Genetic Diversity of Rhipicephalus (Boophilus) microplus for a Global Scenario: A Comprehensive Review
Abstract
1. Introduction
2. Diversity in the Marker Genes
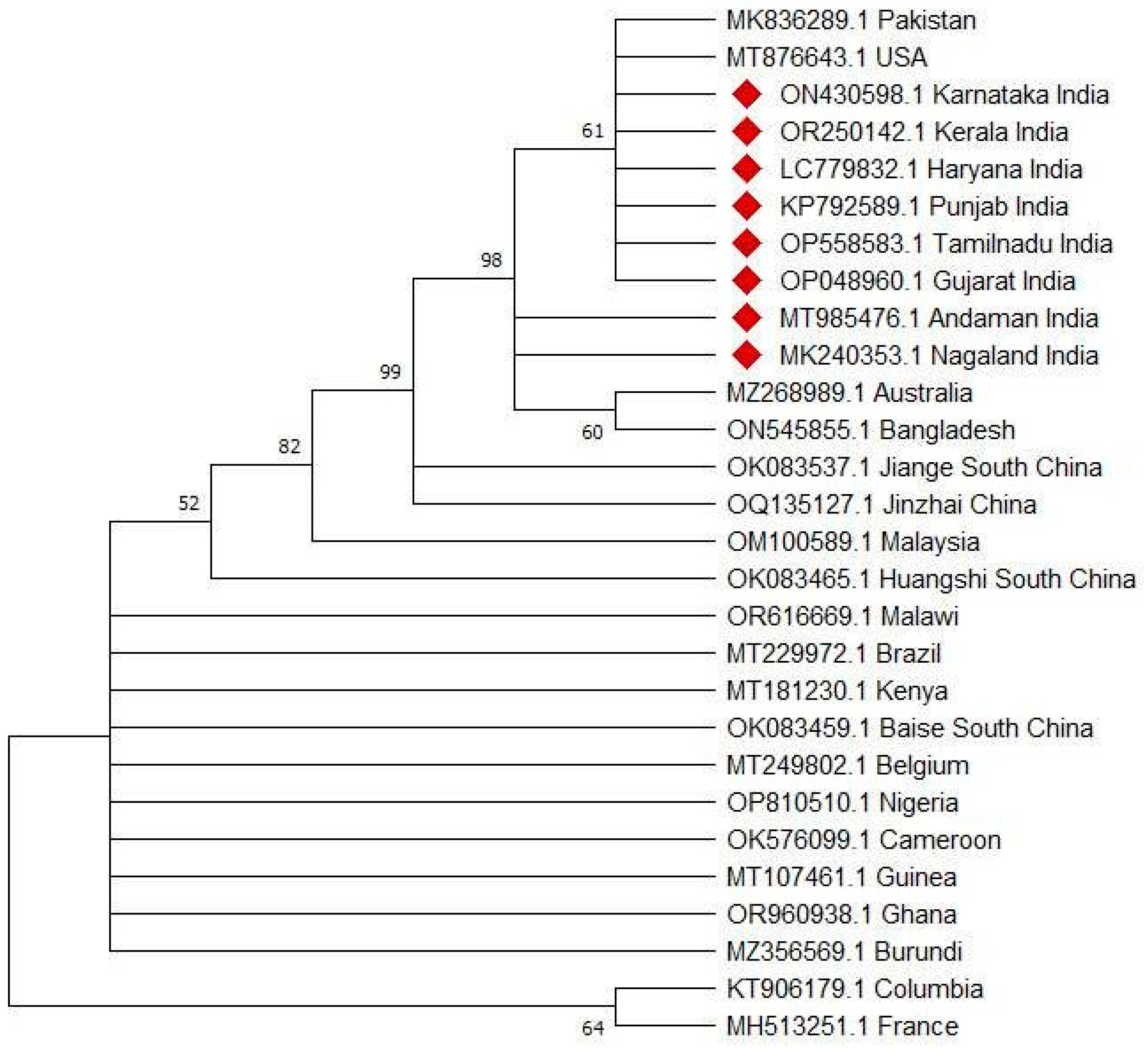
2.1. Cytochrome c Oxidase I (COX1)
2.2. Ribosomal Genes
2.3. Microsatellites
3. Diversity in Bm86 Gene
4. Diversity in Sodium Channel Genes
Sodium Channel: Synthetic Pyrethroid Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. Introduction to Conservation Genetics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Madder, M.; Thys, E.; Achi, L.; Touré, A.; De Deken, R. Rhipicephalus (Boophilus) microplus: A most successful invasive tick species in West-Africa. Exp. Appl. Acarol. 2011, 53, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.D.; Stone, N.E.; Nottingham, R.; Araya-Anchetta, A.; Lewis, J.; Hochhalter, C.; Giles, J.R.; Gruendike, J.; Freeman, J.; Buckmeier, G.; et al. Widespread movement of invasive cattle fever ticks (Rhipicephalus microplus) in southern Texas leads to shared local infestations on cattle and deer. Parasites Vectors 2014, 7, 188. [Google Scholar] [CrossRef]
- Gomes, A.F.; Neves, L. Rhipicephalus microplus (Acarina, Ixodidae) in Angola: Evidence of its establishment and expansion. Exp. Appl. Acarol. 2018, 74, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.H.; Penzhorn, B.L.; Bryson, N.R.; Stoltsz, W.H.; Masibigiri, T. Displacement of Boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province, South Africa. Exp. Appl. Acarol. 2004, 32, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Adakal, H.; Biguezoton, A.; Zoungrana, S.; Courtin, F.; de Clercq, E.M.; Madder, M. Alarming spread of the Asian cattle tick Rhipicephalus microplus in West Africa-another three countries are affected: Burkina Faso, Mali and Togo. Exp. Appl. Acarol. 2013, 61, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Nyangiwe, N.; Harrison, A.; Horak, I.G. Displacement of Rhipicephalus decoloratus by Rhipicephalus microplus (Acari: Ixodidae) in the Eastern Cape Province, South Africa. Exp. Appl. Acarol. 2013, 61, 371–382. [Google Scholar] [CrossRef]
- Haque, M.; Singh, N.K.; Rath, S.S.; Ghosh, S. Epidemiology and seasonal dynamics of ixodid ticks of dairy animals of Punjab state, India. Indian J. Anim. Sci. 2011, 81, 661. [Google Scholar]
- Etiang, P.; Atim, S.A.; Nkamwesiga, J.; Nalumenya, D. Identification and distribution of Rhipicephalus microplus in selected high-cattle density districts in Uganda: Signaling future demand for novel tick control approaches. BMC Vet. Res. 2024, 20, 119. [Google Scholar] [CrossRef] [PubMed]
- Baffi, M.A.; de Souza, G.R.L.; de Sousa, C.S.; Ceron, C.R.; Bonetti, A.M. Esterase enzymes involved in pyrethroid and organophosphate resistance in a Brazilian population of Rhipicephalus (Boophilus) microplus (Acari, Ixodidae). Mol. Biochem. Parasitol. 2008, 160, 70. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Kumar Mishra, A.; Raghavendra, R.J. Randomly amplified polymorphic DNA-polymerase chain reaction fingerprinting of Babesia bigemina strains of India. Vet. Arhiv. 2008, 78, 545–551. [Google Scholar]
- Bhat, S.A.; Singh, N.K.; Singh, H.; Rath, S.S. Molecular prevalence of Babesia bigemina in Rhipicephalus microplus ticks infesting cross-bred cattle of Punjab, India. Parasite Epidemiol. Control 2017, 2, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Soulsby, E.J. Helminths, Arthropods and Protozoa of Domesticated Animals, 7th ed.; Baillière Tindall: London, UK, 1982; 809p. [Google Scholar]
- Walker, A.R.; Bouttour, A.; Camicas, J.L.; Estrada–Peña, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003. [Google Scholar]
- Barker, S.C.; Walker, A.R. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 2014, 18, 1–144. [Google Scholar] [CrossRef] [PubMed]
- Burger, T.D.; Shao, R.; Barker, S.C. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol. Phylogenet. Evol. 2014, 76, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Low, V.L.; Tay, S.T.; Kho, K.L.; Koh, F.X.; Tan, T.K.; Lim, Y.A.; Ong, B.L.; Panchadcharam, C.; Norma-Rashid, Y.; Sofian-Azirun, M. Molecular characterisation of the tick Rhipicephalus microplus in Malaysia: New insights into the cryptic diversity and distinct genetic assemblages throughout the world. Parasites Vectors 2015, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Naranjo, V.; Mangold, A.J.; Thompson, C.; Estrada-Peña, A.; Guglielmone, A.A.; Jongejan, F.; De La Fuente, J. Allopatric speciation in ticks: Genetic and reproductive divergence between geographic strains of Rhipicephalus (Boophilus) microplus. BMC Evol. Biol. 2009, 9, 46. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Venzal, J.M.; Nava, S.; Mangold, A.; Guglielmone, A.A.; Labruna, M.B.; De La Fuente, J. Reinstatement of Rhipicephalus (Boophilus) australis (Acari: Ixodidae) with redescription of the adult and larval stages. J. Med. Entomol. 2012, 49, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Csordas, B.G.; Garcia, M.V.; Cunha, R.C.; Giachetto, P.F.; Blecha, I.M.; Andreotti, R. New insights from molecular characterization of the tick Rhipicephalus (Boophilus) microplus in Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F. Invited Review: Using genome scans of DNA polymorphism to infer adaptive population divergence. Mol. Ecol. 2005, 14, 671–688. [Google Scholar] [CrossRef]
- Baron, S.; van der Merwe, N.A.; Maritz-Olivier, C. The genetic relationship between Rhipicephalus microplus and Rhipicephalus decoloratus ticks in South Africa and their population structure. Mol. Phylogenet. Evol. 2018, 129, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.L.; Wu, W.J.; Shih, C.M. Species identification of Ixodes granulatus (Acari: Ixodidae) based on internal transcribed spacer 2 (ITS2) sequences. Exp. Appl. Acarol. 2011, 54, 51–63. [Google Scholar] [CrossRef]
- Chitimia, L.; Lin, R.Q.; Cosoroaba, I.; Wu, X.Y.; Song, H.Q.; Yuan, Z.G.; Zhu, X.Q. Genetic characterization of ticks from southwestern Romania by sequences of mitochondrial cox1 and nad5 genes. Exp. Appl. Acarol. 2010, 52, 305–311. [Google Scholar] [CrossRef]
- Li, L.H.; Zhang, Y.; Wang, J.Z.; Li, X.S.; Yin, S.Q.; Zhu, D.; Xue, J.B.; Li, S.G. High genetic diversity in hard ticks from a China-Myanmar border county. Parasites Vectors 2018, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.C.; Estrada-Peña, A.; Krücken, J.; Rehman, A.; Nijhof, A.M. Morphological and phylogenetic analyses of Rhipicephalus microplus ticks from Bangladesh, Pakistan and Myanmar. Ticks Tick Borne Dis. 2018, 9, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Kanduma, E.G.; Emery, D.; Githaka, N.W.; Nguu, E.K.; Bishop, R.P.; Šlapeta, J. Molecular evidence confirms occurrence of Rhipicephalus microplus Clade A in Kenya and sub-Saharan Africa. Parasites Vectors 2020, 13, 432. [Google Scholar] [CrossRef]
- Zeb, J.; Shams, S.; Ayaz, S.; Israr, U.D.; Khan, A.; Adil, N.; Ullah, H.; Raza, A. Epidemiology of ticks and molecular characterization of Rhipicephalus microplus in cattle population in North-Western Pakistan. Int. J. Acarol. 2020, 46, 335–343. [Google Scholar] [CrossRef]
- Guzman, P.E.; Fernández Cuétara, C.; Cano Argüelles, A.L.; Fuentes Castillo, A.; García Martínez, Y.; Rodríguez Fernández, R.; Fernández Afonso, Y.; Bello Soto, Y.; González Alfaro, Y.; Méndez, L.; et al. Characterization of two Cuban colonies of Rhipicephalus microplus ticks. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100591. [Google Scholar] [CrossRef]
- De, A.K.; Bhattacharya, D.; Sawhney, S.; Bala, P.; Sunder, J.; Sujatha, T.; Ponraj, P.; Chakurkar, E.B. Molecular characterization of Rhipicephalus microplus in Andaman and Nicobar Islands, India: An insight into genetic assemblages. J. Genet. 2022, 101, 46. [Google Scholar] [CrossRef]
- Yousseu, F.; Simo Tchetgna, H.; Kamgang, B.; Djonabaye, D.; McCall, P.J.; Ndip, R.N.; Wondji, C.S. Infestation rates, seasonal distribution, and genetic diversity of ixodid ticks from livestock of various origins in two markets of Yaoundé, Cameroon. Med. Vet. Entomol. 2022, 36, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, A.D.; Nehra, A.K.; Vohra, S. Phylogeography and demographic dynamics of Rhipicephalus microplus from North India. Infect. Genet. Evol. 2023, 112, 105464. [Google Scholar] [CrossRef]
- Zheng, Z.; Zeng, W.; Wang, S.; Tan, W.; Lu, X.; Kairullayev, K.; Mi, L.; Hazihan, W.; Liu, G.; Yang, M.; et al. Application of DNA barcodes in the genetic diversity of hard ticks (Acari: Ixodidae) in Kazakhstan. Exp. Appl. Acarol. 2024, 92, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Gaut, B.S. Evolution of genes and taxa: A primer. Plant Mol. Biol. 2000, 42, 1–6. [Google Scholar] [CrossRef]
- Cruickshank, R.H. Molecular markers for the phylogenetics of mites and ticks. Syst. Appl. Acarol. 2002, 7, 3–14. [Google Scholar] [CrossRef]
- Tabor, A.E. A Review of Australian Tick Vaccine Research. Vaccines 2021, 16, 1030. [Google Scholar] [CrossRef] [PubMed]
- Bishop, L.J.; Stutzer, C.; Maritz-Olivier, C. More than Three Decades of Bm86: What We Know and Where to Go. Pathogens 2023, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, B.C.; Kumar, B.; Nagar, G.; Manjunathachar, H.V.; de la Fuente, J.; Ghosh, S. Analysis of genetic diversity in Indian strains of Rhipicephalus microplus based on bm86 gene sequence. Vaccines 2021, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Kaewmongkol, S.; Kaewmongkol, N.G.; Inthong, N.; Lakkitjaroen, T.; Sirinarumitr, C.M.; Berry, N.N.; Jonsson, R.W.; Stich, S.; Jittapalapong, S. Variation among Bm86 sequences in Rhipicephalus (Boophilus) microplus ticks collected from cattle across Thailand. Exp. Appl. Acarol. 2015, 66, 247–256. [Google Scholar] [CrossRef] [PubMed]
- García-García, J.C.; Gonzalez, I.L.; González, D.M.; Valdés, M.; Méndez, L.; Lamberti, J.; D’Agostino, B.; Citroni, D.; Fragoso, H.; Ortiz, M.; et al. Sequence variations in the Boophilus microplus Bm86 locus and implications for immunoprotection in cattle vaccinated with this antigen. Exp. Appl. Acarol. 1999, 23, 883–895. [Google Scholar] [CrossRef]
- Kumar, B.; Murugan, K.; Ray, D.D.; Ghosh, S. Efficacy of rBm86 against Rhipicephalus (Boophilus) microplus (IVRI-I line) and Hyalomma anatolicum anatolicum (IVRI-II line) infestations on bovine calves. Parasitol. Res. 2012, 111, 629–635. [Google Scholar] [CrossRef]
- Johnson, N. Controlling ticks and tick borne disease transmission. In Ticks Biology, Ecology, and Diseases; Academic Press-Elsevier: Cambridge, MA, USA, 2023; pp. 193–215. [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Black, W.C., 4th; Klompen, J.S.; Keirans, J.E. Phylogenetic relationships among tick subfamilies (Ixodida: Ixodidae: Argasidae) based on the 18S nuclear rDNA gene. Mol. Phylogenet. Evol. 1997, 7, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.J.; Bargues, M.D.; Mas-Coma, S. 18S rRNA gene sequences and phylogenetic relationships of European hard-tick species (Acari: Ixodidae). Parasitol. Res. 1998, 84, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zahler, M.; Filippova, N.A.; Morel, P.C.; Gothe, R.; Rinder, H. Relationships between species of the Rhipicephalus sanguineus group: A molecular approach. J. Parasitol. 1997, 83, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.C. Distinguishing species and populations of Rhipicephaline ticks with ITS 2 ribosomal RNA. J. Parasitol. 1998, 84, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Bello Soto, Y.; Rodríguez-Mallon, A. Genetic and biological characterization of a Cuban tick strain from Rhipicephalus sanguineus complex and its sensitivity to different chemical acaricides. Int. J. Acarol. 2016, 42, 18–25. [Google Scholar] [CrossRef]
- Rooman, M.; Assad, Y.; Tabassum, S.; Sultan, S.; Ayaz, S.; Khan, M.F.; Khan, S.N.; Ali, R. A cross-sectional survey of hard ticks and molecular characterization of Rhipicephalus microplus parasitizing domestic animals of Khyber Pakhtunkhwa, Pakistan. PLoS ONE 2021, 16, e0255138. [Google Scholar] [CrossRef] [PubMed]
- Dobson, S.J.; Barker, S.C. Phylogeny of the hard ticks (Ixodidae) inferred from 18S rRNA indicates that the genus Aponomma is paraphyletic. Mol. Phylogenet. Evol. 1999, 11, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.E.; Klompen, J.S.; Black, W.C. Comparison of the mitochondrial 12S and 16S ribosomal DNA genes in resolving phylogenetic relationships among hard ticks (Acari: Ixodidae). Ann. Entomol. Soc. Am. 1999, 92, 117–129. [Google Scholar] [CrossRef]
- Black, W.C., 4th; Piesman, J. Phylogeny of hard-and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef] [PubMed]
- Murrell, A.; Campbell, N.J.; Barker, S.C. Mitochondrial 12S rDNA indicates that the Rhipicephalinae (Acari: Ixodida: Ixodidae) is paraphyletic. Mol. Phylogenet. Evol. 1999, 12, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Beati, L.; Keirans, J.E. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J. Parasitol. 2001, 87, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Gemayel, R.; Vinces, M.D.; Legendre, M.; Verstrepen, K.J. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu. Rev. Genet. 2010, 44, 445–477. [Google Scholar] [CrossRef] [PubMed]
- Kanduma, E.G.; Mwacharo, J.M.; Sunter, J.D.; Nzuki, I.; Mwaura, S.; Kinyanjui, P.W.; Kibe, M.; Heyne, H.; Hanotte, O.; Skilton, R.A.; et al. Micro- and minisatellite-expressed sequence tag (EST) markers discriminate between populations of Rhipicephalus appendiculatus. Ticks Tick Borne Dis. 2012, 3, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.R.; Burke, J.M. EST-SSRs as a resource for population genetic analyses. Heredity 2007, 99, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chigagure, N.N.; Baxter, G.D.; Barker, S.C. Microsatellite loci of the cattle tick Boophilus microplus (Acari: Ixodidae). Exp. Appl. Acarol. 2000, 24, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Koffi, B.B.; Risterucci, A.M.; Joulia, D.; Durand, P.; Barre, N.; de Meeûs, T.; Chevillon, C. Characterization of polymorphic microsatellite loci within a young Boophilus microplus metapopulation. Mol. Ecol. Notes 2006, 6, 502–504. [Google Scholar] [CrossRef]
- Sungirai, M.; Baron, S.; Van der Merwe, N.A.; Moyo, D.Z.; De Clercq, P.; Maritz-Olivier, C.; Madder, M. Population structure and genetic diversity of Rhipicephalus microplus in Zimbabwe. Acta. Trop. 2018, 180, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Oberholster, T. Characterisation of the Genetic Diversity of the Southern Cattle Tick, Rhipicephalus microplus, Populations from South Africa. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2014. Available online: http://hdl.handle.net/2263/43208 (accessed on 13 June 2024).
- de la Fuente, J.; Rodriguez, M.; Garcia-Garcia, J.C. Immunological control of ticks through vaccination with Boophilus microplus gut antigens. Ann. N. Y. Acad. Sci. 2000, 916, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Rajput, Z.I.; Hu, S.H.; Chen, W.J.; Arijo, A.G.; Xiao, C.W. Importance of ticks and their chemical and immunological control in livestock. J. Zhejiang Univ. Sci. B 2006, 7, 912–921. [Google Scholar] [CrossRef]
- Rodriguez-Vivas, R.I.; Trees, A.J.; Rosado-Aguilar, J.A.; Villegas-Perez, S.L.; Hodgkinson, J.E. Evolution of acaricide resistance: Phenotypic and genotypic changes in field populations of Rhipicephalus (Boophilus) microplus in response to pyrethroid selection pressure. Int. J. Parasitol. 2011, 41, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Robbertse, L.; Baron, S.; van der Merwe, N.A.; Madder, M.; Stoltsz, W.H.; Maritz-Olivier, C. Genetic diversity, acaricide resistance status and evolutionary potential of a Rhipicephalus microplus population from a disease-controlled cattle farming area in South Africa. Ticks Tick Borne Dis. 2016, 7, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Chevillon, C.; de Garine-Wichatitsky, M.; Barre, N. Understanding the genetic, demographical and/or ecological processes at play in invasions: Lessons from the southern cattle tick Rhipicephalus microplus (Acari: Ixodidae). Exp. Appl. Acarol. 2013, 59, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, F.D.; Lovis, L.; Martins, J.R. Acaricide Resistance Mechanisms in Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Vet. 2012, 21, 1–6. [Google Scholar] [CrossRef]
- Coles, T.B.; Dryden, M.W. Insecticide/acaricide resistance in fleas and ticks infesting dogs and cats. Parasites Vectors 2014, 7, 8. [Google Scholar] [CrossRef]
- Wakeling, E.N.; Neal, A.P.; Atchison, W.D. Pyrethroids and Their Effects on Ion Channels. In Pesticides—Advances in Chemical and Botanical Pesticides; Arcler Education Incorporated: Burlington, ON, Canada; In Tech: London, UK, 2012. [Google Scholar] [CrossRef][Green Version]
- Vatsya, S.; Yadav, C.L. Evaluation of acaricide resistance mechanisms in populations of Rhipicephalus (Boophilus) microplus collected from India. Int. J. Acarol. 2011, 37, 405–410. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, R.; Kumar, S.; Nagar, G.; Singh, N.K.; Rawat, S.S.; Dhakad, M.L.; Rawat, A.K.S.; Raya, D.D.; Ghosh, S. Deltamethrin and cypermethrin resistance status of Rhipicephalus (Boophilus) microplus collected from six agro-climatic regions of India. Vet. Parasitol. 2012, 188, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nagar, G.; Sharma, A.K.; Kumar, S.; Ray, D.D.; Chaudhuri, P.; Ghosh, S. Survey of pyrethroids resistance in Indian strains of Rhipicephalus (Boophilus) microplus: Identification of C190A mutation in the domain II of the para-sodium channel gene. Acta. Trop. 2013, 125, 237–245. [Google Scholar] [CrossRef]
- Godara, R.; Katoch, R.; Rafiqi, S.I.; Yadav, A.; Nazim, K.; Sharma, R.; Singh, N.K.; Katoch, M. Synthetic Pyrethroid Resistance in Rhipicephalus (Boophilus) microplus ticks from North-Western Himalayas, India. Trop. Anim. Health Prod. 2019, 51, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Davey, R.B.; George, J.E. Characterization of Pyrethroid Resistance and Susceptibility to Coumaphos in Mexican Boophilus microplus (Acari: Ixodidae). J. Med. Entomol. 1999, 36, 533–538. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, A.C.; Davey, R.B.; Ivie, G.W.; George, J.E. Identification of a point mutation in the para-type sodium channel gene from a pyrethroid-resistant cattle tick. Biochem. Biophys. Res. Commun. 1999, 261, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.; Corley, S.W.; Jackson, L.A.; Lew-Tabor, A.E.; Moolhuijzen, P.M.; Jonsson, N.N. Identification of a mutation in the para-sodium channel gene of the cattle tick Rhipicephalus (Boophilus) microplus associated with resistance to synthetic pyrethroid acaricides. Int. J. Parasitol. 2009, 39, 775–779. [Google Scholar] [CrossRef]
- Jonsson, N.N.; Cutullè, C.; Corley, S.W.; Seddon, J.M. Identification of a mutation in the para-sodium channel gene of the cattle tick Rhipicephalus microplus associated with resistance to flumethrin but not to cypermethrin. Int. J. Parasitol. 2010, 40, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Klafke, G.M.; Miller, R.J.; Tidwell, J.P.; Thomas, D.B.; Sanchez, D.; Feria Arroyo, T.P.; Pérez de León, A.A. High-resolution melt (HRM) analysis for detection of SNPs associated with pyrethroid resistance in the southern cattle fever tick, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 100–111. [Google Scholar] [CrossRef]
- Stone, N.E.; Olafson, P.U.; Davey, R.B.; Buckmeier, G.; Bodine, D.; Sidak-Loftis, L.C.; Giles, J.R.; Duhaime, R.; Miller, R.J.; Mosqueda, J.; et al. Multiple mutations in the para-sodium channel gene are associated with pyrethroid resistance in Rhipicephalus microplus from the United States and Mexico. Parasites Vectors 2014, 7, 456. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Klafke, G.M.; Miller, R.J. Voltage-gated sodium channel gene mutations and pyrethroid resistance in Rhipicephalus microplus. Ticks Tick Borne Dis. 2020, 11, 101404. [Google Scholar] [CrossRef] [PubMed]
- Nagar, G.; Sharma, A.K.; Kumar, S.; Saravanan, B.C.; Kumar, R.; Gupta, S.; Kumar, S.; Ghosh, S. Molecular mechanism of synthetic pyrethroid and organophosphate resistance in field strains of Rhipicephalus microplus tick collected from a northern state of India. Exp. Appl. Acarol. 2018, 75, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, D.; Kumar, B.; Kumar, S.; Sharma, A.K.; Fular, A.; Bisht, N.; Srivastava, S.; Boruah, R.R.; Nagar, G.; Shakya, M.; et al. Characterization of acaricide resistance in Rhipicephalus microplus populations infesting cattle in northeastern India and assessment of local plant extracts for tick management. Vet. Parasitol. 2020, 277, 109011. [Google Scholar] [CrossRef]
- Bisht, N.; Kumar, S.; Sharma, A.K.; Nandi, A.; Singh, K.; Fular, A.; Nagar, G.; Ghosh, S. Comparative susceptibility of Rhipicephalus microplus collected from the northern state of India to coumaphos, malathion, deltamethrin, ivermectin, and fipronil. Trop. Anim. Health Prod. 2021, 53, 460. [Google Scholar] [CrossRef] [PubMed]
- Fular, A.; Sharma, A.K.; Upadhaya, D.; Nandi, A.; Nagar, G.; Bisht, N.; Shakya, M.; Kumar, S.; Kumar, S.; Kumar, R.; et al. Evaluation of acaricidal resistance status of Rhipicephalus microplus ticks from the hilly state (Uttarakhand) of India and evaluation of efficacy of a natural formulation for the management of resistant ticks. Exp. Appl. Acarol. 2021, 85, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kumar, R.; Nagar, G.; Kumar, S.; Sharma, A.K.; Srivastava, A.; Kumar, S.; Ajith Kumar, K.G.; Saravanan, B.C. Survey of acaricides resistance status of Rhipiciphalus (Boophilus) microplus collected from selected places of Bihar, an eastern state of India. Ticks Tick Borne Dis. 2015, 6, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A.K.; Nagar, G.; Rawat, S.S.; Tiwari, S.S.; Kumar, R.; Dhakad, M.L.; Sharma, R.K.; Saxana, R.K.; Mehraniya, R.S.; et al. Characterization of acaricide resistance in tick strains collected from Rajasthan, India. Indian J. Anim. Sci. 2016, 86, 14–23. [Google Scholar] [CrossRef]
- Rosario-Cruz, R.; Guerrero, F.D.; Miller, R.J.; Rodriguez-Vivas, R.I.; Tijerina, M.; Dominguez-Garcia, D.I.; Hernandez-Ortiz, R.; Cornel, A.J.; McAbee, R.D.; Alonso-Diaz, M.A. Molecular survey of pyrethroid resistance mechanisms in Mexican field populations of Rhipicephalus (Boophilus) microplus. Parasitol. Res. 2009, 105, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Davey, R.B.; George, J.E. First report of permethrin-resistant Boophilus microplus (Acari: Ixodidae) collected within the United States. J. Med. Entomol. 2007, 44, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; He, H.; Temeyer, K.B.; Jones, S.; Green, P.; Barker, S.C. A survey of Rhipicephalus microplus populations for mutations associated with pyrethroid resistance. J. Econ. Entomol. 2009, 102, 373–380. [Google Scholar] [CrossRef]
- Rodriguez-Vivas, R.I.; Alonso-Díaz, M.A.; Rodríguez-Arevalo, F.; Fragoso-Sanchez, H.; Santaaria, V.M.; Rosario-Cruz, R. Prevalence and potential risk factors for organohosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the state of Yucatan, Mexico. Vet. Parasitol. 2006, 136, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lovis, L.; Guerrero, F.D.; Miller, R.J.; Bodine, D.M.; Betschart, B.; Sager, H. Distribution patterns of three sodium channel mutations associated with pyrethroid resistance in Rhipicephalus (Boophilus) microplus populations from North and South America, South Africa and Australia. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.C.; Duarte, F.C.; Martins, J.R.; Klafke, G.M.; Fiorini, L.C.; de Barros, A.T. Characterization of the pyrethroid resistance profile of Rhipicephalus (Boophilus) microplus populations from the states of Rio Grande do Sul and Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2013, 22, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, R.; Guerrero, F.D.; Soares, M.A.; Barros, J.C.; Miller, R.J.; Pérez de León, A. Acaricide resistance of Rhipicephalus (Boophilus) microplus in State of Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2011, 20, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Domingues, L.N.; Brasil, B.D.S.A.F.; de Paiva Bello, A.C.P.; da Cunha, A.P.; de Barros, A.T.M.; Leite, R.C.; Silaghi, C.; Pfister, K.; Friche Passos, L.M. Survey of Pyrethroid and Organophosphate Resistance in Brazilian Field Populations of Rhipicephalus (Boophilus) microplus: Detection of C190A Mutation in Domain II of the Para-Type Sodium Channel Gene. Vet. Parasitol. 2012, 189, 327–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cruz-Valdés, T.; Grostieta, E.; Chagoya-Fuentes, J.L.; Bravo-Ramos, J.L.; Ojeda-Chi, M.; Lammoglia-Villagómez, M.A.; Rojas-Ronquillo, R.; Cabrera-Núñez, A.; Aguilar-Tipacamú, G.; Colunga-Salas, P.; et al. Identification of the G184C, C190A and T2134A mutations in the para-sodium channel gene of the southern cattle tick Rhipicephalus (Boophilus) microplus associated with resistance to cypermethrin in northern Veracruz, Mexico. Vet. Parasitol. Reg. Stud. Rep. 2023, 39, 100838. [Google Scholar] [CrossRef] [PubMed]
- Wyk, R.D.j.; Baron, S.; Maritz-Olivier, C. An integrative approach to understanding pyrethroid resistance in Rhipicephalus microplus and R. decoloratus ticks. Ticks Tick Borne Dis. 2016, 7, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Yessinou, R.E.; Akpo, Y.; Sidick, A.; Adoligbe, C.; Youssao Abdou Karim, I.; Akogbeto, M.; Farougou, S. Evidence of multiple mechanisms of alpha-cypermethrin and deltamethin resistance in ticks Rhipicephalus microplus in Benin, West Africa. Ticks Tick Borne Dis. 2018, 9, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Villar, D.; Klafke, G.M.; Rodríguez-Durán, A.; Bossio, F.; Miller, R.; Pérez de León, A.A.; Cortés-Vecino, J.A.; Chaparro-Gutiérrez, J.J. Resistance profile and molecular characterization of pyrethroid resistance in a Rhipicephalus microplus strain from Colombia. Med. Vet. Entomol. 2020, 34, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bandara, K.J.; Karunaratne, S.P. Mechanisms of acaricide resistance in the cattle tick Rhipicephalus (Boophilus) microplus in Sri Lanka. Pestic. Biochem. Physiol. 2017, 139, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rivera, E.; Holguín, C.; Daniel, A.U.M. New polymorphism in the sodium channel gene of Rhipicephalus microplus tick (Ixodida: Ixodidae) resistant to pyrethroids. Rev. Biol. Trop. 2019, 67, 935–944. [Google Scholar] [CrossRef]
| State | Strains | Amino Acid Changes with Respect to IVRI-I Bm86 Conserved Sequence |
|---|---|---|
| Assam | Nagaon | N442D, I590V, K595N |
| Barpeta | N442D, A499T, D500N, G562D, H563R, I590V, K595N | |
| Kamrup | N442D, D500N, G562D, E568Q, K595N, A614P | |
| Sonitpur | N442D, A499T, D500N, G562Y, H563R, R567G, I590V, K595N | |
| Morigaon | N442D, A499T, D500N, G562D, I590V | |
| Dibrugarh | N442D, A499T, D500N, G562D, I590V, K595N | |
| Rajasthan | Alwar | N442D, A499T, D500N, E508K, G562D, H563R, R567G, I590V, K595N |
| Sikar | N442D, A499T, D500N, G562D, H563R, R567G, I590V, K595N | |
| Jaipur | N442D, A499T, D500N, G562D, H563R, R567G, I590V, K595N | |
| Chittorgarh | N442D, A499T, D500N, K521R, G562D, H563R, S566F, I590V, K595N | |
| Pratapgarh | N442D, D500N, G562D, K595N, A614P | |
| Bharatpur | N442D, D500N, G562D, K595N, A614P | |
| Banswara | N442D, D500N, G562D, K595N, A614P | |
| Bhilwara | N442D, A499T, D500N, D519G, G562D, H563R, I590V, K595N | |
| Churu | N442D, A499T, D500N, G562D, H563R, R567G, I590V, N593D, K595N | |
| Dausa | N442D, A499T, D500S, K521R, K554I, G562D, R567G, I590V, E603D, A614S | |
| Udaipur | N442D, N459F, A499T, D500N, K86R, G562D, H563R, S566F, I590V, K595N | |
| Dungarpur | N442D, L459F, A499T, D500N, D84G, K86R, G562D, S566F, K595N | |
| Maharashtra | Jalgaon | N442D, L459F, A499T, D500N, E89G, G562D, H563R, S566F, I590V, K595N |
| Nashik | N442D, D500N, G562D, E133Q, K595N, A614P | |
| Dhule | N442D, A499T, D500N, G562D, I590V, K595N, A614P | |
| Ahmednagar | N442D, A499T, D500N, E524G, G562D, E568Q, K595N, A614P | |
| Raigad | N442D, C464R, D500N, G562D, H563R, R567G, I590V, K595N | |
| Pune | N442D, A499T, D500N, G562D, H563R, S566F, I590V, K595N, K601T, D618N | |
| Aurangabad | N442D, D500N, E508K, G562Y, H563R, R567G, I590V, K595N | |
| Satara | N442D, A499T, D500N, G562Y, H563R, R567G, I590V, K601T, D616N | |
| Solapur | N442D, A499T, D500N, G562Y, H563R, R567G, I590V, K601T, D616N | |
| Madhya Pradesh | Khandwa | N442D |
| Shajapur | N442D, A499T, D500N, G562D, H563R, R567G, I590V, K595N | |
| Barwani | N442D, A499T, D500N, G562D, H563R, S566F, R567G, I590V, K595N | |
| Mandsaur | N442D, A499T, D500N, G562D, H563R, R567G, I590V, K595N | |
| Indore | N442D, A499T, D500N, G562D, H563R, R567G, I590V, K595N | |
| Ujjain | N442D, A499T, D500N, G562D, H563R, R567G, I590V, K595N | |
| Uttar Pradesh | Pilibhit | N442D, D500N, D618N |
| Raebareli | N7D, D500N, G562D, H563R, S566F, I590V, K595N, E166T | |
| Lucknow | N442D, D500N, G562D, H563R, S566F, I590V, K595N, E166T | |
| Haryana | Panipat | N442D, A499T, D500N, G562D, H563R, R567G, S575G, I590V, K595N |
| Sonipat | N442D, F460I, S482F, A499D, D500N, G562D, R567G, S575G, I590V, K595N | |
| Kurukshetra | N442D, D500N, G562D, H563R, R567G, S575G, I590V, K595N | |
| Yamuna Nagar | N442D | |
| Kaithal | N442D, F460Y, G467D | |
| Ambala | N442D, A499T, D500N, E508K, G562D, H563R, R567E, D578G, I590V, K595N, V597A | |
| Karnal | N442D, A499T, D500N, G562D, H563R, R567G, I590V, K595N | |
| Hisar | N442D, A499T, D500N, G562D, H563R, S566F, I590V, K595N | |
| Fatehabad | N442D, T469K, E473G, A608S | |
| Bhiwani | N442D, A499T, D500N, G562D, H563R, I590V, K595N | |
| Rohtak | N442D, A499T, D500N | |
| Uttarakhand | Mukteswar | N442D, A499T, D500N, G562Y, H563R, R567G, E577V, I590V, K595N |
| Haridwar | N442D, A499T, D500N, G562Y, H563R, R567G, E577V, I590V, K595N | |
| New Tehri | N442D, A499T, D500N, G562Y, H563R, R567G, E577V, I590V, K595N, K601T | |
| Uttarkashi | N442D, A499T, D500N, E508K, G562Y, H563R, R567G, E577V, I590V, K595N | |
| Dehradun | N442D, A499T, D500N, G562Y, H563R, R567G, E568Q, I590V, K595N | |
| Almora | N442D, A499T, D500N, G562Y, H563R, R567G, E577V, I590V, K595N, K601T | |
| Gujarat | Ahmedabad | N442D, V483M, A499T, D500N, G562D, H563R, I590V, K595N, D616N |
| Junagadh | N442D, V483M, A499T, D500N, G562D, H563R, R567G, I590V, K595N, D616N | |
| Porbandar | N442D, V483M, A499T, D500N, G562D, H563R, I590V, K595N, D616N | |
| Jamnagar | N442D, V483M, A499T, D500N, K86R, G12D, H563R, I590V, K595N, D616N | |
| Somnath | N442D, V483M, A499T, D500N, G562D, H563R, I590V, K595N, D616N | |
| Bhavnagar | N442D, V483M, A499T, D500N, E508K, G562D, H563R, I590V, K595N, D616N | |
| Anand | N442D, V483M, A499T, D500N, G562D, H563R, I590V, K595N, D616N | |
| Punjab | Muktsar | N442D, A499T, D500N, I590V |
| Firozpur | N442D, A499T, D500N, K595N | |
| Ludhiana | N442D, A499T, D500N, H128D | |
| Mansa | N442D, A499T, D500N, I590V | |
| Moga | N442D, A499T, D500N, G562D |
| Location | Position of Nucleotide Substitution | Position of Amino Acid Substitution | Country | References |
|---|---|---|---|---|
| Domain III S6 | T2134A | F1550I | Mexico and USA | [75,76,80,88,89,90,91,92] |
| Domain II S4-5 linker | C190A | L64I | Australia | [77,78,90,92] |
| Brazil | [92,93,94,95,96] | |||
| Mexico | [80] | |||
| USA | [92,97] | |||
| Africa | [98] | |||
| Argentina | [92] | |||
| Columbia | [99] | |||
| India | [73,81,82,86,87] | |||
| Domain II S4-5 linker | G215T | G72V | Australia | [78,92] |
| Sri Lanka | [100] | |||
| Mexico | [79] | |||
| Domain II S4-5 linker | T170C | M57T | Mexico | [79,80] * |
| USA | [80] | |||
| Colombia | [99] | |||
| Domain III S6 | T2134C | F1550L | Colombia | [99,101] * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sankar, M.; Kumar, B.; Manjunathachar, H.V.; Parthasarathi, B.C.; Nandi, A.; Neethu, C.K.S.; Nagar, G.; Ghosh, S. Genetic Diversity of Rhipicephalus (Boophilus) microplus for a Global Scenario: A Comprehensive Review. Pathogens 2024, 13, 516. https://doi.org/10.3390/pathogens13060516
Sankar M, Kumar B, Manjunathachar HV, Parthasarathi BC, Nandi A, Neethu CKS, Nagar G, Ghosh S. Genetic Diversity of Rhipicephalus (Boophilus) microplus for a Global Scenario: A Comprehensive Review. Pathogens. 2024; 13(6):516. https://doi.org/10.3390/pathogens13060516
Chicago/Turabian StyleSankar, Muthu, Binod Kumar, Haranahally Vasanthachar Manjunathachar, Balasamudram Chandrasekhar Parthasarathi, Abhijit Nandi, Chemmangat Kunnath Subramanian Neethu, Gaurav Nagar, and Srikant Ghosh. 2024. "Genetic Diversity of Rhipicephalus (Boophilus) microplus for a Global Scenario: A Comprehensive Review" Pathogens 13, no. 6: 516. https://doi.org/10.3390/pathogens13060516
APA StyleSankar, M., Kumar, B., Manjunathachar, H. V., Parthasarathi, B. C., Nandi, A., Neethu, C. K. S., Nagar, G., & Ghosh, S. (2024). Genetic Diversity of Rhipicephalus (Boophilus) microplus for a Global Scenario: A Comprehensive Review. Pathogens, 13(6), 516. https://doi.org/10.3390/pathogens13060516








