modeling groups recommended a rapid response to cVDPV2 outbreaks using any available OPV2 vaccine (including mOPV2) and SAGE endorsed this recommendation, some countries in the African region delayed their responses to cVDPV2 outbreaks after 2020 to wait for nOPV2 because of its perceived higher safety profile. Multiple modeling groups also published studies that highlighted the late, small, and low quality of oSIAs in some areas as a primary reason for OPV2 cessation failure.
During the past 5 years, modeling studies suggested an expected reduction in overall incremental net benefits of the global polio eradication efforts, decreased chances of a successful polio eradication endgame, expectations of increased costs of polio immunization prospectively due to the shift from OPV to IPV in RI associated with the increase in recommended IPV doses in RI, and the complexity of continued use of different formulations of OPV. However, commitment to “finishing the job” remains strong, in spite of a less favorable economic impact. Consistent with this, discussions by GPEI and SAGE trended more toward a focus on polio epidemiological studies, equity (particularly gender equity [154]), and integration of GPEI activities into national immunization programs. The increased focus on statistical analyses of polio epidemiological data reflects the reality of the increased incidence of polio cases reported since 2020 [104].
Similar to the prior review [1], we looked for areas of disagreement about recommendations from the modeling groups. We could not formally document some known differences from internal modeling group discussions because they involved unpublished results. For example, published modeling emphasized the importance of rapidly responding to cVDPV2 outbreaks using the available mOPV2 given the limited availability of nOPV2 [25,34], which unpublished modeling by one other group supported and unpublished modeling by another group did not support (it suggested that countries should wait to use nOPV2 before using mOPV2 in oSIAs). In addition, the communications to countries related to the roll out and promotion of nOPV2 in 2021 promised a better vaccine than mOPV2, which effectively disincentivized the use of mOPV2, devalued the available mOPV2 stockpile, and created challenges with respect to using any Sabin OPV in SIAs to increase and maintain population immunity.
With respect to Pakistan and Afghanistan, modeling performed by KRI, IC, and IDM did not lead to the accelerated achievement of WPV1 eradication in Pakistan and Afghanistan. We could not assess the agreement between these since some of the analyses remain unpublished, but studies by KRI and IC agreed with the need to quickly restart immunization in Pakistan and Afghanistan following disruptions caused by COVID-19 [32,47]. In addition, insights from modeling did not succeed in preventing the failure of OPV2 cessation, despite repeated warnings of its likely failure as early as 2017 in Cessation Risk Task Team meetings that occurred between June 2016 and April 2021, which included all of the modeling groups along with representatives from most GPEI core partners. Modeling support of stockpile needs prior to OPV2 cessation did not account for shifts in GPEI leadership and governance and the resulting adoption of lower prioritization of SIAs [104], decentralization of decision making, or the apparent willingness to accept increased polio cases to wait for a different vaccine (i.e., nOPV2). Notably, prior to OPV2 cessation, prospective modeling assumed aggressive oSIAs with mOPV2 would either successfully stop all type 2 poliovirus transmission or countries would restart the use of tOPV in RI [36], and thus developed estimates of demand for a time-limited OPV stockpile [40]. Instead, global efforts moved toward increased use of IPV, the development and deployment of new vaccine tools, including nOPV, and the associated extension of the polio endgame. Given the current epidemiological situation, we can anticipate that future publications of polio modeling studies may support another update of the systematic review.
Limitations of this review include our inability to evaluate whether and how national and regional decision makers interact with each other, GPEI, and other global health policy makers. Although we described some work by the modeling teams used by national and regional decision makers (e.g., New York modeling, the African Regional Certification Commission), these represent anecdotal examples, not the results of a systematic process to collect information from decision makers at all levels about their use of modeling results. Modeling results represent only one of the factors that may influence decisions.
5. Conclusions
The representatives from some modeling groups continued to serve as members on the SPWG and the modeling groups continue to increase and to publish their work at an increasing rate. By combining and updating results from prior review [1], for the full time period of 2000–2024.25 we identified 246 polio publications by the modeling groups KRI (124), IC (64), LSHTM (29), IDM/BMGF (27), and GIT (2), plus 46 publications by others (including 32 poliovirus transmission modeling papers and 14 economic analyses). With the initial target for achieving global polio eradication in 2000, the increasing number of modeling groups and rate of publications contrast notably with expectations of successful eradication and dissolution of the GPEI long before 2024. In spite of this, the GPEI core partners remain committed to delivering on the promise of polio eradication, and this suggests that opportunities and support for polio modeling will continue for the foreseeable future.
Author Contributions
Conceptualization, funding acquisition, and original draft preparation K.M.T.; Methodology, formal analysis, review and editing, and visualization, K.M.T. and K.B. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support for this report under Cooperative Agreement Number 5NU2RGH001915-04-00 funded by the Centers for Disease Control and Prevention. The views expressed are solely those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or Department of Health and Human Services.
Acknowledgments
We thank Nicholas Grassly, Isobel Blake, Kathleen O’Reilly and Hil Lyons for comments related to our descriptions of polio publications from their respective groups.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ACPE, Advisory Committee on Polio Eradication; AFP, acute flaccid paralysis; BMGF, Bill and Melinda Gates Foundation; bOPV, bivalent OPV; cVDPV, circulating VDPV; DEB, differential equation-based; DES, discrete event simulation; EUL, emergency use listing; GIT, Georgia Institute of Technology; GPEI, Global Polio Eradication Initiative; IB, individual-based; IC, Imperial College; IDM, Institute for Disease Modeling; IPV, inactivated poliovirus vaccine; iVDPV, immunodeficiency-associated VDPV; KRI, Kid Risk, Inc.; LPV, live poliovirus; LSHTM, London School of Hygiene and Tropical Medicine; mOPV, monovalent OPV; NA, not available; nOPV, novel OPV; OPV, oral poliovirus vaccine; OPV#, type # OPV (# = 1, 2, or 3); oSIA, outbreak response SIA; PRC, GPEI polio research committee; pSIA, preventive SIA; RI, routine immunization; QUIVER, Quantitative Immunization and Vaccine Related Research Advisory Committee; SACEMA, the South African Centre for Epidemiological Modelling and Analysis; SAGE, Strategic Advisory Group of Experts on immunization for the World Health Organization; SC, stochastic compartmental; SIA, supplemental immunization activity; SPWG, SAGE polio working group; tOPV, trivalent OPV; VAPP, vaccine-associated paralytic polio; VDPV, vaccine-derived poliovirus; VICP, Vaccine Injury Compensation Program (United States); WHA, World Health Assembly; WHO, World Health Organization; WPV, wild poliovirus; WPV#, type # WPV (# = 1, 2, or 3).
References
- Thompson, K.M.; Kalkowska, D.A. Review of poliovirus modeling performed from 2000 to 2019 to support global polio eradication. Expert Rev. Vaccines 2020, 19, 661–686. [Google Scholar] [CrossRef]
- Badizadegan, K.; Goodson, J.L.; Rota, P.A.; Thompson, K.M. The potential role of using vaccine patches to induce immunity: Platform and pathways to innovation and commercialization. Expert Rev. Vaccines 2020, 19, 175–194. [Google Scholar] [CrossRef]
- Thompson, K.M.; Orenstein, W.A.; Hinman, A.R. Performance of the United States Vaccine Injury Compensation Program (VICP): 1988–2019. Vaccine 2020, 38, 2136–2143. [Google Scholar] [CrossRef]
- Thompson, K.M.; Orenstein, W.A.; Hinman, A.R. An opportunity to incentivize innovation to increase vaccine safety in the United States by improving vaccine delivery using vaccine patches. Vaccine 2020, 38, 4060–4065. [Google Scholar] [CrossRef]
- Ochalek, J.; Claxton, K.; Lomas, J.; Thompson, K.M. Valuing health outcomes: Developing better defaults based on health opportunity costs. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 729–736. [Google Scholar] [CrossRef]
- Thompson, K.M. Modeling and managing poliovirus risks: We are where we are. Risk Anal. 2021, 41, 223–228. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Pallansch, M.A.; Wassilak, S.G.F.; Cochi, S.L.; Thompson, K.M. Global transmission of live polioviruses: Updated dynamic modeling of the polio endgame. Risk Anal. 2021, 41, 248–265. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A. Reflections on modeling poliovirus transmission and the polio eradication endgame. Risk Anal. 2021, 41, 229–247. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Thompson, K.M. Insights from modeling preventive supplemental immunization activities as a strategy to eliminate wild poliovirus transmission in Pakistan and Afghanistan. Risk Anal. 2021, 41, 266–272. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Franka, R.; Higgins, J.; Kovacs, S.D.; Forbi, J.C.; Wassilak, S.G.F.; Pallansch, M.A.; Thompson, K.M. Modeling poliovirus transmission in Borno and Yobe, Northeast Nigeria. Risk Anal. 2021, 41, 289–302. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Thompson, K.M. Modeling undetected live poliovirus circulation after apparent interruption of transmission: Borno and Yobe in Northeast Nigeria. Risk Anal. 2021, 41, 303–311. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Pallansch, M.A.; Cochi, S.L.; Kovacs, S.D.; Wassilak, S.G.F.; Thompson, K.M. Updated characterization of post-OPV cessation risks: Lessons from 2019 serotype 2 outbreaks and implications for the probability of OPV restart. Risk Anal. 2021, 41, 320–328. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A. Potential future use, costs, and value of poliovirus vaccines. Risk Anal. 2021, 41, 349–363. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Thompson, K.M. Expected implications of globally coordinated cessation of serotype 3 oral poliovirus vaccine (OPV) before serotype 1 OPV. Risk Anal. 2021, 41, 312–319. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Pallansch, M.A.; Wilkinson, A.; Bandyopadhyay, A.S.; Konopka-Anstadt, J.L.; Burns, C.C.; Oberste, M.S.; Wassilak, S.G.F.; Badizadegan, K.; Thompson, K.M. Updated characterization of outbreak response strategies for 2019–2029: Impacts of using a novel type 2 oral poliovirus vaccine strain. Risk Anal. 2021, 41, 329–348. [Google Scholar] [CrossRef]
- Thompson, K.M. Poliovirus vaccine options: Another step forward. Lancet 2020, 397, 2–3. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A. An updated economic analysis of the Global Polio Eradication Initiative. Risk Anal. 2021, 41, 393–406. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Thompson, K.M. Health and economic outcomes associated with polio vaccine policy options: 2019–2029. Risk Anal. 2021, 41, 364–375. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A.; Badizadegan, K. A health economic analysis for oral poliovirus vaccine to prevent COVID-19 in the United States. Risk Anal. 2021, 41, 376–386. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Voorman, A.; Pallansch, M.A.; Wassilak, S.G.F.; Cochi, S.L.; Badizadegan, K.; Thompson, K.M. The impact of disruptions caused by the COVID-19 pandemic on global polio eradication. Vaccine 2021, 41, A12–A18. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A.; Badizadegan, K. Hypothetical emergence of poliovirus in 2020: Part 1. consequences of policy decisions to respond using nonpharmaceutical interventions. Expert Rev. Vaccines 2021, 20, 465–481. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A.; Badizadegan, K. Hypothetical emergence of poliovirus in 2020: Part 2. exploration of the potential role of vaccines in control and eradication. Expert Rev. Vaccines 2021, 20, 449–460. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Pallansch, M.A.; Cochi, S.L.; Thompson, K.M. Updated Characterization of Poliovirus Transmission in Pakistan and Afghanistan and the Impacts of Different Outbreak Response Vaccine Options. J. Infect. Dis. 2021, 224, 1529–1538. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Pallansch, M.A.; Cochi, S.L.; Thompson, K.M. Modeling Poliovirus Surveillance and Immunization Campaign Quality Monitoring Costs for Pakistan and Afghanistan for 2019–2023. Open Forum Infect. Dis. 2021, 8, ofab264. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Pallansch, M.A.; Wassilak, S.G.F.; Cochi, S.L.; Thompson, K.M. Serotype 2 oral poliovirus vaccine (OPV2) choices and the consequences of delaying outbreak response. Vaccine 2023, 41 (Suppl. 1), A136–A141. [Google Scholar] [CrossRef]
- Thompson, K.M. Polio eradication: What kind of world do we want? Lancet Infect. Dis. 2022, 22, 161–163. [Google Scholar] [CrossRef]
- Thompson, K.M. Effectiveness of a new vaccine for outbreak response and the increasingly complicated polio endgame. Lancet Glob. Health 2022, 10, e1697–e1698. [Google Scholar] [CrossRef]
- Thompson, K.M.; Badizadegan, K. Health economic analyses of secondary vaccine effects: A systematic review and policy insights. Expert Rev. Vaccines 2022, 21, 297–312. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A.; Badizadegan, K. Polio health economics: Assessing the benefits and costs of polio, non-polio, and integrated activities of the Global Polio Eradication Initiative. Gates Open Res. 2022, 6, 5. [Google Scholar] [CrossRef]
- Badizadegan, K.; Kalkowska, D.A.; Thompson, K.M. Polio by the numbers—A global perspective. J. Infect. Dis. 2022, 226, 1309–1318. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A.; Badizadegan, K. Health economic analysis of vaccine options for the polio eradication endgame: 2022-2036. Expert Rev. Vaccines 2022, 21, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Badizadegan, K.; Thompson, K.M. Modeling scenarios for ending poliovirus transmission in Pakistan and Afghanistan. Risk Anal. 2023, 43, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Badizadegan, K.; Thompson, K.M. Modeling undetected live type 1 wild poliovirus circulation after apparent interruption of transmission: Pakistan and Afghanistan. Risk Anal. 2023, 43, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Wassilak, S.G.F.; Pallansch, M.A.; Burns, C.C.; Wiesen, E.; Durry, E.; Badizadegan, K.; Thompson, K.M. Outbreak response strategies with type 2-containing oral poliovirus vaccines. Vaccine 2023, 41 (Suppl. 1), A142–A152. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Badizadegan, K.; Thompson, K.M. Outbreak management strategies for cocirculation of multiple poliovirus types. Vaccine 2023, 41, 3718–3727. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Kalkowska, D.A.; Badizadegan, K. Looking back at prospective modeling of outbreak response strategies for managing global type 2 oral poliovirus vaccine (OPV2) cessation. Front. Public Health 2023, 11, 1098419. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.; Wassilak, S.; Wiesen, E.; F Estivariz, C.; Burns, C.; Badizadegan, K.; Thompson, K. Complexity of options related to restarting oral poliovirus vaccine (OPV) in national immunization programs after OPV cessation. Gates Open Res. 2023, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Lauring, A.S.; Pollard, A.J.; Andino, R.; Bandyopadhyay, A.S.; Berkley, S.; Bhutta, Z.A.; Routh, J.; Benn, C.S. Polio eradication: Addressing the hurdles on the last mile. Cell 2023, 186, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M. Polio endgame complexity: Updating expectations for nOPV2. Lancet Infect. Dis. 2023, 23, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Kalkowska, D.A.; Badizadegan, K. Oral polio vaccine stockpile modeling: Insights from recent experience. Expert Rev. Vaccines 2023, 22, 813–825. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Wassilak, S.G.F.; Wiesen, E.; Burns, C.C.; Pallansch, M.A.; Badizadegan, K.; Thompson, K.M. Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences. Risk Anal. 2023, 44, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Wiesen, E.; Wassilak, S.G.F.; Burns, C.C.; Pallansch, M.A.; Badizadegan, K.; Thompson, K.M. Worst-case scenarios: Modeling uncontrolled type 2 polio transmission. Risk Anal. 2023, 44, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Badizadegan, K.; Kalkowska, D.A.; Thompson, K.M. Health economic analysis of antiviral drugs in the global polio eradication endgame. Med. Decis. Mak. 2023, 43, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Kalkowska, D.A.; Routh, J.A.; Brenner, I.R.; Rosenberg, E.S.; Zucker, J.R.; Langdon-Embry, M.; Sugerman, D.E.; Burns, C.C.; Badizadegan, K. Modeling poliovirus transmission and responses in New York State. J. Infect. Dis. 2024, 229, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Badizadegan, K.; Routh, J.A.; Burns, C.C.; Rosenberg, E.S.; Brenner, I.R.; Zucker, J.R.; Langdon-Embry, M.; Thompson, K.M. Modeling undetected poliovirus circulation following the 2022 outbreak in the United States. Expert Rev. Vaccines 2023, 23, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Kalkowska, D.A.; Kidd, S.E.; Burns, C.C.; Badizadegan, K. Trade-offs of different poliovirus vaccine options for outbreak response in the United States and other countries that only use inactivated poliovirus vaccine (IPV) in routine immunization. Vaccine 2024, 42, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Jafari, H.; Safdar, R.M.; Ahmed, J.A.; Mahamud, A.; Bandyopadhyay, A.S.; Shukla, H.; Quddus, A.; Zaffran, M.; Sutter, R.W.; et al. Modelling the spread of serotype-2 vaccine derived-poliovirus outbreak in Pakistan and Afghanistan to inform outbreak control strategies in the context of the COVID-19 pandemic. Vaccine 2023, 41, A93–A104. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Usman, A.; Javaid, A.; Wahdan, A.; Parker, E.P.K.; Ahmed, J.A.; Shah, N.; Agbor, J.; Mahamud, A.; Safdar, R.M. Quantifying movement patterns and vaccination status of high risk mobile populations in Pakistan and Afghanistan to inform poliovirus risk and vaccination strategy. Vaccine 2021, 39, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Babji, S.; Manickavasagam, P.; Chen, Y.H.; Jeyavelu, N.; Jose, N.V.; Praharaj, I.; Syed, C.; Kaliappan, S.P.; John, J.; Giri, S.; et al. Immune predictors of oral poliovirus vaccine immunogenicity among infants in South India. NPJ Vaccines 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, D.; Pons-Salort, M.; Shaw, A.G.; Grassly, N.C. The role of genetic sequencing and analysis in the polio eradication programme. Virus Evol. 2020, 6, veaa040. [Google Scholar] [CrossRef] [PubMed]
- Hamisu, A.W.; Blake, I.M.; Sume, G.; Braka, F.; Jimoh, A.; Dahiru, H.; Bonos, M.; Dankoli, R.; Mamuda Bello, A.; Yusuf, K.M.; et al. Characterizing environmental surveillance sites in Nigeria and their sensitivity to detect poliovirus and other enteroviruses. J. Infect. Dis. 2022, 225, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.V.; Bandyopadhyay, A.; Gumede-Moeletsi, N.; Mach, O.; Mkanda, P.; Ndoutabé, M.; Okiror, S.O.; Ramirez-Gonzalez, A.; Touray, K.; Wanyoike, S.; et al. Risk factors for spread of vaccine-derived type 2 polioviruses in Africa following global withdrawal of trivalent oral poliovirus vaccine and impact of outbreak response with monovalent vaccine: A retrospective analysis of surveillance data. Lancet Infect. Dis. 2022, 22, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.J.; Cooper, L.V.; Bandyopadhyay, A.S.; Blake, I.M.; Grassly, N.C. The origins and risk factors for serotype-2 vaccine-derived poliovirus (VDPV2) emergences in Africa during 2016–2019. J. Infect. Dis. 2023, 228, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Grassly, N.C.; Andrews, N.; Cooper, G.; Stephens, L.; Waight, P.; Jones, C.E.; Heath, P.T.; Calvert, A.; Southern, J.; Martin, J.; et al. Effect of maternal immunisation with multivalent vaccines containing inactivated poliovirus vaccine (IPV) on infant IPV immune response: A phase 4, multi-centre randomised trial. Vaccine 2023, 41, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.V.; Erbeto, T.B.; Danzomo, A.A.; Abdullahi, H.W.; Boateng, K.; Adamu, U.S.; Shuaib, F.; Modjirom, N.; Gray, E.J.; Bandyopadhyay, A.S.; et al. Effectiveness of poliovirus vaccines against circulating vaccine-derived type 2 poliomyelitis in Nigeria between 2017 and 2022: A case-control study. Lancet Infect. Dis. 2024, 24, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.V.; Blake, I.M. First Africa-based clinical trial for novel type 2 oral poliovirus vaccine. Lancet 2024, 403, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Cooper, L.V.; Zipursky, S. One billion doses and WHO prequalification of nOPV2: Implications for the global polio situation and beyond. PLOS Glob. Public Health 2024, 4, e0002920. [Google Scholar] [CrossRef] [PubMed]
- Kurji, F.D.; Bandyopadhyay, A.S.; Zipursky, S.; Cooper, L.V.; Gast, C.; Toher, M.; Clemens, R.; Clemens, S.A.C.; Prasad, R.; Azhari, A. Novel oral polio vaccine type 2 use for polio outbreak response: A global effort for a global health emergency. Pathogens 2024, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.G.; Majumdar, M.; Troman, C.; O’Toole, A.; Benny, B.; Abraham, D.; Praharaj, I.; Kang, G.; Sharif, S.; Alam, M.M.; et al. Rapid and sensitive direct detection and identification of poliovirus from stool and environmental surveillance samples by use of nanopore sequencing. J. Clin. Microbiol. 2020, 58, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.G.; Cooper, L.V.; Gumede, N.; Bandyopadhyay, A.S.; Grassly, N.C.; Blake, I.M. Time taken to detect and respond to polio outbreaks in Africa and the potential impact of direct molecular detection and nanopore sequencing. J. Infect. Dis. 2022, 226, 453–462. [Google Scholar] [CrossRef]
- Akello, J.O.; Bujaki, E.; Shaw, A.G.; Khurshid, A.; Arshad, Y.; Troman, C.; Majumdar, M.; O’Toole, A.; Rambaut, A.; Alam, M.M.; et al. Comparison of eleven RNA extraction methods for poliovirus direct molecular detection in stool samples. Microbiol. Spectr. 2023, 11, e0425222. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.G.; Mampuela, T.K.; Lofiko, E.L.; Pratt, C.; Troman, C.; Bujaki, E.; O’Toole, A.; Akello, J.O.; Aziza, A.A.; Lusamaki, E.K.; et al. Sensitive poliovirus detection using nested PCR and nanopore sequencing: A prospective validation study. Nat. Microbiol. 2023, 8, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.G.; Troman, C.; Akello, J.O.; O’Reilly, K.M.; Gauld, J.; Grow, S.; Grassly, N.; Steele, D.; Blazes, D.; Kumar, S.; et al. Defining a research agenda for environmental wastewater surveillance of pathogens. Nat. Med. 2023, 29, 2155–2157. [Google Scholar] [CrossRef] [PubMed]
- Grassly, N.C. Polio’s detection in London is a wake-up call. BMJ 2022, 377, o1589. [Google Scholar] [CrossRef] [PubMed]
- Klapsa, D.; Wilton, T.; Zealand, A.; Bujaki, E.; Saxentoff, E.; Troman, C.; Shaw, A.G.; Tedcastle, A.; Majumdar, M.; Mate, R.; et al. Sustained detection of type 2 poliovirus in London sewage between February and July, 2022, by enhanced environmental surveillance. Lancet 2022, 400, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Cavestany, R.L.; Blake, I.M.; Macklin, G.; Cooper, L.V.; Grassly, N.C.; Nery, A.L.; Mach, O. Use of inactivated poliovirus vaccine for poliovirus outbreak response. Lancet Infect. Dis. 2023, 24, e328–e342. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, C.; Pearson, C.A.B.; Koopman, J.S.; Hladish, T.J. Effect of population partitioning on the probability of silent circulation of poliovirus. Bull. Math. Biol. 2022, 84, 62. [Google Scholar] [CrossRef] [PubMed]
- Macklin, G.R.; O’Reilly, K.M.; Grassly, N.C.; Edmunds, W.J.; Mach, O.; Santhana Gopala Krishnan, R.; Voorman, A.; Vertefeuille, J.F.; Abdelwahab, J.; Gumede, N.; et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science 2020, 368, 401–405. [Google Scholar] [CrossRef]
- O’Reilly, K.M.; Grassly, N.C.; Allen, D.J.; Bannister-Tyrrell, M.; Cameron, A.; Carrion Martin, A.I.; Ramsay, M.; Pebody, R.; Zambon, M. Surveillance optimisation to detect poliovirus in the pre-eradication era: A modelling study of England and Wales. Epidemiol. Infect. 2020, 148, e157. [Google Scholar] [CrossRef]
- Macklin, G.R.; Goel, A.K.; Mach, O.; Tallis, G.; Ahmed, J.A.; O’Reilly, K.M.; Grassly, N.C.; Diop, O.M. Epidemiology of type 2 vaccine-derived poliovirus outbreaks between 2016 and 2020. Vaccine 2023, 41, A19–A24. [Google Scholar] [CrossRef]
- Auzenbergs, M.; Fountain, H.; Macklin, G.; Lyons, H.; O’Reilly, K.M. The impact of surveillance and other factors on detection of emergent and circulating vaccine derived polioviruses. Gates Open Res. 2021, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Macklin, G.R. Final frontiers of the polio eradication endgame. Curr. Opin. Infect. Dis. 2020, 33, 404–410. [Google Scholar] [CrossRef]
- Macklin, G.R.; Peak, C.; Eisenhawer, M.; Kurji, F.; Mach, O.; Konz, J.; Gast, C.; Bachtiar, N.S.; Bandyopadhyay, A.S.; Zipursky, S.; et al. Enabling accelerated vaccine roll-out for Public Health Emergencies of International Concern (PHEICs): Novel oral polio vaccine type 2 (nOPV2) experience. Vaccine 2023, 41 (Suppl. 1), A122–A127. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.M. Population immunity and polio eradication. Pathogens 2024, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, K.M.; Allen, D.J.; Fine, P.; Asghar, H. The challenges of informative wastewater sampling for SARS-CoV-2 must be met: Lessons from polio eradication. Lancet Microbe 2020, 1, e189–e190. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Gast, C.; Brickley, E.B.; Rüttimann, R.; Clemens, R.; Oberste, M.S.; Weldon, W.C.; Ackerman, M.E.; Connor, R.I.; Wieland-Alter, W.F.; et al. A randomized phase 4 study of immunogenicity and safety after monovalent oral type 2 Sabin poliovirus vaccine challenge in children vaccinated with inactivated poliovirus vaccine in Lithuania. J. Infect. Dis. 2021, 223, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Brickley, E.B.; Connor, R.I.; Wieland-Alter, W.; Weiner, J.A.; Ackerman, M.E.; Arita, M.; Gast, C.; De Coster, I.; Van Damme, P.; Bandyopadhyay, A.S.; et al. Intestinal antibody responses to 2 novel live attenuated type 2 oral poliovirus vaccines in healthy adults in Belgium. J. Infect. Dis. 2022, 226, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Connor, R.I.; Brickley, E.B.; Wieland-Alter, W.F.; Ackerman, M.E.; Weiner, J.A.; Modlin, J.F.; Bandyopadhyay, A.S.; Wright, P.F. Mucosal immunity to poliovirus. Mucosal Immunol. 2022, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Famulare, M.; Wong, W.; Haque, R.; Platts-Mills, J.A.; Saha, P.; Aziz, A.B.; Ahmed, T.; Islam, M.O.; Uddin, M.J.; Bandyopadhyay, A.S.; et al. Multiscale model for forecasting Sabin 2 vaccine virus household and community transmission. PLoS Comput. Biol. 2021, 17, e1009690. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Gauld, J.; Famulare, M. From vaccine to pathogen: Modeling Sabin 2 vaccine virus reversion and evolutionary epidemiology in Matlab, Bangladesh. Virus Evol. 2023, 9, vead044. [Google Scholar] [CrossRef]
- Brouwer, A.F.; Eisenberg, M.C.; Shulman, L.M.; Famulare, M.; Koopman, J.S.; Kroiss, S.J.; Hindiyeh, M.; Manor, Y.; Grotto, I.; Eisenberg, J.N.S. The role of time-varying viral shedding in modelling environmental surveillance for public health: Revisiting the 2013 poliovirus outbreak in Israel. J. R. Soc. Interface 2022, 19, 20220006. [Google Scholar] [CrossRef] [PubMed]
- Valesano, A.L.; Taniuchi, M.; Fitzsimmons, W.J.; Islam, M.O.; Ahmed, T.; Zaman, K.; Haque, R.; Wong, W.; Famulare, M.; Lauring, A.S. The early evolution of oral poliovirus vaccine Is shaped by strong positive selection and tight transmission bottlenecks. Cell Host Microbe 2021, 29, 32–43.e34. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.P.; Cullen, A.C.; Chabot-Couture, G. Disease surveillance investments and administration: Limits to information value in Pakistan polio eradication. Risk Anal. 2021, 41, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Voorman, A.; O’Reilly, K.; Lyons, H.; Goel, A.K.; Touray, K.; Okiror, S. Real-time prediction model of cVDPV2 outbreaks to aid outbreak response vaccination strategies. Vaccine 2023, 41, A105–A112. [Google Scholar] [CrossRef] [PubMed]
- Voorman, A.; Lyons, H.; Bennette, C.; Kovacs, S.; Makam, J.K.; F Vertefeuille, J.; Tallis, G. Analysis of population immunity to poliovirus following cessation of trivalent oral polio vaccine. Vaccine 2023, 41, A85–A92. [Google Scholar] [CrossRef] [PubMed]
- Voorman, A.; Lyons, H.; Shuaib, F.; Adamu, U.S.; Korir, C.; Erbeto, T.; Bandyopadhyay, A.S.; Okiror, S. Impact of supplementary immunization activities using novel oral polio vaccine type 2 during a large outbreak of circulating vaccine-derived poliovirus in Nigeria. J. Infect. Dis. 2024, 229, 805–812. [Google Scholar] [CrossRef]
- Sun, Y.; Keskinocak, P.; Steimle, L.N.; Kovacs, S.D.; Wassilak, S.G. A compartmental simulation model to improve interventions for controlling poliovirus outbreaks. In Proceedings of the 2023 Winter Simulation Conference (WSC), San Antonio, TX, USA, 10–13 December 2023; pp. 1184–1195. [Google Scholar]
- Sun, Y.; Keskinocak, P.; Steimle, L.N.; Kovacs, S.D.; Wassilak, S.G. Modeling the spread of circulating vaccine-derived poliovirus type 2 outbreaks and interventions: A case study of Nigeria. Vaccine X 2024, 18, 100476. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Gambhirrao, N.; Patel, R.; Zhowandai, A.; Rychtář, J.; Taylor, D. A game-theoretical analysis of poliomyelitis vaccination. J. Theor. Biol. 2020, 499, 110298. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.R.; Huppert, A.; Fitzpatrick, M.C.; Pandey, A.; Velan, B.; Singer, B.H.; Bauch, C.T.; Galvani, A.P. Prosocial polio vaccination in Israel. Proc. Natl. Acad. Sci. USA 2020, 117, 13138–13144. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Maimaiti, H.; Sun, X.; Huang, Z.; Liu, J.; Yang, J.; Li, Z.; Bai, Q.; Lu, Y. Cost-effectiveness of three poliovirus immunization schedules in Shanghai, China. Vaccines 2021, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; Wang, J.; Che, X.; Du, J.; Zhang, X.; Gu, W.; Zhang, X.; Jiang, W. Cost-effectiveness of various immunization schedules with inactivated Sabin strain polio vaccine in Hangzhou, China. Front. Public Health 2022, 10, 990042. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Aaby, P.; Avidan, M.S.; Benn, C.S.; Bertozzi, S.M.; Blatt, L.; Chumakov, K.; Khader, S.A.; Kottilil, S.; Nekkar, M.; et al. One vaccine to counter many diseases? Modeling the economics of oral polio vaccine against child mortality and COVID-19. Front. Public Health 2022, 10, 967920. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Macias-Diaz, J.E.; Shahid, N.; Raza, A.; Rafiq, M. A dynamically consistent computational method to solve numerically a mathematical model of polio propagation with spatial diffusion. Comput. Methods Programs Biomed. 2022, 218, 106709. [Google Scholar] [CrossRef] [PubMed]
- Alrawajeh, F.A.; Allehiany, F.M.; Raza, A.; Abdelmohsen, S.A.M.; Cheema, T.N.; Rafiq, M.; Mohsin, M. Bio-inspired computational methods for the polio virus epidemic model. CMC-Comput. Mater. Contin. 2022, 72, 2357–2374. [Google Scholar] [CrossRef]
- Liu, X.; Rahman, M.U.; Arfan, M.; Tchier, F.; Ahmad, S.; Inc, M.; Akinyemi, L. Fractional mathematical modeling to the spread of polio with the role of vaccination under non-singular kernal. Fractals-Complex Geom. Patterns Scaling Nat. Soc. 2022, 30, 17. [Google Scholar] [CrossRef]
- Naveed, M.; Baleanu, D.; Raza, A.; Rafiq, M.; Soori, A.H. Treatment of polio delayed epidemic model via computer simulations. CMC-Comput. Mater. Contin. 2022, 70, 3415–3431. [Google Scholar] [CrossRef]
- Raza, A.; Baleanu, D.; Khan, Z.U.; Mohsin, M.; Ahmed, N.; Rafiq, M.; Anwar, P. Stochastic analysis for the dynamics of a poliovirus epidemic model. CMES-Comput. Model. Eng. Sci. 2022, 136, 257–275. [Google Scholar] [CrossRef]
- Bornaa, C.S.; Seidu, B.; Makinde, O.D. Mathematical analysis of the impact of vaccination and poor sanitation on the dynamics of poliomyelitis. Int. J. Nonlinear Sci. Numer. Simul. 2023, 24, 161–169. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Ahmed, N.; Akgül, A.; Satti, A.M.; Iqbal, Z.; Raza, A.; Rafiq, M.; Anjum, R.; Zakarya, M.; Park, C. Analysis of the fractional polio model with the Mittag-Leffler kernels. Alex. Eng. J. 2023, 64, 957–967. [Google Scholar] [CrossRef]
- Karaagac, B.; Owolabi, K.M. Numerical analysis of polio model: A mathematical approach to epidemiological model using derivative with Mittag-Leffler Kernel. Math. Methods Appl. Sci. 2023, 46, 8175–8192. [Google Scholar] [CrossRef]
- Wang, X.J.; Lin, G. Spreading speeds in two reaction-diffusion models for polio disease. Commun. Nonlinear Sci. Numer. Simul. 2023, 118, 18. [Google Scholar] [CrossRef]
- World Health Assembly. Global Eradication of Poliomyelitis by the Year 2000; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Thompson, K.M.; Badizadegan, K. Evolution of global polio eradication strategies: Targets, vaccines, and supplemental immunization activities (SIAs). Expert Rev. Vaccines, 2024; In press. [Google Scholar]
- World Health Organization. Vaccines & Biologicals Annual Report 1999; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Fine, P.E.M. Herd immunity: History, theory, practice. Epidemiol. Rev. 1993, 15, 265–302. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.; Carneiro, I.A. Transmissibility and persistence of oral polio vaccine viruses: Implications for the global poliomyelitis eradication initiative. Am. J. Epidemiol. 1999, 150, 1001–1021. [Google Scholar] [CrossRef]
- Eichner, M.; Hadeler, K.P. Deterministic models for the eradication of poliomyelitis: Vaccination with the inactivated (IPV) and attenuated (OPV) polio virus vaccine. Math. Biosci. 1995, 127, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Eichner, M.; Hadeler, K.P.; Dietz, K. Stochastic models for the eradication of poliomyelitis: Minimum population size for polio virus persistence. In Models for Infectious Human Diseases: Their Structure and Relation to Data; Isham, V., Medley, G.F., Eds.; Cambridge University Press: New York, NY, USA, 1996; pp. 315–327. [Google Scholar]
- Bart, K.J.; Foulds, J.; Patriarca, P. Global eradication of poliomyelitis: Benefit-cost analysis. Bull. World Health Organ. 1996, 74, 35–45. [Google Scholar] [PubMed]
- O’Reilly, K.M.; Verity, R.; Durry, E.; Asghar, H.; Sharif, S.; Zaidi, S.Z.; Wadood, M.Z.M.; Diop, O.M.; Okayasu, H.; Safdar, R.M.; et al. Population sensitivity of acute flaccid paralysis and environmental surveillance for serotype 1 poliovirus in Pakistan: An observational study. BMC Infect. Dis. 2018, 18, 176. [Google Scholar] [CrossRef] [PubMed]
- Macklin, G.R.; Grassly, N.C.; Sutter, R.W.; Mach, O.; Bandyopadhyay, A.S.; Edmunds, W.J.; O’Reilly, K.M. Vaccine schedules and the effect on humoral and intestinal immunity against poliovirus: A systematic review and network meta-analysis. Lancet Infect. Dis. 2019, 19, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.; Sutter, R.W.; Orenstein, W.A. Stopping a polio outbreak in the post-eradication era. Dev. Biol. 2001, 105, 129–147; discussion 149–150. [Google Scholar]
- Wringe, A.; Fine, P.E.; Sutter, R.W.; Kew, O.M. Estimating the extent of vaccine-derived poliovirus infection. PLoS ONE 2008, 3, e3433. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.F.; Connor, R.I.; Wieland-Alter, W.F.; Hoen, A.G.; Boesch, A.W.; Ackerman, M.E.; Oberste, M.S.; Gast, C.; Brickley, E.B.; Asturias, E.J.; et al. Vaccine-induced mucosal immunity to poliovirus: Analysis of cohorts from an open-label, randomised controlled trial in Latin American infants. Lancet Infect. Dis. 2016, 16, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Brickley, E.B.; Strauch, C.B.; Wieland-Alter, W.F.; Connor, R.I.; Lin, S.; Weiner, J.A.; Ackerman, M.E.; Arita, M.; Oberste, M.S.; Weldon, W.C.; et al. Intestinal immune responses to type 2 oral polio vaccine (OPV) challenge in infants previously immunized with bivalent OPV and either high-dose or standard inactivated polio vaccine. J. Infect. Dis. 2018, 217, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Brickley, E.B.; Connor, R.I.; Wieland-Alter, W.F.; Collett, M.S.; Hartford, M.; Van Der Avoort, H.; Boesch, A.W.; Weiner, J.A.; Ackerman, M.E.; McKinlay, M.A.; et al. Intestinal antibody responses to a live oral poliovirus vaccine challenge among adults previously immunized with inactivated polio vaccine in Sweden. BMJ Glob. Health 2019, 4, e001613. [Google Scholar] [CrossRef] [PubMed]
- Brickley, E.B.; Wieland-Alter, W.; Connor, R.I.; Ackerman, M.E.; Boesch, A.W.; Arita, M.; Weldon, W.C.; O’Ryan, M.G.; Bandyopadhyay, A.S.; Wright, P.F. Intestinal immunity to poliovirus following sequential trivalent inactivated polio vaccine/bivalent oral polio vaccine and trivalent inactivated polio vaccine-only immunization schedules: Analysis of an open-label, randomized, controlled trial in Chilean infants. Clin. Infect. Dis. 2018, 67, S42–S50. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.M. Gaps in our knowledge about transmission of vaccine-derived polioviruses. Bull. World Health Organ. 2000, 78, 358–359. [Google Scholar] [PubMed]
- Nathanson, N.; Fine, P.E.M. Virology. Poliomyelitis eradication—A dangerous endgame. Science 2002, 296, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.; Ritchie, S. Perspective: Determinants of the severity of poliovirus outbreaks in the post eradication era. Risk Anal. 2006, 26, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.M.; Oblapenko, G.; Sutter, R.W. Polio control after certification: Major issues outstanding. Bull. World Health Organ. 2004, 82, 47–52. [Google Scholar] [PubMed]
- Fine, P.E. Poliomyelitis: Very small risks and very large risks. Lancet Neurol. 2004, 3, 703. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.; Griffiths, U.K. Global poliomyelitis eradication: Status and implications. Lancet 2007, 369, 1321–1322. [Google Scholar] [CrossRef]
- Fine, P.E. Polio: Measuring the protection that matters most. J. Infect. Dis. 2009, 200, 673–675. [Google Scholar] [CrossRef]
- Fine, P. Commentary: An unexpected finding that needs confirmation or rejection. BMJ 2000, 321, 1439. [Google Scholar] [PubMed]
- Brickley, E.B.; Wright, P.F. Maximising the impact of inactivated polio vaccines. Lancet Infect. Dis. 2017, 17, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Voorman, A.; Hoff, N.A.; Doshi, R.H.; Alfonso, V.; Mukadi, P.; Muyembe-Tamfum, J.J.; Wemakoy, E.O.; Bwaka, A.; Weldon, W.; Gerber, S.; et al. Polio immunity and the impact of mass immunization campaigns in the Democratic Republic of the Congo. Vaccine 2017, 35, 5693–5699. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, V.H.; Voorman, A.; Hoff, N.A.; Weldon, W.C.; Gerber, S.; Gadoth, A.; Halbrook, M.; Goldsmith, A.; Mukadi, P.; Doshi, R.H.; et al. Poliovirus immunity among adults in the Democratic Republic of the Congo: A cross-sectional serosurvey. BMC Infect. Dis. 2022, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Voorman, A.; Habib, M.A.; Hussain, I.; Muhammad Safdar, R.; Ahmed, J.A.; Weldon, W.C.; Ahmed, I.; Umer, M.; Partridge, J.; Soofi, S.B. Immunity and field efficacy of type 2-containing polio vaccines after cessation of trivalent oral polio vaccine: A population-based serological study in Pakistan. Vaccine X 2020, 5, 100067. [Google Scholar] [CrossRef] [PubMed]
- VanderEnde, K.; Voorman, A.; Khan, S.; Anand, A.; Snider, C.J.; Goel, A.; Wassilak, S. New analytic approaches for analyzing and presenting polio surveillance data to supplement standard performance indicators. Vaccine X 2020, 4, 100059. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Strategic Advisory Group of Experts on Immunization Meetings. Available online: https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization/meetings (accessed on 28 March 2024).
- Global Polio Eradication Initiative. Progress 1999: Every Child Counts. Available online: https://iris.who.int/bitstream/handle/10665/66964/WHO_POLIO_00.03.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. Progress 2000. Available online: https://iris.who.int/bitstream/handle/10665/66763/WHO_POLIO_01.03.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. Progress 2001. Available online: https://iris.who.int/bitstream/handle/10665/67417/WHO_POLIO_02.08.pd (accessed on 13 November 2023).
- Global Polio Eradication Initiative. Progress 2002. Available online: https://iris.who.int/bitstream/handle/10665/67931/WHO_POLIO_03.02.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. Progress 2003. Available online: https://iris.who.int/bitstream/handle/10665/68653/WHO_POLIO_04.02.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2004 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/69055/WHO_POLIO_05.03.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2005 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/70869/WHO_Polio_06.02_eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2006 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/70868/WHO_Polio_07.02_eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2007 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/70867/WHO_Polio_08.02_eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2008 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/70866/WHO_POLIO_09.03_eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2009 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/70865/WHO_Polio_10.05_eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2010 Annual Report: Every Last Child. Available online: https://iris.who.int/bitstream/handle/10665/70849/WHO_POLIO_11.02_eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2011 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/276243/WHO-POLIO-12.02-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2012 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/276241/WHO-POLIO-13.03-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2013 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/276239/WHO-POLIO-14.02-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2014 Annual Report: On the Threshold of a Polio Free World. Available online: https://iris.who.int/bitstream/handle/10665/276238/WHO-POLIO-15.01-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2015 Annual Report: Eradication within Reach. Available online: https://iris.who.int/bitstream/handle/10665/250145/WHO-POLIO-16.01-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2016 Annual Report: Eradication within Reach. Available online: https://iris.who.int/bitstream/handle/10665/276236/WHO-POLIO-17.03-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2017 Annual Report: Securing a Lasting World Free of All Polioviruses. Available online: https://iris.who.int/bitstream/handle/10665/276237/WHO-POLIO-18.01-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2018 Annual Report: To Achieve Lasting Success. Available online: https://polioeradication.org/wp-content/uploads/2016/07/Annual-report-2018.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2019 Annual Report and Semi-Annual Status Updates, January–June and July–December 2019. Available online: https://iris.who.int/bitstream/handle/10665/336693/9789240013117-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2020 Annual Report and Semi-Annual Status Updates, January–June and July–December 2020. Available online: https://iris.who.int/bitstream/handle/10665/344329/9789240030763-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2021 Annual Report. Available online: https://iris.who.int/bitstream/handle/10665/364215/9789240058934-eng.pdf (accessed on 13 November 2023).
- Global Polio Eradication Initiative. 2022 Annual Report. Available online: https://polioeradication.org/wp-content/uploads/2023/06/GPEI_2022_Expenditure_Report.pdf (accessed on 13 November 2023).
- Polio Oversight Board. Meeting of the Polio Oversight Board, Global Polio Eradication Initiative, 12 December 2014. Available online: https://polioeradication.org/wp-content/uploads/2016/07/POB_Minutes_Mtg20141212.pdf (accessed on 10 April 2023).
- Polio Oversight Board. Meeting of the Polio Oversight Board (Call), 13 January 2016. Available online: https://polioeradication.org/wp-content/uploads/2016/07/POB_Minutes_Mtg20160113.pdf (accessed on 10 April 2023).
- Polio Oversight Board. Meeting of the Polio Oversight Board (POB)—Teleconference, 18 December 2020. Available online: https://polioeradication.org/wp-content/uploads/2021/02/POB-minutes-20201218.pdf (accessed on 10 April 2024).
- Kalkowska, D.A.; Pallansch, M.A.; Thompson, K.M. Updated modelling of the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus (iVDPV) excreters. Epidemiol. Infect. 2019, 147, e295. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative; World health organization. Polio Endgame Strategy 2019–2023: Eradication, Integration, Certification and Containment; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Global Polio Eradication Initiative. Polio Post-Certification Strategy: A Risk Mitigation Strategy for a Polio-Free World; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Cochi, S.L.; Wassilak, S.G.F.; Thompson, K.M. An economic analysis of poliovirus risk management policy options for 2013–2052. BMC Infect. Dis. 2015, 15, 389. [Google Scholar] [CrossRef] [PubMed]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Wassilak, S.G.; Cochi, S.L.; Thompson, K.M. Characterization of outbreak response strategies and potential vaccine stockpile needs for the polio endgame. BMC Infect. Dis. 2016, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Cochi, S.L.; Ehrhardt, D.T.; Farag, N.H.; Hadler, S.C.; Hampton, L.M.; Martinez, M.; Wassilak, S.G.F.; Thompson, K.M. Modeling poliovirus transmission in Pakistan and Afghanistan to inform vaccination strategies in undervaccinated subpopulations. Risk Anal. 2018, 38, 1701–1717. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Duintjer Tebbens, R.J.; Thompson, K.M. Environmental surveillance system characteristics and impacts on confidence about no undetected serotype 1 wild poliovirus circulation. Risk Anal. 2019, 39, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Duintjer Tebbens, R.J.; Pallansch, M.A.; Thompson, K.M. Modeling undetected live poliovirus circulation after apparent interruption of transmission: Pakistan and Afghanistan. Risk Anal. 2019, 39, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Duintjer Tebbens, R.J.; Thompson, K.M. Evaluation of proactive and reactive strategies for polio eradication activities in Pakistan and Afghanistan. Risk Anal. 2019, 39, 389–401. [Google Scholar] [CrossRef] [PubMed]
- African Regional Commission for the Certification of Poliomyelitis Eradication. Certifying the interruption of wild poliovirus transmission in the WHO African region on the turbulent journey to a polio-free world. Lancet Glob. Health 2020, 8, e1345–e1351. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report from the Twentieth Meeting of the Global Commission for Certification of Poliomyelitis Eradication, Geneva, Switzerland, 17–18 October 2019. Available online: http://polioeradication.org/wp-content/uploads/2016/07/20th-meeting-of-the-Global-Commission-for-the-Certification-of-Eradication-of-Poliomyelitis-17-18-October-2019.pdf (accessed on 31 December 2019).
- Global Polio Eradication Initiative; World Health Organization. Strategy for the Response to Type 2 Circulating Vaccine-Derived Poliovirus 2020–2021: An Addendum to the Polio Endgame Strategy 2019-2023; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Cochi, S.L.; Wassilak, S.G.; Linkins, J.; Sutter, R.W.; Aylward, R.B.; Thompson, K.M. Economic analysis of the Global Polio Eradication Initiative. Vaccine 2010, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, K.; Benn, C.S.; Aaby, P.; Kottilil, S.; Gallo, R. Can existing live vaccines prevent COVID-19? Science 2020, 368, 1187–1188. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Arbess, D.J. An Old Vaccine May Help against Coronavirus: A Tablet for Polio Boosts Innate Immunity, Which Fights Other Viruses. Available online: https://www.wsj.com/articles/an-old-vaccine-may-help-against-coronavirus-11593557168 (accessed on 1 July 2020).
- Thompson, K.M.; Kalkowska, D.A.; Badizadegan, K. No role for reintroducing OPV into the United States with respect to controlling COVID-19 [Response to the letter to the Editor by Chumakov et al.]. Risk Anal. 2021, 41, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative; World Health Organization. Polio Eradication Strategy 2022–2026: Delivering on a Promise; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Thompson, K.M.; Duintjer Tebbens, R.J. Eradication versus control for poliomyelitis: An economic analysis. Lancet 2007, 369, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report from the Twenty-Second Meeting of the Global Commission for Certification of Poliomyelitis Eradication, Geneva, Switzerland, 3 June 2022. Available online: https://polioeradication.org/wp-content/uploads/2022/09/22nd-GCC-report-20220907.pdf (accessed on 12 September 2022).
- Kidd, S.; Clark, T.; Routh, J.; Cineas, S.; Bahta, L.; Brooks, O. Use of inactivated polio vaccine among U.S. qdults: Updated recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1327–1330. [Google Scholar] [CrossRef]
- Bunimovich-Mendrazitsky, S.; Stone, L. Modeling polio as a disease of development. J. Theor. Biol. 2005, 237, 302–315. [Google Scholar] [CrossRef]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Kew, O.M.; Caceres, V.M.; Sutter, R.W.; Thompson, K.M. A dynamic model of poliomyelitis outbreaks: Learning from the past to help inform the future. Am. J. Epidemiol. 2005, 162, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Duintjer Tebbens, R.J. The case for cooperation in managing and maintaining the end of poliomyelitis: Stockpile needs and coordinated OPV cessation. Medscape J. Med. 2008, 10, 190. [Google Scholar] [PubMed]
- Thompson, K.M.; Tebbens, R.J.; Pallansch, M.A.; Kew, O.M.; Sutter, R.W.; Aylward, R.B.; Watkins, M.; Gary, H.E., Jr.; Alexander, J.; Jafari, H.; et al. The risks, costs, and benefits of possible future global policies for managing polioviruses. Am. J. Public Health 2008, 98, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Grassly, N.C.; Fraser, C.; Wenger, J.; Deshpande, J.M.; Sutter, R.W.; Heymann, D.L.; Aylward, R.B. New strategies for the elimination of polio from India. Science 2006, 314, 1150–1153. [Google Scholar] [CrossRef]
- Grassly, N.C.; Wenger, J.; Durrani, S.; Bahl, S.; Deshpande, J.M.; Sutter, R.W.; Heymann, D.L.; Aylward, R.B. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: A case-control study. Lancet 2007, 369, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.E.; Aylward, R.B.; Gasasira, A.; Donnelly, C.A.; Abanida, E.A.; Koleosho-Adelekan, T.; Grassly, N.C. Effectiveness of immunization against paralytic poliomyelitis in Nigeria. N. Engl. J. Med. 2008, 359, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.E.; Aylward, R.B.; Gasasira, A.; Donnelly, C.A.; Mwanza, M.; Corander, J.; Garnier, S.; Chauvin, C.; Abanida, E.; Pate, M.A.; et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N. Engl. J. Med. 2010, 326, 2360–2369. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Hagedorn, B.; Lyons, H. Projection of costs of polio eradication compared to permanent control. J. Infect. Dis. 2020, 221, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative. Investment Case 2022–2026; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Status of polio outbreaks in 2005. Polio Erad. Initiat.—Polio News 2005, 25, 2–3. [Google Scholar]
- Thompson, K.M.; Duintjer Tebbens, R.J.; Pallansch, M.A. Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal. 2006, 26, 1541–1556. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M. Poliomyelitis and the role of risk analysis in global infectious disease policy and management. Risk Anal. 2006, 26, 1419–1421. [Google Scholar] [CrossRef] [PubMed]
- Dowdle, W.; van der Avoort, H.; de Gourville, E.; Delpeyroux, F.; Desphande, J.; Hovi, T.; Martin, J.; Pallansch, M.; Kew, O.; Wolff, C. Containment of polioviruses after eradication and OPV cessation: Characterizing risks to improve management. Risk Anal. 2006, 26, 1449–1469. [Google Scholar] [CrossRef]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Kew, O.M.; Caceres, V.M.; Jafari, H.; Cochi, S.L.; Sutter, R.W.; Aylward, R.B.; Thompson, K.M. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal. 2006, 26, 1471–1505. [Google Scholar] [CrossRef] [PubMed]
- Grassly, N.C.; Jafari, H.; Bahl, S.; Sethi, R.; Deshpande, J.M.; Wolff, C.; Sutter, R.W.; Aylward, R.B. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J. Infect. Dis. 2012, 205, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Duintjer Tebbens, R.J. Modeling the dynamics of oral poliovirus vaccine cessation. J. Infect. Dis. 2014, 210 (Suppl. 1), S475–S484. [Google Scholar] [CrossRef] [PubMed]
- Pons-Salort, M.; Molodecky, N.A.; O’Reilly, K.M.; Wadood, M.Z.; Safdar, R.M.; Etsano, A.; Vaz, R.G.; Jafari, H.; Grassly, N.C.; Blake, I.M. Population immunity against serotype-2 poliomyelitis leading up to the global withdrawal of the oral poliovirus vaccine: Spatio-temporal modelling of surveillance data. PLOS Med. 2016, 13, e1002140. [Google Scholar] [CrossRef]
- Grassly, N.C. Immunogenicity and effectiveness of routine immunization with 1 or 2 doses of inactivated poliovirus vaccine: Systematic review and meta-analysis. J. Infect. Dis. 2014, 210 (Suppl. 1), S439–S446. [Google Scholar] [CrossRef] [PubMed]
- Macklin, G.; Liao, Y.; Takane, M.; Dooling, K.; Gilmour, S.; Mach, O.; Kew, O.M.; Sutter, R.W.; iVDPV Working Group. Prolonged excretion of poliovirus among individuals with primary immunodeficiency disorder: An analysis of the World Health Organization registry. Front. Immunol. 2017, 8, 1103. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, M.; Famulare, M.; Zaman, K.; Uddin, M.J.; Upfill-Brown, A.M.; Ahmed, T.; Saha, P.; Haque, R.; Bandyopadhyay, A.S.; Modlin, J.F.; et al. Community transmission of type 2 poliovirus after cessation of trivalent oral polio vaccine in Bangladesh: An open-label cluster-randomised trial and modelling study. Lancet Infect. Dis. 2017, 17, 1069–1079. [Google Scholar] [CrossRef]
- Parker, E.P.; Molodecky, N.A.; Pons-Salort, M.; O’Reilly, K.M.; Grassly, N.C. Impact of inactivated poliovirus vaccine on mucosal immunity: Implications for the polio eradication endgame. Expert Rev. Vaccines 2015, 14, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Mangal, T.D.; Aylward, R.B.; Grassly, N.C. The potential impact of routine immunization with inactivated poliovirus vaccine on wild-type or vaccine-derived poliovirus outbreaks in a posteradication setting. Am. J. Epidemiol. 2013, 178, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Shirreff, G.; Wadood, M.Z.; Vaz, R.G.; Sutter, R.W.; Grassly, N.C. Estimated effect of inactivated poliovirus vaccine campaigns, Nigeria and Pakistan, January 2014–April 2016. Emerg. Infect. Dis. 2017, 23, 258–263. [Google Scholar] [CrossRef]
- Grassly, N.C.; Wadood, M.Z.; Safdar, R.M.; Mahamud, A.S.; Sutter, R.W. Effect of inactivated poliovirus vaccine campaigns, Pakistan, 2014–2017. Emerg. Infect. Dis. 2018, 24, 2113–2115. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Duintjer Tebbens, R.J. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 2006, 26, 1423–1440. [Google Scholar] [CrossRef] [PubMed]
- Duintjer Tebbens, R.J.; Sangrujee, N.; Thompson, K.M. The costs of future polio risk management policies. Risk Anal. 2006, 26, 1507–1531. [Google Scholar] [CrossRef] [PubMed]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Kew, O.M.; Sutter, R.W.; Bruce Aylward, R.; Watkins, M.; Gary, H.; Alexander, J.; Jafari, H.; Cochi, S.L.; et al. Uncertainty and sensitivity analyses of a decision analytic model for posteradication polio risk management. Risk Anal. 2008, 28, 855–876. [Google Scholar] [CrossRef] [PubMed]
- Sangrujee, N.; Duintjer Tebbens, R.J.; Caceres, V.M.; Thompson, K.M. Policy decision options during the first 5 years following certification of polio eradication. MedGenMed 2003, 5, 35. [Google Scholar] [PubMed]
- Duintjer Tebbens, R.J.; Thompson, K.M. Modeling the potential role of inactivated poliovirus vaccine to manage the risks of oral poliovirus vaccine cessation. J. Infect. Dis. 2014, 210 (Suppl. 1), S485–S497. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Duintjer Tebbens, R.J.; Thompson, K.M. Modeling strategies to increase population immunity and prevent poliovirus transmission in the high-risk area of northwest Nigeria. J. Infect. Dis. 2014, 210 (Suppl. 1), S412–S423. [Google Scholar] [CrossRef] [PubMed]
- Pons-Salort, M.; Burns, C.C.; Lyons, H.; Blake, I.M.; Jafari, H.; Oberste, M.S.; Kew, O.M.; Grassly, N.C. Preventing vaccine-derived poliovirus emergence during the polio endgame. PLoS Pathog. 2016, 12, e1005728. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).