Vertical Intrauterine Bovine and Ovine Papillomavirus Coinfection in Pregnant Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Samples
2.3. DNA Extraction
2.4. Droplet Digital Polymerase Chain Reaction (ddPCR)
2.5. RNA Extraction and One-Step Reverse Transcription (RT)-ddPCR
2.6. Antibodies
2.7. Western Blot (WB) and Densitometric Analysis
2.8. Statistical Analysis
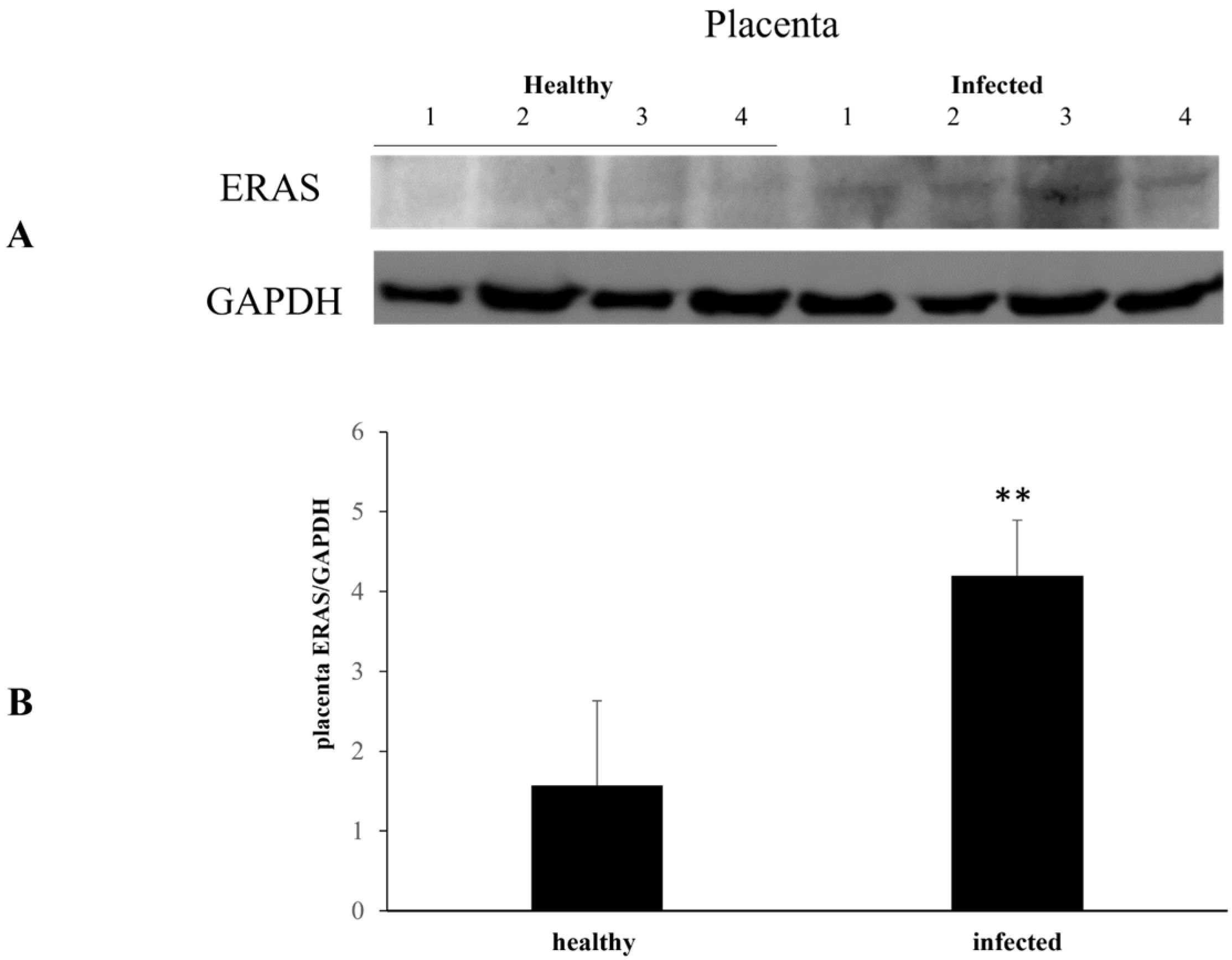
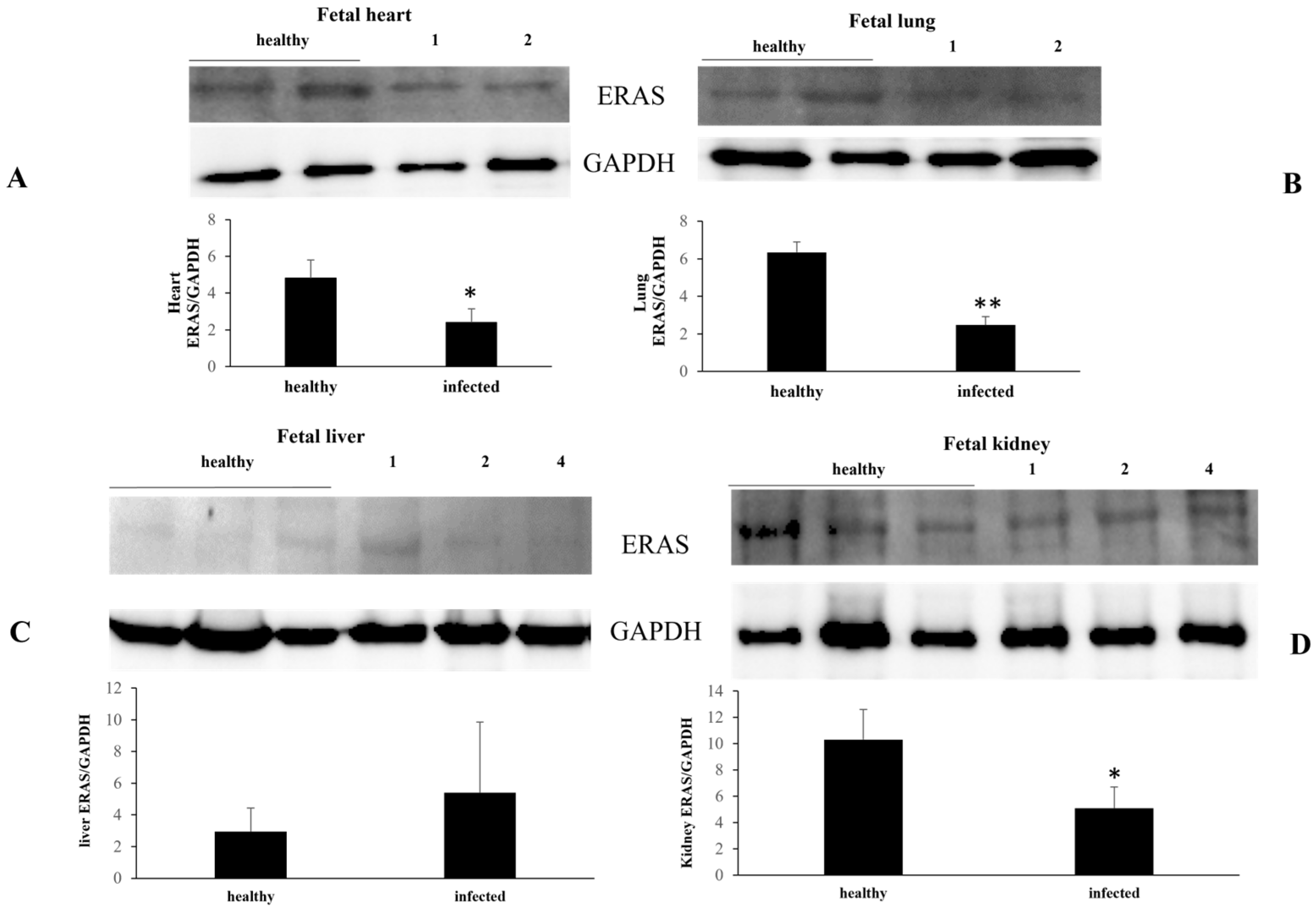
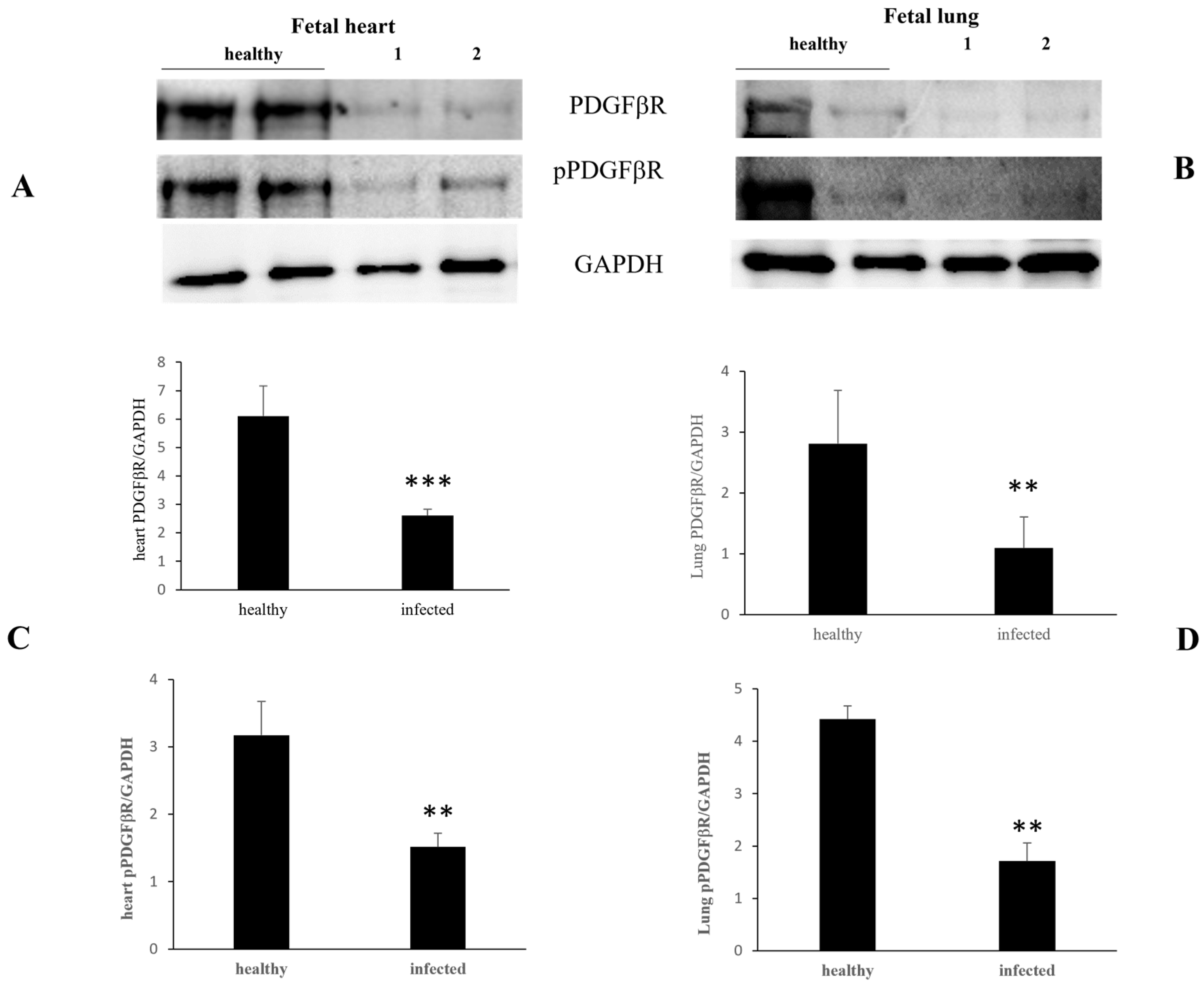
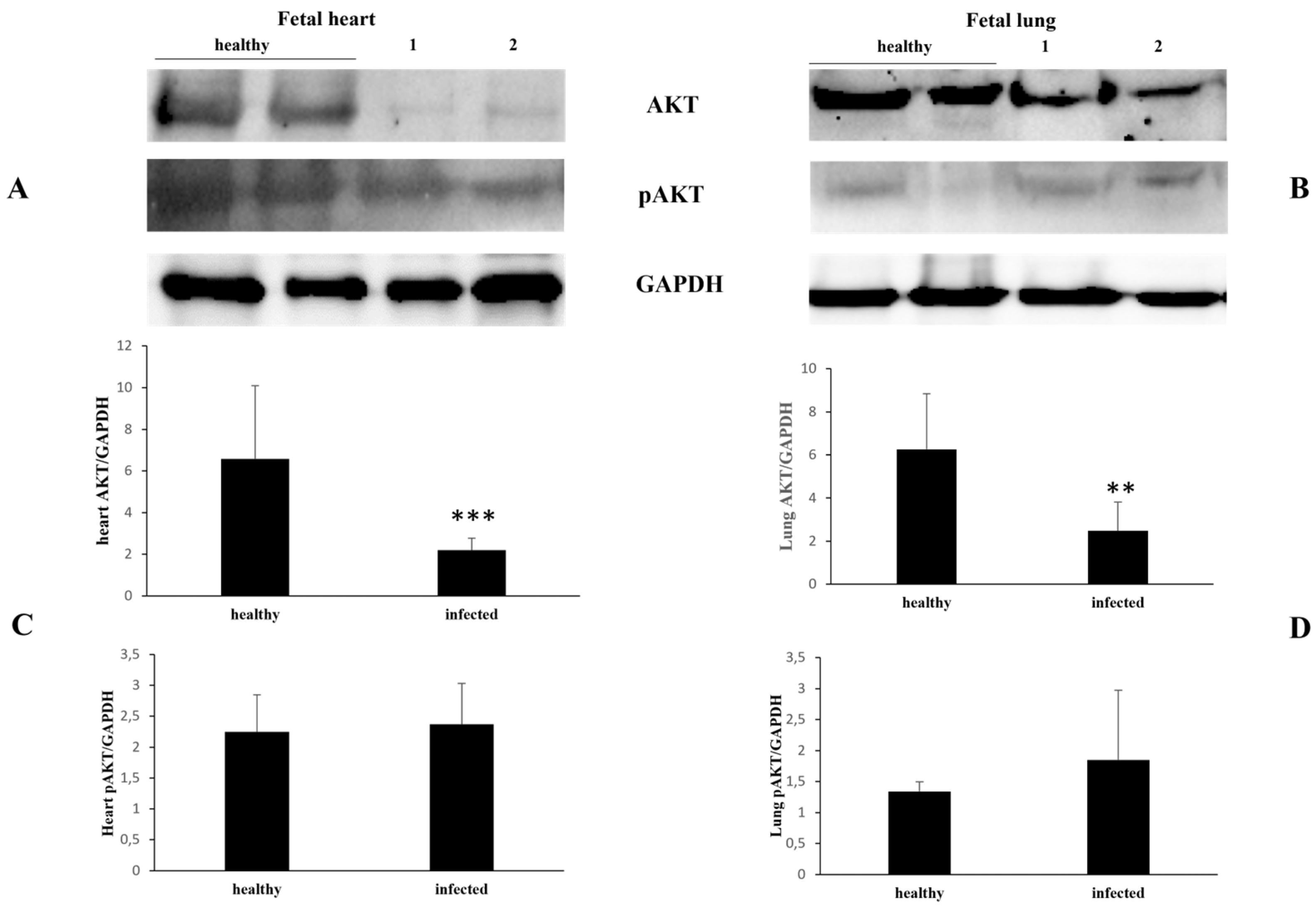
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency on Cancer Research (IARC). Human papillomaviruses. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2007; Volume 90. [Google Scholar]
- Ambühl, L.M.M.; Leonhard, A.K.; Zakhary, C.W.; Jørgensen, A.; Blaakær, J.; Dybkær, K.; Baandrup, U.; Uldbjerg, N.; Sørensen, S. Human papillomavirus infects placental trophoblast and Hofbauer cells, but appears not to play a causal role in miscarriage and preterm labor. Acta Obstet. Gynecol. Scand. 2017, 96, 1188–1196. [Google Scholar] [CrossRef]
- Chilaka, V.N.; Navti, O.B.; Beloushi, M.A.; Ahmed, B.; Kone, J.C. Human papillomavirus (HPV) in pregnancy—An update. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 340–348. [Google Scholar] [CrossRef]
- Værnesbranden, M.R.; Wiik, J.; Sjøborg, K.; Staff, A.C.; Carlsen, K.C.L.; Haugen, G.; Hedlin, G.; Hilde, K.; Nordlund, B.; Nystrand, C.F.; et al. Maternal human papillomavirus infections at mid-pregnancy and delivery in a Scandinavian mother-child cohort study. Int. J. Infect. Dis. 2021, 108, 574–581. [Google Scholar] [CrossRef]
- Ardekani, A.; Sepidarkish, M.; Mollalo, A.; Afradiasbagharani, P.; Rouholamin, S.; Rezaeinejad, M.; Farid-Mojtahedi, M.; Mahjour, S.; Almukhtar, M.; Shiadeh, M.N.; et al. Worldwide prevalence of human papillomavirus among pregnant women: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2374. [Google Scholar] [CrossRef]
- Tramontano, L.; Sciorio, R.; Bellaminutti, S.; Esteves, S.C.; Petignat, P. Exploring the potential impact of human papillomavirus on infertility and assisted reproductive technology outcomes. Reprod. Biol. 2023, 23, 100753. [Google Scholar] [CrossRef]
- Daudt, C.; Da Silva, F.R.C.; Lunardi, M.; Alves, C.B.D.T.; Weber, M.N.; Cibulski, S.P.; Alfieri, A.F.; Alfieri, A.A.; Canal, C.W. Papillomaviruses in ruminants: An update. Transbound. Emerg. Dis. 2018, 65, 1381–1395. [Google Scholar] [CrossRef]
- Campo, M.S.; Jarrett, W.F.H.; Barron, R.J.; O’Neil, B.W.; Smith, K.T. Association of bovine papillomavirus type 2 and bracken fern with bladder cancer in cattle. Cancer Res. 1992, 52, 6898–6904. [Google Scholar]
- Roperto, S.; Borzacchiello, G.; Brun, R.; Leonardi, L.; Maiolino, P.; Martano, M.; Paciello, O.; Papparella, S.; Restucci, B.; Russo, V.; et al. A review of bovine urothelial tumours and tumour-like lesions of the urinary bladder. J. Comp. Pathol. 2010, 142, 95–108. [Google Scholar] [CrossRef]
- Roperto, S.; Russo, V.; Leonardi, L.; Borzacchiello, G.; Martano, M.; Corrado, F.; Riccardi, M.G.; Roperto, F. Bovine papillomavirus type 13 expression in the urothelial bladder tumours of cattle. Transbound. Emerg. Dis. 2016, 63, 628–634. [Google Scholar] [CrossRef]
- Roperto, S.; Russo, V.; De Falco, F.; Taulescu, M.; Roperto, F. Congenital papillomavirus infection in cattle: Evidence for transplacental transmission. Vet. Microbiol. 2019, 230, 95–100. [Google Scholar] [CrossRef]
- Russo, V.; Roperto, F.; De Biase, D.; Cerino, P.; Urraro, C.; Munday, J.S.; Roperto, S. Bovine papillomavirus type 2 infection associated with papillomatosis of the amniotic membrane in water buffaloes (Bubalus bubalis). Pathogens 2020, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; de Carvalho, C.; Brunner, O.; Birgel-Junior, E.H.; Dellalibera, A.M.M.P.; Benesi, F.J.; Gregory, L.; Beçak, W.; Stocco dos Santos, R.C. Viral DNA sequences in peripheral blood and vertical transmission of the virus: A discussion about BPV-1. Braz. J. Microbiol. 2003, 34 (Suppl. S1), 76–78. [Google Scholar] [CrossRef]
- Yaguiu, A.; Dagli, M.L.Z.; Birgel, E.H., Jr.; Alves Reis, B.C.A.; Ferraz, O.P.; Pituco, E.M.; Freitas, A.C.; Beçak, W.; Stocco, R.C. Simultaneous presence of bovine papillomavirus and bovine leukemia virus in different bovine tissues: In situ hybridization and cytogenetic analysis. Genet. Mol. Res. 2008, 71, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Roperto, S.; Borzacchiello, G.; Esposito, I.; Riccardi, M.; Urraro, C.; Lucà, R.; Corteggio, A.; Tatè, R.; Cermola, M.; Paciello, O.; et al. Productive infection of bovine papillomavirus type 2 in the placenta of pregnant cows affected with urinary bladder tumors. PLoS ONE 2012, 7, e33569. [Google Scholar] [CrossRef] [PubMed]
- Stocco dos Santos, R.C.; Lindsey, C.J.; Ferraz, O.P.; Pinto, J.R.; Mirandola, S.R.; Benesi, F.J.; Birgel, E.H.; Bragança Pereira, A.; Beçak, W. Bovine papillomavirus transmission and chromosomal aberrations: An experimental model. J. Gen. Virol. 1998, 79, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Gallina, L.; Mazza, F.; Mariella, J.; Castagnetti, G.; Scagliarini, A. Molecular detection of bovine papillomavirus DNA in the placenta and blood of healthy mares and respective foals. Vet. Sci. 2019, 6, 14. [Google Scholar] [CrossRef]
- PaVE: The Papillomavirus Episteme. Available online: http://pave.niaid.nih.gov (accessed on 16 May 2023).
- Alberti, A.; Pirino, S.; Pintore, F.; Addis, M.F.; Chessa, B.; Cacciotto, C.; Cubeddu, T.; Anfossi, A.; Benenati, G.; Coradduzza, E.; et al. Ovis aries papillomavirus 3: A prototype of a novel genus in the family Papillomaviridae associated with ovine squamous cell carcinoma. Virology 2010, 407, 352–359. [Google Scholar] [CrossRef]
- Tore, G.; Cacciotto, C.; Anfossi, A.G.; Dore, G.M.; Antuofermo, E.; Scagliarini, A.; Burrai, G.P.; Pau, S.; Zedda, M.T.; Masala, G.; et al. Host cell tropism, genome characterization, and evolutionary features of OaPV4, a novel Deltapapillomavirus identified in sheep fibropapilloma. Vet. Microbiol. 2017, 204, 151–158. [Google Scholar] [CrossRef]
- Gibbs, C.J.; Smale, E.P.; Lawman, M.J. Warts in sheep. Identification of a papillomavirus and transmission of infection to sheep. J. Comp. Pathol. 1975, 85, 327–334. [Google Scholar] [CrossRef]
- Vanselow, B.A.; Spradbrow, P.B.; Jackson, A.R.B. Papillomaviruses, papillomas and squamous cell carcinomas in sheep. Vet. Rec. 1982, 110, 561–562. [Google Scholar] [CrossRef]
- Trenfield, K.; Spradbrow, P.B.; Vanselow, B.A. Detection of papillomavirus DNA in precancerous lesions of the ears of sheep. Vet. Microbiol. 1990, 25, 103–116. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Cutarelli, A.; Cuccaro, B.; Catoi, C.; De Carlo, E.; Roperto, S. Evidence of a novel cross-species transmission by ovine papillomaviruses. Transbound. Emerg. Dis. 2022, 69, 3850–3857. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Cuccaro, B.; De Tullio, R.; Alberti, A.; Cutarelli, A.; De Carlo, E.; Roperto, S. Possible etiological association of ovine papillomaviruses with bladder tumors in cattle. Virus Res. 2023, 328, 199084. [Google Scholar] [CrossRef] [PubMed]
- Roperto, S.; Russo, V.; Urraro, C.; Restucci, B.; Corrado, F.; De Falco, F.; Roperto, F. ERAS is constitutively expressed in full term placenta of pregnant cows. Theriogenology 2017, 103, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Hoch, R.V.; Soriano, P. Roles of PDGF in animal development. Development 2003, 130, 4769–4784. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, S.H.; Chen, C.Y.; Chen, P.C.; Chen, C.P. Human placenta-derived multipotent mesenchymal stromal cells involved in placental angiogenesis via the PDGF.BB and Stat3 pathways. Biol. Reprod. 2015, 93, 1–10. [Google Scholar] [CrossRef]
- De Falco, F.; Perillo, A.; Del Piero, F.; Del Prete, C.; Zizzo, N.; Marcus, I.; Roperto, F. ERAS is constitutively expressed in the tissues of adult horses and may be a key player in basal autophagy. Front. Vet. Sci. 2022, 9, 818294. [Google Scholar] [CrossRef]
- Russo, V.; Roperto, F.; Esposito, I.; Ceccarelli, D.M.; Zizzo, N.; Leonardi, L.; Capparelli, R.; Borzacchiello, G.; Roperto, S. ERas protein is overexpressed and binds to the activated platelet-derived growth factor® receptor in bovine urothelial tumour cells associated with papillomavirus infection. Vet. J. 2016, 212, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Karabadzhak, A.G.; Petti, L.M.; Barrera, F.N.; Edwards, A.P.B.; Moya-Rodríguez, A.; Polikanov, Y.S.; Freites, J.A.; Tobias, D.J.; Engelman, D.M.; DiMaio, D. Two transmembrane dimers of the bovine papillomavirus E5 oncoprotein clamp the PDGF® receptor in an active dimeric conformation. Proc. Natl. Acad. Sci. USA 2017, 114, E7262–E7271. [Google Scholar] [CrossRef]
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial vertical transmission during human pregnancy. Cell Host Microbe 2017, 21, 561–567. [Google Scholar] [CrossRef]
- Ambühl, L.M.M.; Baandrup, U.; Dybkær, K.; Blaakær, J.; Uldbjerg, N.; Sørensen, S. Human papillomavirus infection as a possible cause of spontaneous abortion and spontaneous preterm delivery. Infect. Dis. Obstet. Gynecol. 2016, 2016, 3086036. [Google Scholar] [CrossRef] [PubMed]
- Silasi, M.; Cardenas, I.; Racicot, K.; Kwon, J.Y.; Aldo, P.; Mor, G. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef]
- Watanabe, S.; Vasudevan, S.G. Clinical and experimental evidence for transplacental vertical transmission of flaviviruses. Antivir. Res. 2023, 210, 105512. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Oguejiofor, C.F.; Thomas, C.; Cheng, Z. Importance of viral disease in dairy cow fertility. Engineering 2020, 6, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Castellsagué, X.; Drudis, T.; Cañadas, M.P.; Goncé, A.; Ros, R.; Pérez, J.M.; Quintana, M.J.; Muñoz, J.; Albero, S.; de Sanjosé, G.; et al. Human papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: A prospective study in Spain. BMC Infect. Dis. 2009, 9, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Zouridis, A.; Kalampokas, T.; Panoulis, K.; Salakos, N.; Deligeoroglou, E. Intrauterine HPV transmission: A systematic review of the literature. Arch. Gynecol. Obstet. 2018, 298, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Megli, C.J.; Coyne, C.B. Infections at the maternal-fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Meenu, M.N.; Patel, K.; Singh, R.; Gupta, P.; Purwar, S.; Mukhopadhyay, S.; Mishra, N.; Gupta, S.; Rawat, S.K.; et al. Presence of entry receptors and viral markers suggest a low level of placental replication of hepatitis B virus in a proportion of pregnant women infected with chronic hepatitis B. Sci. Rep. 2022, 12, 17795. [Google Scholar] [CrossRef] [PubMed]
- Cladel, N.M.; Jiang, P.; Li, J.J.; Peng, X.; Cooper, T.K.; Majerciak, V.; Balogh, K.K.; Meyer, T.J.; Brendle, S.A.; Budgeon, L.R.; et al. Papillomavirus can be transmitted through the blood and produce infections in blood recipients: Evidence from two animal models. Emerg. Microbes Infect. 2019, 8, 1108–1121. [Google Scholar] [CrossRef]
- Syrjänen, S.; Syrjänen, K. HPV-associated benign squamous cell papillomas and in the upper aero-digestive tract and their malignant potential. Viruses 2021, 13, 1624. [Google Scholar] [CrossRef]
- Smith, E.M.; Ritchie, J.M.; Yankowitz, J.; Swarnavel, S.; Wang, D.; Haugen, T.H.; Turek, L.D. Human papillomavirus prevalence and types in newborns and parents. Concordance and mode of transmission. Sex Transm. Dis. 2004, 31, 57–62. [Google Scholar] [CrossRef]
- Koskimaa, H.M.; Waterboer, T.; Pawlita, M.; Grénman, S.; Syrjänen, K.; Syrjänen, S. Human papillomavirus genotypes present in the oral mucosa of newborns and their concordance with maternal cervical human papillomavirus genotypes. J. Pediatr. 2012, 160, 837–843. [Google Scholar] [CrossRef]
- Suominen, N.T.; Luukkaala, T.H.; Laprise, C.; Haataja, M.A.; Grénman, S.E.; Syrjänen, S.M.; Louvanto, K. Human papillomavirus concordance between parents and their newborn offspring: Results from the Finnish family human papillomavirus study. J. Infect. Dis. 2024, 229, 448–456. [Google Scholar] [CrossRef]
- Sajiki, Y.; Konnai, S.; Nishimori, A.; Okagawa, T.; Maekawa, N.; Goto, S.; Nagano, M.; Kohara, J.; Kitano, N.; Takahashi, T.; et al. Intrauterine infection with bovine leukemia virus in pregnant dam with high viral load. J. Vet. Med. Sci. 2017, 79, 2036–2038. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wei, Z.; Tao, X.; Du, Y.; Wu, J.; Yu, Y.; Yu, H.; Zhao, H. PLAC8 promotes the autophagic activity and improves the growth priority of human trophoblast cells. FASEB J. 2021, 35, e21351. [Google Scholar] [CrossRef] [PubMed]
- Rossello, R.A.; Pfenning, A.; Howard, J.T.; Hochgeschwender, U. Characterization and genetic manipulation of primed stem cells into a functional naïve state with ESRRB. World J. Stem Cells 2016, 8, 355–366. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, D.; Tian, Q.; Chen, X.; Jiang, B.; Chou, B.K.; Hu, P.; Cheng, L.; Gao, P.; Li, J.; et al. Stimulation of somatic cell reprogramming by Eras-Akt-Fox01 signaling axis. Stem Cells 2014, 32, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.A.; Yu, Y.; Ma, H.X.; Wang, X.X.; Lu, X.; Zhai, Y.; Zhang, X.; Wang, H.; Li, L. The roles of ERAS during cell lineage specification of mouse early embryonic development. Open Biol. 2015, 5, 150092. [Google Scholar] [CrossRef]
- Nakhaei-Rad, S.; Nakhaeizadeh, H.; Götze, S.; Kordes, C.; Sawitza, I.; Hoffmann, M.J.; Franke, M.; Schulz, W.A.; Schelle, J.; Peekorz, R.P.; et al. The role of embryonic stem cell-expressed RAS (ERAS) in the maintenance of quiescent hepatic stellate cells. J. Biol. Chem. 2016, 291, 8399–8413. [Google Scholar] [CrossRef]
- Van den Akker, N.M.S.; Winkel, L.C.J.; Nisancioglu, M.H.; Maas, S.; Wisse, L.J.; Armulik, A.; Poelmann, M.H.; Lie-Venema, H.; Betsholtz, C.; Groot, A.C.G. PDGF-B signaling is important for murine cardiac development: Its role in developing atrioventricular valves, coronaries, and cardiac innervation. Dev. Dyn. 2008, 237, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef] [PubMed]
- Bjarnegärd, M.; Enge, M.; Norlin, J.; Gustafsdottir, S.; Fredriksson, S.; Abramsson, A.; Takemoto, M.; Gustafsson, E.; Fässler, R.; Betsholtz, C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development 2013, 131, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Gimple, R.C.; Zhong, C.; Wu, Q.; Yang, K.; Pager, B.C.; Godugu, B.; Qiu, Z.; Zhao, L.; Zhang, G.; et al. PDGF signaling inhibits mitophagy in glioblastoma stem cell through N6-methyladenosine. Dev. Cell. 2022, 57, 1466–1481. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Yang, K.; Rich, J.N. Growth factor receptor signaling induces mitophagy through epitranscriptomic regulation. Autophagy 2023, 19, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Li, A.; Li, S.; Zhang, C.; Fang, Z.; Sun, Y.; Peng, Y.; Wang, X.; Zhang, M. FPR2 serves a role in recurrent spontaneous abortion by regulating trophoblast function via the PI3K/AKT signaling pathway. Mol. Med. Rep. 2021, 24, 838. [Google Scholar] [CrossRef]
- Wolf-Jäckel, G.A.; Hansen, M.S.; Larsen, G.; Holm, E.; Agerholm JSJensen, T.K. Diagnostic studies of abortion in Danish cattle 2015–2017. Acta Vet. Scand. 2020, 62, 1. [Google Scholar] [CrossRef]




| Forward 5′ 3′ | Reverse 5′ 3′ | Probe (FAM) | Region | |
|---|---|---|---|---|
| BPV1 | ACTTCTGATCACTGCCATT | ATAGAAACCATAGATTTGGCA | TGAAGTGTTTCTGTTTGTGA | ORF E5 |
| BPV2 | TACAGGTCTGCCCTTTTAAT | AACAGTAAACAAATCAAATCCA | AACAACAAAGCCAGTAACC | 3′UTR E5 |
| BPV13 | CTGTGTGGATTTGATTTGTT | CAGGGGGAATACAAATTCT | TGAAGTGTTTCTGTTTGTGA | 3′UTR E5 |
| BPV14 | CTTTGTTATTGTATATGAGTCTGT | ACTCTTGACGGTTTAAAAGTA | ATCTTGCCAGTGATCCTG | 3′UTR E5 |
| OaPV1 | CCTGATTCTATGACTGTAAGAGGC | CTCCCCACAGAAGTCCAAG | TGCAACAGCAGAGTCCCATCAGAAG | ORF E5 |
| OaPV2 | AGTTCCCGCTCTGATTTACC | ATGGCGGACGTATACTTGTTC | ATTGCCAGCAGTCTCCTCAGTCATTC | L1 |
| OaPV3 | AGCCCACACTCCCTGATATAG | TTCAGTCTTTGACAGCACCTC | AGCAACCAGCACTGTACACGCTAT | E7 |
| OaPV4 | GGGTTCTATGGTGTCTGCTTAG | GCTCAAAATGGTCTACTGTTGC | CAGGAATGCTCTGTGCAGGGTATAGTG | E5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Falco, F.; Cutarelli, A.; Leonardi, L.; Marcus, I.; Roperto, S. Vertical Intrauterine Bovine and Ovine Papillomavirus Coinfection in Pregnant Cows. Pathogens 2024, 13, 453. https://doi.org/10.3390/pathogens13060453
De Falco F, Cutarelli A, Leonardi L, Marcus I, Roperto S. Vertical Intrauterine Bovine and Ovine Papillomavirus Coinfection in Pregnant Cows. Pathogens. 2024; 13(6):453. https://doi.org/10.3390/pathogens13060453
Chicago/Turabian StyleDe Falco, Francesca, Anna Cutarelli, Leonardo Leonardi, Ioan Marcus, and Sante Roperto. 2024. "Vertical Intrauterine Bovine and Ovine Papillomavirus Coinfection in Pregnant Cows" Pathogens 13, no. 6: 453. https://doi.org/10.3390/pathogens13060453





