Abstract
Rickettsioses, often underreported, pose public health challenges. Rickettsia asembonensis is a potential emerging pathogen that was previously detected in humans, animals, and a variety of arthropods. While its pathogenicity in humans remains unclear, it poses a potential public health threat. Here, we present an extended epidemiological, diagnostic, and genetic analysis of the information provided in a preliminary report on the investigation of rickettsiae in Peru. In particular, we report the detection of R. asembonensis in blood specimens collected from four human patients with an acute undifferentiated fever of a seven- to nine-day duration, all of whom tested negative for other vector-borne pathogens. Additionally, we describe the replicative capacity of the R. asembonensis isolates in cell cultures.
1. Introduction
Rickettsioses are neglected infectious diseases found across various locations throughout the Americas, resulting in its underreporting and under-recognition [1]. Rickettsia asembonensis is an emerging flea-borne agent, closely related to Rickettsia felis and other R. felis-like organisms, that belongs serologically to the spotted fever group of rickettsiae [SFGR] but genetically to the transitional group of rickettsiae [2]. R. asembonensis was first identified in Kenya in 2013, and since then, multiple studies have reported its global detection in a variety of arthropods collected from domestic, peri-domestic, and wildlife animals [2,3,4,5,6,7,8,9]. Although its pathogenicity and role as a human pathogen has not yet been elucidated, its detection in both human [10,11,12,13] and animal [14,15,16] specimens suggests a potential public health threat.
Rickettsial diseases have been extensively described across various regions of Peru through the identification of group-specific antibodies (typhus group rickettsiae [TGR] and SFGR) and/or rickettsial DNA in samples collected from human cases with febrile illness [13,17,18,19,20,21,22]. R. asembonensis has predominantly been detected in flea species of the genus Ctenocephalides collected from domestic or backyard animals belonging to individuals with an acute undifferentiated fever (AUF) or asymptomatic individuals in the Amazon basin and multiple other Peruvian urban and border areas [17,22,23]. However, it was not until 2018 that the detection of R. asembonensis in cultured samples from humans presenting with AUF in Peru was preliminarily reported [13]. Briefly, R. asembonensis was detected in four human leukocyte samples collected from the same number of cases in various Peruvian locations by using multiple cell lines, immunofluorescence assays (IFAs), and sequencing of a PCR-amplified short fragment of the gltA gene [13]. The lack of detailed methodology, epidemiological information, the use of complementary tests on primary samples and cultures, and the preliminary genetic analysis restricted to a set of four genetic references are constraints that have not been addressed to date. Here, our aim was to comprehensively provide and expand on the epidemiological information and genetic analysis that were preliminarily reported.
2. Materials and Methods
In 2009, a blood sample was obtained from an 11-year-old girl (case 1), and in 2010, three other blood samples were collected from a 23-year-old male (case 2), a 23-year-old female (case 3), and a 41-year-old female (case 4). All four cases described symptoms of AUF within the previous 7 to 9 days and were subsequently enrolled in the study “Pathogen investigation in human cases with acute undifferentiated febrile illness in Peru” at four distinct geographic locations in Peru (Table 1). The acute blood samples collected from each case were sent to the Peruvian National Institute of Health (PNIH) for the detection and characterization of multiple pathogens using advanced techniques not available at the enrollment sites. At PNIH, human serum/plasma and leukocytes were isolated from blood samples and were routinely screened for infections caused by various vector-borne viral (e.g., dengue virus serotypes 1–4) and bacterial (e.g., Leptospira spp.) pathogens, yielding negative results [13]. Since Rickettsia species detection was not included in the routine diagnostic procedures during the study period, the investigation of Rickettsia was conducted on human leukocytes (primary samples) preserved at −80 °C. Figure 1 depicts the workflow used in this study.

Table 1.
Peruvian localities from which the cases were detected, and the laboratory results.
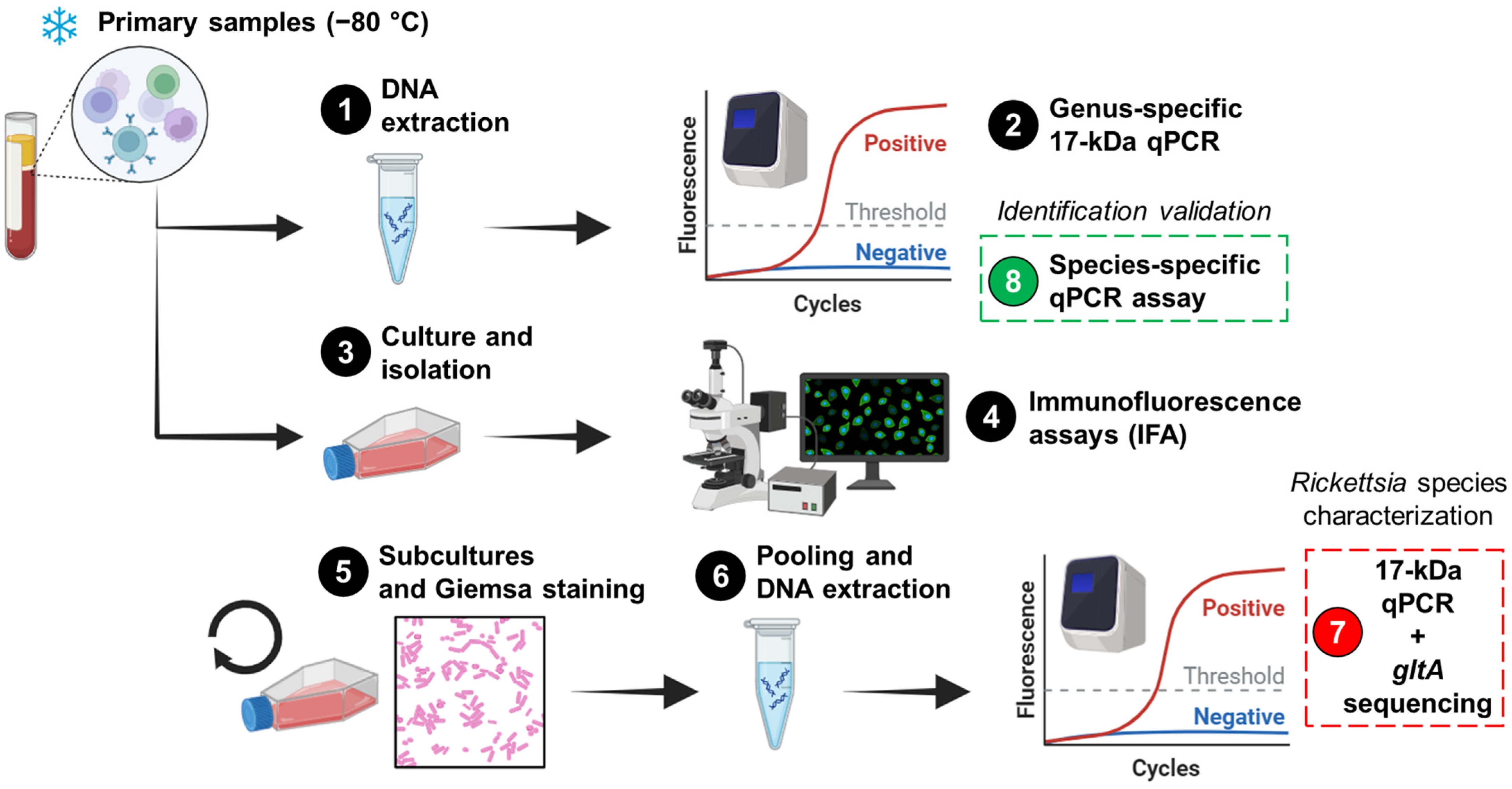
Figure 1.
The workflow for the detection and characterization of Rickettsia in cultures and primary samples. The workflow comprises eight steps, starting with human blood leukocytes that were previously stored at −80 °C. The penultimate step (Step 7) involved the characterization of the agent in cultures, while the final step (Step 8) was performed to validate the results in primary samples. The figure was created in https://www.biorender.com/ (Accessed on 2 April 2024).
2.1. DNA Extraction
2.2. Culture and Immunofluorescence Assay
Primary samples were inoculated into Vero E6 (African green monkey; ATCC CRL-1586), canine macrophage-like DH82 (ATCC CRL-3590), Aedes albopictus clone C6/36 (ATCC CRL-1660), murine macrophage-like J774A.1 (ATCC TIB-67), and/or human monocyte-like THP-1 (ATCC TIB-202) cells (Step 3; Figure 1) as described elsewhere [24]. Infected cells were maintained in Eagle’s Minimum Essential Medium (Merck; Darmstadt, Germany) supplemented with Earle′s salts, L-glutamine, non-essential amino acids, sodium pyruvate, and 5% fetal bovine serum (Merck; De Soto, KS, USA). Infections were conducted in 80% confluent monolayers within T-12.5 flasks, and inoculated flasks were incubated at 34 °C with 5% CO2 for 15 days. The presence of Rickettsia was assessed using an immunofluorescence assay (IFA) with Rickettsia-specific IgG antibodies (PanBio; Columbia, MD, USA) (Step 4; Figure 1). To increase the bacterial load and visualize at least 50% positive cells by IFA (Step 5; Figure 1), multiple subcultures were performed (Table 1). In these subcultures, Giemsa staining and light microscopy were used to monitor the infection (Step 5; Figure 1). However, it is important to highlight that the staining step was not critical for proceeding with the pooling of harvested subcultures or subsequent steps. When 20–50% IFA positivity was observed in each cell line, the cultures were harvested. Subsequently, 100 µL of each harvested culture was pooled, and genomic DNA was extracted (Step 6; Figure 1). IFA-negative cultures were excluded.
2.3. Molecular Assays
A genus-specific quantitative real-time PCR (qPCR) [25] and a species-specific qPCR for R. asembonensis, targeting the conserved 17 kDa surface antigen gene and the ompB gene [26], respectively, were performed as previously described using the 7500 Real-Time PCR System. The genus-specific 17 kDa qPCR assay was used on primary samples (Step 2; Figure 1) and pooled harvested subcultures (Step 7; Figure 1), while the species-specific qPCR was used only on primary samples (Step 8; Figure 1). Cycle threshold (Ct) values were recorded, and positive and negative controls were included in each run. Nested PCRs were performed to amplify regions of the conserved 17 kDa surface antigen and gltA genes, as well as the variable ompB gene, using previously described primers and conditions [27,28]. These PCRs were applied to primary samples to generate amplicons for subsequent sequencing.
2.4. Sequencing and Phylogenetic Analysis
PCR products were separated by electrophoresis on 2% agarose gels, purified, and then sequenced using the Big Dye terminator kit v3.1 (Thermo Fisher Scientific; Waltham, MA, USA) on the 3500XL genetic analyzer (Step 7; Figure 1). Sequences were analyzed and assembled in the BioEdit Sequence Alignment Editor v7.0.5.3, and consensus sequences were further analyzed using the nucleotide Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi; Accessed on 7 February 2024). Multiple Rickettsia species sequences were downloaded from the National Center for Biotechnology Information (NCBI) database (Table 2), and subsequently included in a phylogenetic analysis. The model was inferred by using the Maximum Likelihood method based on the Tamura 3-parameter model with a discrete Gamma distribution and 1000 bootstrap replicates. The phylogenetic analysis was conducted using Molecular Evolutionary Genetics Analysis (MEGA) X v10.2.6 software (https://www.megasoftware.net/; Accessed on 13 July 2023).

Table 2.
Reference sequences of Rickettsia species and identity level against R. asembonensis isolated from human blood. Similarity values obtained by comparing the partial sequence of gltA from the Peruvian isolate CA2053 (accession number MW655895) and other sequences from multiple Rickettsia species and strains with BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi; Accessed on 7 February 2024).
3. Results
All four primary samples were subjected to rickettsial DNA screening and displayed amplification signals by a genus-specific 17 kDa qPCR assay [25] (range of Ct values: 35.3 to 39.1). Water negative control samples were consistently negative. Based on the available sample volume, samples were cultured in at least two of the five cell lines described in Table 1 for the characterization of Rickettsia agents. All cultured samples were IFA-positive with the first attempt; and in the subcultures, coccobacillary microorganisms were identified by Giemsa staining. Subsequently, harvested cells were pooled and total genomic DNA was extracted [13].
The Rickettsia species characterization was based on the following approach: (1) the detection of the 17 kDa antigen gene by qPCR [25], and (2) the sequencing and analysis of a segment of the gltA gene amplified by PCR [27]. From cultures, the Ct values derived from the 17 kDa qPCR assay were in the range of 25.3 to 29.1, and the sequencing of 379 nucleotides from the gltA gene confirmed the presence of R. asembonensis (GenBank accession numbers: MW655895–MW655898). Notably, all sequences described here were identical, and uninoculated control cultures and no-template controls tested negative by qPCR. A detailed comparative analysis with other Rickettsia sequences is summarized in Table 2, and the phylogenetic analysis is shown in Figure 2.
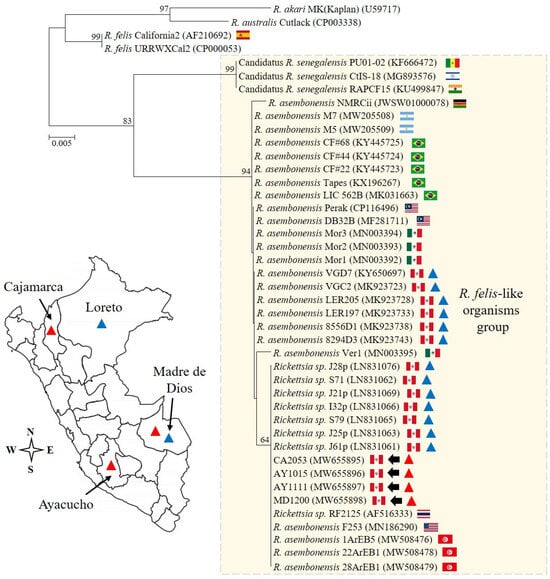
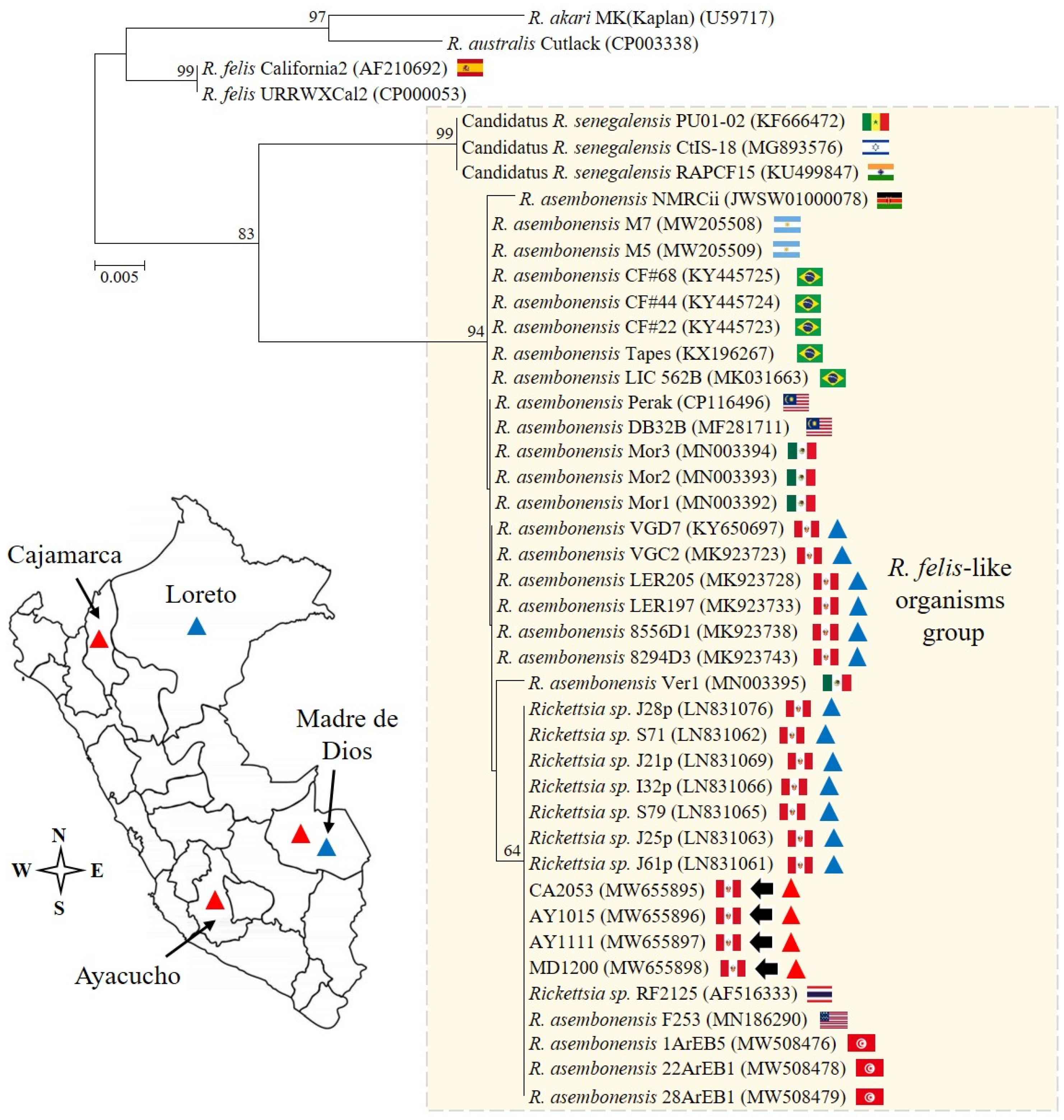
Figure 2.
The phylogenetic analysis and detection sites. The analysis was performed including 28.8% (379/1314) of the full open reading frame of the conserved gltA gene from Rickettsia asembonensis strains and other Rickettsia species of the transitional group. The scale bar represents the number of substitutions per site, and the four sequences described in this study are marked by arrows. The genetic information reported for Peru is summarized in the genetic tree and map. Specifically, R. asembonensis detected in arthropods and humans is represented with blue and red triangles, respectively.
To validate our findings, a species-specific qPCR assay for R. asembonensis that targets a portion of the ompB gene was used on primary samples [26]. Amplification curves, although with high Ct values (36.3 and 38.9) suggestive of a low bacterial load, were observed in two of the four primary samples (Table 1). Importantly, the two primary samples that were negative for ompB gene amplification were positive for 17 kDa antigen gene amplification (samples from case 1 and case 4, Table 1). The detection limit for the genus-specific 17 kDa qPCR assay has been reported as three copies per reaction [25], whereas the detection limit for the species-specific qPCR has not been previously established [26]. These discrepant results may be attributed to a bacterial load below the detection limit for species-specific qPCR. Despite multiple attempts, we failed to generate ompB gene and 17 kDa antigen gene amplicons by standard nested PCRs from primary samples [28], as reported elsewhere for other genes [12]. The low bacterial load in primary samples likely limited our ability to generate PCR amplicons for sequencing.
4. Discussion
Our findings are consistent with those previously reported in two fundamental aspects. First, we successfully detected R. asembonensis in acute blood samples obtained from individuals exhibiting febrile illness and testing negative for pathogens other than Rickettsia [10,11,12]. Second, the methodology relying on partial sequencing of the gltA gene, as utilized here, has previously been used to confirm the presence of R. asembonensis in humans [10,11]. It is important to note that, compared to previous reports of R. asembonensis in humans [10,11,12], here, we describe not only its molecular detection but also its replicative capacity in multiple cell lines. In light of our findings, though it is not possible to conclusively assert that R. asembonensis was the causative agent of the patients’ diseases, we suggest further investigations to assess the pathogenicity of this novel agent. In subsequent investigations, the assessment of seroconversions/four-fold rise in titers through the examination of paired blood samples, a more complete culture, and genetic typing/detection (including more variable genes other than ompB and other conserved genes other than gltA and 17 kDa antigen) may contribute to a more robust determination of a rickettsial infection specifically occurring at the time of sample collections.
Furthermore, it is important to highlight that our study encompasses a diagnostic and genetic analysis that supports the close genetic relationship between R. asembonensis documented here and strains previously identified in various geographic areas, vectors, and humans (Figure 2 and Figure 3). Noteworthy is that the gltA sequences reported in humans [10,11] and in vectors collected in Peru (Figure 3) are closely related. The sequence documented by Kho et al. [10] differs by only one amino acid (one base pair: guanine to adenine) compared to the sequences described herein, and the genetic regions compared between our sequences and those in vectors are identical. Understanding the biological implication of these findings was beyond the scope of this study; however, future research aimed at exploring the implications of our findings could greatly contribute to advancing our comprehension of the pathological role of R. asembonensis.
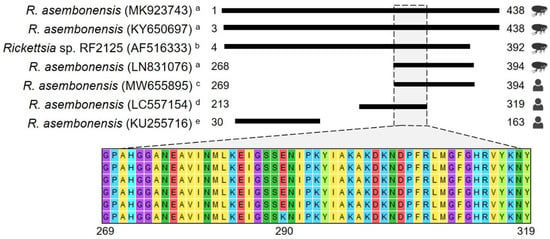
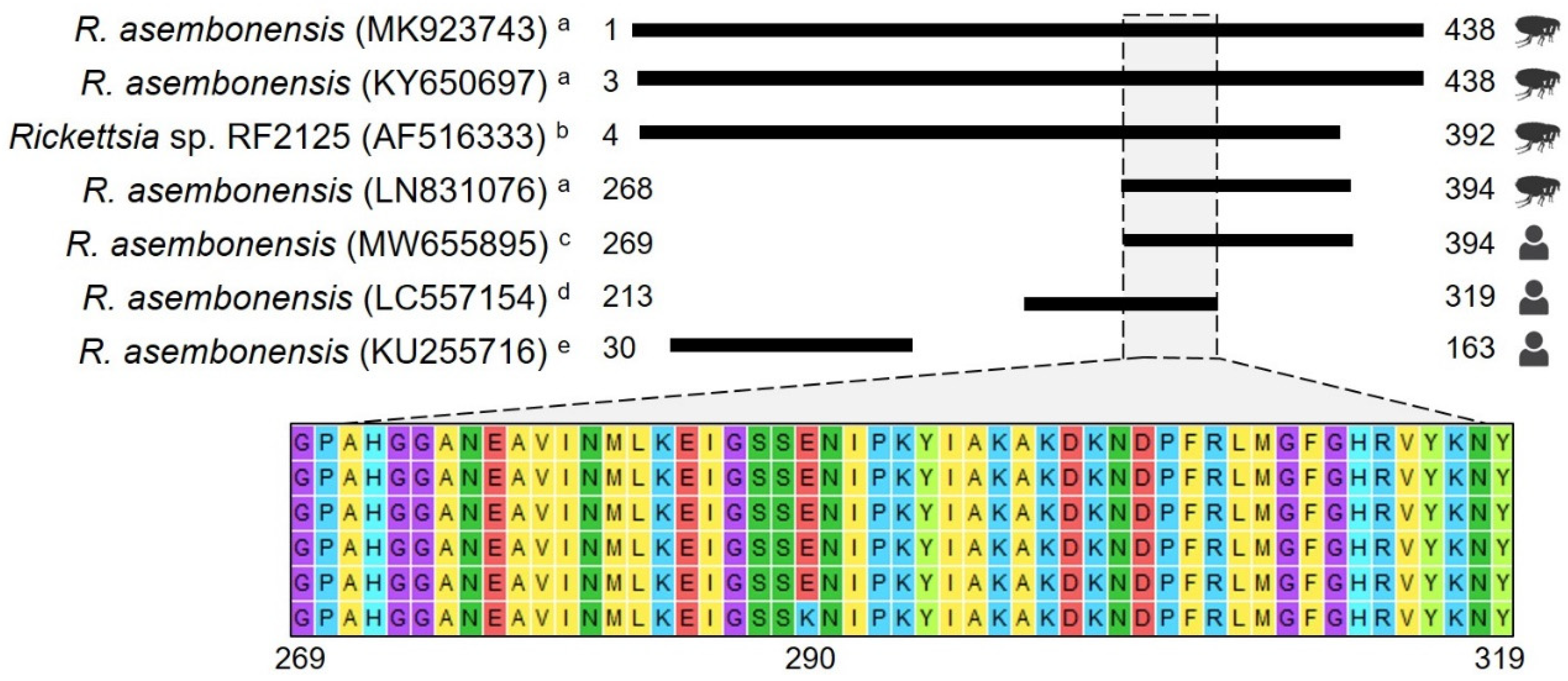
Figure 3.
Amino acid alignment using gltA gene sequences. The figure depicts amino acid (aa) alignments using information deposited in GenBank for the full ORF of the gltA gene (438 aa). GenBank accession numbers are presented in parentheses, and numbers next to black boxes represent the aa positions. The first four sequences were obtained from arthropods collected in Peru a and Thailand b, and the last three were obtained from human samples c–e. The Thailand sequence was included because it is considered the potential first formal report of R. asembonensis. Since no differences were observed between sequences reported for arthropods among the studies published in Peru a, MK923743 [29], LN831076 [23], and KY650697 [30] were selected to represent other sequences reported elsewhere. MW655895 is one of those reported in this study. LC557154 d is one of two sequences reported by Moonga et al. [11], and KU255716 e is the only sequence reported by Tay et al. [12].
5. Conclusions
Our study adds to the epidemiological and genetic knowledge of R. asembonensis in humans and provides evidence that validates the presence of this agent in human samples.
Author Contributions
Conceptualization, S.L. and R.P.-S.; Methodology, S.L., R.P.-S., O.C.-R. and A.L.R.; Software, S.L.; Validation, R.P.-S., O.C.-R. and A.L.R.; Formal Analysis, S.L. and R.P.-S.; Investigation, S.L., R.P.-S., O.C.-R. and A.L.R.; Resources, R.P.-S. and O.C.-R.; Data Curation, S.L. and O.C.-R.; Writing—Original Draft Preparation, S.L.; Writing—Review & Editing, R.P.-S., O.C.-R. and A.L.R.; Visualization, S.L. and R.P.-S.; Supervision, A.L.R.; Project Administration, R.P.-S. and O.C.-R.; Funding Acquisition, R.P.-S., O.C.-R. and A.L.R. All authors have read and agreed to the published version of the manuscript.
Funding
The study and its procedures were funded by Instituto Nacional de Salud del Perú (OC-050-13). SL is supported by a competitive award received from Universidad de Cartagena–UNIMOL, as part of a PhD scholarship (BU-179211001).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Scientific Committee and the Ethics Committee of the Peruvian National Institute of Health (protocol code N° 874-2013-DG-OGITT-OPE/INS; date of approval: 22 November 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, and the consent was applied by employees of the Peruvian National Institute of Health.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author. Also, the data presented in this study are available in the National Center for Biotechnology Information at https://www.ncbi.nlm.nih.gov/nucleotide/ (Accessed on 14 June 2022), reference numbers MW655895.1; MW655896.1; MW655897.1; and MW655898.1.
Acknowledgments
We thank the field staff for their effort and support in data collection. We also thank all the individuals who participated in the study and provided samples to the Instituto Nacional de Salud del Perú.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Salje, J.; Weitzel, T.; Newton, P.N.; Varghese, G.M.; Day, N. Rickettsial infections: A blind spot in our view of neglected tropical diseases. PLoS Negl. Trop. Dis. 2021, 15, e0009353. [Google Scholar] [CrossRef] [PubMed]
- Maina, A.N.; Jiang, J.; Luce-Fedrow, A.; St John, H.K.; Farris, C.M.; Richards, A.L. Worldwide presence and features of flea-borne Rickettsia asembonensis. Front. Vet. Sci. 2018, 5, 334. [Google Scholar] [CrossRef] [PubMed]
- Kolo, A.O.; Sibeko-Matjila, K.P.; Maina, A.N.; Richards, A.L.; Knobel, D.L.; Matjila, P.T. Molecular detection of zoonotic rickettsiae Anaplasma spp. in domestic dogs and their ectoparasites in Bushbuckridge, South Africa. Vector Borne Zoonotic Dis. 2016, 16, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Martínez Díaz, H.-C.; Gil-Mora, J.; Betancourt-Ruiz, P.; Silva-Ramos, C.R.; Matiz-González, J.M.; Villalba-Perez, M.-A.; Ospina-Pinto, M.C.; Ramirez-Hernández, A.; Olaya-M, L.-A.; Bolaños, E.; et al. Molecular detection of tick-borne rickettsial pathogens in ticks collected from domestic animals from Cauca, Colombia. Acta Trop. 2023, 238, 106773. [Google Scholar] [CrossRef] [PubMed]
- Souza, U.A.; Webster, A.; Dall’Agnol, B.; Peters, F.B.; Favarini, M.O.; Schott, D.; Zitelli, L.C.; Mazim, F.D.; Kasper, C.B.; Ott, R.; et al. Ticks, mites, fleas, and vector-borne pathogens in free-ranging neotropical wild felids from southern Brazil. Ticks Tick. Borne Dis. 2021, 12, 101706. [Google Scholar] [CrossRef] [PubMed]
- Durães, L.S.; Bitencourth, K.; Ramalho, F.R.; Nogueira, M.C.; Nunes, E.C.; Gazêta, G.S. Biodiversity of potential vectors of rickettsiae and epidemiological mosaic of spotted fever in the state of Paraná, Brazil. Front. Public Health 2021, 9, 577789. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Vizzoni, V.F.; Costa, A.P.; Costa, F.B.; Moraes-Filho, J.; Labruna, M.B.; Gazêta, G.S.; de Maria Seabra Nogueira, R. First report of a Rickettsia asembonensis related infecting fleas in Brazil. Acta Trop. 2017, 172, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Colonia, C.B.; Ramírez-Hernández, A.; Gil-Mora, J.; Agudelo, J.C.; Castaño-Villa, G.J.; Pino, C.; Betancourt-Ruiz, P.; Pérez Cárdenas, J.E.; Blanton, L.S.; Hidalgo, M. Flea-borne Rickettsia species in fleas, Caldas department, Colombia. J. Infec Dev. Ctries. 2020, 14, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Urdapilleta, M.; Pech-May, A.; Lamattina, D.; Burgos, E.F.; Balcazar, D.E.; Ferrari, W.A.O.; Lareschi, M.; Salomón, O.D. Ecology of fleas and their hosts in the trifinio of north-east Argentina: First detection of Rickettsia asembonensis in Ctenocephalides felis felis in Argentina. Med. Vet. Entomol. 2022, 36, 20–29. [Google Scholar] [CrossRef]
- Kho, K.L.; Koh, F.X.; Singh, H.K.; Zan, H.A.; Kukreja, A.; Ponnampalavanar, S.; Tay, S.T. Spotted fever group rickettsioses and murine typhus in a Malaysian teaching hospital. Am. J. Trop. Med. Hyg. 2016, 95, 765–768. [Google Scholar] [CrossRef]
- Moonga, L.C.; Hayashida, K.; Mulunda, N.R.; Nakamura, Y.; Chipeta, J.; Moonga, H.B.; Namangala, B.; Sugimoto, C.; Mtonga, Z.; Mutengo, M.; et al. Molecular detection and characterization of Rickettsia asembonensis in human blood, Zambia. Emerg. Infect. Dis. 2021, 27, 2237–2239. [Google Scholar] [CrossRef]
- Tay, S.T.; Kho, K.L.; Vythilingam, I.; Ooi, C.H.; Lau, Y.L. Investigation of possible rickettsial infection in patients with malaria. Trop. Biomed. 2019, 36, 257–262. [Google Scholar] [PubMed]
- Palacios-Salvatierra, R.; Caceres-Rey, O.; Vasquez-Dominguez, A.; Mosquera-Visaloth, P.; Anaya-Ramirez, E. Rickettsia species in human cases with non-specific acute febrile syndrome in Peru. Rev. Peru. Med. Exp. Salud Publica 2018, 35, 630–635. [Google Scholar] [CrossRef]
- Tay, S.T.; Koh, F.X.; Kho, K.L.; Sitam, F.T. Rickettsial infections in monkeys, Malaysia. Emerg. Infect. Dis. 2015, 21, 545–547. [Google Scholar] [CrossRef]
- Low, V.L.; Azhar, S.S.; Tan, T.K.; Bathmanaban, P.; AbuBakar, S.; Chandrawathani, P.; Nizamuddin, H.N.Q.; Hanim, M.S.F.; Akma, N.H.; Norlizan, M.N.; et al. First report of Rickettsia asembonensis in small ruminants. Vet. Res. Commun. 2022, 46, 979–983. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen, H.Q.; Stenos, J.; Nguyen, T.V.; Ng-Nguyen, D. Molecular detection of Rickettsia sp. genotype RF2125 from household dogs in the central highlands of Vietnam. Res. Vet. Sci. 2023, 163, 104989. [Google Scholar] [CrossRef]
- Salmon-Mulanovich, G.; Simons, M.P.; Flores-Mendoza, C.; Loyola, S.; Silva, M.; Kasper, M.; Rázuri, H.R.; Canal, L.E.; Leguia, M.; Bausch, D.G.; et al. Seroprevalence and risk factors for Rickettsia and Leptospira infection in four ecologically distinct regions of Peru. Am. J. Trop. Med. Hyg. 2019, 100, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- del Valle-Mendoza, J.; Vasquez-Achaya, F.; Aguilar-Luis, M.A.; Martins-Luna, J.; Bazán-Mayra, J.; Zavaleta-Gavidia, V.; Silva-Caso, W.; Carrillo-Ng, H.; Tarazona-Castro, Y.; Aquino-Ortega, R.; et al. Unidentified dengue serotypes in DENV positive samples and detection of other pathogens responsible for an acute febrile illness outbreak 2016 in Cajamarca, Peru. BMC Res. Notes 2020, 13, 467. [Google Scholar] [CrossRef] [PubMed]
- Ricapa-Antay, F.; Diaz-Melon, K.; Silva-Caso, W.; Del Valle, L.J.; Aguilar-Luis, M.A.; Vasquez-Achaya, F.; Palomares-Reyes, C.; Weilg, P.; Li, D.; Manrique, C.; et al. Molecular detection and clinical characteristics of Bartonella bacilliformis, Leptospira spp., and Rickettsia spp. in the Southeastern Peruvian Amazon basin. BMC Infect. Dis. 2018, 18, 618. [Google Scholar] [CrossRef]
- Anaya-Ramirez, E.; Palacios-Salvatierra, R.; Mosquera, P.; Alvarez, C.; Peralta, C.; Gonzales, R.; Sakuray, S. Prevalence of antibodies to rickettsiae and ehrlichiae in four border departments of Peru. Rev. Peru. Med. Exp. Salud Publica 2017, 34, 268–272. [Google Scholar]
- Kocher, C.; Jiang, J.; Morrison, A.C.; Castillo, R.; Leguia, M.; Loyola, S.; Ampuero, J.S.; Cespedes, M.; Halsey, E.S.; Bausch, D.G.; et al. Serologic evidence of scrub typhus in the peruvian Amazon. Emerg. Infect. Dis. 2017, 23, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Kocher, C.; Morrison, A.C.; Leguia, M.; Loyola, S.; Castillo, R.M.; Galvez, H.A.; Astete, H.; Flores-Mendoza, C.; Ampuero, J.S.; Bausch, D.G.; et al. Rickettsial disease in the Amazon basin. PLoS Negl. Trop. Dis. 2016, 10, e0004843. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Salvatierra, R.; Anaya-Ramirez, E.; Juscamayta-Lopez, J.; Caceres-Rey, O.; Mendoza-Uribe, L.; Mosquera-Visaloth, P.; Conceicao-Silva, F. Epidemiological and molecular profile of rickettsiosis in Peruvian border locations. Rev. Peru. Med. Exp. Salud Publica 2017, 34, 76–84. [Google Scholar] [CrossRef]
- Ammerman, N.C.; Beier-Sexton, M.; Azad, A.F. Laboratory maintenance of Rickettsia rickettsii. Curr. Protoc. Microbiol. 2008, 11, 3A-5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Stromdahl, E.Y.; Richards, A.L. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from humans in the United States. Vector Borne Zoonotic Dis. 2012, 12, 175–182. [Google Scholar] [CrossRef]
- Jiang, J.; Maina, A.N.; Knobel, D.L.; Cleaveland, S.; Laudisoit, A.; Wamburu, K.; Ogola, E.; Parola, P.; Breiman, R.F.; Njenga, M.K.; et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013, 13, 550–558. [Google Scholar] [CrossRef]
- Santibáñez, S.; Portillo, A.; Santibáñez, P.; Palomar, A.M.; Oteo, J.A. Usefulness of rickettsial PCR assays for the molecular diagnosis of human rickettsioses. Enferm. Infecc. Microbiol. Clin. 2013, 31, 283–288. [Google Scholar] [CrossRef]
- Jiang, J.; Blair, P.J.; Felices, V.; Moron, C.; Cespedes, M.; Anaya, E.; Schoeler, G.B.; Sumner, J.W.; Olson, J.G.; Richards, A.L. Phylogenetic analysis of a novel molecular isolate of spotted fever group Rickettsiae from northern Peru: Candidatus Rickettsia andeanae. Ann. N. Y Acad. Sci. 2005, 1063, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Loyola, S.; Torre, A.; Flores-Mendoza, C.; Kocher, C.; Salmon-Mulanovich, G.; Richards, A.L.; Leguia, M. Molecular characterization by multilocus sequence typing and diversity analysis of Rickettsia asembonensis in Peru. Vector Borne Zoonotic Dis. 2022, 22, 170–177. [Google Scholar] [CrossRef]
- Loyola, S.; Flores-Mendoza, C.; Torre, A.; Kocher, C.; Melendrez, M.; Luce-Fedrow, A.; Maina, A.N.; Richards, A.L.; Leguia, M. Rickettsia asembonensis characterization by multilocus sequence typing of complete genes, Peru. Emerg. Infect. Dis. 2018, 24, 931–933. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).